- 1The Japan Drug Information Institute in Pregnancy, National Center for Child Health and Development, Tokyo, Japan
- 2Department of Pharmacy, National Center for Child Health and Development, Tokyo, Japan
- 3Medical Affairs Department, Maruho Co., Ltd, Osaka, Japan
Background: Recurrent herpes simplex (RHS) is a disease caused by reactivation of the herpes simplex virus. Patients with RHS are treated with anti-herpes virus medication. Amenamevir is one such medication used for RHS in Japan and is administered as patient-initiated therapy (PIT); this involves initiation at the discretion of the patient, based on early symptoms. However, there are insufficient data on the transfer of amenamevir into breast milk among breastfeeding patients with RHS.
Objectives: This study aims to assess the transfer of amenamevir into breast milk and evaluate the infant's risk of drug exposure.
Methods: This study is a single-arm, open-label, interventional multicenter study. Patients who experience RHS during breastfeeding will be recruited and treated with amenamevir. The concentration of amenamevir in breast milk will be determined by liquid chromatography-mass spectrometry. The primary outcome is relative infant dose (RID) calculated by Cave[AUC(0–24 h)]. The secondary outcome is RID calculated by Cmax and Cave[AUC(0–48 h)].
Discussion: This study will provide evidence of the transfer profile of amenamevir into breast milk during PIT. If the RID of amenamevir is less than 10%, such therapy is generally considered to be safe, and use of PIT with amenamevir may consequently lead to a new standard therapy for breastfeeding patients.
1 Introduction
Herpes simplex is a disease caused by primary infection or reactivation of the herpes simplex virus (HSV), which causes blisters or erosive lesions on the skin and mucous membranes (1). The disease occurs most commonly in individuals in their twenties, and the prevalence of HSV in adults is estimated at about 50% (2).
HSV is classified into HSV-1 and HSV-2, with latency in the trigeminal nerve or a dorsal root ganglion after primary infection. HSV-1 recurs mainly on the face, particularly around the lips, and HSV-2 recurs mainly on the lower body, particularly around the genitals (1). The frequency of recurrence is generally reported to be higher with HSV-2 than HSV-1, although some patients will get monthly recurrences, while others will experience this once every few years. In women, there may be a tendency for the condition to recur more easily due to the fact that progesterone secreted in the latter half of the menstrual cycle has an immunosuppressive effect (3). This is because stress caused by premenstrual syndrome disrupts the autonomic nervous system (4), and because the immune system is weakened during pregnancy, so that the mother's immune system does not recognize the fetus as a “foreign body” (5).
In Japan, the oral antiviral drugs acyclovir, valacyclovir, famciclovir, and amenamevir are clinically used for the treatment of recurrent herpes simplex (RHS). As of February 2025, acyclovir and valacyclovir are listed as safe for use during breastfeeding on a website (supervised by the US National Institutes of Health) that provides expert information on the use of drugs during breastfeeding (6). This is based on a number of recent medical studies.
In Japan, amenamevir received marketing approval in 2017 for the indication of herpes zoster (7, 8) and in February 2023 for the treatment of RHS, with approval of patient-initiated therapy (PIT), where medication can be initiated at the patient's discretion, based on early symptoms (9). Approximately 80% of patients with RHS can recognize early symptoms (tingling, itching, etc.) before skin lesions appear. PIT facilitates the suppression of viral replication from an early stage by exposing the virus to high concentrations of the medication soon after symptom onset, a strategy referred to as “early short-term treatment”. However, in Japan, only famciclovir (approved in 2019) and amenamevir are approved for PIT (9, 10).
The oral treatment of herpes simplex can be expected to work if administered as soon as possible after onset of disease symptoms. There is also a guideline stating that a sufficient clinical effect cannot be obtained if administration is not started within a day after rash onset (11). Acyclovir and valacyclovir, while safe for nursing mothers, are not approved for PIT. In some cases, it is difficult for breastfeeding women to visit to a medical institution immediately after rash onset, as bringing the infant can be challenging, and making childcare arrangements at short notice is often not a realistic option. In other cases, the patient suffers from significant stress because of the risk of infecting the infant. In this respect, PIT would be a particularly convenient treatment for breastfeeding women as they can take pre-prescribed medication as soon as they recognize early symptoms. The transfer of amenamevir into breastmilk was reported in mice (12), and we thus cannot currently recommend its use in breastfeeding women due to insufficient data regarding transfer into breast milk (13). Therefore, when patients who develop RHS during breastfeeding are taking amenamevir for PIT, treatment benefits and the benefits for the infant of continued breastfeeding must both be considered.
This study aims to evaluate the transfer of amenamevir into breast milk by administering a single dose of 1,200 mg to patients with RHS during lactation, followed by milk sampling and blood sampling, measurement of amenamevir concentration in milk and plasma, and evaluation of adverse events. This study could provide a new treatment option for patients with RHS during lactation.
2 Methods and analysis
2.1 Trial design
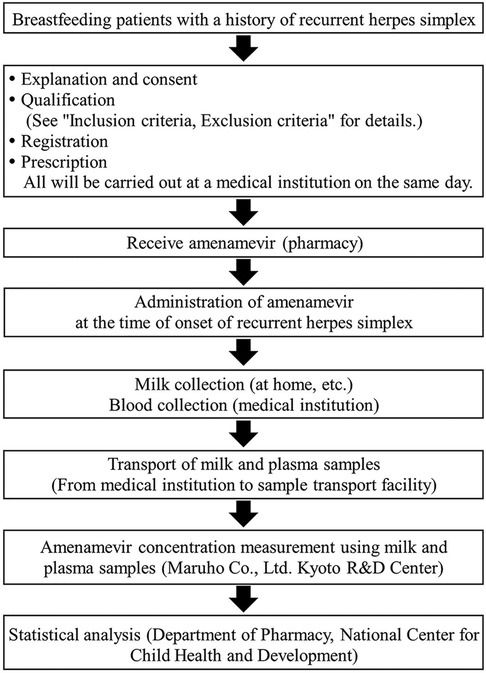
This study is a single-arm, open-label, multicenter interventional study. The subjects are patients who experience RHS during breastfeeding and are treated with amenamevir. The National Center for Child Health and Development (NCCHD) and four hospitals in Japan are registered as participants. This study is under the control of the principal investigator from NCCHD and sub-investigators from registered hospitals. All investigators are clinical doctors.
2.2 Recruitment and selection
Registrations are promoted through in-hospital public relations (posters, pamphlets), direct interviews with patients at medical institutions, and completed questionnaires. Patients with a background that could potentially be relevant are also investigated based on their past medical history. Patients who have a history of herpes simplex and are candidates for enrollment will receive an explanation of the study, together with a supplementary informed consent document during their regular check-up visits. Clinical research coordinators (CRC) from the contracting institution will support the explanations. Patients who meet all of the inclusion criteria and who do not meet any of the exclusion criteria will be eligible for enrollment. The inclusion criteria are described below.
(1) Patients who have had a diagnosis of RHS;
(2) In the past year, patients with one or more recurrences of herpes simplex;
(3) Patients who are breastfeeding within 1 year after childbirth, at the time of informed consent;
(4) Patients who are aged from 18 to 47 years at the time of informed consent;
(5) Patients with a body weight ≥40 kg and ≤70 kg at the time of informed consent;
(6) Patients who are assessed by the principal investigator or a sub-investigator as able to stop breastfeeding for 48 h after taking amenamevir; and
(7) Patients who provide voluntary, written consent to participate in the study.
Rationales for the inclusion criteria are described below.
(1–3) To target breastfeeding women with recurrent herpes simplex;
(4) Weaning of infants begins by 6 months after birth in 87.7% of cases, and 34.7% of cases are completely weaned by 12 months (14). Based on this report, we set the time limit to avoid patients dropping out due to weaning during the study;
(5) To target breastfeeding women in general; the minimum age is set taking into consideration when late teens attain reproductive age (15) and can provide informed consent on their own. The upper age limit is based on the end of the reproductive age, which is in the early 40s, taking into account the latest possible pregnancy age. Assuming the latest pregnancy age is 45 years, adding approximately one year of pregnancy and one year of breastfeeding results in an age of 47 years;
(6) Based on the general weight range for women aged 18 to 47 in Japan (16);
(7) Continuation or discontinuation of breastfeeding while taking amenamevir should be considered while taking account of the expected therapeutic benefits and the benefits of breastfeeding. However, considering the unknown actual amount of exposure through breast milk and its effects, in this study, the safety of the infants was prioritized by organizing cessation of breastfeeding for 48 h after administration. The half-life of amenamevir is approximately 7 h, and it has been confirmed that blood concentrations decrease sufficiently after 48 h in healthy adults (12).
(8) To honor ethical considerations that apply to patients.
The exclusion criteria are described below.
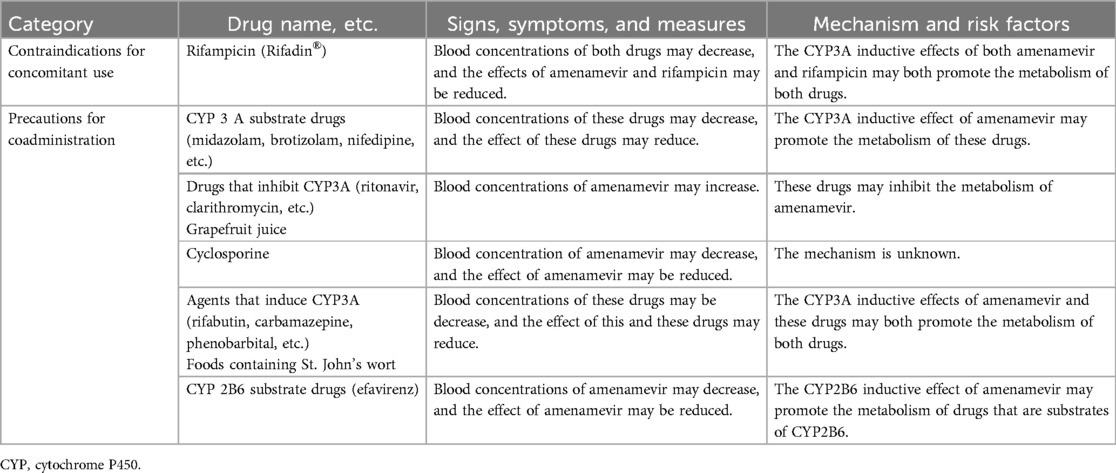
(1) Patients administered drugs/beverages listed in the section “Contraindications to concomitant use” or “Precautions for concomitant use” in the package insert of amenamevir, within the 14 days prior to the time of obtaining informed consent;
(2) Patients requiring dietary restrictions associated with comorbidities,;
(3) Patients with extreme dietary restriction due to weight loss;
(4) Patient with a history of amenamevir hypersensitivity;
(5) Patients with comorbidity or a history of diabetes, hepatitis B or C, tuberculosis, mastitis, or malignant tumors;
(6) Patients with a history of HIV infection or AIDS comorbidities
(7) Patients who are pregnant or have childbearing potential and intend to become pregnant during the study period; or
(8) Other patients who are considered ineligible by the principal investigator or a sub-investigator.
Rationales for the exclusion criteria are below.
(1) To avoid drug interactions and maintain an appropriate plasma drug concentration, thereby ensuring efficacy and safety;
(1–3) There is a report that indicates the impact of diet on the composition of breast milk;
(4–7) To ensure the safety of those subjects;
(8) In order for the investigator or sub-investigator to make a comprehensive decision taking into account the effects of amenamevir on blood drug levels and milk drug levels, and factors related to patients safety.
2.3 Screening and consent
We will use an informed consent form to explain the study to patients and obtain their voluntary written consent to participate in the study. In the unlikely event that the informed consent form (ICF) is revised due to a change in the study plan or other reasons after the patients begin participation in this study, the revised ICF must be rigorously reviewed by a certified review board. After the revised form is approved, the details of the change will be explained to patients, and adequate time will be given them to consider whether they are willing to continue their participation. We will therefore once again obtain their consent for continued participation. In addition, for patients who do not have an opportunity to visit the hospital and for whom it is difficult to obtain face-to-face re-consent, an explanatory document and ICF will be mailed, and the changes will be explained by telephone or web interview, to obtain consent.
2.4 Intervention
Patients are prescribed 1,200 mg of amenamevir and carry the medicine with them, to take at any time. Patients take the prescribed amenamevir as PIT, taking medication at their own discretion based on early symptoms of RHS. Amenamevir should be taken after a meal, and within six hours of the earliest symptoms of RHS. Patients should avoid breastfeeding for 48 h after taking amenamevir. To avoid effects on plasma and breast milk concentrations of amenamevir due to drug interactions and other factors, the use of drugs and food items listed in the “contraindications or precautions for concomitant use” sections of the packaging insert is prohibited (Table 1). A schematic representation of this study is shown in Figure 1.
To improve medication adherence, CRC calls patients monthly to check that they received the medicine and to explain when and how to take it. If any of the following conditions are found to apply, the patient will be discontinued from participation in this study.
(1) In the event of withdrawal of consent;
(2) If it is found that the patient is ineligible based on the inclusion or exclusion criteria;
(3) If breast milk production stops;
(4) Discontinuation of the entire study; or
(5) Other than the above, when the principal investigator or a sub-investigator decides that discontinuation is warranted.
2.5 Outcomes
The primary outcome is the relative infant dose (RID) of amenamevir calculated by Cave[AUC(0–24 h)].
The secondary outcomes are as follows:
• RID calculated by Cmax
• RID calculated by Cave[AUC(0–48 h)]
• Plasma concentration of amenamevir
• Frequency and rate of adverse events
RID is defined as follows:
TID is defined as follows:
Drug concentration in milk uses Cave[AUC(0–24 h)], Cave[AUC(0–48 h)] or Cmax. The infant feeding volume is set at 150 ml/kg/day in this study (17).
2.6 Data collection and measurement
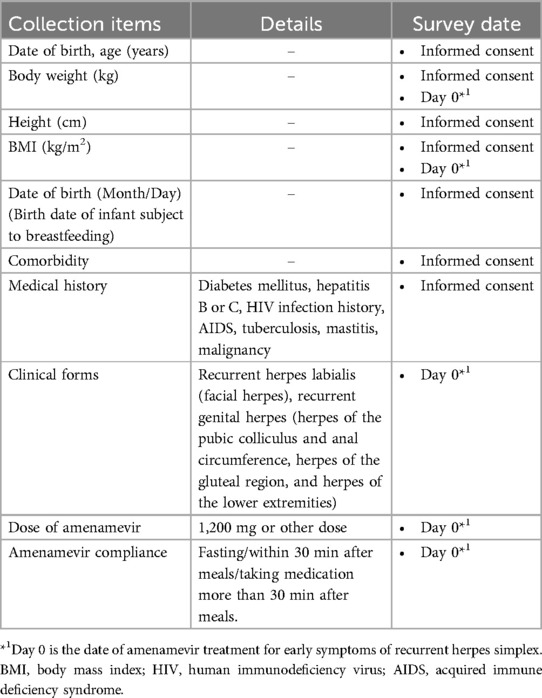
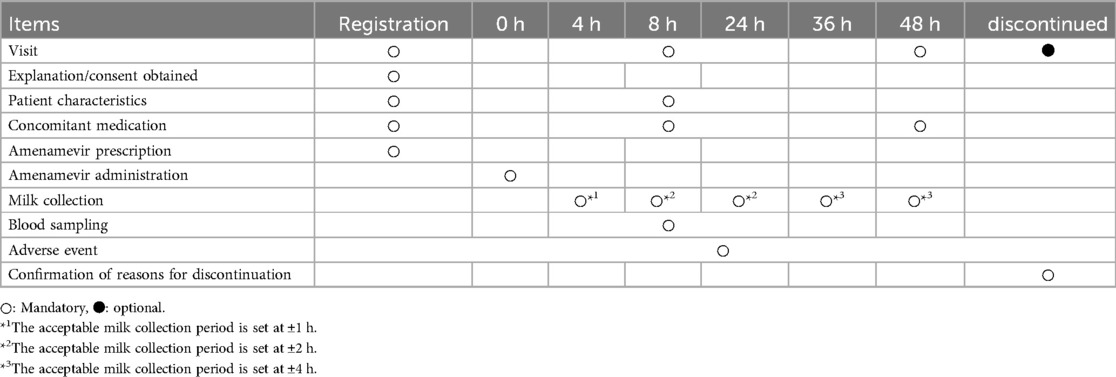
Detailed patient characteristics (e.g., body weight, clinical-disease type) are collected (Table 2). After administration of amenamevir, approximately 3 ml of milk is collected into individual milk storage containers at 4, 8, 24, 36, and 48 h (Table 3). The acceptable milk collection period is set at ±1, ±2, ±2, ±4, and ±4 h, respectively. The time at which a specimen of breast milk is collected is entered on both a recording sheet and the container. In addition to the required collection times mentioned above, we also request optional breast milk collection at any time within the 24 h following administration to enhance the accuracy of analysis. Patients submit breast milk samples when they visit the hospital. Blood will be drawn once in hospital (blood volume: 3 ml) within 5 to 24 h after taking the drug.
Participant recruitment occurred from January to November 2024, and data collection will continue until April 2025. Monitoring will be conducted throughout the entire data collection period.
For laboratory testing, 3 ml of whole blood treated with sodium heparin will be centrifuged for 5 min at 2,000 × g and the supernatant will immediately be transferred to a clean tube using a pipette, within 44 h of blood collection. Milk and plasma samples will be stored at −80℃. Amenamevir milk and plasma concentration measurements will be performed by liquid chromatography-tandem mass spectrometry (LC/MS/MS), and peak identification, calculation of peak areas and peak area ratios will be calculated. Preparation of calibration curves and calculation of quantitative values will be performed using the LC/MS/MS operation software Chromeleon 7.2.10 (Thermo Fisher Scientific Inc., Tokyo, Japan) at Maruho Co., Ltd. Kyoto R&D Center. In order to confirm the reproducibility of the quantitative values from the initial analysis, an incurred samples reanalysis (ISR) of milk and plasma samples will be performed. The ISR should be performed on a different day from the initial analysis, within a period during which sample stability is ensured. If the deviation is within ± 20% for more than two-thirds of the samples that underwent ISR, reproducibility is adjudged to be present.
2.7 Number of breast milk samples
For calculation of RID, in order to reduce the burden on patients as much as possible, mandatory sampling times of breast milk should be set to the minimum number required. In a study conducted on healthy adults, the tmax and t1/2 of amenamevir in plasma were 3.83 ± 0.41 h and 7.06 ± 0.29 h, respectively. Based on these data, breast milk sampling times should be set at approximately 4 h, 8 h, and 24 h, corresponding to the tmax, t1/2, and disappearance as the minimum necessary time for calculating AUC0–24 h. Additionally, 36 h and 48 h were established as mandatory milk collection points for calculating AUC0–48 h.
2.8 The need for blood sampling
In this study, blood will be sampled 5–24 h after amenamevir administration. Amenamevir concentration in the plasma will then be measured. If RID is markedly higher or lower than expected, the patient's plasma drug concentration will be compared to data obtained from healthy adults in clinical trials (12). Verifying that the plasma concentration of amenamevir in these patients is in the range of the mean ± standard deviation plasma concentrations established for healthy adults will allow identification of major differences. The blood sampling time was set between 5 and 24 h to ensure that amenamevir can be detected in plasma without affecting breast milk sampling at 4 h, when the amenamevir concentration is assumed to be highest. If the plasma drug concentration is different from that of healthy adults, we will again need to check dietary contents, their intake status, and concomitant medications, etc., as these may affect the pharmacokinetics of amenamevir. These measures will be taken to ensure data quality is maintained.
2.9 Statistical methods
The analysis population will comprise the full analysis set (FAS) and per protocol set (PPS), with the primary analysis population in this study being the PPS. The FAS consists of all patients enrolled in this trial except for the following patients:
• Patients who have not received the protocol treatment
• Patients whose data are not collected after the protocol treatment starts
• Patients subsequently found to violate eligibility criteria after enrollment
The PPS consists of the population excluding the following patients from the FAS:
• Patients who deviated from the study protocol, affecting the proper assessment of the primary endpoint
Background patient information (e.g., medical history) will be described individually. In addition, the frequency of each category will be calculated and summary statistics (number of cases, mean, standard deviation, median, maximum, and minimum) will be calculated.
Pharmacokinetic parameters (AUC0–24 h, AUC0–48 h, and Cmax) will be determined for each patient using the drug concentration in milk obtained by measurement. AUC0–24 h and AUC0–48 h will be calculated using the linear-log trapezoidal calculation method, with Cmax the actual measured value. TID and RID will also be calculated for each patient. The infant feeding volume is set at 150 ml/kg/day in this study (14).
Even if milk samples are not obtained at all mandatory time points in the target population, the primary and secondary endpoints will be evaluated without excluding the target population from the analysis. If it is considered possible to calculate the AUC by confirming the linearity of the elimination of amenamevir from breast milk, the primary and secondary endpoints will be evaluated. If this is impossible, all or some of the secondary endpoints will be evaluated. As the transfer of the drug into breast milk has been evaluated in few cases so far, the data obtained from all subjects will be disclosed as a data set, including the time of collection and the concentration at each time point.
Statistical analysis will be performed at the Department of Pharmacy, National Center for Child Health and Development.
Adverse events will be calculated with reference to MedDRA/J (ver. 27.1) based on the number of cases with adverse events of a given severity, study drug, and causality, as well as the percentage of the total and the number of occurrences.
2.10 Sample size
Sample size considerations include variability in the pharmacokinetics of the drug, study design, and variation in lactation physiology, which are determined according to the main objective; for exclusively breast milk studies, which are exploratory, a minimum of 6 to 8 subjects is considered sufficient (18). Furthermore, in clinical studies during lactation, the number of cases is often limited due to ethical and practical constraints. Recent reviews of pharmacokinetic studies on drug transfer into breast milk have not prevented research due to the difficulty in recruiting subjects and the small number of cases; indeed, studies based on data from fewer than ten cases have also been included for consideration (19). Considering that a small number of cases would result in sparse data, a study was performed to determine how many cases were needed, and the results showed that if there are samples which have two time points per case, the minimum number of cases will be 5 (20). Based on the above theory, the target number of evaluation cases will be set at 5. The frequency of recurrence varies from once every month to once every few years. Therefore, the selection criteria include a history of recurrence in the past year. In addition, during clinical trials of amenamevir, about 40% of patients did not have a recurrence during the study period. Furthermore, there is a possibility that patients will forget to take amenamevir because there will be a period between enrollment and recurrence. There is also a possibility that breast milk production may stop during the study period. Taking these into consideration, the target number of enrolled cases will be set at 20. Note that, as the average number of feeds per day for infants aged 3 months or older is generally 6 to 8, it was not deemed necessary to set the number of cases after taking into account the timing of milk sample collection.
2.11 Adverse event reporting and harms
For adverse events, the date of onset, severity, seriousness, predictability, outcome and outcome date, causal relationship to amenamevir, and causal relationship to the study will be assessed by the principal investigator or a sub-investigator and recorded on a case report form.
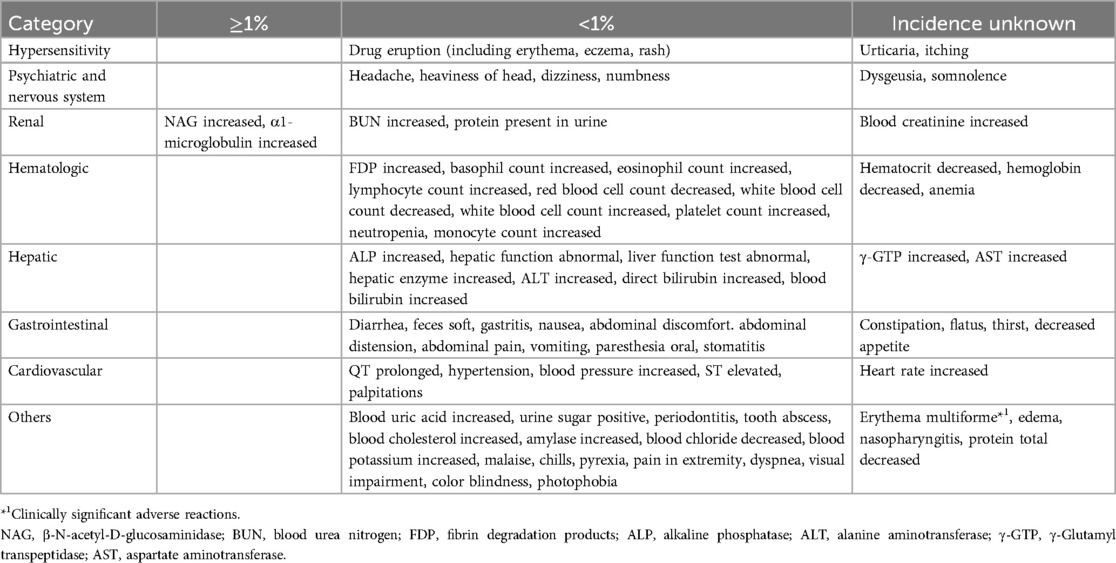
Illnesses occurring in patients will be evaluated from the time of consent until 48 h after taking amenamevir or at the time of study discontinuation. Adverse events occurring in patients will be evaluated from the time of taking amenamevir until 48 h after taking amenamevir or at the time of study discontinuation. However, if treatment for an adverse event that occurred during the study period persists even after 48 h have passed since taking amenamevir, the follow-up period will be until the completion of treatment. In principle, follow-up will be conducted until the adverse event that occurred has recovered or improved, or until the principal investigator and co-investigator determine that it is no longer necessary or possible to follow up on the adverse event that occurred. Among the adverse events that may occur in patients, information on adverse reactions accumulated from clinical trials is shown in Table 4 (21). The half-life of amenamevir is approximately 7 h, and it has been confirmed that blood concentrations decrease sufficiently after 48 h (12). Since patients will stop breastfeeding for 48 h after taking the medication, amenamevir will not be transferred to the infant, and therefore adverse events on the infant will not be evaluated. However, if, for various reasons, an illness occurs in a breastfed child within 48 h after taking amenamevir, the same measures as above will be taken, but the record will be in the illness report for the drug. The period for collecting information on the child's illnesses, etc. will be from the time of breastfeeding within 48 h after taking amenamevir to 48 h later.
3 Discussion
The results of this study will provide essential data on the transfer profile of amenamevir in breast milk during PIT. Although there have been reports of acyclovir and valacyclovir being transferred to breast milk during 5-day treatment (22, 23), there have been no reports of drugs administered by PIT being transferred into breast milk. For the assessment of infant safety, an RID level of 10% or less is generally considered to be safe (24, 25). If the RID of amenamevir is identified, this could provide a benchmark for deciding whether to administer amenamevir to patients. Therefore, these findings are anticipated to contribute significantly to the development of safe and effective treatment options for breastfeeding women with RHS.
Finally, PIT is a rational approach for patients, particularly breastfeeding mothers, enabling timely and effective treatment while balancing infant safety. By addressing the practical challenges faced by these patients, such as the difficulty of accessing immediate medical care, this study aligns with patient-centered care principles. As this study targets a small number of breastfeeding women (5 to 6 cases), it will not be possible to evaluate inter-individual variability in amenamevir concentrations in breast milk. In addition, as collections may be sparse, it may not be possible to calculate accurate values for amenamevir transfer into breast milk. Furthermore, as the participants in the study will not breastfeed within 48 h after taking amenamevir, it is impossible to evaluate the influence of the drug on infants when acquired by breastfeeding.
Ethics statement
The studies involving humans were approved by the Certified Review Board of the National Center for Child Health and Development. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. MG: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. JS: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TT: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Maruho Co., Ltd. provides the necessary funding for the conduct of this study and is involved in the design of research plans, the design of statistical analyses, and the implementation of measurements using milk and plasma samples, but is not directly involved in the implementation of data management, statistical analyses, monitoring and audits. In addition, test kits for milk collection and breast pumps (if not owned by the study person) are provided to patients free of charge.
Acknowledgments
We wish to thank all patients participating in this study. We also wish to express our sincere appreciation to Dr. Kazunori Kinoshita at Seijo Kinoshita Hospital, Dr. Kouya Wataba at Seijo maternity clinic, Dr. Satoshi Hayashi at Tokyo Mother's Clinic, Dr. Hiroaki Aoki at Aoki Obstetrics and Gynecology Clinic. Additionally, we would like to thank Mariko Takagai, an assistant at the National Center for Child Health and Development, who provided invaluable support during the initiation of this study.
Conflict of interest
This study represents industry-initiated collaborative clinical research and TT is an employee of Maruho Co., Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
: AIDS, acquired immune deficiency syndrome; AUC, area under the curve; Cave, average plasma concentration; Cmax, maximum plasma concentration; CRB, certified review board; CRC, clinical research coordinators; FAS, full analysis set; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HSV-1, herpes simplex virus Type 1; HSV-2, herpes simplex virus type 2; ICF, informed consent form; ISR, incurred samples reanalysis; LC/MS/MS, liquid chromatography-tandem mass spectrometry; NCCHD, national center for child health and development; PIT, patient-initiated therapy; PPS, per protocol set; RHS, recurrent herpes simplex; RID, relative infant dose; TID, theoretical infant daily dose; T1/2, biological half-life; Tmax, time to peak drug concentration.
References
1. Spruance SL, Overall JC, Kern ER, Krueger GG, Pliam V, Miller W. The natural history of recurrent herpes simplex labialis-implications for antiviral therapy. N Engl J Med. (1977) 297(2):69–75. doi: 10.1056/NEJM197707142970201
2. Doi Y, Ninomiya T, Hata J, Yonemoto K, Tanizaki Y, Arima H, et al. Seroprevalence of herpes simplex virus 1 and 2 in a population-based cohort in Japan. J Epidemiol. (2009) 19(2):56–62. doi: 10.2188/jea.JE20080061
3. Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, et al. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. (2004) 13(9):635–8. doi: 10.1191/0961203304lu1094oa
4. Tamaki M. Reviewing past and recent research on premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD). J Jp Soc Psychosom Obstet Gynecol. (2023) 28(2):167–75. doi: 10.18977/jspog.28.2_167
5. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. (2012) 490:102–6. doi: 10.1038/nature11462
6. Drugs and Lactation Database (LactMed®). Available at: https://www.ncbi.nlm.nih.gov/books/NBK501922/ (Accessed February 28, 2025).
7. Kawashima M, Nemoto O, Honda M, Watanabe D, Nakayama J, Imafuku S, et al. Amenamevir, a novel helicase–primase inhibitor, for treatment of herpes zoster: a randomized, double-blind, valaciclovir-controlled phase 3 study. J Dermatol. (2017) 44(11):1219–27. doi: 10.1111/1346-8138.13948
8. Shiraki K, Yasumoto S, Toyama N, Fukuda H. Amenamevir, a helicase-primase inhibitor, for the optimal treatment of herpes zoster. Viruses. (2021) 13(8):1547. doi: 10.3390/v130815477
9. Maruho Co., Ltd. (2023). Available at: www.maruho.co.jp/english/information/20230224.html (Accessed December 18, 2024).
10. Kawashima M, Kato T, Fujii C, Kato R. Single-day, patient-initiated Famciclovir treatment for recurrent herpes simplex: a randomized, placebo-controlled, double-blind, parallel-group, multicenter phase 3 study. Jpn J Dermatol. (2018) 35(3):488–96. doi: 10.3812/jocd.35.488
11. Japanese Society for Sexually Transmitted Infections. Sexually Transmitted Disease Diagnosis and Treatment Guidelines 2020. Tokyo: Shindan to chiryo sha Inc (2020). p. 68.
12. Amenalief® Tablet 200 mg. Interview Form. (2024). Available at: https://www.maruho.co.jp/medical/pdf/products/amenalief/amenalief_if.pdf (Accessed December 18, 2024).
13. Pharmaceuticals and Medical Devices Agency. (2023). Available at: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/730155_6250046F1028_1_06 (Accessed December 18, 2024).
14. Ministry of Health, Labour and Welfare. (2015). Available at: https://www.mhlw.go.jp/file/06-Seisakujouhou-11900000-Koyoukintoujidoukateikyoku/0000134207.pdf (Accessed March 10, 2025).
15. Katagiri Y, Nagano T, Miyazaki Y, Otani A, Takahashi A, Morita M. Psychosomatic changes for women in reproductive age. Jpn J Psychosom Med. (2014) 54:666–72. doi: 10.15064/jjpm.54.7_666
16. Statistics Bureau of Japan. (2025). Available at: https://www.e-stat.go.jp/dbview?sid=0003224177 (Accessed March 10, 2025).
17. Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. (1998) 48(6):1375–86. doi: 10.1093/ajcn/48.6.1375
18. Center for Drug Evaluation and Research. Guidance Document, Clinical Lactation Studies: Considerations for Study Design. Silver Spring, MD: U.S. Food and Drug Administration (2019). p. 9.
19. Gong C, Bertagnolli LN, Boulton DW, Coppola P. A literature review of drug transport mechanisms during lactation. CPT Pharmacometrics Syst Pharmacol. (2024) 13(11):1870–80. doi: 10.1002/psp4.13195
20. Iftekhar M, John D. Population pharmacokinetics with a very small sample size. Drug Metabol Drug Interact. (2009) 24(2-4):259–74. doi: 10.1515/DMDI.2009.24.2-4.259
21. Amenalief® Tablet 200 mg. Package Insert. (2023). Available at: https://www.maruho.co.jp/medical/pdf/products/amenalief/amenalief_te.pdf (Accessed March 18, 2025)
22. Taddio A, Klein J, Koren G. Acyclovir Excretion in human breast milk. Ann Pharmacother. (1994) 28(5):585–7. doi: 10.1177/106002809402800506
23. Sheffield JS, Fish DN, Hollier LM, Cadematori S, Nobles BJ, Wendel GD. Acyclovir Concentrations in human breast milk after valaciclovir administration. Am J Obstet Gynecol. (2002) 186(1):100–2. doi: 10.1067/mob.2002.119183
24. Nice FJ, Luo AC. Medications and breastfeeding: current concept. J Am Pharm Assoc. (2012) 52(1):86–94. doi: 10.1331/JAPhA.2012.10139
Keywords: breastfeeding, recurrent herpes simplex (RHS), amenamevir, milk transfer, relative infant dose (RID), patient initiated therapy (PIT)
Citation: Koinuma S, Goto M, Saito J, Murashima A and Takeshita T (2025) Transfer of amenamevir into breast milk in breastfeeding patients with recurrent herpes simplex: study protocol for a single-arm, open-label study. Front. Pediatr. 13:1551335. doi: 10.3389/fped.2025.1551335
Received: 16 January 2025; Accepted: 25 March 2025;
Published: 10 April 2025.
Edited by:
Catherine M. T. Sherwin, University of Western Australia, AustraliaReviewed by:
A. Alwin Prem Anand, University of Tübingen, GermanyKazuhiro Itoh, National Hospital Organization Awara Hospital, Japan
Philip Anderson, University of California, San Diego, United States
Copyright: © 2025 Koinuma, Goto, Saito, Murashima and Takeshita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sachi Koinuma, a29pbnVtYS1zQG5jY2hkLmdvLmpw
†Present Address: Atsuko Murashima, Japan Drug Information in Pregnancy Society, Department of Rheumatology and Applied Immunology, Saitama Medical University, Saitama, Japan
 Sachi Koinuma
Sachi Koinuma Mikako Goto
Mikako Goto Jumpei Saito
Jumpei Saito Atsuko Murashima1,†
Atsuko Murashima1,† Tomoyoshi Takeshita
Tomoyoshi Takeshita