
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 13 February 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1528167
Objective: Washed microbiota transplantation (WMT) has emerged as a promising therapeutic strategy for autism spectrum disorder (ASD), though the factors that influence its efficacy remain poorly understood. This study explores the impact of gastrointestinal (GI) symptoms on the effectiveness of WMT in ASD.
Methods: Clinical data encompassing ASD symptoms, GI disturbances, and sleep disorders were collected from patients with ASD undergoing WMT. The therapeutic impact of WMT and the contributing factors to its efficacy were assessed.
Results: WMT significantly reduced scores on the Aberrant Behavior Checklist (ABC), Childhood Autism Rating Scale (CARS), and Sleep Disturbance Scale for Children (SDSC), alongside a significant reduction in the incidence of constipation, abnormal stool forms, and diarrhea (all p < 0.05). After six courses of WMT, substantial reductions were observed in ABC, CARS, and SDSC scores, with increased treatment courses correlating with greater improvement (p < 0.05). Multiple linear regression analysis revealed that WMT efficacy was enhanced in patients with pre-existing GI symptoms (diarrhea: β = 0.119, p < 0.001; abnormal stool form: β = 0.201, p < 0.001) and those receiving a higher number of treatment courses (β = 0.116, p < 0.001). Additionally, the analysis indicated that treatment outcomes were more favorable in patients who had not undergone adjunct interventions (β = −0.041, p = 0.002), had a longer disease duration (β = 0.168, p = 0.007), and exhibited more severe disease symptoms (β = 0.125, p < 0.001).
Conclusion: WMT significantly alleviates both ASD and GI symptoms, along with sleep disturbances, in affected individuals. Six treatment courses resulted in notable improvement, with increased course numbers further improving therapeutic outcomes. Furthermore, pre-treatment GI symptoms, such as diarrhea and abnormal stool forms, may influence the effectiveness of WMT. Notably, patients who did not receive additional interventions, had a prolonged disease duration, and presented with more severe symptoms experienced markedly improved treatment responses.
Autism spectrum disorder (ASD) is a multifaceted neurodevelopmental condition that impacts the central nervous system, resulting in impairments in communication, behavior, and social interactions (1). The global prevalence of ASD was approximately 1% in 2022 (2), with a continually rising incidence, and a male-to-female ratio of 4–5:1 (3). Despite this prevalence, no definitive cure for ASD currently exists.
The microbiota-gut-brain axis (MGBA) represents the complex bidirectional communication between the brain and the gastrointestinal (GI) tract (4). The stability of the gut microbiota plays a pivotal role in this axis, as dysbiosis can negatively affect not only the GI system but also psychiatric health (5, 6). Patients with ASD often present with disturbances in gut microbiota composition (7), and research suggests that fecal microbiota transplantation (FMT) may alleviate some of the symptoms of ASD (8, 9, 10). Washed microbiota transplantation (WMT), an advanced form of FMT (11), involves microfiltration of microbiota preparations, utilizing an automated purification system to remove fecal particles, parasite eggs, and fungi. This process also eliminates metabolites with pro-inflammatory effects from the fecal microbiota supernatant, resulting in fewer FMT-related adverse events compared to traditional manual FMT preparation (12). Furthermore, WMT has been shown to improve symptoms of ASD (13, 14). However, its therapeutic efficacy remains variable among patients with ASD.
GI symptoms are prevalent comorbidities in ASD, including diarrhea, constipation, and abnormal stool forms (15, 16), with the incidence of these symptoms being six times higher in children with ASD compared to their typically developing peers (17). These GI disturbances can exacerbate behavioral issues, such as rigidity, hyperactivity, and social withdrawal (18, 19). Moreover, the severity of symptoms and disease duration have been shown to influence the effectiveness of FMT in conditions like ulcerative colitis (UC) and inflammatory bowel disease (IBD) (20, 21). Given these considerations, the present study aims to investigate the factors that influence the effectiveness of WMT in ASD treatment, with a particular focus on GI symptoms.
Patients from the First Affiliated Hospital of Guangdong Pharmaceutical University who met the DSM-5 criteria for a diagnosis of ASD and received at least two consecutive WMT courses between June 2019 and June 2024 were recruited for the study. Inclusion criteria were as follows: (1) age between 2 and 18 years; (2) confirmed ASD diagnosis; (3) signed informed consent from the patients’ parents or guardians; and (4) willingness of the patients' parents or guardians to complete questionnaires related to ASD and GI symptoms. Exclusion criteria included: (1) severe heart, lung, liver, or kidney disease; (2) a history of brain injury, cerebral palsy, head tumors, or major brain malformations; and (3) participation in other clinical trials within the last month. The study was approved by the institutional review board of our hospital (#2020-14). Informed consent was obtained from the parents or guardians of all included patients before enrollment.
Healthy donors underwent thorough screening for WMT, as previously outlined (13). This screening process included a questionnaire and a physical examination by a clinician. Stool and blood samples were tested to rule out any potential infectious or communicable diseases (22). Informed consent was also obtained from the parents or guardians of the healthy donors before initiating the WMT procedures. WMT was routinely administered to hospitalized patients at our institution using fresh microbiota, as described in prior research (23). In brief, a homogeneous fecal suspension was prepared with a ratio of 100 g of feces to 500 ml of normal saline. The fecal suspension was then filtered using a microfiber filtration instrument (GenFMTer; FMM Medical, Nanjing, China) to remove food particles, inflammatory substances, and fungi. This process enhances the safety of the microbiota suspensions. Each WMT treatment involved the administration of a fecal suspension containing approximately 5.0 × 1013 bacteria, delivered daily (60–90 ml) for 6 consecutive days via a transendoscopic enteral tube. According to the Nanjing Consensus on the Methodology of Washed Microbiota Transplantation (24), only fresh, healthy microbiota were used, with preparation and transplantation completed within 1 h. Patients were instructed to remain in the left lateral position for 2 h post-injection and to consume light meals for at least 2 days after WMT. Follow-up WMT treatments were scheduled monthly.
Patients were assessed at baseline, prior to the first course of WMT treatment, and before each subsequent course. Clinical characteristics were gathered from the patients' electronic medical records. The following questionnaires were employed to assess ASD- and GI-related symptoms. The Aberrant Behavior Checklist (ABC) (25) is a validated and reliable instrument widely used to assess ASD symptoms (26). It consists of 57 items across five key domains: stereotypy, irritability, lethargy, inappropriate speech, and hyperactivity. The Childhood Autism Rating Scale (CARS) (27) is a 15-item tool used to diagnose ASD and assess the overall severity of symptoms. Patients with scores between 30 and 36 are considered to have mild to moderate symptoms, while those with scores greater than 36 are categorized as having severe symptoms. The CARS is completed by pediatricians or other qualified professionals (28). The Sleep Disturbance Scale for Children (SDSC) is a 34-item questionnaire completed by parents, with scores ranging from 34 to 170. Scores above 39 suggest the presence of a sleep disorder (29). The Bristol Stool Form Scale (BSFS) is used to evaluate stool consistency (30), where scores of 1 or 2 indicate hard stool, 6 or 7 represent soft stool, and scores of 3–5 reflect normal stool consistency. The Rome III criteria were applied to define constipation (31), while diarrhea was characterized by the occurrence of three or more loose or watery stools (scores of 5–7 on the BSFS) per day.
All statistical analyses were performed using SPSS 23.0 (IBM Corp., Armonk, NY, United States) and Prism 8 software (GraphPad Prism Inc., San Diego, CA, United States). Continuous data were expressed as means (standard deviation) or medians (interquartile range), depending on the distribution, while categorical data were presented as n (%). Categorical variables were analyzed using Chi-square and Fisher's exact tests. The normality of continuous variables was tested using the Shapiro–Wilk test. For comparing differences in continuous variables between independent groups, the unpaired Student's t-test (for normal distribution) or Mann–Whitney U-test (for non-normal distribution) was applied. Paired data were analyzed using the paired Student's t-test (for normal distribution) or Wilcoxon signed-rank test (for non-normal distribution). Multivariate linear regression analysis was conducted to identify potential influencing factors. A p-value of less than 0.05 was considered statistically significant.
Based on the inclusion and exclusion criteria, a total of 129 patients with ASD (110 males and 19 females) were included in the analysis (Table 1). These 129 patients collectively completed 566 treatment courses. Specifically, 80 patients underwent four treatment courses, while 11 patients received seven courses. Additionally, 97 patients were treated with supplementary therapies such as behavioral, communication, or educational therapy, or medication, whereas 32 patients were exclusively administered WMT without additional interventions. Among the 566 WMT courses, only 14 resulted in adverse effects, which included fever (1.00%), diarrhea (0.50%), abdominal pain (0.70%), and vomiting (0.50%) (Table 2). All adverse effects were mild and resolved following symptomatic treatment, with no serious adverse events reported.
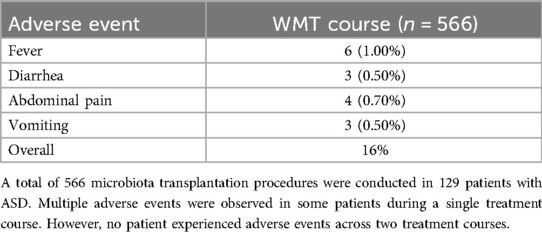
Table 2. Treatment-related adverse events observed during the washed microbiota transplantation procedure.
The improvement in ASD symptoms after WMT was primarily assessed using the ABC, CARS, and SDSC. The post-treatment scores from these questionnaires were compared to baseline scores, as shown in Figure 1. The results demonstrated that WMT led to a significant reduction in the ABC (Figure 1A), CARS (Figure 1B), and SDSC scores (Figure 1C), indicating improvement in ASD symptoms.
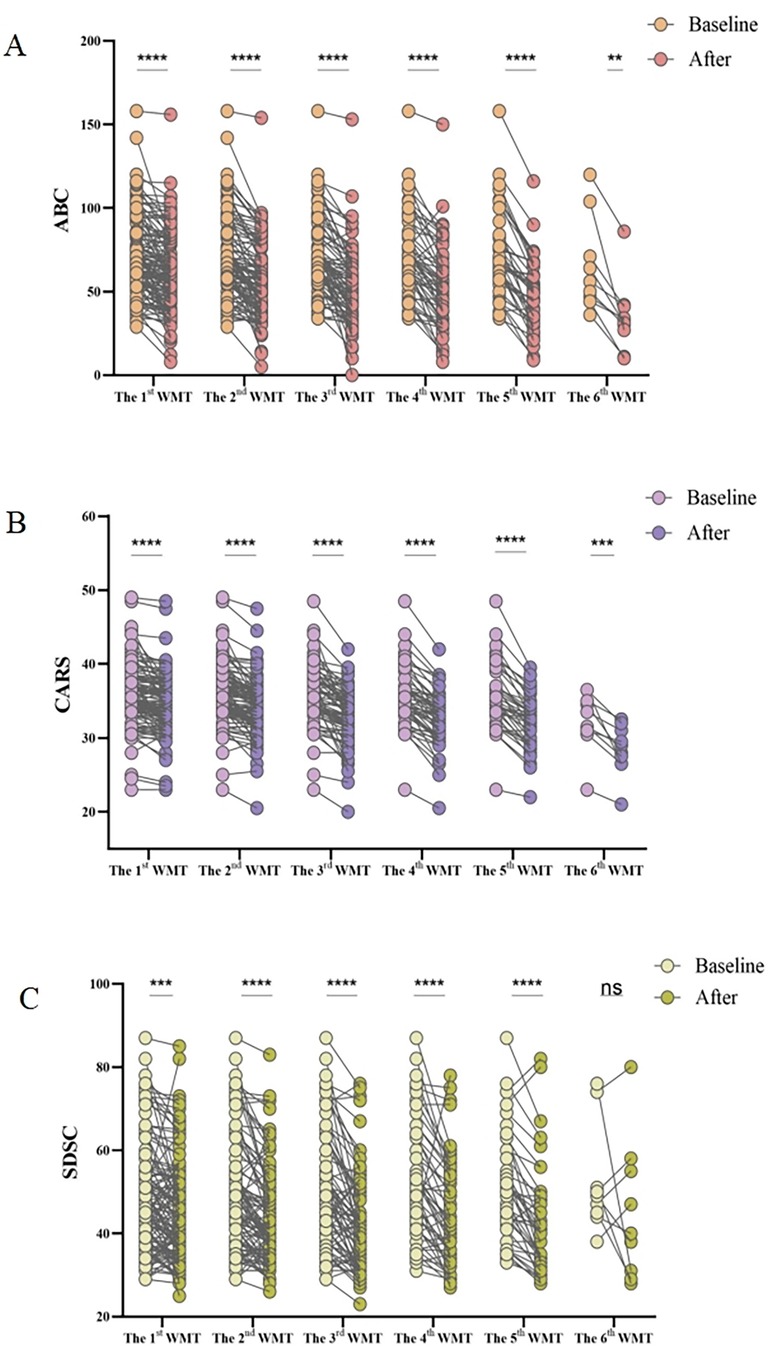
Figure 1. Effect of WMT on ASD symptoms and sleep disorders. Changes in the ABC (A), CARS (B), and SDSC (C) scores. Paired data obtained after each treatment course were compared with the primary baseline data. ABC, Aberrant Behavior Checklist; ASD, autism spectrum disorder; CARS, Childhood Autism Rating Scale; SDSC, Sleep Disturbance Scale for Children; WMT, washed microbiota transplantation. Statistical analysis was performed using the paired Student's t-test or the Wilcoxon signed-rank test. *Denotes statistical significance at p < 0.05; ** at p < 0.01; *** at p < 0.001; and **** at p < 0.0001. nsindicates non-significance at p > 0.05.
Furthermore, the relationship between the number of WMT courses and treatment efficacy was explored. Figure 2 illustrates the changes in questionnaire scores (ΔCARS, ΔABC, and ΔSDSC) before and after each increase in the number of WMT courses. The findings suggested that a greater number of treatment courses correlated with a more substantial reduction in symptom severity. Specifically, after six courses of WMT, there was a significant decrease in CARS scores, indicating that increasing the number of treatment courses led to more pronounced improvements in ASD symptoms (Figure 2A). For both the ABC and SDSC scores, significant reductions were observed after three courses, with further treatment leading to increasingly notable improvements (Figures 2B,C).
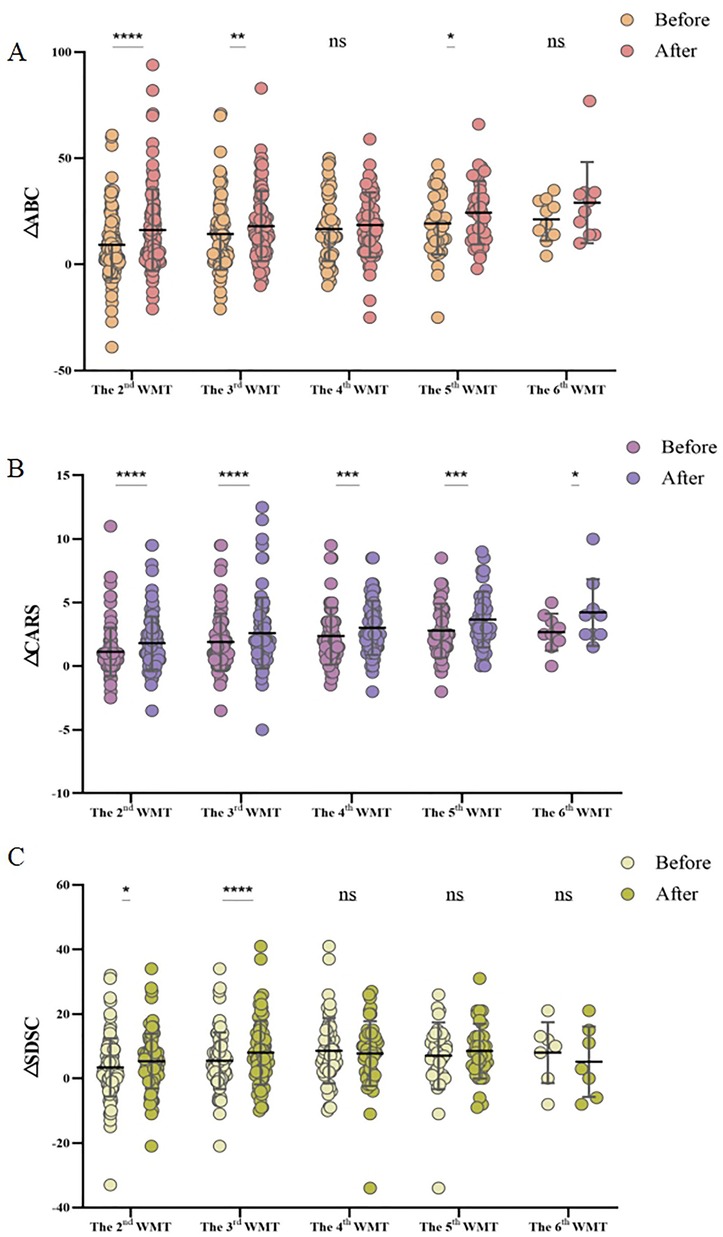
Figure 2. Effect of the number of WMT treatment courses on the ABC (A), CARS (B), and SDSC (C) scores. MABC: the difference between the ABC scores after WMT and at primary baseline; MCARS: the difference between the CARS scores following WMT and at primary baseline; and MSDSC: the difference between the SDSC scores after WMT and at primary baseline. ABC, Aberrant Behavior Checklist; CARS, Childhood Autism Rating Scale; SDSC, Sleep Disturbance Scale for Children; WMT, washed microbiota transplantation. The paired Student's t-test and Wilcoxon signed-rank test were employed for data comparison. Horizontal lines in the graphs represent the mean ± standard deviation. * denotes statistical significance at p < 0.05; ** at p < 0.01; *** at p < 0.001; and **** at p < 0.0001, nsindicates non-significance at p > 0.05.
GI symptoms, such as constipation, diarrhea, and abnormal stool form, are common comorbidities in patients with ASD. To evaluate the effect of WMT on GI symptoms, the incidence of these symptoms before and after each treatment course was compared. Compared to the baseline incidence of constipation, the occurrence of constipation was significantly reduced after the first WMT course (0.37 vs. 0.17, p < 0.0001, Figure 3A). By the sixth treatment course, all patients were free of constipation. Similarly, the incidence of abnormal stool form significantly decreased after the first WMT course (0.31 vs. 0.15, p < 0.0001, Figure 3C). With increasing WMT courses, the proportion of abnormal stool form continued to decline. However, the proportion of patients experiencing diarrhea did not significantly change across the six treatment courses (Figure 3B).
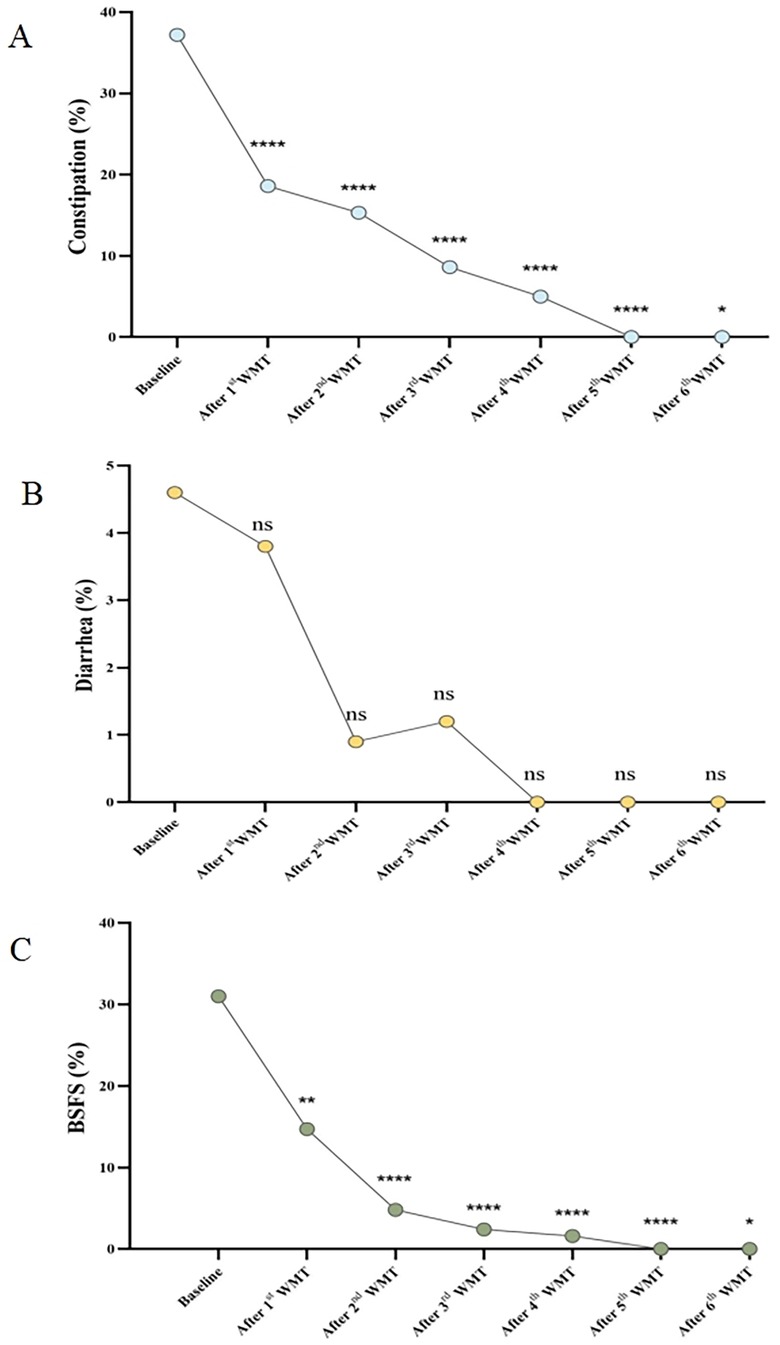
Figure 3. Effect of WMT on GI symptoms. (A) Changes in the proportion of patients with constipation. (B) Changes in the proportion of patients with diarrhea. (C) Changes in the proportion of patients with abnormal stool form according to the BSFS score. Data collected after each WMT treatment course were compared with the primary baseline data. WMT, washed microbiota transplantation; and BSFS, Bristol Stool Form Scale (abnormal stool form: scores of 1, 2, and 6; normal stool form: scores of 3, 4, and 5). Statistical significance was evaluated using the Wilcoxon signed-rank test, Fisher's exact test, and Chi-square test. *Denotes statistical significance at p < 0.05; ** at p < 0.01; *** at p < 0.001; and **** at p < 0.0001. nsindicates non-significance at p > 0.05.
To assess the impact of GI symptoms on WMT efficacy, changes in the ΔABC, ΔCARS, and ΔSDSC scores (Figure 4) were compared between patients with and without GI symptoms before and after each treatment course. The results indicated that patients without constipation showed a significantly better response to WMT. Although, in the univariate analysis, patients with diarrhea and abnormal stool form tended to exhibit greater improvement in autism symptoms compared to those without these symptoms, the differences were not statistically significant.
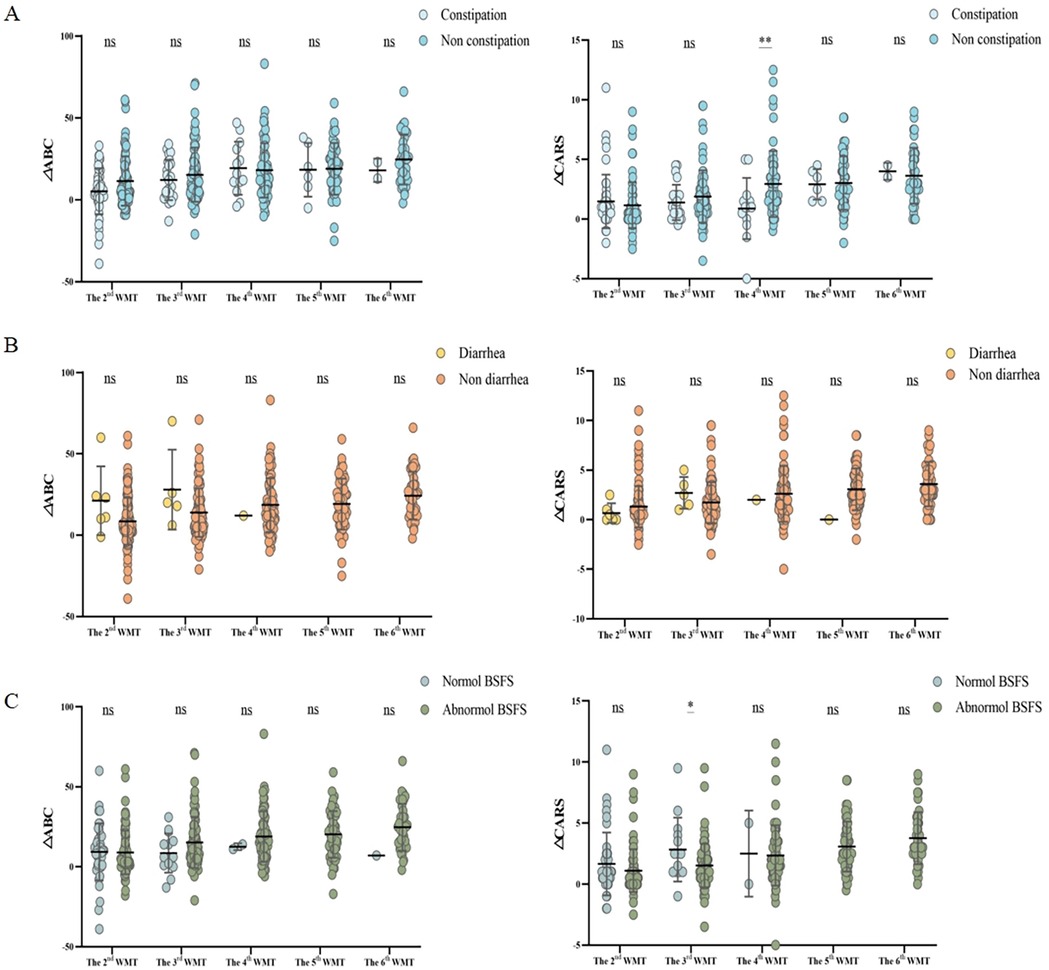
Figure 4. Effect of the number of WMT treatment courses on gastrointestinal symptoms according to the ABC and CARS scores. Changes in the proportion of patients with (A) constipation, (B) diarrhea, and (C) abnormal stool form (based on the BSFS score). ΔABC: the difference between the ABC scores after WMT and at primary baseline; ΔCARS: the difference between the CARS scores following WMT and at primary baseline; WMT, washed microbiota transplantation; ABC, Aberrant Behavior Checklist; CARS, Childhood Autism Rating Scale; and BSFS, Bristol Stool Form Scale (abnormal stool form: scores of 1, 2, and 6; normal stool form: scores of 3, 4, and 5). Student's t-tests were employed to compare the data. Horizontal lines in the graphs represent the mean ± standard deviation. * denotes statistical significance at p < 0.05; ** at p < 0.01; *** at p < 0.001; and **** at p < 0.0001. nsindicates non-significance at p > 0.05.
Multiple linear regression analyses were performed to evaluate the influence of various factors on WMT efficacy in ASD treatment (Tables 3, 4). The results revealed that patients with ASD and GI symptoms, such as diarrhea and abnormal stool form, demonstrated more pronounced therapeutic responses, as did those without constipation. Additionally, a positive correlation was found between the number of treatment courses and improved efficacy, with a greater number of WMT courses leading to better outcomes. Longer disease duration was also associated with enhanced efficacy. Moreover, patients who did not receive additional interventions and those with more severe symptoms tended to show better therapeutic responses. Males and patients with a lower BMI also exhibited relatively improved outcomes. However, age did not significantly influence WMT efficacy in ASD treatment. In summary, patients with ASD and co-existing GI symptoms prior to WMT treatment may experience more favorable outcomes.
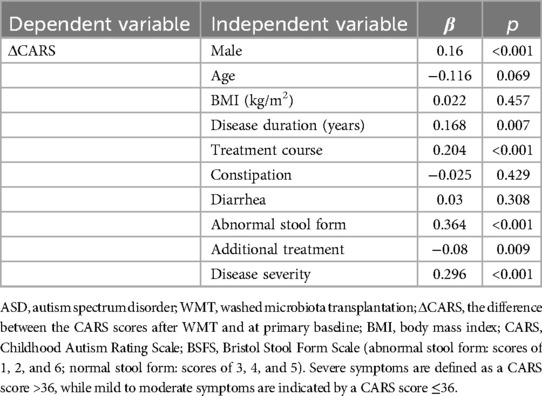
Table 3. Multiple linear regression analysis of the influence of different factors on the efficacy of washed microbiota transplantation in patients with ASD based on their CARS scores.
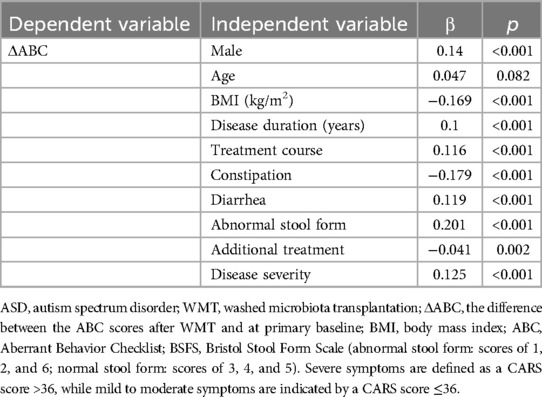
Table 4. Multiple linear regression analysis of the influence of different factors on the efficacy of washed microbiota transplantation in patients with ASD based on their ABC scores.
This study demonstrates that WMT significantly improves both ASD-related symptoms and sleep disorders. Six courses of WMT were particularly effective in treating ASD, with the therapeutic effect improving as the number of courses increased. Notably, patients who did not receive additional interventions, as well as those with severe symptoms and prolonged disease duration, exhibited the most significant improvements. Additionally, WMT alleviated constipation, diarrhea, and abnormal stool form. To our knowledge, this study is the first to suggest that pre-treatment GI symptoms, including diarrhea and diarrhea abnormal stool form, may influence the efficacy of WMT in ASD therapy.
Recent research has recognized FMT as a promising therapeutic option for ASD treatment (32). An open-label study found that patients with ASD who received FMT exhibited significant improvements in their autism symptoms (33). Further studies have shown that microbiota transfer therapy can substantially reduce both GI and ASD symptoms, with these improvements persisting for at least 8 weeks post-treatment (9, 34). WMT, an enhanced version of FMT, has also been proven to significantly improve ASD, GI symptoms, and sleep disturbances (13, 14, 35). In line with these findings, our study adds to the growing body of evidence, offering validation with a larger sample size.
The duration of FMT has been previously linked to its efficacy. For instance, Hui et al. demonstrated that multiple FMT infusions significantly improved the clinical remission rate of diarrhea in patients with recurrent Clostridium difficile infection (CDI) (36). Similarly, multiple courses of WMT have been shown to outperform single or double courses in patients with hyperlipidemia (37). Additionally, a study indicated that three FMT courses led to significant improvements in ASD-related symptoms and sleep disorders, with additional courses further improving outcomes (13). Consistent with these studies, our research found that WMT was effective after six courses, with treatment benefits increasing as the number of courses grew. These results underscore the potential for improved outcomes in patients adhering to FMT-based therapies. However, further studies are needed to determine the optimal number of treatment courses for maximal benefit.
Gut microbiota may influence autism symptoms through several mechanisms. Notably, gut microbiota modulates social behavior in individuals with autism via neurotransmitters, particularly 5-hydroxytryptamine (5-HT), a monoamine neurotransmitter that regulates mood, behavior, and neural development (38, 39). Xiao et al. observed that 5-HT levels were elevated in fecal samples from GF mice following FMT (40). Proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) have been implicated in the development of severe ASD-related neurobehavioral symptoms (41, 42), with elevated peripheral blood levels observed in patients with ASD (43). Avolio et al. demonstrated that FMT reduced IL-6 and TNF-α levels in the gut and brain of autism mouse models (44). Additionally, fecal samples from individuals with autism exhibit reduced levels of short-chain fatty acids (SCFAs) (45), influencing brain development and social behavior (46). Several studies have shown that FMT significantly increases SCFA levels in patients with ASD (47).
GI symptoms, including constipation, diarrhea, and abnormal stool form, are prevalent in individuals with ASD (7, 48). Numerous clinical studies have shown that FMT not only alleviates autism symptoms but also significantly improves GI symptoms (9, 33, 49). Consistent with these findings, the present study demonstrates that WMT improves GI symptoms in patients with autism. However, the precise mechanisms by which WMT ameliorates GI symptoms remain unclear. Both inflammatory responses and intestinal barrier dysfunction are believed to contribute to these symptoms. FMT has been shown to reduce serum IL-6 levels in patients with UC, suggesting its potential to mitigate inflammatory diseases by decreasing cytokine-mediated inflammation (50). Furthermore, FMT reduces intestinal inflammation and enhances intestinal barrier function, which helps alleviate CDI-induced diarrhea (51). These mechanisms may similarly underlie the therapeutic effects of WMT in improving GI symptoms in patients with autism.
Although FMT has been established as an effective treatment for ASD, its therapeutic efficacy can vary, resulting in differing clinical outcomes among patients (9, 52). Previous short-term WMT studies reported better outcomes in patients without constipation (35), aligning with our findings that increased treatment courses lead to improved efficacy. Many studies have also indicated that the effectiveness of FMT is influenced by the disease duration and treatment period. For instance, Moayyedi et al. demonstrated that FMT was more beneficial in patients with a shorter duration of UC (23). In contrast, a longer disease duration in patients with autism corresponded to improved efficacy. Given the potential for delayed autism diagnosis, this observation warrants further exploration. Furthermore, multiple courses of WMT have been found to be more effective than single or double courses in treating patients with hyperlipidemia (37). Numerous studies have highlighted a strong correlation between GI symptoms and ASD severity (53, 54), with other research showing that patients with GI symptoms tend to have higher CARS and ABC scores (33), reflecting more severe disease. Additionally, WMT was shown to significantly reduce the incidence of diarrhea in patients with CDI (55) and markedly improve abnormal stool form in patients with ASD (33). These findings suggest that WMT may alleviate autism symptoms by improving GI function.
However, several limitations remain. First, the potential influence of confounding variables, such as genetic factors related to autism and the varying cognitive functioning of patients (high-functioning vs. low-functioning), were not considered, which may have affected WMT's efficacy in treating autism. Secondly, while animal and clinical studies have demonstrated that FMT enhances short-chain fatty acid production and reduces inflammatory markers, the impact of FMT on other metabolites or proteomic elements remains uncertain, due to the insufficient fecal samples for analysis in our study. Lastly, the study was limited by its small sample size and single-center design. Therefore, larger, multicenter studies are needed to validate our findings.
In conclusion, this study demonstrates that WMT can significantly improve ASD, GI symptoms, and sleep disturbances in patients with ASD. Six courses of WMT show enhanced therapeutic outcomes, with efficacy improving as the number of courses increases. Patients without additional treatments, as well as those with more severe conditions and longer disease durations, exhibited better outcomes. Additionally, pre-treatment GI symptoms, such as abnormal stool form and diarrhea, may influence the efficacy of WMT in patients with ASD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
DH: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. CL: Data curation, Writing – original draft. XS: Data curation, Writing – original draft. QW: Methodology, Writing – original draft. LW: Supervision, Writing – review & editing. HZ: Conceptualization, Methodology, Writing – review & editing. XH: Conceptualization, Investigation, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key-Area Research and Development Program of Guangdong Province (grant No.: 2022B1111070006), the Shenzhen Science and Technology Program (grant No.: JCYJ20240813140519025), the Medical Scientific Research Foundation of Guangdong Province (grant No.: B2022209), and the Scientific Research Projects of Guangdong Bureau of Traditional Chinese Medicine (grant No.: 20221232).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Taylor MJ, Rosenqvist MA, Larsson H, Gillberg C, D’Onofrio BM, Lichtenstein P, et al. Etiology of autism spectrum disorders and autistic traits over time. JAMA Psychiatry. (2020) 77:936. doi: 10.1001/jamapsychiatry.2020.0680
2. Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: a systematic review update. Autism Res. (2022) 15:778–90. doi: 10.1002/aur.2696
3. Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. (2017) 56:466–74. doi: 10.1016/j.jaac.2017.03.013
4. Signorini L, Ballini A, Arrigoni R, De Leonardis F, Saini R, Cantore S, et al. Evaluation of a nutraceutical product with probiotics, vitamin D, plus banaba leaf extracts (lagerstroemia speciosa) in glycemic control. EMIDDT. (2021) 21:1356–65. doi: 10.2174/1871530320666201109115415
5. Ng QX, Soh AYS, Venkatanarayanan N, Ho CYX, Lim DY, Yeo W-S. A systematic review of the effect of probiotic supplementation on schizophrenia symptoms. Neuropsychobiology. (2019) 78:1–6. doi: 10.1159/000498862
6. Inchingolo AM, Patano A, Piras F, Mancini A, Inchingolo AD, Paduanelli G, et al. Interconnection between microbiota–gut–brain axis and autism spectrum disorder comparing therapeutic options: a scoping review. Microorganisms. (2023) 11:1477. doi: 10.3390/microorganisms11061477
7. Fattorusso A, Di Genova L, Dell’Isola G, Mencaroni E, Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. (2019) 11:521. doi: 10.3390/nu11030521
8. Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. (2019) 177:1600–18.e17. doi: 10.1016/j.cell.2019.05.004
9. Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. (2017) 5:10. doi: 10.1186/s40168-016-0225-7
10. Goo N, Bae HJ, Park K, Kim J, Jeong Y, Cai M, et al. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. (2020) 262:118497. doi: 10.1016/j.lfs.2020.118497
11. Lu G, Wang W, Li P, Wen Q, Cui B, Zhang F. Washed preparation of faecal microbiota changes the transplantation related safety, quantitative method and delivery. Microb Biotechnol. (2022) 15:2439–49. doi: 10.1111/1751-7915.14074
12. Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. (2020) 11:251–66. doi: 10.1007/s13238-019-00684-8
13. Pan Z-Y, Zhong H-J, Huang D-N, Wu L-H, He X-X. Beneficial effects of repeated washed microbiota transplantation in children with autism. Front Pediatr. (2022) 10:928785. doi: 10.3389/fped.2022.928785
14. Liu N-H, Liu H-Q, Zheng J-Y, Zhu M-L, Wu L-H, Pan H-F, et al. Fresh washed microbiota transplantation alters gut microbiota metabolites to ameliorate sleeping disorder symptom of autistic children. J Microbiol. (2023) 61:741–53. doi: 10.1007/s12275-023-00069-x
15. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. (2018) 392:508–20. doi: 10.1016/S0140-6736(18)31129-2
16. Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. (2009) 39(3):405–13. doi: 10.1007/s10803-008-0637-8
17. Lou M, Cao A, Jin C, Mi K, Xiong X, Zeng Z, et al. Deviated and early unsustainable stunted development of gut microbiota in children with autism spectrum disorder. Gut. (2021) 71(8):1588–99. doi: 10.1136/gutjnl-2021-325115
18. Deng W, Wang S, Li F, Wang F, Xing YP, Li Y, et al. Gastrointestinal symptoms have a minor impact on autism spectrum disorder and associations with gut microbiota and short-chain fatty acids. Front Microbiol. (2022) 13:1000419. doi: 10.3389/fmicb.2022.1000419
19. He J, Gong X, Hu B, Lin L, Lin X, Gong W, et al. Altered gut microbiota and short-chain fatty acids in Chinese children with constipated autism spectrum disorder. Sci Rep. (2023) 13:19103. doi: 10.1038/s41598-023-46566-2
20. Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis. (2014) 8:1569–81. doi: 10.1016/j.crohns.2014.08.006
21. Moayyedi P. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. (2015) 149:102–9. doi: 10.1053/j.gastro.2015.04.001
22. Wu L, Yuan Q, Wu L, Hua-Xiang Xia H, Zhong M, Liu T, et al. Efficacy of washed microbiota transplantation for therapeutic targets of refractory functional constipation and the influencing factors: a single-center, retrospective, 24-week follow-up study. BMC Gastroenterol. (2023) 23:291. doi: 10.1186/s12876-023-02929-7
23. Zhong H-J, Zeng H-L, Cai Y-L, Zhuang Y-P, Liou Y-L, Wu Q, et al. Washed microbiota transplantation lowers blood pressure in patients with hypertension. Front Cell Infect Microbiol. (2021) 11:679624. doi: 10.3389/fcimb.2021.679624
24. Fecal Microbiota Transplantation-Standardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J. (2020) 133:2330–2. doi: 10.1097/CM9.0000000000000954
25. Volkmar FR, Cicchetti DV, Dykens E, Sparrow SS, Leckman JF, Cohen DJ. An evaluation of the autism behavior checklist. J Autism Dev Disord. (1988) 18:81–97. doi: 10.1007/BF02211820
26. Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X, et al. Prevalence of autism spectrum disorder in China: a nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. (2020) 36:961–71. doi: 10.1007/s12264-020-00530-6
27. Randall M, Egberts KJ, Samtani A, Scholten RJ, Hooft L, Livingstone N, et al. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst Rev. (2018) 2018. doi: 10.1002/14651858.CD009044.pub2
28. Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: childhood autism rating scale (CARS). J Autism Dev Disord. (1980) 10:91–103. doi: 10.1007/BF02408436
29. Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The sleep disturbance scale for children (SDSC) construct ion and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. (1996) 5:251–61. doi: 10.1111/j.1365-2869.1996.00251.x
30. Yang B, Tian H, Ye C, Lin Z, Zhao D, Ma C, et al. The efficacy and safety of fecal microbiota transplantation combined with biofeedback for mixed constipation: a retrospective cohort study. Front Med. (2021) 8:746990. doi: 10.3389/fmed.2021.746990
31. Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified bristol stool form scale for children. J Pediatr. (2011) 159:437–41.e1. doi: 10.1016/j.jpeds.2011.03.002
32. Zhang J, Zhu G, Wan L, Liang Y, Liu X, Yan H, et al. Effect of fecal microbiota transplantation in children with autism spectrum disorder: a systematic review. Front Psychiatry. (2023) 14:1123658. doi: 10.3389/fpsyt.2023.1123658
33. Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol. (2021) 11:759435. doi: 10.3389/fcimb.2021.759435
34. Kang D-W, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep. (2019) 9:5821. doi: 10.1038/s41598-019-42183-0
35. Zhang Y, Zhang J, Pan Z, He X. Effects of washed fecal Bacteria transplantation in sleep quality, stool features and autism symptomatology: a Chinese preliminary observational study. NDT. (2022) 18:1165–73. doi: 10.2147/NDT.S355233
36. Hui W, Li T, Liu W, Zhou C, Gao F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: an updated randomized controlled trial meta-analysis. PLoS One. (2019) 14:e0210016. doi: 10.1371/journal.pone.0210016
37. Liang F, Song Y, Lin D, He H, Xu J, He X, et al. Washed microbiota transplantation is associated with improved lipid profiles: long-term efficacy and safety in an observational cohort from South China. Clin Transl Gastroenterol. (2024) 15:e00735. doi: 10.14309/ctg.0000000000000735
38. Jiang C-C, Lin L-S, Long S, Ke X-Y, Fukunaga K, Lu Y-M, et al. Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications. Sig Transduct Target Ther. (2022) 7:229. doi: 10.1038/s41392-022-01081-0
39. Choe KY, Bethlehem RAI, Safrin M, Dong H, Salman E, Li Y, et al. Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse. Neuron. (2022) 110:795–808.e6. doi: 10.1016/j.neuron.2021.11.031
40. Xiao L, Yan J, Yang T, Zhu J, Li T, Wei H, et al. Fecal microbiome transplantation from children with autism spectrum disorder modulates tryptophan and serotonergic synapse metabolism and induces altered behaviors in germ-free mice. mSystems. (2021) 6:e01343–20. doi: 10.1128/mSystems.01343-20
41. Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van De Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. (2011) 25:40–5. doi: 10.1016/j.bbi.2010.08.003
42. Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim V, et al. The maternal interleukin-17a pathway in mice promotes autismlike phenotypes in offspring. Science. (2016) 351(6276):933–9. doi: 10.1126/science.aad0314
43. Zhao H, Zhang H, Liu S, Luo W, Jiang Y, Gao J. Association of peripheral blood levels of cytokines with autism spectrum disorder: a meta-analysis. Front Psychiatry. (2021) 12:670200. doi: 10.3389/fpsyt.2021.670200
44. Avolio E, Olivito I, Rosina E, Romano L, Angelone T, De Bartolo A, et al. Modifications of behavior and inflammation in mice following transplant with fecal microbiota from children with autism. Neuroscience. (2022) 498:174–89. doi: 10.1016/j.neuroscience.2022.06.038
45. Kang D-W, Adams JB, Vargason T, Santiago M, Hahn J, Krajmalnik-Brown R. Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. mSphere. (2020) 5:e00314–20. doi: 10.1128/mSphere.00314-20
46. MacFabe DF, Cain NE, Boon F, Ossenkopp K-P, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. (2011) 217:47–54. doi: 10.1016/j.bbr.2010.10.005
47. Hu C, He T, Zou B, Li H, Zhao J, Hu C, et al. Fecal microbiota transplantation in a child with severe ASD comorbidities of gastrointestinal dysfunctions—a case report. Front Psychiatry. (2023) 14:1219104. doi: 10.3389/fpsyt.2023.1219104
48. Holingue C, Newill C, Lee L-C, Pasricha PJ, Daniele Fallin M. Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence: gastrointestinal symptoms in autism spectrum disorder. Autism Res. (2018) 11:24–36. doi: 10.1002/aur.1854
49. Huang H-L, Xu H-M, Liu Y-D, Shou D-W, Chen H-T, Nie Y-Q, et al. First application of fecal microbiota transplantation in adult asperger syndrome with digestive symptoms—a case report. Front Psychiatry. (2022) 13:695481. doi: 10.3389/fpsyt.2022.695481
50. Wang Y, Ren R, Sun G, Peng L, Tian Y, Yang Y. Pilot study of cytokine changes evaluation after fecal microbiota transplantation in patients with ulcerative colitis. Int Immunopharmacol. (2020) 85:106661. doi: 10.1016/j.intimp.2020.106661
51. Yadegar A, Pakpour S, Ibrahim FF, Nabavi-Rad A, Cook L, Walter J, et al. Beneficial effects of fecal microbiota transplantation in recurrent clostridioides difficile infection. Cell Host Microbe. (2023) 31:695–711. doi: 10.1016/j.chom.2023.03.019
52. Wei J, Chen J, Fang X, Liu T, Yuan Y, Zhang J. Protocol for the safety and efficacy of fecal microbiota transplantation liquid in children with autism spectrum disorder: a randomized controlled study. Front Microbiol. (2023) 14:1236904. doi: 10.3389/fmicb.2023.1236904
53. Hung LY, Margolis KG. Autism spectrum disorders and the gastrointestinal tract: insights into mechanisms and clinical relevance. Nat Rev Gastroenterol Hepatol. (2024) 21:142–63. doi: 10.1038/s41575-023-00857-1
54. Vargason T, McGuinness DL, Hahn J. Gastrointestinal symptoms and oral antibiotic use in children with autism spectrum disorder: retrospective analysis of a privately insured U.S. population. J Autism Dev Disord. (2019) 49:647–59. doi: 10.1007/s10803-018-3743-2
Keywords: autism, washed microbiota transplantation, clinical efficacy, gastrointestinal symptoms, treatment course
Citation: Hu D-X, Lu C-M, Si X-Y, Wu Q-T, Wu L-H, Zhong H-J and He X-X (2025) Effects of gastrointestinal symptoms on the efficacy of washed microbiota transplantation in patients with autism. Front. Pediatr. 13:1528167. doi: 10.3389/fped.2025.1528167
Received: 14 November 2024; Accepted: 24 January 2025;
Published: 13 February 2025.
Edited by:
Batia Weiss, Edmond and Lily Safra Children's Hospital, IsraelReviewed by:
Odelia Chorin, Edmond and Lily Safra Children's Hospital, IsraelCopyright: © 2025 Hu, Lu, Si, Wu, Wu, Zhong and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-Jie Zhong, amF4emhvbmdAMTI2LmNvbQ==; Xing-Xiang He, aGV4aW5neGlhbmdAZ2RwdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.