
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 01 April 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1529725
This article is part of the Research TopicPerspectives And Recent Advances In Rare Liver Diseases In ChildrenView all 3 articles
Wilson's disease (WD) and Duchenne muscular dystrophy (DMD) are rare genetic diseases, and their co-occurrence is even rarer. Here, we describe our experience diagnosing a 6-year-old male Chinese patient presenting with an atypical phenotype and two genetic causative factors who was ultimately diagnosed with coexisting WD and DMD. We used a comprehensive and systematic evaluation of the patient's history, physical examinations, laboratory tests, and genetic testing to make the diagnosis. The patient was treated for one year with therapy to inhibit copper absorption and an anti-inflammatory treatment, and their condition remained stable. This case suggests that the inflammatory response could be a common pathogenesis between these two diseases. It also demonstrates the clinical efficacy of anti-inflammatory therapy for WD with DMD. Furthermore, this case illustrates the importance of taking a detailed history and performing thorough physical examinations to diagnose coexisting hereditary diseases.
Wilson's disease (WD) is a rare autosomal recessive genetic disease with a global incidence of approximately 1/45000–1/35,000 (now, when conducting large molecular genetic studies, the frequency is calculated as 1/7026.) (1). WD is caused by a mutation of the ATP7B gene on chromosome 13, resulting in the inactivation of the ATP7B transporter protein, obstructing copper excretion in bile. Excess copper accumulates in the liver, brain, kidney, muscles, and other tissues, which can cause common liver diseases, cirrhosis, neuropsychiatric disorders, and, rarely, muscle lesions (2).
Duchenne muscular dystrophy (DMD) is a rare X-linked recessive muscular disease occurring in 4.6/100,000 male newborns (3). The loss of dystrophin, encoded by the DMD gene, is the main pathogenesis of DMD, resulting in progressively aggravated skeletal muscle weakness and atrophy, intellectual development disorders, and cardiomyopathy (4).
WD and DMD are rare diseases involving multiple systems. Diagnosing each becomes more challenging if they are both present in the same patient owing to the complexity of the clinical phenotype and the relative inexperience of clinicians in diagnosing and managing these diseases. Therefore, this report describes our experience diagnosing a 6-year-old Chinese patient with WD and DMD.
A 6-year-old male patient was admitted to our department in July 2023 due to elevated transaminase, weakness in both lower limbs, and difficulty squatting for three years. At 3 years old, the child was diagnosed with drug-induced liver injury at a local hospital because of fever, an alanine aminotransferase (ALT) level of 167 U/L, and an aspartate aminotransferase (AST) level of 208 U/L. At 5 years old, when the child was in kindergarten, they were noted to be less physically active (based on running and other physical activities) than other children of the same age. The patient was examined at a local hospital, and the liver function results were: ALT: 133 U/L, AST: 214 U/L, creatine kinase (CK): 12138 U/L, and ceruloplasmin (CP): 107 mg/L (reference, 200–600 mg/L). The patient was diagnosed with WD and treated with penicillamine (250 mg/day) for copper removal. At 6 years old, the patient gradually developed weakness in both lower limbs, swaying from side to side when walking, and was unable to walk long distances and stand up after squatting. The child's birth, past, personal, and family histories were unexceptional (see the timeline in Figure 1).
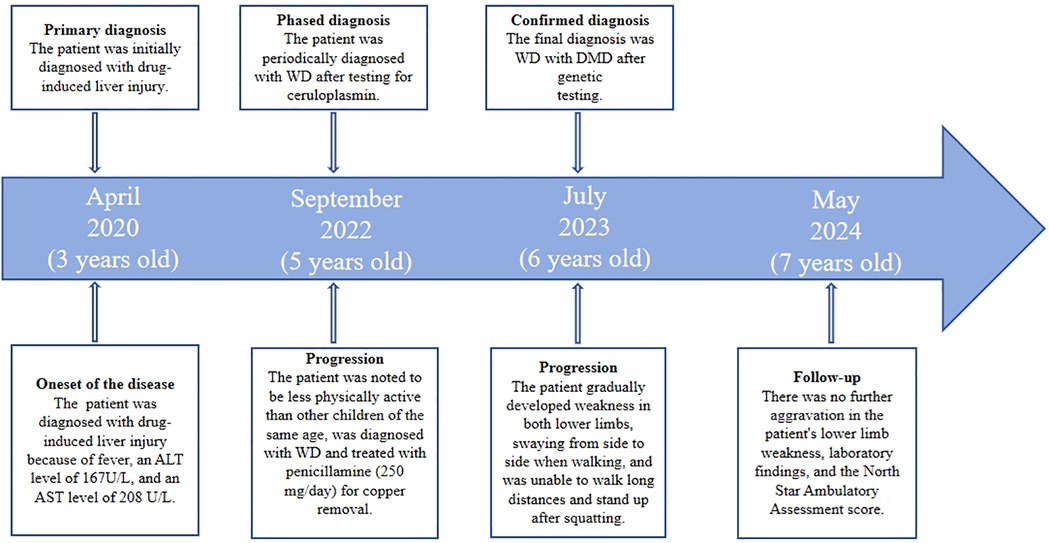
Figure 1. Case report timeline. ALT, alanine aminotransferase; AST, aspartate aminotransferase; DMD, duchenne muscular dystrophy; WD, Wilson's disease.
A physical examination found no obvious abnormalities in the heart, lung, spinal column, or abdomen. A neurological examination showed that the child had mental clarity and clear speech but walked with a “duck step” gait. A cranial nerve examination also showed no obvious abnormalities. The muscle strength of both upper limbs was grade 5, and both lower limbs were grade 4 proximal and grade 4+ distal. The muscle tone of all four limbs was normal, but the radial membrane reflexes and the knee tendon reflexes were weakened bilaterally, and Babinski's sign was not elicited bilaterally. Mild bilateral calf muscle hypertrophy with tenderness (−) (Figure 2C) and Gowers' sign (+) was also present. The Mini-Mental State Examination test score was 28, and the North Star Ambulatory Assessment score (NSAA) was 22 points.
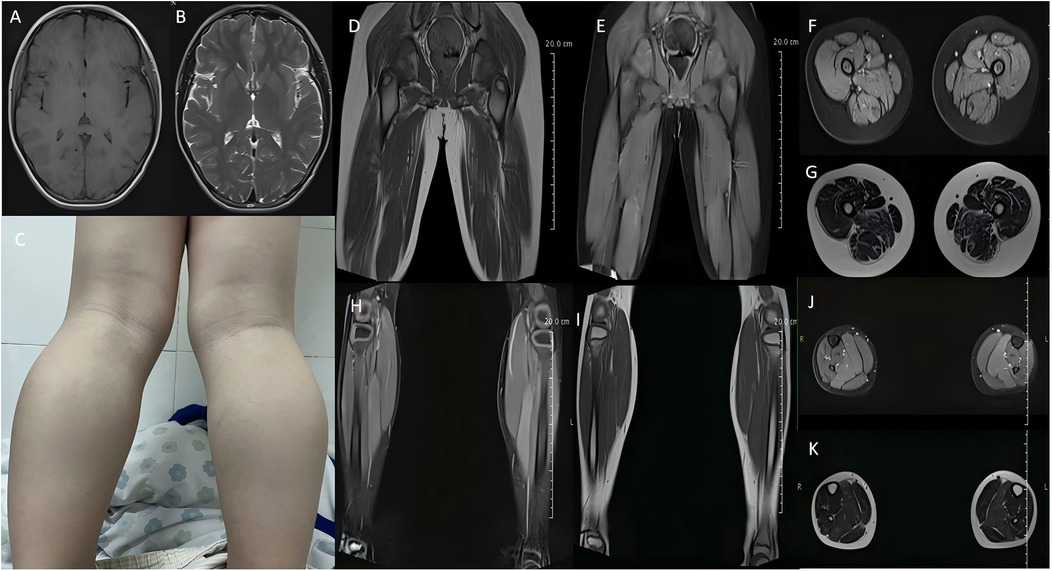
Figure 2. Cranial magnetic resonance imaging (MRI) shows no significant abnormalities (A,B). The patient had bilateral calf muscle hypertrophy (C). MRI of the thigh shows atrophy of thigh muscle groups on both sides, with slightly sparse muscle fibers, slightly enlarged intermuscular gaps, and increased intermuscular fat (D–G). MRI of the calf shows muscle swelling in some muscle groups of the posterior calf group bilaterally (H–K).
Laboratory examinations showed the following: AST: 62 U/L, ALT: 80 U/L, CK: 6,985 U/L, CP: 148.0 mg/L, interleukin (IL)-6: 7.8 pg/ml, and tumor necrosis factor-alpha (TNF-α): 9.55 pg/ml (Table 1). The hepatitis virology results were negative. The basal urine copper level was 92.07 ug/24 h.
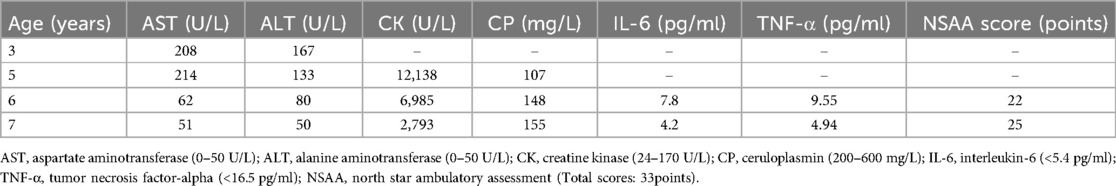
Table 1. Laboratory examination results of the 6-year-old male patient admitted for suspected Wilson's disease and Duchenne muscular dystrophy.
Electrocardiographic and cardiac ultrasonographic findings were normal. Abdominal ultrasonography showed a slightly dense echogenicity in the hepatic region, and elastography showed no or mild liver fibrosis. Auditory and visually evoked potentials showed no abnormalities. Electromyography showed myogenic damage in the right biceps brachii, right deltoid, bilateral anterior tibialis, and bilateral internal femoral muscles. The patient was negative for the corneal Kayser-Fleischer ring (−). Cranial magnetic resonance imaging revealed no significant abnormalities (Figures 2A,B), but magnetic resonance imaging of top the thigh shows atrophy of thigh muscle groups on both sides, with slightly sparse muscle fibers, slightly enlarged intermuscular gaps, and increased intermuscular fat (Figures 2D–G); and magnetic resonance imaging of the calf shows muscle swelling in some muscle groups of the posterior calf group bilaterally (Figures 2H–K).
The ATP7B gene had complex heterozygous variations of c.2333G > T (P.RG778Leu) and c.2804G > T (P.HR935Met) (Figures 3A,B). According to the American College of Medical Genetics (ACMG) guidelines, both mutation sites are pathogenic mutations, and a family verification analysis showed that both mutation sites originated from the patient's parents (Figures 3C,D). Additionally, c.6117G > T (p.Lys2039Asn) hemizygous missense variants were present in the DMD gene (Figure 3E), and the ACMG guidelines state that this mutation site is pathogenic. A phylogenetic analysis showed that the patient's mother had a heterozygous mutation at this site (Figure 3F).

Figure 3. A mutational analysis of 21 exons and the flanking sequences of the ATP7B gene was performed via targeted sequencing and verified by Sanger sequencing. Compound heterozygous missense variants in ATP7B of pre-documented individuals at c.2333G > T (p.Arg778Leu) and c.2804C > T (p.Thr935Met) (A,B) are present, all of which are known pathogenic mutations. Lineage validation reveals that the variants originated from the father (c.2333G > T) (C) and mother (c.2804C > T) (D). A mutation analysis of the exon and flanking sequences of the DMD gene was performed using targeted sequencing and validated by Sanger sequencing. The pre-documented patient had a c.6117G > T (p.Lys2039Asn) hemizygous missense variant in DMD with a known pathogenic mutation (E). Lineage validation confirms that the variant originated from the mother (c.6117G > T) (F). Pedigree of the family (G). Lines within the circle or square indicate carriers of ATP7B gene, and white dots on a black background within the circle indicate DMD gene carrier. The black arrow indicates the proband.
The final diagnosis was WD (presymptomatic) with DMD. Thus, the patient was administered dimercaptopropanesulfonic acid (0.25 g IV) for 14 days during hospitalization to reduce the copper level. After discharge, the patient was treated with zinc gluconate (420 mg/d) to inhibit copper absorption, prednisone acetate (10 mg/kg/week) for anti-inflammation, and glutathione (0.3 g/d) for detoxification and anti-inflammation. The patient underwent a follow-up visit in May 2024 at 7 years old. Worsening of the bilateral lower extremity weakness was not observed, the NSAA score was 25 points, and the laboratory results were: AST: 51 U/L, ALT: 50 U/L, CK: 2,793 U/L, IL-6: 4.2 pg/ml, and TNF-α: 4.94 pg/ml.
WD and DMD are rare genetic disorders, and their co-occurrence is even rarer, occurring in approximately 4.3% of patients diagnosed with a genetic disorder. In such cases, the child's phenotype might be atypical owing to the synergistic effect of the two genetic causative factors, which makes clinical diagnosis challenging. However, the prevalence of coexisting WD and DMD may be underestimated because neurologists tend to overlook elevated ALT levels caused by WD when treating patients with significantly elevated CK levels. On the other hand, DMD is often diagnosed based on elevated ALT and AST levels in liver function tests, which can be easily missed when there are no signs of muscle weakness. Therefore, it is easy to misdiagnose the disease as liver disease, prolonging the duration until a correct diagnosis.
Most individuals with WD develop symptoms between ages 5 and 35 years. Recognizing and diagnosing this treatable disease early is important because it can involve multiple systems and lead to diverse clinical manifestations. Most pediatric patients with WD present with abnormal liver function, making it imperative to evaluate any child with hepatic dysfunction since the early initiation of lifelong therapy is crucial to the patient's prognosis (5). The first symptoms of DMD appear between ages 2 and 3 years (6). The primary early manifestations are muscular atrophy and weakness of the proximal lower extremity and pelvic girdle, pseudohypertrophy of the calf gastrocnemius muscle, a “duck walk,” and Gowers' sign. In patients with no family history, DMD is usually diagnosed at ages 4–5 years (7). Generally, the condition is relatively stable before the age of 7 (6), after which the disease gradually progresses, resulting in difficulties in climbing stairs, falling, and generally losing the ability to walk autonomously around 12 years old.
In this case, the patient had markedly elevated ALT, AST, and CK levels but markedly reduced CP levels at an early stage. However, they were without symptoms; thus, they were diagnosed with WD. However, the cause of the elevated CK level was not further investigated until the patient developed signs and symptoms suggestive of progressive muscular dystrophy, such as bilateral lower extremity weakness and Gowers' sign positivity. In 2023, the patient was examined in our department, where we performed liver function, CP, urine copper, serum inflammatory marker, and electromyography tests, as well as gene analyses, where we found a p.(Arg778Leu) mutation on one allele and a p.(Thr935Met) mutation on the other of the ATP7B gene. Furthermore, we identified the patient had a deletion variant in exons 46–50 of the DMD gene, which confirmed the diagnosis of coexisting WD and DMD (8, 9).
WD and DMD can involve multiple systems; therefore, clarifying the relationship between these diseases is important. The inflammatory response is the body's most common pathological and physiological process, and WD and DMD have been associated with this response (10, 11). In this case, the patient had co-occurring WD and DMD, which resulted in abnormal copper metabolism, copper accumulation in the tissues, and cup-induced oxidative stress damage due to ATP7B gene defects, causing an inflammatory response (10). Levels of inflammatory factors in the serum, such as IL-6 and TNF-α, are abnormally high in patients with WD, attributed to the accumulation and abnormal metabolism of copper in the body, leading to oxidative stress and cellular damage, which triggers an inflammatory response and, consequently, further increases the serum levels of several inflammatory factors (12). A high copper level contributes to the inflammatory response that causes liver lesions (13); it can also trigger muscle lesions (14). Our patient had elevated serum inflammatory markers during their hospitalization in 2023 (IL-6: 7.8 pg/ml and TNF-α:9.55 pg/ml). Therefore, maintaining appropriate copper levels in the body is essential to suppress inflammation and reduce liver and muscle damage.
WD was one of the first liver diseases for which an effective drug therapy was developed (15), and its clinical prognosis is closely related to the timing of treatment. There is still no effective treatment for DMD, and the disease progresses rapidly, eventually causing disability and death. Therefore, finding an effective treatment for patients with DMD is critical. Glucocorticoids are the only treatment shown to slow the progression of DMD through their anti-inflammatory effects (16). Treatment is recommended to start at approximately 4 years old to minimize the rate of muscle function decline (17). For our patient, we administered zinc gluconate to inhibit copper absorption, prednisone (10 mg/kg/week) as an anti-inflammatory treatment (18), and preventative therapy against adverse hormonal reactions.
Motor symptom scores (e.g., the NSAA score) and the CK level in patients with DMD can directly and indirectly reflect the severity of the condition. In this case, after one year of treatment, the ALT, AST, CK, IL-6, and TNF-α levels decreased, the Mini-Mental State Examination score did not change, and the NSAA score decreased compared to the pre-treatment values, similar to a previous report (19). These changes were mainly characterized by an increase in muscle strength and a prolongation of the walking time; hormonal adverse effects did not occur. However, the patient is only seven years old, and the specific treatment plan must be adjusted based on the severity of the patient's clinical symptoms and the expected quality of survival, with multidisciplinary collaboration among the departments of neurology, hepatology, and rehabilitation to develop an optimal individualized plan and clinical efficacy follow-up assessment.
The co-occurrence of WD and DMD is exceedingly rare, and children with WD and DMD may present with an atypical phenotype, given the presence of two genetic causative factors. Therefore, clinicians should perform comprehensive and systematic assessments of the patient's history and physical examinations. As early as possible, whole-exome sequencing combining a variety of molecular genetic tests would be helpful for early diagnosis and treatment to improve their clinical prognosis. Here, we report the case of a Chinese patient who presented with both WD and DMD, highlighting a possible link between these two diseases. However, further clinical and basic research is required to better understand the disease-related mechanisms and associations.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by The studies involving humans were approved by the Medical Research Ethics Committee of the Affiliated Hospital of the Neurology Institute of Anhui University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JW: Data curation, Investigation, Writing – original draft. DS: Conceptualization, Formal analysis, Writing – original draft. YW: Investigation, Methodology, Writing – original draft. MF: Conceptualization, Formal analysis, Writing – original draft. XW: Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article. This research was financially supported by the Scientific Research Program of Higher Education Institutions in Anhui Province (No. 2023AH050849).
The authors thank the patient and their family for placing their trust in us.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ovchinnikova EV, Garbuz MM, Ovchinnikova AA, Kumeiko VV. Epidemiology of Wilson's disease and pathogenic variants of the ATP7B gene leading to diversified protein disfunctions. Int J Mol Sci. (2024) 25(4):2402. doi: 10.3390/ijms25042402
2. Aggarwal A, Bhatt M. Wilson disease. Curr Opin Neurol. (2020) 33(4):534–42. doi: 10.1097/WCO.0000000000000837
3. Salari N, Fatahi B, Valipour E, Kazeminia M, Fatahian R, Kiaei A, et al. Global prevalence of Duchenne and Becker muscular dystrophy: a systematic review and meta-analysis. J Orthop Surg Res. (2022) 17(1):96. doi: 10.1186/s13018-022-02996-8
4. Fortunato F, Farnè M, Ferlini A. The DMD gene and therapeutic approaches to restore dystrophin. Neuromuscul Disord. (2021) 31(10):1013–20. doi: 10.1016/j.nmd.2021.08.004
5. Prashanth LK, Taly AB, Sinha S, Arunodaya GR, Swamy HS. Wilson's disease: diagnostic errors and clinical implications. J Neurol Neurosurg Psychiatry. (2004) 75(6):907–9. doi: 10.1136/jnnp.2003.026310
6. Mercuri E, Bönnemann CG, Muntoni F. Muscular dystrophies. Lancet. (2019) 394(10213):2025–38. doi: 10.1016/S0140-6736(19)32910-1
7. van Dommelen P, van Dijk O, de Wilde JA, Verkerk PH. Short developmental milestone risk assessment tool to identify Duchenne muscular dystrophy in primary care. Orphanet J Rare Dis. (2024) 19(1):192. doi: 10.1186/s13023-024-03208-8
8. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. DMD care considerations working group. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol (2010) 9(2):177–89. doi: 10.1016/S1474-4422(09)70272-8
9. Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. (2003) 23(3):139–42. doi: 10.1034/j.1600-0676.2003.00824
10. Goyal MK, Sinha S, Patil SA, Jayalekshmy V, Taly AB. Do cytokines have any role in Wilson's disease? Clin Exp Immunol. (2008) 154(1):74–9. doi: 10.1111/j.1365-2249.2008.03755
11. Mizunoya W, Upadhaya R, Burczynski FJ, Wang G, Anderson JE. Nitric oxide donors improve prednisone effects on muscular dystrophy in the mdx mouse diaphragm. Am J Physiol Cell Physiol. (2011) 300(5):C1065–77. doi: 10.1152/ajpcell.00482.2010
12. Rajeshwari M, Suri V, Kaur K, Suri A, Garg A, Sharma MC, et al. Intracranial interhemispheric osteochondrolipoma: diagnostic and surgical challenges in an extremely rare entity. Neuropathology. (2016) 36(5):470–4. doi: 10.1111/neup.12294
13. Spee B, Arends B, van den Ingh TS, Penning LC, Rothuizen J. Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs. J Vet Intern Med. (2006) 20(5):1085–92. doi: 10.1892/0891-6640(2006)20[1085:cmaosi]2.0.co;2
14. Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. (2014) 20(3):460–73. doi: 10.1089/ars.2013.5371
15. Roberts EA, Schilsky ML. Division of gastroenterology and nutrition, hospital for sick children, Toronto, Ontario, Canada. A practice guideline on Wilson disease. Hepatology. (2003) 37(6):1475–92. doi: 10.1053/jhep.2003.50252
16. Beytía Mde L, Vry J, Kirschner J. Drug treatment of Duchenne muscular dystrophy: available evidence and perspectives. Acta Myol. (2012) 31(1):4–8.
17. Sarkozy A, Quinlivan R, Bourke JP, Ferlini A, ENMC 263rd Workshop Study Group. 263rd ENMC international workshop: focus on female carriers of dystrophinopathy: refining recommendations for prevention, diagnosis, surveillance, and treatment. Hoofddorp, The Netherlands, 13–15 may 2022. Neuromuscul Disord. (2023) 33(3):274–84. doi: 10.1016/j.nmd.2023.01.003
18. Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. (2011) 77(5):444–52. doi: 10.1212/WNL.0b013e318227b164
19. Moxley RT 3rd, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the quality standards subcommittee of the American academy of neurology and the practice committee of the child neurology society. Neurology. (2005) 64(1):13–20. doi: 10.1212/01.WNL.0000148485.00049.B7
Keywords: Wilson's disease, Duchenne muscular dystrophy, rare liver disease, inflammatory response, copper
Citation: Wang J, Sun D, Wang Y, Fang M and Wang X (2025) Co-occurrence of Wilson's disease and Duchenne muscular dystrophy in a Chinese patient: a case report. Front. Pediatr. 13:1529725. doi: 10.3389/fped.2025.1529725
Received: 17 November 2024; Accepted: 19 March 2025;
Published: 1 April 2025.
Edited by:
Atsushi Asakura, University of Minnesota Twin Cities, United StatesReviewed by:
Amy Brower, Creighton University, United StatesCopyright: © 2025 Wang, Sun, Wang, Fang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xun Wang, bmV1cm9kb2NAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.