
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 27 January 2025
Sec. Pediatric Critical Care
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1530467
Introduction: Neonates with congenital diaphragmatic hernia (CDH) have an associated high mortality and morbidity. The European CDH EURO consortium has developed guidelines for initial and perioperative ventilatory management. There are, however, no recommendations on how to wean these patients from the ventilator. Extubation failure is more frequent in this group of patients than in other neonates. The aim of this study was to describe patient characteristics and risk factors for failed extubation and to evaluate predictive factors for successful weaning.
Methods: We performed a retrospective study in a single centre tertiary pediatric intensive care unit in Stockholm, Sweden. CDH-patients (n = 38), aged 0–28 days, with extubation events were identified from 2017 to 2019. Eight patients (21.1%) needed reintubation within 24 h after the first extubation attempt. Patient demographics, surgical repair with patch, oxygenation saturation index (OSI), rapid shallow breathing index (RSBI), ventilatory settings, fluid balance and sedation on the day of extubation were recorded.
Results: Patients in the failed extubation group (FE) had lower birth weight (p < 0.05), surgical patch repair (p < 0.05), longer length of stay in intensive care (p < 0.05), longer time on the ventilator (p < 0.05) and other comorbidities (p < 0.001). Using logistic regression we identified OSI, RSBI and inspiratory pressure (Pinsp) as factors predicting a successful extubation, AUCROC 0.95 (95% CI: 0.87 to 1.00). Patients in the FE-group had significantly more often pulmonary hypertension requiring treatment (p < 0.05), a higher fraction of inspired oxygen (FiO2) (p < 0.05) and hypercapnia (p < 0.001) prior to extubation and an oxygen demand exceeding 40% two hours after extubation (p < 0.05).
Conclusion: Useful predictors of successful extubation in CDH patients are OSI, RSBI and Pinsp. Low birth weight, patch repair and comorbidity also appear to be important factors. Prospective studies are required to confirm findings in the present study.
Congenital diaphragmatic hernia (CDH) is a rare condition with an incidence of approximately 40 cases per 100,000 births (1). Despite advancements in pediatric critical care, it remains a significant challenge for clinicians, with an overall mortality of 28% over the last 25 years (2). The majority of CDH patients require invasive ventilation immediately after birth to maintain adequate gas exchange (3, 4). This is due to pulmonary hypoplasia, which occurs to varying degrees because of abdominal contents herniating into the thorax during fetal life. The extent of lung hypoplasia depends on both the size of the herniation and the gestational timing of the herniation (5).
Corrective surgery is usually performed after a few days of stabilization (1, 3). Postoperatively, mechanical ventilation is required in almost all patients for a variable length of time. However, it is critical to minimize the duration of invasive ventilation due to the associated risks for ventilator-induced lung injury (6), ventilator-associated pneumonia (7, 8), higher morbidity and mortality and also the increased length of stay (LOS) and hospital costs (9–11).
Weaning refers to the gradual transition from full invasive ventilatory support to spontaneous breathing, with or without non-invasive ventilatory support, such as continuous positive airway pressure (CPAP) or high flow nasal cannula (HFNC). Optimizing the timing of weaning and extubation is important to reduce the risk of complications from invasive ventilation (12) while avoiding the risks of reintubation (13, 14).
Failed extubation (FE) has been well-studied in the pediatric intensive care population, with reported incidences ranging between 5%–10% (9, 15). However, there is limited literature on the incidence of FE specifically in newborns with CDH. A retrospective study of CDH patients by Schroeder et al. reported an incidence of 35% (16). In general, existing weaning protocols are few and specific guidelines for weaning CDH-patients are lacking.
The intensive care of CDH patients after corrective surgery remains highly challenging. It is of outmost importance to avoid desaturation episodes, pulmonary hypertension crisis, trauma to the upper airways, and ventilator induced lung- injury, among other complication.
In Sweden, the care of CDH patients has been centralized since 2017, as mandated by the Swedish National Board of Health and Welfare, and the aim of this study was to investigate the incidence of FE at our high volume CDH centre, and to identify variables that could predict a successful extubation in this particular group of patients.
This is a retrospective, single centre study. Data was collected from all newborn patients with CDH admitted to our PICU at the Astrid Lindgren Childreńs Hospital, Karolinska University Hospital between January 1, 2017 and December 31, 2019. Patients were identified using the Swedish patient administrative system for intensive care units (PAS-IVA, Otimo Data AB, Kalmar, Sweden) and the Swedish Intensive Care Registry (SIR).
We reviewed patient records and data from our patient data management system (Centricity Critical Care, GE Health Care). Inclusion criteria were the presence of CDH, age 0–28 days at admission to PICU and the need for invasive ventilatory support. Exclusion criteria were death during the period of ventilatory support in the PICU, extubation as a part of withdrawal of treatment/end of life and respiratory failure due to neurological disease.
Patients were divided into two groups: (1). Successful extubation (SE; control group) including patients with a successful first extubation attempt defined as no reintubation within 24 h. Second group; Failed extubation (FE; study group) including patients requiring reintubation within 24 h of the first extubation attempt.
Demographic data included variables such as birth weight, sex, gestational age, congenital cardiac malformation, and persistent pulmonary hypertension of the newborn (PPHN) were assessed. Comorbidities other than CDH (neurological, pulmonary, renal, gastrointestinal, ongoing pneumonia or other infections) were also recorded. CDH characteristics were collected [side of the defect, observed/expected lung-head ratio (O/E LHR%), patch or primary repair]. Date and time of start and end of invasive ventilatory support, findings on chest x-ray (performed 0–48 h before extubation), including presence of infiltrates or atelectasis were also collected.
Sedation and analgesia score (Comfort B), sedative and analgesic doses, circulatory and respiratory variables, presence of vasoactive and/or pulmonary hypertension treatment and ventilatory settings were recorded 1 h prior to morning blood gas taken on the day of extubation. PaO2/FiO2%-index (PFI), oxygenation index (OI, calculated as [(FiO2xmean airway pressure)/PaO2 (converted to mmHg)]x100), oxygenation saturation index (OSI, calculated as FiO2x100/SpO2), rapid shallow breathing index [RSBI, calculated as breaths per minute/(tidal volume (ml)/ bodyweight (kg))] were calculated from the obtained data. These data were tested in monovariate analysis for successful/failed extubation and the three variables with best prediction were combined in logistic regression analysis.
Presence and type of non-invasive ventilatory support after extubation was recorded as CPAP, HFNC or other type of support i.e., flow-by oxygen through a mask, low flow nasal cannula or none and also the need of FiO2 > 0.4 2 h post extubation. Reintubation within 24 h was noted.
Mann–Whitney U-test was used for comparison of two unrelated populations. Models' discrimination was evaluated using c-statistics (ROC analyses).
Sensitivity and specificity of the ROC curve were evaluated by the Youden index (17). Calibration belt analyses were performed by RStudio 2022.02.3 Build 492 (18).
Statistical analysis was carried out using MS Excel (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism version 5.04 and version 10.2.0 (GraphPad Software inc. San Diego, CA, USA).
All reported p-values are from two-sided tests. A p-value less than 0.05 is considered significant.
The study was approved by the Swedish Ethical Review Authority (Dnr 2021-05551-01).
CDH patients (n = 54) were evaluated during the study period; 38 patients fulfilled the inclusion criteria and 16 did not meet the inclusion criteria (eight patients had never been to the PICU, four patients were older than one month on admission to the PICU, one patient was born in 2016, two patients did not have an extubation attempt in the PICU (foreign patients transferred back to referring hospital still on the ventilator), one patient was never on the ventilator during PICU care (Figure 1).
Eight patients (21.1%) required reintubation within 24 h of the first extubation attempt, and two of these eight patients subsequently failed a second extubation attempt. No patient failed more than twice.
Demographic data at the time of the first extubation attempt are presented in Table 1. Patients who failed extubation had significantly lower birth weight, surgical patch repair, longer LOS, longer time on mechanical ventilation and more associated comorbidities.
Treatment and scores at the first extubation attempt are presented in Table 2. There were significantly more females in the FE group compared to the SE group (87.5% vs. 7.7%, respectively), and other comorbidities except heart malformations were more common in the FE group (50% vs. 10%, respectively). No differences were found in gestational age, side of the defect, PIM3, ECMO-treatment or cardiac malformations. Analgosedation was similar between the FE and SE groups: intravenous opioid treatment (8.3 vs. 2.4 µg/kg/h, respectively), and midazolam (21.6 vs. 1.5 µg/kg/h, respectively), nor was there any difference in the comfort B score. After extubation, 57.8% received CPAP (87.5%) in FE group and 50% in SE group, p = 0.1056) and 36.8% received HFNC (0% in FE- group and 46.7% in SE-group, p = 0.0335). Oxygen requirement more than 40% two hours after extubation was significantly higher in the FE group as compared to the SE group (62.5% and 16.7%, respectively, p = 0.0186).
Physiological parameters at first extubation attempt are summarized in Table 3. Three factors with the lowest p-values in separate monovariate analysis for successful extubation, (OSI, RSBI and Pinsp), combined in logistic regression, proved to have a high discriminating ability to identify patients in the FE and SE groups. The area of the ROC curve (AUCROC) was 0.95 (95% CI: 0.87 to 1.00; p < 0.001), Figure 2. The calibration belt analysis with internal calibration showed a p-value of 0.799, i.e., the slope of the observed risk and predicted probability did not deviate from unity. All data were within the 95% confidence interval, Figure 3.
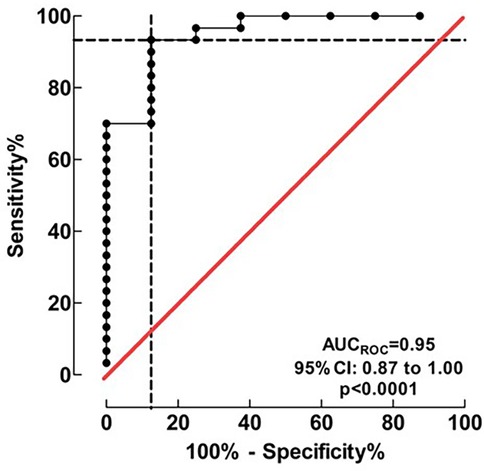
Figure 2. Discrimination of the logistic regression model for successful extubation. Three factors (OSI, RSBI and Pinsp), combined in the logistic regression model, proved a high discriminating ability to identify patients in the FE and SE groups. AUCROC value was 0.95 (95% CI: 0.87 to 1.00) indicating an excellent accuracy of discrimination.
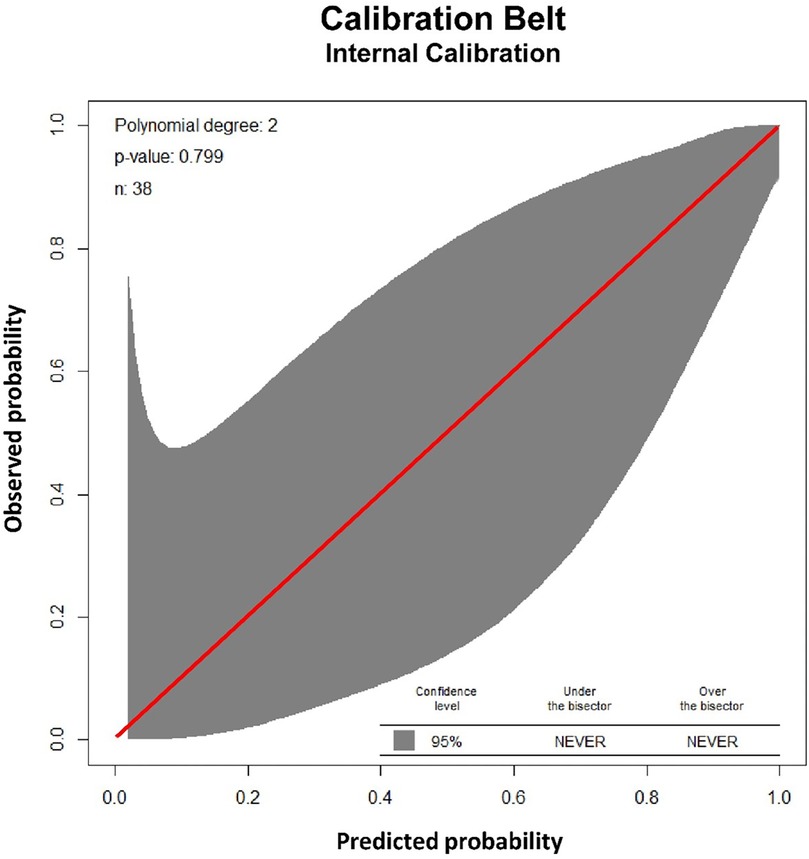
Figure 3. Validation of the logistic regression model for successful extubation. The calibration belt analysis of the logistic model including three factors (oxygenation saturation index, rapid shallow breathing and peak inspiratory pressure) using internal calibration showed a p-value of 0.799, i.e., the slope of the observed risk and predicted probability did not deviate from unity. All data were within the 95% confidence interval (shadow area).
Our study shows that it is possible to discriminate between successful and failed extubation within the first 24 h in CDH patients. Using logistic regression we identified OSI, RSBI and Pinsp as three predictive factors for successful extubation. Using these three factors in combination might be a useful tool for achieving successful extubation. The three factors reflect oxygenation, tidal volume and inspiratory pressure required to achieve effective ventilation. We used OSI because there were no missing data compared to 24% missing data for OI (not surprisingly 8 out of 9 were in the SE group, reflecting that not all patients still had an arterial line at the time for extubation). Furthermore, OSI is strongly correlated with OI (19, 20). Using a calibration plot belt we could see good agreement between our predictive model and FE (p = 0.799, Figure 2), which served as a model validation.
Mechanical ventilation is a cornerstone in neonatal and pediatric critical care. However, it is generally thought to be important to wean patients from the ventilator as early as possible to avoid associated complications. The timing of weaning is largely dependent on clinician judgement as several extubation readiness protocols have been shown to be inadequate (21, 22). Our interest was in this particular group of patients with CDH and mechanical ventilation with a suspected high incidence of FE. To our knowledge, guidelines for weaning CDH patients from mechanical ventilation are scarce or non-existent.
All infants in the FE group had a patch repair as compared to 60% in the SE group, significantly longer PICU stay (11.6 vs. 26.1 days) and also significantly longer total invasive ventilation time (140.7 vs. 340.2 h). These findings are consistent with a previous study by Schroeder et al. and Brindle et al. (16, 23), showing higher morbidity in patients requiring patch repair (16). In addition, this study also reported that ECMO treatment and low estimated lung area to head circumference ratio (LHR) were indicators of FE, whereas in our cohort 37.5% needed ECMO treatment in FE group and 10% non ECMO in SE group (p = 0.0940) not reaching statistical significance. Regarding LHR variable we had missing data in 20 patients and therefore cannot draw any conclusions in comparison.
When analysing the type of ventilatory support after extubation we found that 87.5% were on CPAP and 62.5% had an O2 demand above 40% in the FE group, the latter may reflect which is likely due to be too early extubation attempts and may be an indication for the clinician to be cautious. Our recommendation is to assess readiness for extubation by evaluating OSI, RSBI and Pinsp. This ensures the patient is physiologically stable and ready for extubation. We recommend CPAP as the first line of respiratory support after extubation, to help maintain airway patency and support breathing in the immediate post-extubation period, particularly in patients with CDH. Step-wise change to HFNC after an evaluation of the patient´s respiratory status is clinical practice in transition after CPAP treatment in our unit.
Failed extubation in pediatric ICU patients has been reported in other studies to be around 5%–10% (9, 15). There is no standard definition for the observation time for failed extubation, ranging from 24, 48 to 72 h. Our clinical practice and Swedish national quality registry define FE as occurring within 24 h. Gaies et al. (24) reported in their large study on almost 1,500 pediatric cardiac patients that 71% of FE events occurred within 24 h and Edmunds et al. (15) reported that 70% of their cohort of 632 general PICU patients failed within 24 h, although the studies had different definitions, <48 and <72 h, respectively. We believe that our results are highly relevant for this cohort of patients, but this highlights the need for a consistent definition and design of prospective multi centre studies for more comparable results. It is also important to note that zero failed extubations are likely to prolong ventilator time and subsequent complications and is probably not something to aim for. Further research is needed to assess the appropriate number of failed extubations in CDH patients.
This study has limitations. It is a retrospective, high volume CDH specialized single-centre study with a small number of patients. The low sample size may affect the statistical analysis and findings must therefore be evaluated with caution even though we have strong significance. Our centre has been a national referral centre in Sweden since 2017 and is therefore experienced in the care of CDH patients. This may, of course, affect the generalizability of our findings for unexperienced low volume hospitals and can also affect the numbers of failed extubations. Some of the data are not measurements, but scores that are determined by the nursing staff, for example analgosedation scores such as Comfort B. Some parameters are not prioritized in the initial management of a newborn with CDH, for example birth weight, making it difficult to assess weight gain and fluid overload. Echocardiographic findings, such as signs of pulmonary hypertension and cardiac dysfunction were not recorded, only the presence or absence pulmonary hypertension medications and vasoactive agents. On the other hand the data collected is very robust due to the long experience of patient data management systems in our unit.
A high incidence of FE in CDH patients (21%) was associated with OSI, RSBI and Pinsp among other factors. To our knowledge, this is the first study combining prognostic factors into a model to predict extubation failure in CDH-patients. These findings may contribute to the management of CDH-patients, and prospective studies are underway to confirm these findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Swedish Ethical Review Authority (Dnr 2021-05551-01). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from Laboratory results from clinical routine. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AR: Methodology, Writing – original draft, Writing – review & editing, Data curation, Formal Analysis. CM-B: Validation, Writing – original draft, Writing – review & editing. UF: Conceptualization, Validation, Writing – original draft, Writing – review & editing. SE: Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. JB: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was partly funded by ALF grants from dep of pediatric perioperative medicine Karolinska University Hospital, Region Stockholm.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wild KT, Hedrick HL, Ades AM, et al. Update on management and outcomes of congenital diaphragmatic hernia. J Intensive Care Med. (2023) 39(12):8850666231212874. doi: 10.1177/08850666231212874
2. Gupta VS, Harting MT, Lally PA, et al. Mortality in congenital diaphragmatic hernia: a multicenter registry study of over 5000 patients over 25 years. Ann Surg. (2023) 277(3):520–7. doi: 10.1097/SLA.0000000000005113
3. Canadian Congenital Diaphragmatic Hernia C, Puligandla PS, Skarsgard ED, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ. (2018) 190(4):E103–12. doi: 10.1503/cmaj
4. Snoek KG, Reiss IK, Greenough A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus—2015 update. Neonatology. (2016) 110(1):66–74. doi: 10.1159/000444210
5. Le Duc K, Mur S, Sharma D, et al. Antenatal assessment of the prognosis of congenital diaphragmatic hernia: ethical considerations and impact for the management. Healthcare. (2022) 10(8):1433. doi: 10.3390/healthcare10081433
6. Duncan KV, Polites S, Krishnaswami S, Scottoline BP. Congenital diaphragmatic hernia management: a systematic review and care pathway description including volume-targeted ventilation. Adv Neonatal Care. (2021) 21(5):E138–43. doi: 10.1097/ANC.0000000000000863
7. Antalova N, Klucka J, Rihova M, Polackova S, Pokorna A, Stourac P. Ventilator-associated pneumonia prevention in pediatric patients: narrative review. Children. (2022) 9(10):1540. doi: 10.3390/children9101540
8. Kohbodi GA, Rajasurya V, Noor A. Ventilator-Associated pneumonia. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2025). https://www.ncbi.nlm.nih.gov/books/NBK507711/
9. Ng P, Tan HL, Ma YJ, et al. Tests and indices predicting extubation failure in children: a systematic review and meta-analysis. Pulm Ther. (2023) 9(1):25–47. doi: 10.1007/s41030-022-00204-w
10. Ramnarayan P, Blackwood B, Khemani RG. What’s new in paediatric ventilator liberation? Intensive Care Med. (2022) 48(11):1635–7. doi: 10.1007/s00134-022-06865-0
11. Epstein SK. Extubation failure: an outcome to be avoided. Crit Care. (2004) 8(5):310–2. doi: 10.1186/cc2927
12. Sood S, Ganatra HA, Perez Marques F, Langner TR. Complications during mechanical ventilation-A pediatric intensive care perspective. Front Med. (2023) 10:1016316. doi: 10.3389/fmed.2023.1016316
13. Ing C, Chui I, Ohkawa S, Kakavouli A, Sun L. Incidence and causes of perioperative endotracheal reintubation in children: a review of 28,208 anesthetics. Paediatr Anaesth. (2013) 23(7):621–6. doi: 10.1111/j.1460-9592.2012.03920.x
14. Snoek KG, Capolupo I, van Rosmalen J, et al. Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomized clinical trial (the VICI-trial). Ann Surg. (2016) 263(5):867–74. doi: 10.1097/SLA.0000000000001533
15. Edmunds S, Weiss I, Harrison R. Extubation failure in a large pediatric ICU population. Chest. (2001) 119(3):897–900. doi: 10.1378/chest.119.3.897
16. Schroeder L, Reutter H, Gembruch U, Berg C, Mueller A, Kipfmueller F. Clinical and echocardiographic risk factors for extubation failure in infants with congenital diaphragmatic hernia. Paediatr Anaesth. (2018) 28(10):864–72. doi: 10.1111/pan.13470
17. Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3(1):32–5. doi: 10.1002/1097-0142(1950)3:1%3C32::aid-cncr2820030106%3E3.0.co;2-3
18. Finazzi S, Poole D, Luciani D, Cogo PE, Bertolini G. Calibration belt for quality-of-care assessment based on dichotomous outcomes. PLoS One. (2011) 6(2):e16110. doi: 10.1371/journal.pone.0016110
19. DesPrez K, McNeil JB, Wang C, Bastarache JA, Shaver CM, Ware LB. Oxygenation saturation Index predicts clinical outcomes in ARDS. Chest. (2017) 152(6):1151–8. doi: 10.1016/j.chest.2017.08.002
20. Muniraman HK, Song AY, Ramanathan R, et al. Evaluation of oxygen saturation Index compared with oxygenation Index in neonates with hypoxemic respiratory failure. JAMA Netw Open. (2019) 2(3):e191179. doi: 10.1001/jamanetworkopen.2019.1179
21. Fu M, Hu Z, Yu G, et al. Predictors of extubation failure in newborns: a systematic review and meta-analysis. Ital J Pediatr. (2023) 49(1):133. doi: 10.1186/s13052-023-01538-0
22. Sangsari R, Saeedi M, Maddah M, Mirnia K, Goldsmith JP. Weaning and extubation from neonatal mechanical ventilation: an evidenced-based review. BMC Pulm Med. (2022) 22(1):421. doi: 10.1186/s12890-022-02223-4
23. Brindle ME, Brar M, Skarsgard ED, Canadian Pediatric Surgery N. Patch repair is an independent predictor of morbidity and mortality in congenital diaphragmatic hernia. Pediatr Surg Int. (2011) 27(9):969–74. doi: 10.1007/s00383-011-2925-1
Keywords: congenital diaphragmatic hernia, neonatal, weaning, extubation failure, paediatric intensive care
Citation: Rannebro A, Mesas-Burgos C, Fläring U, Eksborg S and Berner J (2025) Prognostic factors for successful extubation in newborns with congenital diaphragmatic hernia. Front. Pediatr. 13:1530467. doi: 10.3389/fped.2025.1530467
Received: 19 November 2024; Accepted: 6 January 2025;
Published: 27 January 2025.
Edited by:
Adnan Bhutta, Riley Hospital for Children, United StatesReviewed by:
Saleem Islam, Aga Khan University, PakistanCopyright: © 2025 Rannebro, Mesas-Burgos, Fläring, Eksborg and Berner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Berner, am9uYXMuYmVybmVyQGtpLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.