
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 19 February 2025
Sec. Pediatric Critical Care
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1545230
Introduction: Currently, there are no clear guidelines regarding the use of recruitment maneuvers in pediatric patients with severe respiratory failure who are on extracorporeal membrane oxygenation (ECMO), nor is there consensus on how they should be performed.
Methods: In this report, we describe pulmonary recruitment maneuvers performed in four children with respiratory failure on ECMO (three on VA-ECMO and one on VV-ECMO), all of whom were monitored using electrical impedance tomography (EIT) between March and December 2024.
Results: Our findings demonstrate that EIT is a feasible tool for evaluating lung recruitability in children with severe respiratory failure on ECMO. Additionally, EIT aids in distinguishing between responders and non-responders to recruitment maneuvers.
Severe respiratory failure is associated with high morbidity and mortality especially when extracorporeal membrane oxygenation (ECMO) implantation is necessary (1, 2). To date, there are no clear guidelines on whether standardized recruitment maneuver (RM) should be applied in severe cases of respiratory failure requiring ECMO (3).
Some studies have evaluated the use of RM in adult and pediatric ARDS demonstrating that it can be feasible and effective in some patients (4–8). However, approximately 40% of patients did not respond to recruitment maneuvers, making it challenging to determine which patients would benefit from RM. Recent studies have shown that electrical impedance tomography (EIT) can be a valuable tool for optimizing mechanical ventilation in patients with ARDS on ECMO (9). Given the limited ability to monitor oxygenation changes during ECMO, EIT proves particularly valuable in this regard.
To the best of our knowledge, there is no existing data on the use of EIT to monitor lung recruitability in children with respiratory failure on ECMO.
We describe four patients in whom we assessed lung recruitability within the first 3 days on ECMO using electrical impedance tomography (EIT). The placement of the belts was consistent across all patients. The Enlight 2100 device (Timpel, São Paulo, Brazil) was used in all cases, along with neonatal or pediatric belts, depending on the patient’s thoracic circumference. The belts were positioned above the nipple line whenever possible, and their placement remained unchanged throughout the entire examination. Proximal flow sensors specific to the device were used to calculate changes in tidal volumes and lung compliance. Table 1 presents patient characteristics and changes in respiratory parameters.
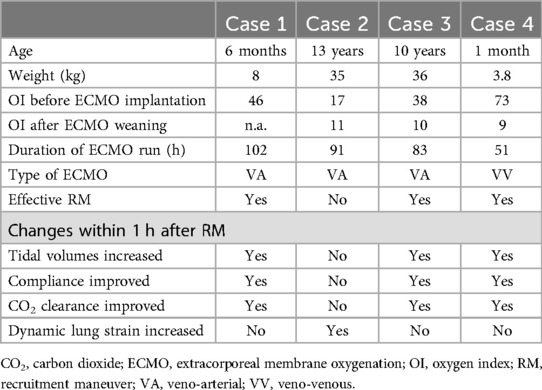
Table 1. Patient characteristics are provided for all cases, including changes in respiratory parameters after recruitment maneuvers.
Several studies have investigated the use of electrical impedance tomography (EIT) for assessing dynamic lung strain. Lung strain is defined as the difference in lung tissue deformation between two distinct pressure levels (e.g., varying levels of PEEP). While CT scans are considered the gold standard, EIT has demonstrated a reasonable agreement with CT scans when evaluating dynamic lung strain (10). The assessment of dynamic lung strain can be performed across different ventilatory modes, with high lung strain potentially contributing to ventilator-induced lung injury (11). Therefore, limiting dynamic lung strain during recruitment maneuvers is crucial. Most EIT devices are capable of displaying impedance values derived from EIT pixels at various time points within the respiratory cycle. These values, which reflect changes in lung impedance rather than those measured by flow sensors (as in conventional ventilators that calculate tidal volume or pressure), offer comparable insights. Notably, values based on impedance changes typically feature a small ‘z’ at the end of their abbreviation.
Care guidelines were followed for the manuscript preparation (12).
This was a 6-month-old female patient (8 kg) with a partial cavopulmonary connection (PCPC) due to an unbalanced atrioventricular septal defect (AVSD). She developed pediatric ARDS secondary to viral pneumonia and required urgent veno-arterial ECMO implantation during cardiopulmonary resuscitation due to severe respiratory failure, with the lowest arterial pH recorded at <6.7. The first chest x-ray after ECMO implantation showed diffuse lung infiltrates, and nearly no tidal volumes could be generated on mechanical ventilation, which was set in pressure-controlled mode with a PEEP of 10 cmH2O and driving pressures of 15 cmH2O.
A recruitment maneuver (RM) was performed using incremental and decremental PEEP titration, with EIT (P1-belt, Timpel, São Paulo, Brazil) used to monitor the intervention. We first increased PEEP by 2 cmH2O every 5 min up to a maximum of 24 cmH2O with fixed driving pressures of 10 cmH2O, followed by a gradual reduction back to the baseline PEEP of 10 cmH2O. Lung recruitability was assessed by observing changes in both EIT signals and conventional ventilator parameters, including tidal volumes and lung compliance.
During incremental PEEP titration, tidal volumes and lung compliance remained stable, which was interpreted as a sign of ongoing lung recruitment. Dynamic EIT images did not show any increase in dynamic lung strain in any region of interest. The patient remained hemodynamically stable throughout the procedure, and no adjustments to the ECMO settings were required.
With RM, tidal volumes (VT) increased from 0.5 ml/kg to 2.5 ml/kg during the decremental PEEP titration. EIT signals indicated a substantial improvement in ventilation (Figure 1). Post-intervention blood gas analysis showed improved gas exchange, and FiO2 and ECMO sweep gas flow were reduced by 30%. A chest x-ray performed immediately after the RM demonstrated a significant reduction in atelectasis.
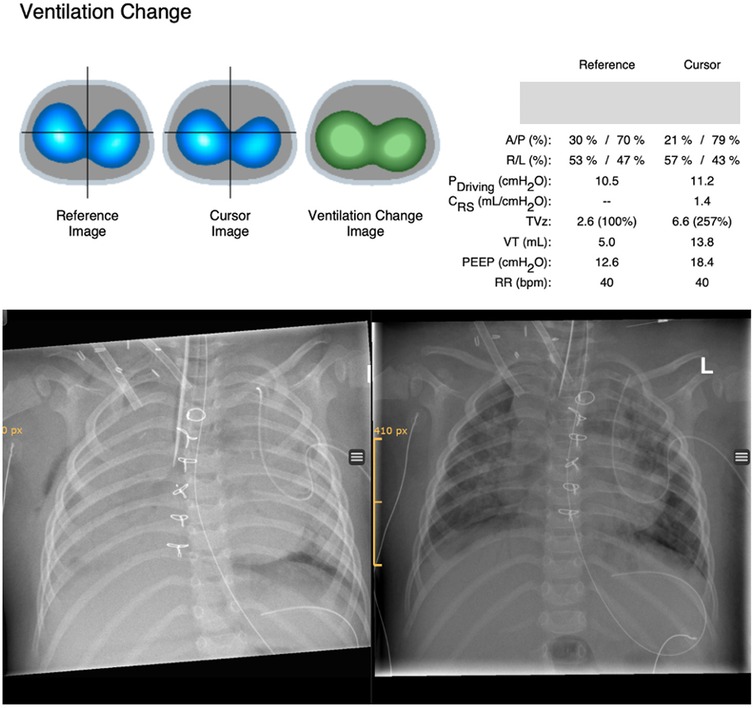
Figure 1. Ventilation changes derived from EIT are shown above. The reference image shows ventilation distribution before recruitment and the cursor image ventilation distribution after recruitment. The ventilation change image compares ventilation distribution before and after recruitment (reference image vs. cursor image) based on EIT pixels and displays changes in colors (green, gain in ventilation; red, loss in ventilation). Ventilator settings for paired time points and associated changes of different ventilatory and EIT parameters are shown on the right side comparing the same reference and cursor time points. Ventilation change after recruitment on ECMO with incremental/decremental PEEP titration up to 24 cmH2O showed a significant gain of ventilation (green) and improvement in tidal volumes. Chest x-ray before recruitment maneuver (left picture) and after recruitment (right picture). A/P, anterior/posterior; R/L, right/left; Pdriving, driving pressure; Crs, compliance respiratory system; TVz, tidal volume based on EIT signals; VT, tidal volume in milliliters.
However, due to significant neurological impairment following the cardiopulmonary resuscitation, confirmed by two independent pediatric neurologists, therapy was discontinued with parental consent, despite the improvements in pulmonary function.
This was a 13-year-old female patient with a body weight of 35 kg who received double lung transplantation due to drug-resistant pulmonary arterial hypertension. The day after transplantation, donor lungs showed decreased compliance with <0.5 ml/cmH2O/kg. The donor had a car accident, and lung contusion of the donor organ was suspected. We performed a recruitment maneuver as outlined before, starting from a baseline PEEP of 9 cmH2O and with a constant driving pressure of 10 cmH2O. We stopped at a maximum PEEP of 15 cmH2O because of the continuous worsening of lung compliance and dynamic EIT signals without recruitment effect over time. The patient remained hemodynamically stable throughout the procedure. Upon returning to the baseline PEEP of 9 cmH2O, EIT signals showed no improvement in ventilation. Decreasing PEEP to 6 cmH2O also yielded no benefit.
Evaluation of regional ventilation distribution revealed reduced ventilation in the posterior lung, which was refractory to recruitment. These findings were consistent with a subsequent CT scan, which showed consolidation in the dorsal lung (Figure 2). Based on these results, we classified the patient as non-recruitable and opted for gentle ventilation (PEEP 8 cmH2O, driving pressure 10 cmH2O, RR 10 /min) and pronation (16 h/day).
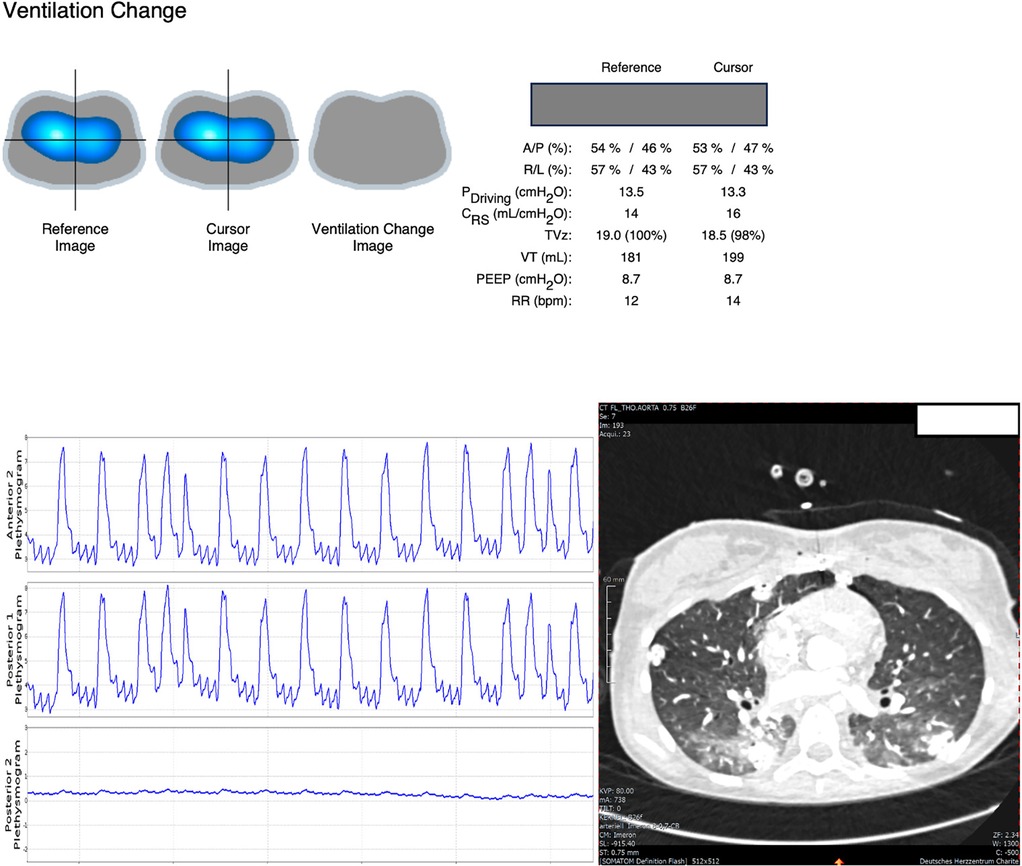
Figure 2. EIT signals showed no benefit of PEEP titration (neither gain nor loss in ventilation as shown in the ventilation change image on top after RM) but indicated substantial loss in posterior ventilation which is reflected by very little amplitude in the posterior plethysmogram. CT scan showed opacities in the dorsal lung.
The patient was successfully weaned from ECMO 3 days later, with improved lung compliance.
This was a 10-year-old boy with a severe bacterial infection that led to multiorgan failure, lung hemorrhage, and acute heart decompensation. Veno-arterial (VA) ECMO was implanted on-site during cardiopulmonary resuscitation, and the patient was subsequently transferred to our unit. On Day 2 of ECMO, lung compliance had decreased to <0.5 ml/cmH2O/kg with a FiO2 of 80% on ECMO.
A standardized recruitment maneuver (RM) was performed, as described previously, using a constant driving pressure of 12 cmH2O and a maximum PEEP of 24 cmH2O, followed by a stepwise return to baseline PEEP of 10 cmH2O. During incremental PEEP titration, EIT signals did not show increased dynamic lung strain in the dynamic images, and tidal volumes as well as lung compliance remained stable.
During decremental PEEP titration, a clear recruitment effect was observed, with increased tidal volumes and improved compliance when comparing baseline PEEP (10 cmH2O) to PEEP at 22 cmH2O, with a more pronounced effect after reducing PEEP from 22 to 16 cmH2O. In contrast, decreasing PEEP from 18 to 14 cmH2O resulted in a decrease in tidal volumes and compliance, indicating the onset of de-recruitment (Figure 3).
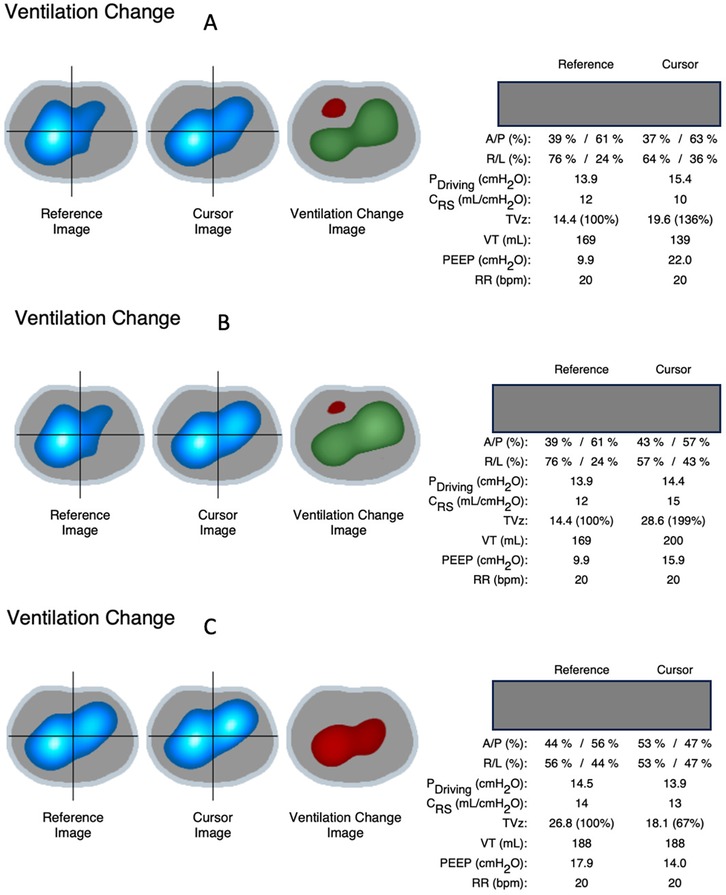
Figure 3. Ventilation change images based on EIT signals showed a gain in ventilation indicated by green zones (and in TVz on the right side) when increasing PEEP from 10 cmH2O to 22 cmH2O (A) and even more after reducing PEEP from 22 cmH2O to 16 cmH2O (B) decreasing PEEP from 18 to 14 cmH2O (C) resulted in decreased ventilation instead (red zone and TVz), indicating onset of de-recruitment in between. A/P, anterior/posterior; R/L, right/left; TVz, tidal volume; Pdriving, driving pressure; Crs, compliance respiratory system; TVz, tidal volume based on EIT signals; VT, tidal volume in milliliters.
After a brief re-recruitment with PEEP at 20 cmH2O, we decided to maintain PEEP at 16 cmH2O, which was considered the “best PEEP” at that time, with a constant driving pressure of 12 cmH2O for the following hours. Immediately after the RM, FiO2 and ECMO sweep gas flow were reduced based on blood gas analysis, reflecting improved ventilation as indicated by enhanced lung compliance and tidal volumes.
Despite ongoing heart failure, the pulmonary ECMO was successfully weaned 2 days later, and the patient was subsequently placed on a ventricular assist device.
This was a 1-month-old boy (3.8 kg) admitted to a children's hospital with tachypnea and fatigue. Following deterioration on nasal CPAP, the patient was transferred to another hospital for invasive ventilation. Intubation required multiple attempts, and the subsequent chest x-ray showed bilateral lung consolidation. The patient was then transferred to a tertiary neonatal intensive care unit, where antibiotic therapy was initiated (Klebsiella pneumonia was later isolated). Despite peak inspiratory pressures up to 44 cmH2O (PEEP 12 cmH2O) and 100% FiO2, oxygen saturation remained at 70%, and arterial pH was approximately 7.0. High doses of inotropic support (noradrenaline and adrenaline, both at 0.2 µg/kg/min) were required. Right ventricular function was preserved, and veno-venous ECMO was initiated on-site, with the patient subsequently transferred to our pediatric CICU.
Forty hours after ECMO initiation, tidal volumes were <0.5 ml/kg, and driving pressures were approximately 15 cmH2O. Lung recruitability was assessed using EIT during an incremental/decremental PEEP titration as previously described. Due to very low lung compliance and the absence of signs of increased dynamic lung strain on dynamic EIT images during HFOV, we decided to perform a recruitment maneuver on HFOV in the prone position. Ventilation change images could not be generated by the EIT device during conventional ventilation due to tidal volumes being <1 ml.
With a fixed amplitude of 50 cmH2O and a frequency of 13 Hz, we increased the mean airway pressure (Paw) by 2 cmH2O every 3–5 min, up to a maximum of 36 cmH2O, and then gradually reduced Paw to 24 cmH2O, which was considered optimal based on improvements in dynamic EIT images and oscillatory tidal volumes, as well as the carbon dioxide transport coefficient (DCO2). Even at a mean airway pressure of 36 cmH2O, the patient remained hemodynamically stable: arterial blood pressure slightly improved, heart rate did not increase, and central venous pressure rose only by 1 mmHg. Dynamic EIT signals did not show increased dynamic lung strain with Paw >30 cmH2O.
Oscillatory tidal volumes increased from <0.5 to 7 ml, and DCO2 improved from 20 to 60 to 700 immediately following the recruitment maneuver. Post-RM blood gas samples showed improved ventilation and gas exchange, leading to a reduction in FiO2 and ECMO sweep gas flow. ECMO was successfully weaned 16 h later on HFOV after the patient was repositioned supine, with Paw at 15 cmH2O, frequency of 14 Hz, and amplitude of 40 cmH2O.
The patient was transferred to the NICU the next day on conventional ventilation (PEEP of 6 cmH2O, pressure support of 12 cmH2O, and FiO2 of 0.3). Chest x-rays before and after the recruitment maneuver are shown in Figure 4. A short video of dynamic EIT images during HFOV at a Paw of 36 cmH2O is available in the online supplement.
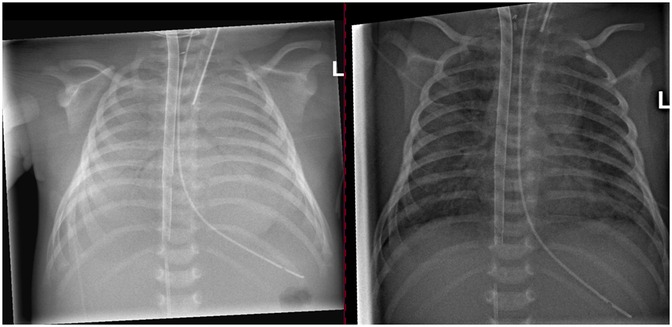
Figure 4. Chest x-ray prior to the recruitment maneuver on the left side and after recruitment on the right side.
This case series shows that (1) evaluation of lung recruitability with EIT is feasible. (2) In recruitable lung disease, recruitment maneuver might improve lung aeration and thereby shorten ECMO duration. (3) In non-recruitable lung disease, increases in dynamic lung strain can be detected early with EIT, thereby facilitating gentle ventilation by avoiding unnecessary lung strain.
EIT played a crucial role in monitoring dynamic lung strain during recruitment and in assessing how patients respond to the intervention (responder/non-responder).
Recently, several studies in both adult and pediatric populations have suggested that adjusting ventilation settings based on EIT findings may improve patient outcomes compared to standard treatment alone (13–15).
The recruitment maneuver in patients with recruitable lung disease led to a significant improvement in lung compliance and initiated ECMO weaning, which was successfully achieved within 48 h in Cases 3 and 4. In Case 1, lung compliance also improved markedly, but ECMO weaning was not attempted due to poor neurological prognosis. It remains unclear why no homogenization effect was observed in Case 1, despite all other parameters indicating successful recruitment. While EIT is a useful tool for assessing regional lung behavior, it is important to recognize that it may have limitations in evaluating the behavior of the entire lung, as it provides data from only a cross-sectional slice of the lung.
In Case 2, an aggressive recruitment maneuver was avoided in the context of non-recruitable lung disease, as it might have been harmful.
The advantage of EIT over conventional monitoring is the ability to continuously monitor dynamic lung strain during the maneuver, providing real-time insights into lung recruitment and potential overdistension.
Additionally, EIT was valuable in identifying the “best PEEP” levels after recruitment to prevent de-recruitment.
Our study's limitations include a small sample size and an uncontrolled design. We did not collect blood gases to obtain pCO2 values for each PEEP level during the recruitment maneuver, as this would have required 15 blood gas samples per patient and intervention. Additionally, we did not report individual lung compliance values for every PEEP level in each patient, as compliance remained stable during incremental PEEP titration in patients who responded positively or decreased only in Case 2, where the patient's lung was not recruitable. Instead, we chose to compare baseline settings after the recruitment maneuver to more clearly illustrate the changes following RM with identical ventilator settings. Hemodynamics did not change in any of the reported cases, individual hemodynamic values for every PEEP level have not been collected. However, given the rarity of these cases, we believe that integrating EIT into clinical practice could help personalize respiratory care at the bedside. These findings may also serve as a basis for designing larger, controlled studies on the topic.
In conclusion, EIT monitoring of lung recruitability in children with severe respiratory failure on ECMO is feasible. EIT may aid in distinguishing between recruitable and non-recruitable lung disease. Recruitment maneuvers in recruitable lung disease may shorten ECMO duration by optimizing lung aeration. Ventilation strategies that reduce ECMO and invasive mechanical ventilation duration could potentially lower the risk of impaired neurocognitive outcomes (16).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study involving humans was approved by Ethikkommission der Charité, a corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Charitéplatz 1, 10117 Berlin, Germany. This study was conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
J-CC: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. AK: Investigation, Writing – review & editing. AS: Investigation, Writing – review & editing. OM: Funding acquisition, Methodology, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by a grant of Deutsche Herzstiftung e.V. to author J-CC. There was no involvement in the clinical work or the interpretation of data by the funding party.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1545230/full#supplementary-material
ARDS, acute respiratory distress syndrome; AVSD, atrioventricular septal defect; ECMO, extracorporeal membrane oxygenation; EIT, electrical impedance tomography; Paw mean airway pressure; PCPC, partial cavopulmonary connection; RM, recruitment maneuver; VA, veno-arterial; VT, tidal volume; VV, veno-venous.
1. Khemani RG, Smith LS, Zimmerman JJ, Erickson S. Pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. (2015) 16(5 Suppl 1):S23–40. doi: 10.1097/PCC.0000000000000432
2. Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care. (2021) 25(1):38. doi: 10.1186/s13054-021-03463-2
3. Kneyber MCJ, Cheifetz IM. Mechanical ventilation during pediatric extracorporeal life support. Curr Opin Pediatr. (2023) 35(5):596–602. doi: 10.1097/MOP.0000000000001277
4. Smallwood CD, Walsh BK, Arnold JH, Gouldstone A. Equilibration time required for respiratory system compliance and oxygenation response following changes in positive end-expiratory pressure in mechanically ventilated children. Crit Care Med. (2018) 46(5):e375–9. doi: 10.1097/CCM.0000000000003001
5. van der Zee P, Gommers D. Recruitment maneuvers and higher PEEP, the so-called open lung concept, in patients with ARDS. Crit Care. (2019) 23(1):73. doi: 10.1186/s13054-019-2365-1
6. Hess DR. Recruitment maneuvers and PEEP titration. Respir Care. (2015) 60(11):1688–704. doi: 10.4187/respcare.04409
7. de Jager P, Kamp T, Dijkstra SK, Burgerhof JGM, Markhorst DG, Curley MAQ, et al. Feasibility of an alternative, physiologic, individualized open-lung approach to high-frequency oscillatory ventilation in children. Ann Intensive Care. (2019) 9(1):9. doi: 10.1186/s13613-019-0492-0
8. de Jager P, Koopman AA, Markhorst DG, Kneyber MCJ. Lung behavior during a staircase high-frequency oscillatory ventilation recruitment maneuver. Intensive Care Med Exp. (2024) 12(1):42. doi: 10.1186/s40635-024-00623-w
9. Franchineau G, Bréchot N, Lebreton G, Hekimian G, Nieszkowska A, Trouillet J-L, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med. (2017) 196(4):447–57. doi: 10.1164/rccm.201605-1055OC
10. Brito R, Morais CCA, Lazo MT, Guiñez DV, Gajardo AIJ, Arellano DH, et al. Dynamic relative regional lung strain estimated by computed tomography and electrical impedance tomography in ARDS patients. Crit Care. (2023) 27(1):457. doi: 10.1186/s13054-023-04748-4
11. Jain SV, Kollisch-Singule M, Satalin J, Searles Q, Dombert L, Abdel-Razek O, et al. The role of high airway pressure and dynamic strain on ventilator-induced lung injury in a heterogeneous acute lung injury model. Intensive Care Med Exp. (2017) 5(1):25. doi: 10.1186/s40635-017-0138-1
12. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
13. Songsangvorn N, Xu Y, Lu C, Rotstein O, Brochard L, Slutsky AS, et al. Electrical impedance tomography-guided positive end-expiratory pressure titration in ARDS: a systematic review and meta-analysis. Intensive Care Med. (2024). doi: 10.1007/s00134-024-07362-2
14. Di Nardo M, Fischer M, Ranieri VM. Electrical impedance tomography to set positive end expiratory pressure during pediatric extracorporeal membrane oxygenation for respiratory failure… is it feasible? ASAIO J. (2022) 68(8):e136–8. doi: 10.1097/MAT.0000000000001612
15. Clausen JC, De Luca D, Schleiger A, Emeis M, Miera O. Neonatal ARDS treated with electrical impedance tomography-guided recruitment maneuvers and surfactant-vehicled budesonide. Respir Care. (2024) 2:19. doi: 10.4187/respcare.12384
Keywords: lung recruitment, respiratory failure, children, ECMO, case report, EIT, HFOV
Citation: Clausen J-C, Krauß A, Schulz A and Miera O (2025) Monitoring lung recruitability during recruitment maneuvers in children with respiratory failure on ECMO using electrical impedance tomography: case report. Front. Pediatr. 13:1545230. doi: 10.3389/fped.2025.1545230
Received: 14 December 2024; Accepted: 3 February 2025;
Published: 19 February 2025.
Edited by:
Michael Hermon, Medical University of Vienna, AustriaReviewed by:
Farhan A. Rashid Shaikh, Rainbow Children’s Hospital, IndiaCopyright: © 2025 Clausen, Krauß, Schulz and Miera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan-Christoph Clausen, amFuLWNocmlzdG9waC5jbGF1c2VuQGRoemMtY2hhcml0ZS5kZQ==
†ORCID:
Jan-Christoph Clausen
orcid.org/0009-0009-3153-8923
Annemarie Krauß
orcid.org/0000-0001-7768-1199
Antonia Schulz
orcid.org/0000-0002-9155-9509
Oliver Miera
orcid.org/0000-0002-7643-1611
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.