
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 17 March 2025
Sec. General Pediatrics and Pediatric Emergency Care
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1524617
 Frederick Dun-Dery1
Frederick Dun-Dery1 Jianling Xie2
Jianling Xie2 Roger Zemek3,4
Roger Zemek3,4 Kathleen Winston1
Kathleen Winston1 Brett Burstein5,6
Brett Burstein5,6 Vikram Sabhaney7
Vikram Sabhaney7 Jason Emsley8
Jason Emsley8 Jocelyn Gravel9
Jocelyn Gravel9 April Kam10
April Kam10 Ahmed Mater11
Ahmed Mater11 Darcy Beer12
Darcy Beer12 Robert Porter13
Robert Porter13 Gabrielle Freire14
Gabrielle Freire14 Naveen Poonai15,16,17
Naveen Poonai15,16,17 Anne Moffatt18
Anne Moffatt18 Simon Berthelot19
Simon Berthelot19 Marina I. Salvadori20
Marina I. Salvadori20 Deepti Reddy21
Deepti Reddy21  Bruce Wright22
Bruce Wright22 Stephen B. Freedman23* on behalf of Pediatric Emergency Research Canada (PERC) COVID Study Group
Stephen B. Freedman23* on behalf of Pediatric Emergency Research Canada (PERC) COVID Study Group
Objective: It remains unclear whether emerging mental health concerns in children infected with SARS-CoV-2 are a direct result of the infection or due to the indirect effects of the pandemic. Therefore, we sought to assess the frequency of new diagnoses of anxiety and/or depression among children diagnosed with and without SARS-CoV-2 infection who were tested in pediatric emergency departments.
Methods: A prospective cohort study with 6- and 12-month follow-ups was conducted across 14 Canadian tertiary-care pediatric emergency departments of the Pediatric Emergency Research Canada (PERC) network. The study included children aged <18 years who were tested for SARS-CoV-2 infection between August 2020 and February 2022. The primary outcome was the diagnosis of anxiety and/or depression reported during follow-up. The surveys incorporated a modified version of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Long-COVID Pediatric Questionnaire.
Results: Among the participants who were eligible for 6- and 12-month follow-ups, 64.7% (268/414) of SARS-CoV-2-positive and 71.9% (743/1,033) of SARS-CoV-2-negative participants completed follow-up at these time points, respectively. The median age was 7.0 [inter-quartile range (IQR): 5.0–11.0] years, and 54.2% (548/1,011) were male. New diagnoses of anxiety and/or depression reported on either survey did not differ significantly between test-positive (4.1%, 11/268) and test-negative (2.8%; 21/743) participants [difference = 1.3% (95% CI: −1.3 to 4.2)]. There was a higher prevalence of new diagnoses of anxiety and/or depression among SARS-CoV-2-negative participants aged ≥12 years relative to those aged <12 years [8.7% (13/149) vs. 1.3% (8/594); difference = 7.4%; 95% CI of the difference = 3.0–12.5], but not among SARS-CoV-2-positive participants [4.4% (2/45) vs. 4.0% (9/223); difference = 0.4%; 95% CI of the difference = −5.6 to 9.4]. At 6 or 12 months, SARS-CoV-2-positive participants were more likely to experience confusion and/or lack of concentration, abdominal pain, and insomnia.
Conclusions: Although no association was found between SARS-CoV-2 infection and new diagnoses of anxiety and/or depression, SARS-CoV-2-positive participants were more likely to experience confusion/lack of concentration, abdominal pain, and insomnia. This finding, in the context of an increased prevalence of new diagnoses of anxiety and depression, underscores the impacts of societal changes on the mental health of children. Our finding that some non-specific symptoms were more frequently reported by SARS-CoV-2-positive participants emphasizes the need for further investigation of the underlying pathophysiologic mechanisms.
The COVID-19 pandemic has posed significant challenges to mental health, particularly among children and adolescents (1). A substantial body of literature has emerged, suggesting that adolescents have experienced a significant increase in psychological distress manifesting as depression and anxiety (2). According to the 2021 Adolescent Behaviors and Experiences Survey, 37% of US high school students reported poor mental health during the pandemic, with 20% considering and 9.0% attempting suicide in the preceding year (3).
The long-term effects of COVID-19, including potential mental health issues, are a growing public health concern (4). Although the prevalence of mental health conditions increased in children and adolescents during the COVID-19 pandemic (4–7), it remains unclear whether this is due to the direct effects of SARS-CoV-2 infection itself, the broader social and situational context of the pandemic, or a combination of both (8). Reviews have called for further research to determine whether the neuropsychiatric symptoms reported in children with the post-COVID-19 condition are a result of COVID-19 infection; stress, anxiety, and behavioral changes related to public health restrictions imposed to mitigate the spread of the SARS-CoV-2 virus; or other societal influences (9).
Both adult (10–12) and pediatric (13) electronic health record cohort studies have demonstrated an increased incidence of neurologic and psychiatric diagnoses following SARS-CoV-2 infection (11, 12). Although few prospective studies that include test-negative controls have addressed this question, a national, cross-sectional study conducted in Denmark found that although SARS-CoV-2-positive participants were more likely to have chronic symptoms, SARS-CoV-2-negative controls had lower quality of life scores, including the emotional, social and school functioning subscales (14).
SARS-CoV-2 may affect brain function by binding to angiotensin-converting enzyme type 2 receptors present in the central nervous system (15). In addition to the potential impact of direct viral infection on neural cells, SARS-CoV-2 infection may also lead to depression through an indirect neuroinflammatory immune response, i.e., cytokine storm (16), which involves the production of interleukin 1β and interleukin 6 (17). Cytokines can lead to depression in various ways including hyperactivation of the hypothalamic–pituitary–adrenal axis which can result in neurotoxicity and neurodegeneration which are associated with the development of depression (18). In addition, other etiologies of depression in SARS-CoV-2-infected children include chronic physical symptoms (19), invalidation by healthcare providers (20), isolation from peers (21), and school absenteeism (22).
Although COVID-19 pandemic restrictions have generally been removed, the SARS-CoV-2 virus continues to circulate (23). Given the concerns regarding the direct neuropsychiatric effects of SARS-CoV-2 infection, it remains important to distinguish between the direct impact of SARS-CoV-2 infection and the indirect effects of the COVID-19 pandemic on mental health (24). Such evaluations must differentiate the direct effects of infection from those due to the situational context created by the pandemic. To address these knowledge gaps, we sought to determine if children who tested positive for SARS-CoV-2 were more likely to be diagnosed with anxiety and/or depression over the subsequent 12 months compared to those who tested negative.
This study is a secondary analysis of a prospective cohort study of participants recruited from 14 Pediatric Emergency Research Canada (PERC) tertiary-care pediatric emergency departments (EDs) (25) between August 2020 and February 2022. Research ethics board approval was obtained at all participating institutions (Supplementary Table S1), and informed oral consent was obtained along with participant assent as per institutional policy. Study findings are reported as per the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (26).
We recruited children aged <18 years who underwent SARS-CoV-2 testing in a participating ED. Specimen collection was performed at the treating physician's discretion and/or per institutional policy. Collected specimens were analyzed using nucleic acid amplification approaches as determined by the local laboratory. We excluded participants <4 years of age as mental health disorders are less often diagnosed in younger children (27). Participants who neither spoke nor understood French and/or English were also excluded. The follow-up surveys were added to the study protocol on 1 November 2021; participants enrolled >12 months before this date were deemed ineligible. We excluded participants who reported seeking support from a mental health specialist (e.g., psychiatrist, psychologist, social worker, or counselor) prior to the onset of the COVID-19 pandemic.
To enable the identification of potentially eligible participants, team members received a list of all children tested daily. Research assistants phoned the caregivers of SARS-CoV-2-positive children first, then those who tested negative, starting with the test performed earliest on each day. This approach was adopted to minimize selection bias should the number of potentially eligible children exceed the capacity of the research team. During the consenting process, we limited the information provided about the rationale for conducting long-term follow-up to the following sentence: “Because of what we have learned about the long-term consequences of COVID infection in children, we have been asked by the Public Health Agency of Canada to collect additional outcome data 6 and 12 months following the emergency department visit.”
The primary outcome was a reported diagnosis of anxiety and/or depression in the 6- and/or 12-month follow-up surveys. These timepoints were selected as it was a priori determined that the comprehensive follow-up surveys would be administered at those time points. Secondary outcomes focused on non-specific symptoms, which we sought to compare between children infected and uninfected with SARS-CoV-2. This comparison enabled us to determine if the development of symptoms was more common among infected children compared to uninfected children, thus permitting an assessment of symptoms as a manifestation of the direct effects of SARS-CoV-2 infection vs. being more a reflection of the effects of the lockdowns, school closures, and limitations of leisure activities (28). We sought to compare the reporting of any of the following symptoms (as composite and individual measures) at the 6- and 12-month surveys: headache, dizziness, syncope, palpitations, chest pain, abdominal pain, nausea, balance problems, myalgias, visual concerns, tremors, paresthesia, concentration challenges, insomnia, hypersomnia, fatigue, and poor appetite.
Data were collected as soon as possible following the index ED visit and via caregiver completion of surveys at 6- and 12-month follow-ups. A medical record review was performed to classify SARS-CoV-2 status based on the result of the test performed at the index ED visit and any additional tests performed during the subsequent 14 days. The 6- and 12-month follow-up surveys included a modified version of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Long-COVID Pediatric Questionnaire (Supplementary Table S2) (29). This questionnaire has become the gold standard tool used by leading investigations into the assessment of long-COVID symptoms in the pediatric population (30).
To meet the study's primary outcome definition, caregivers had to respond yes to specific questions about their child receiving a new diagnosis of anxiety or depression since their index ED visit. We defined exposure status (i.e., SARS-CoV-2-positive/negative) based on detecting SARS-CoV-2 on a swab specimen collected from the nares, nasopharynx, or oral cavity at the index ED visit or within 14 days thereafter. Children who tested negative constituted the control group. Hospitalization status was defined by admission to a hospital at the index ED visit or within the subsequent 14 days (31). The approach to testing and reporting of variants differed across participating institutions. When variant testing was performed, that result was used. However, when variant testing was either not performed or was inconclusive, the SARS-CoV-2 variant was imputed following established methods (32).
Pooled estimates obtained in the first year of the COVID-19 pandemic suggest that depression and anxiety symptoms doubled compared to pre-pandemic levels (33). Our estimate of the baseline rate for new diagnoses of anxiety or depression comes from a scoping review of US children, which showed that 10% of children and adolescents received mental health services in the past year, as reported by their parents (34). Based on earlier analyses of our cohort, we anticipated having four SARS-CoV-2-negative controls per case (32). Thus, to have 90% power with an alpha of 0.05, we required 684 SARS-CoV-2-negative and 171 SARS-CoV-2-positive participants to identify a doubling in the prevalence of mental health conditions from 10% to 20%.
We summarized participant demographic and clinical characteristics using descriptive statistics. For the primary outcome of new mental health diagnoses, and for the secondary outcomes of reporting individual symptoms, we used Chi-square tests and Fisher's exact tests for comparisons between SARS-CoV-2-positive and SARS-CoV-2-negative groups. The Wald test was used to obtain the 95% CI of the difference between proportions, and the Agresti-Caffo approach was used when the event was rare (n < 20) (35). P-values obtained from unadjusted bivariate analyses were adjusted via the Benjamini–Hochberg approach for multiple comparisons (36). We also conducted stratified analyses by participant age and diagnosis (i.e., anxiety and depression).
We did not perform multiple imputations to address missing data for the predictor and outcome variables as the missing at-random assumption is unlikely to be true. All analyses were two-sided, and statistical significance was defined by P < 0.05. Analyses were performed using SPSS Statistics for Windows, version 29 (IBM Corporation), and R-4.3.2. Data were analyzed from 26 February 2024 to 31 March 2024.
A total of 7,421 children were screened, and 2,337 (31.5%) met the inclusion criteria for the analysis, of whom 21.4% (500/2,337) and 78.6% (1,837/2,337) were SARS-CoV-2-positive and SARS-CoV-2-negative, respectively. Among SARS-CoV-2-positive participants eligible for 6- and 12-month follow-ups, 68.9% (157/228) and 54.8% (227/414) completed follow-up at these time points, respectively. Among SARS-CoV-2-negative participants, completion rates were 56.6% (278/491) and 65.3% (675/1,033), respectively (Figure 1). Participants who completed vs. those who did not complete either the 6- or 12-month survey differed with respect to age, COVID-19 vaccination status, number of symptoms at baseline, and SARS-CoV-2 variant (Supplementary Table S3). Follow-up was completed at either of these time points by 64.7% (268/414) and 71.9% (743/1,033), of SARS-CoV-2-positive and negative participants, respectively.
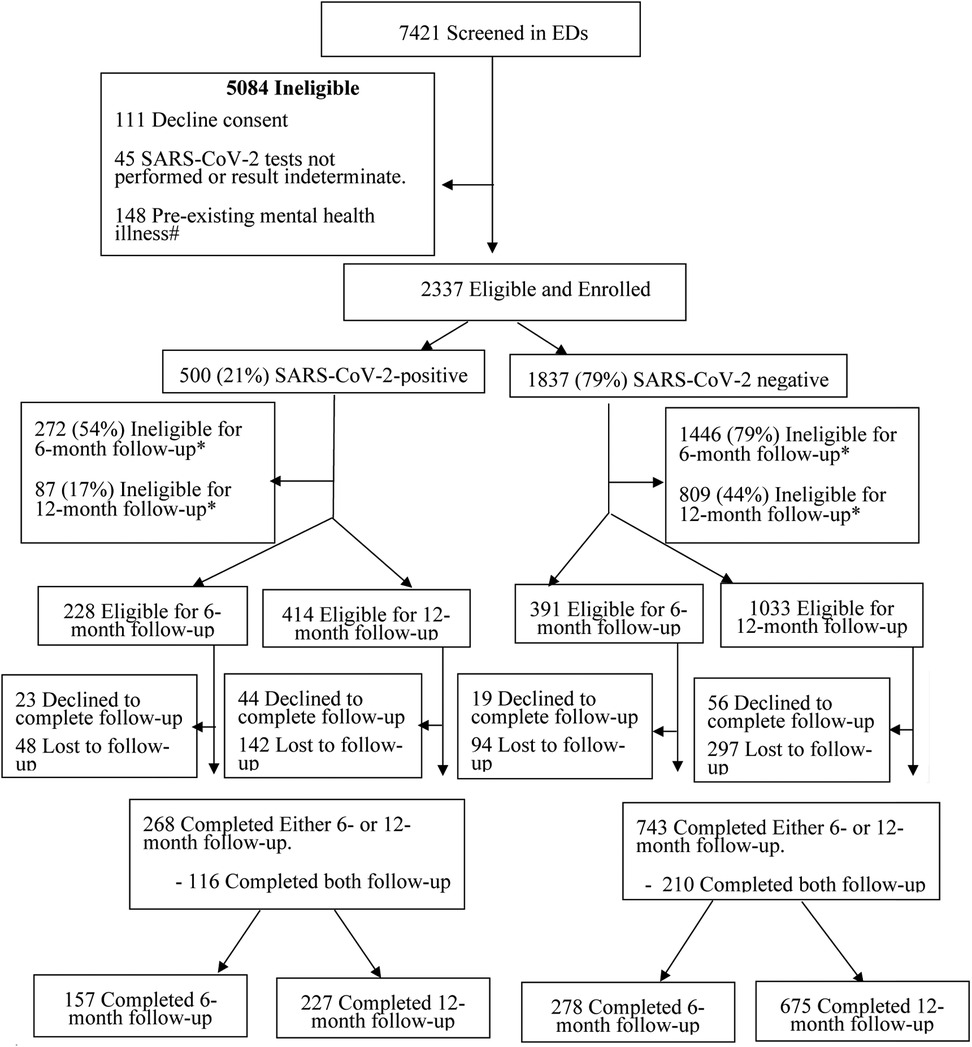
Figure 1. Flow diagram of participants from index emergency department enrolment visit to 12-month follow-up. #Participants who reported seeking support from a mental health specialist (e.g., psychiatrist, psychologist, social worker, and counselor) prior to the onset of the COVID-19 pandemic were excluded. *Had passed the 6- and/or 12-month follow-up survey time windows.
Participant median age was 7 [inter-quartile range (IQR): 5–11] years, 54.2% (548/1,011) were male, 44.8% (448/999) self-identified as White, 12.2% (123/1,011) were hospitalized, and 1% (10/1,006) had a severe outcome (Table 1). Fifty-three percent (396/743) of SARS-CoV-2-negative participants were enrolled during the transmission periods of the wild-type and alphavirus subtypes, while 25.4% of SARS-CoV-2-positive participants were enrolled during the same period.
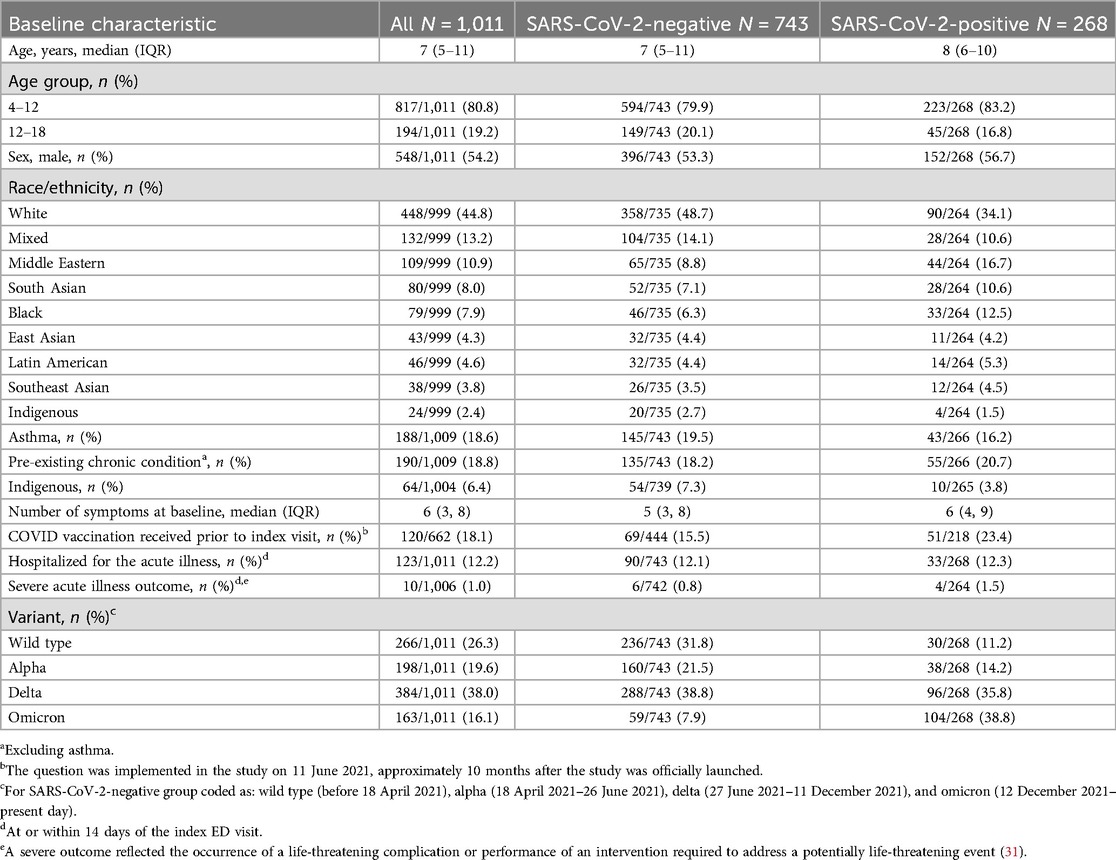
Table 1. Baseline characteristics, stratified by SARS-CoV-2 test outcome, inclusive of all participants that completed either the 6- or 12-month follow-up surveys.
A new diagnosis of anxiety and/or depression provided by a healthcare professional reported by caregivers at the 6- and/or 12-month surveys did not differ between SARS-CoV-2-positive (4.1%, 11/268) and SARS-CoV-2-negative participants (2.8%; 21/743) [difference = 1.3% (95% CI: −1.3 to 4.2); Table 2]. Among participants aged 4–12 years, the percentage difference between SARS-CoV-2-positive and SARS-CoV-2-negative participants was 2.7% (95% CI: 0.1–5.8; adjusted P = 0.22). Among those aged 12–18 years of age, there was no association between SARS-CoV-2 test status and a new diagnosis of anxiety or depression (−4.3% SARS-CoV-2-positive relative to negative; 95% CI: −11.3 to 5.5). Although the prevalence of new diagnoses of anxiety or depression was greater among SARS-CoV-2-negative participants aged ≥12 years relative to those aged <12 years [8.7% (13/149) vs. 1.3% (8/594); difference = 7.4%; 95% CI of the difference = 3.0–12.5], there was no such difference among SARS-CoV-2-positive participants [4.4% (2/45) vs. 4.0% (9/223); difference = 0.4%; 95% CI of the difference = −5.6 to 9.4].
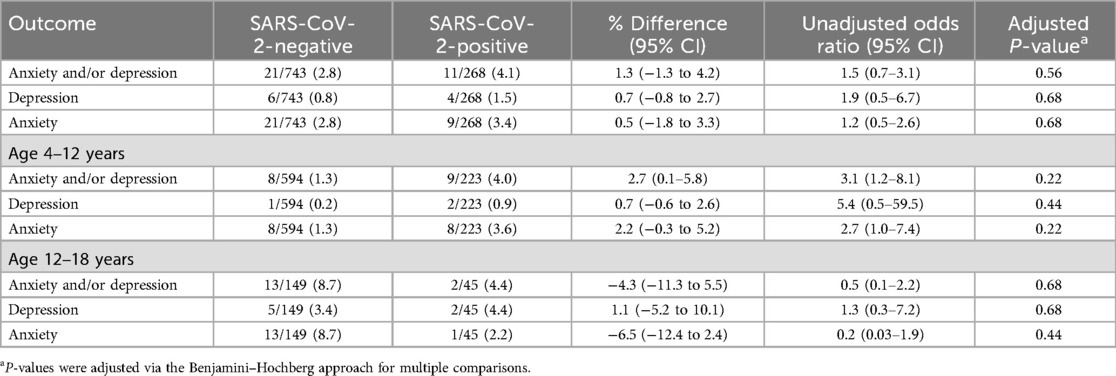
Table 2. Primary outcomes based on index SARS-CoV-2 emergency department index visit test result status.
At 6 and/or 12 months, 28.7 (77/268) and 21.3% (158/743) of SARS-CoV-2-positive and SARS-CoV-2-negative participants, respectively, reported any ongoing symptoms (difference = 7.5%; 95% CI: 1.3, 13.6, p = 0.09; Figure 2, Table 3A). SARS-CoV-2-positive participants were more likely to report experiencing confusion/lack of concentration, abdominal pain, and insomnia. When analyzed by individual time point (i.e., 6 and 12 months separately), the only symptom that differed between groups was abdominal pain at 12 months which was more common among SARS-CoV-2-positive participants (OR = 2.7, 95% CI: 1.6–4.8, adjusted P = 0.007; Supplementary Tables S4, S5). When participants were grouped based on the diagnosis of anxiety and/or depression during the follow-up period, nearly all symptoms were more common among those with anxiety and/or depression (Table 3B). The symptoms with the greatest between-group absolute percent differences were abdominal pain (31.6%), headache (26.8%), poor appetite (22.9%), muscle pain (22.1%), and joint pain (20.0%).
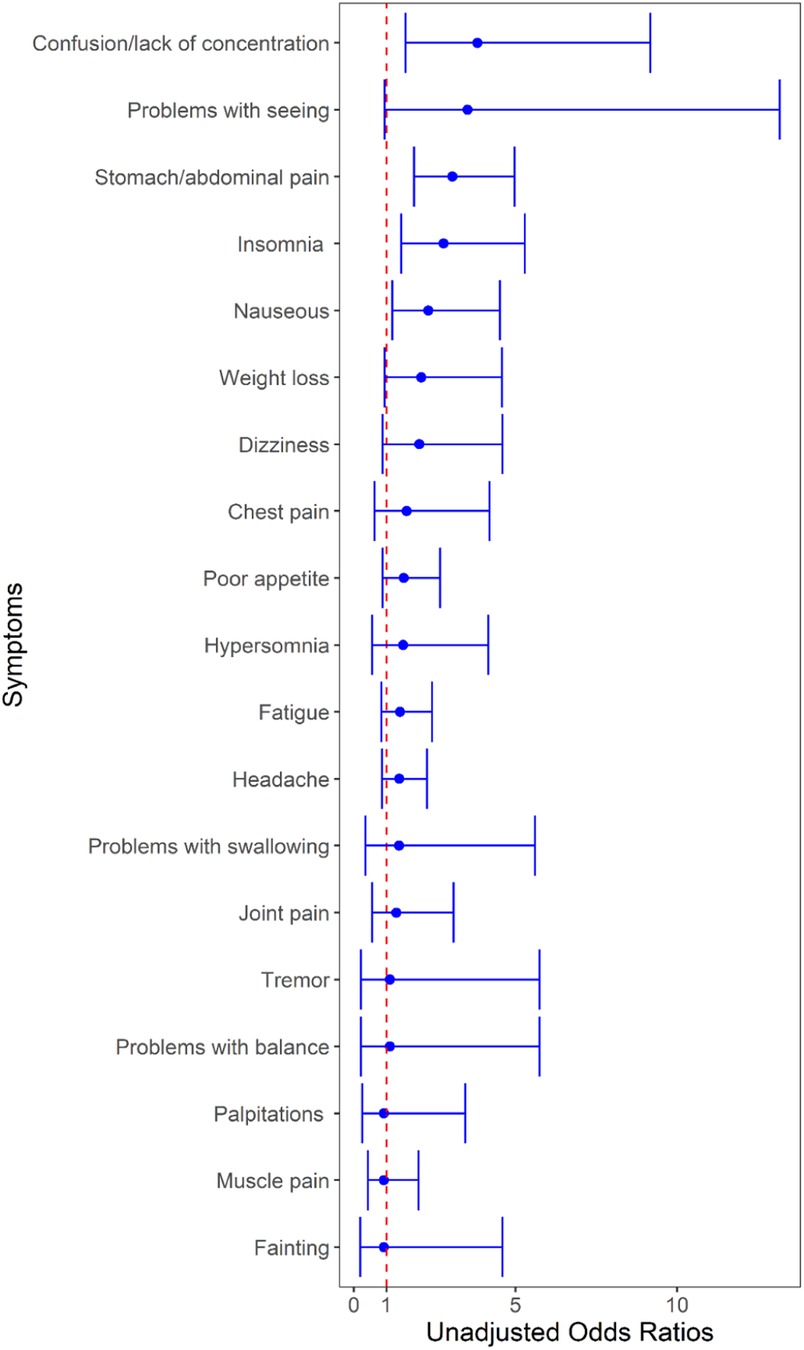
Figure 2. Unadjusted odds of having individual symptoms at 6- and/or 12-month follow-up based on index emergency department visit SARS-CoV-2 test result. The region to the right of the red dotted line indicates an increased risk associated with a positive test. Each line in the forest plot represents a different symptom. The blue dot marks the unadjusted odds ratio (OR) for each symptom in children who tested positive for SARS-CoV-2 compared to those who tested negative. The horizontal blue lines through the dots represent the 95% confidence intervals for the OR. The red vertical dashed line represents the line of no effect—a reference point for determining the significance of the results.
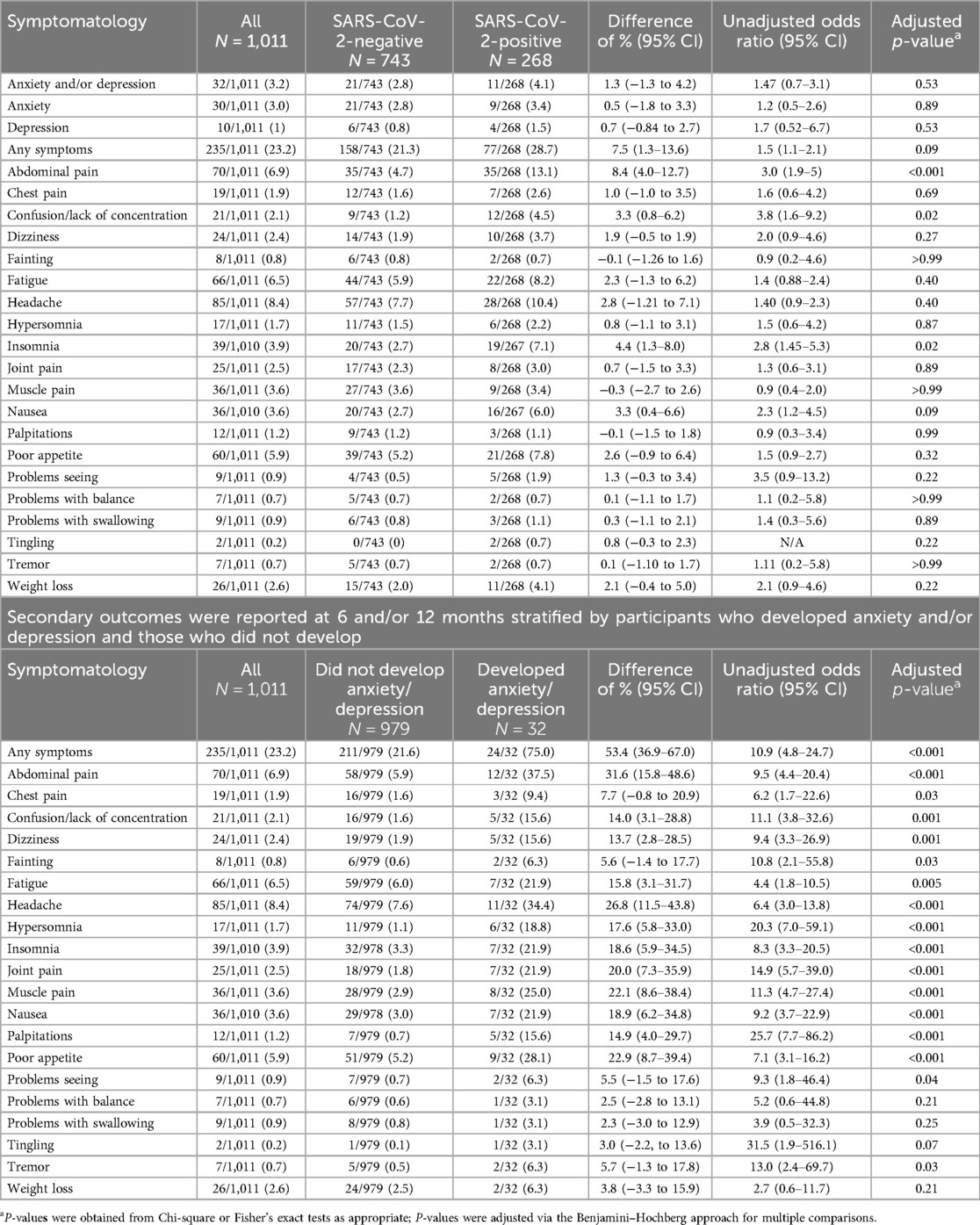
Table 3. Secondary outcomes reported at 6- and/or 12-months stratified by the index ED visit SARS-CoV-2 test.
In this pan-Canadian prospective cohort study of children aged 4–18 years, there was no association between SARS-CoV-2 infection and a new diagnosis of anxiety and/or depression at 6- and/or 12-month follow-ups. Although subgroup analyses based on age identified no differences between groups based on SARS-CoV-2 test status, among SARS-CoV-2-negative children, a greater proportion of teenagers (i.e., ≥12 years) relative to younger participants (4–12 years) received a new diagnosis of anxiety and/or depression. During follow-up, SARS-CoV-2-positive participants were more likely to report experiencing confusion/lack of concentration, abdominal pain, and insomnia.
The prevalence of mental health disorders in children and adolescents had been increasing during the pre-COVID-19 pandemic period with several United States-based pediatric health organizations declaring a national state of emergency in children's mental health (37). Between 2009 and 2019, feelings of sadness or hopelessness increased among high school students by 40% (38) and the suicide rate among individuals aged 10–24 years increased by 57% (39). Moreover, although 13%–20% of children living in the United States experience mental illness annually (40), 50% do not receive adequate treatment. In addition, anxiety and depression are some of the most common disorders affecting 10% and 20% of children aged 3–17 years and 12–17 years, respectively (34).
Posttraumatic stress disorder (PTSD) is a complex and severe mental disorder that is estimated to occur in 10% of the US population at least once during their lifetime (41). Emerging evidence indicates that children and adolescents are more susceptible to the adverse impacts of traumatic events such as infectious disease pandemics (42–44). The COVID-19 pandemic had the potential to trigger PTSD in children due to the imposition of quarantines, school closures, and lockdowns which led to an increase in screen time in children and youth (45, 46). The development of PTSD has been further exacerbated by the emergence of concerning diseases in children and youth such as the post-COVID-19 condition and the multisystem inflammatory syndrome (47). Common symptoms of PTSD in adolescents, which are important to monitor for following traumatic events, include anxiety and depression (48).
Understanding the true prevalence of the post-COVID-19 condition in children remains a challenge, particularly because subjective neuropsychiatric symptoms such as depression, anxiety, and cognitive deficits are prominent complaints among children that can arise from many etiologies (4). Although some reports state that 10%–20% (4) of children with COVID-19 experience chronic sequelae, recent studies with robust methodologic designs, including control groups, report much lower absolute increased risks (49, 50). An electronic health record retrospective cohort study (i.e., ICD-10-CM based) that included 659,286 children, reported a 3.7% increased prevalence of post-acute sequelae of infection among SARS-CoV-2-positive children compared with SARS-CoV-2-negative controls (51). Of particular note, the authors reported an association between prior SARS-CoV-2 infection and receipt of mental health treatment [adjusted hazard ratio (aHR): 1.6; 95% CI: 1.5–1.8] and anxiety symptoms (aHR: 1.3; 95% CI: 1.1–1.6) (51). In a nationwide cohort study conducted in Denmark including over 115,000 children, SARS-CoV-2-positive children were 0.8% more likely to report symptoms lasting >4 weeks (52). Data collection, which employed a questionnaire, revealed that children in the control group were more likely to report numerous symptoms including concentration difficulties, headaches, muscle and joint pains, cough, nausea, diarrhea, and fever. Although in previous analyses of this cohort (53, 54), we reported a low but detectable increased frequency of the post-COVID-19 condition, in this study, we report no statistical association between SARS-CoV-2 infection and new diagnoses of anxiety and/or depression. This may be due to our smaller sample size which may not have permitted the detection of small effect sizes. As such, we did find a small but not statistically significant increase in new diagnoses of anxiety and/or depression (1.3% (95% CI: −1.3 to 4.2) and similar results in relation to many of the individual secondary outcome symptoms.
In a nationally representative cross-sectional Canadian survey, 37%, 34%, and 29% of children and youth self-reported increased symptoms of anxiety, irritability, and mood disorders during the pandemic, respectively (55). The increase in the prevalence of these symptoms highlights the need to differentiate the direct effects of SARS-CoV-2 infection from those of the societal impacts of the pandemic itself. In our cohort, we previously reported the prevalence of the post-COVID-19 condition at 12 months was 0.5% greater among SARS-CoV-2-positive compared to SARS-CoV-2-negative children (53). Our current study, which focuses directly on mental health diagnoses, identified no difference between groups with respect to this subset of conditions.
We did find that confusion, concentration difficulties, insomnia, and abdominal pain were more common among test-positive children. Although recurrent abdominal pain has a prevalence of 14% in the pediatric population (56), and frequently is present in the absence of concomitant anxiety and depression, concentration difficulties (57) and insomnia (58) are commonly associated with the presence of anxiety and depression. The mechanism by which SARS-CoV-2 infection causes chronic abdominal pain remains poorly understood (59), but it may be associated with the neurocognitive symptoms seen in individuals with the post-COVID-19 condition. It is hypothesized that SARS-CoV-2 damages both cerebral blood vessels (60) and the intestinal wall (61) by binding to angiotensin-converting enzyme 2 receptors. This leads to cytokine production (62) which can compromise the brain's neurovascular unit and the intestinal barrier, thereby increasing permeability to harmful substances. The latter are hypothesized to be produced by intestinal microbiota which flourish during a period of post-SARS-CoV-2 infection dysbiosis (63). Thus SARS-CoV-2 may produce its neurocognitive effects by affecting the neurovascular unit that protects the gut and the brain while simultaneously leading to the increased production of neurotoxic and neuroinflammatory substances in the gut.
Our results are generally aligned with those reported in a nationwide cohort study conducted in Demark during the spring of 2021 (52) which included over 33,000 children. However, despite a high prevalence of symptoms lasting >4 weeks among SARS-CoV-2-positive children (28%), this differed from that among control children by only 0.8%. When comparing the responses related to individual symptoms, SARS-CoV-2-infected children were more likely to report chest pain, dizziness, fatigue, loss of smell and taste, muscle weakness, and respiratory problems. On the other hand, concentration difficulties, cough, diarrhea, fever, headache, muscle and joint pain, and nausea were more commonly reported by uninfected children.
Previous research has indicated that among children with COVID-19 infection, the prevalence of anxiety is higher than in the general population (64). However, as this study lacked a control group, symptoms among infected children could not be compared to those without infection (64). In keeping with our results, which found a greater prevalence of anxiety and/or depression among older relative to younger SARS-CoV-2-negative study participants, the impact of the pandemic on new mental health diagnoses has been reported to be more pronounced in adolescents relative to preadolescents (65). This finding suggests that pandemic restrictions may have been more impactful in the development of mental health disorders than infection itself (66). Potential societal events of the pandemic that may have impacted the mental health of adolescents include school closures, social distancing, economic hardship, and increased time spent on social media (67). Alternatively, it is possible that healthcare providers are less comfortable assigning diagnoses of anxiety and depression to younger children or that such children are less likely to verbalize and/or parents to recognize symptoms of anxiety and depression (68). In addition, clinicians should be cognizant of the association between abdominal pain and functional gastrointestinal disorders and the presence of anxiety and depression, with somatic symptoms reflecting the presence of mental health disorders (69).
Our study has some limitations that should be considered. A significant number of consented participants were lost to follow-up, and those who did complete follow-up were younger, more likely to have been vaccinated, and presented later in the pandemic, thereby potentially introducing bias (Supplementary Table S3). The difference in vaccination rates reflects the fact that many of the excluded children were enrolled early in the study and thus were ineligible for the 6- and/or 12-month follow-up surveys. At the time of their enrollment, near the beginning of the pandemic, vaccines were not available, particularly among younger children.
Our reliance on caregiver-reported data may have led to inaccuracies. Previous research has demonstrated that parents tend to report externalizing problems more precisely than children; however, it is unclear which group reports internalizing symptoms better (70). This may reflect the fact that children value their behavior more positively than parents (71). Although parent–adolescent agreement on emotional and behavioral problems has been reported to be high, adolescents tend to report more problems than their parents (72) and were found to be more sensitive to pain and mental health problems (73). As such our use of parental report may have underestimated the prevalence of anxiety and depression in our teenage population.
We were unable to cross-reference our findings with administrative datasets as most mental healthcare in Canada is provided privately and thus is unavailable in provincially held data repositories. Antibody testing was not performed on participants, and thus we cannot exclude the possibility of infection during or before our follow-up period among control participants. Additionally, as numerous viral illnesses are associated with the development of chronic anxiety and/or depressive symptoms, this may have been the case for some control children (74). As our study was limited to those who speak English or French and we excluded children with pre-existing mental health diagnoses, our findings may not reflect the experiences of the excluded children and their families. In addition, our study population was limited to children seeking ED care, and as such children tend to be sicker than those treated by primary care physicians or who did not seek care at all. This may have biased our SARS-CoV-2 cohort to the inclusion of those with more severe disease (75). The bias toward more severe COVID-19 disease in the acute phase may have further biased our results toward identifying an association between infection and long-term neuropsychiatric symptoms among infected children as hospitalization and severe COVID-19 are associated with an increased risk of developing the post-COVID-19 condition (76).
We could not perform a regression analysis to determine if the association between SARS-CoV-2 infection and new diagnoses of anxiety and/or depression persisted after adjustment for confounders (e.g., sex, chronic conditions, and variants) due to the small number of outcome events. This may be particularly relevant as a greater proportion of SARS-CoV-2-positive participants were male (Table 1), and they were less likely to experience anxiety and depression (77). Lastly, due to pandemic restrictions, there were challenges in accessing healthcare, and people also avoided seeking care. As such, mental healthcare provider access was reduced, and formal diagnoses of anxiety or depression may have been delayed or missed in some participants. Importantly, we did not incorporate symptom severity into our analysis, and we excluded children with pre-existing mental health conditions. Future studies should integrate measures of severity to further our understanding of the effects of SARS-CoV-2 infection on children with pre-existing diagnoses (i.e., whether SARS-CoV-2 infection exacerbated symptoms).
In this prospective cohort study with 6- and 12-month follow-ups, there was no association between SARS-CoV-2 infection itself and new diagnoses of anxiety and/or depression in children tested for acute infection in an ED setting. This finding, in the context of an increased prevalence of such diagnoses during the pandemic, as noted in our study population of older SARS-CoV-2-negative participants, underscores the complex impacts of broad societal changes on the mental health of children. However, our finding that some non-specific symptoms, such as confusion, abdominal pain, and insomnia were more frequently reported by SARS-CoV-2-positive participants, emphasizes the need for further investigation of the precise pathophysiologic mechanisms underlying the development of chronic symptoms in children.
Data will only be made available by the authors to requestors with evidence of research ethics board approval and establishment of a data sharing agreement.
This study involving humans was approved by the Conjoint Health Research Ethics Board of the University of Calgary and the Research Ethics Board of all participating study institutions (Supplementary Table S1). This study was conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the minimal risk associated with this study, the need to limit in-person interaction with SARS-CoV-2-infected individuals, and the large number of participants.
FD-D: Conceptualization, Investigation, Methodology, Writing – original draft. JX: Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. RZ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. KW: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft. BB: Data curation, Writing – review & editing. VS: Data curation, Writing – review & editing. JE: Data curation, Writing – review & editing. JG: Data curation, Writing – review & editing. AK: Data curation, Writing – review & editing. AMa: Data curation, Writing – review & editing. DB: Data curation, Writing – review & editing. RP: Data curation, Writing – review & editing. GF: Data curation, Writing – review & editing. NP: Data curation, Writing – review & editing. AMo: Data curation, Writing – review & editing. SB: Data curation, Writing – review & editing. MS: Conceptualization, Methodology, Writing – review & editing. DR: Project administration, Writing – review & editing. BW: Conceptualization, Methodology, Writing – review & editing. SF: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft.
The authors declare financial support was received for the research and/or publication of this article. Support for this study was provided by the Public Health Agency of Canada’s Immunization Partnership Fund (Emerging Issues Fund) and their Emerging Issues Fund (Enhanced Surveillance), and the Canadian Institute of Health Research (Operating Grant: Emerging COVID-19 Research Gaps and Priorities Funding Opportunity). BB and SB are recipients of a career award from the Quebec Health Research Fund (FRQ-S). SF is supported by the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness. FD is supported by the University of Calgary, Eyes High Post-Doctoral Fellowship. None of the funders played any role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript and decision to submit the manuscript for publication. The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada.
We would like to thank Tanya Borthwick for her administrative assistance throughout the conduct of the study; Alissa Kazakoff, BSc (University of Calgary), for her assistance with study management; and Richard Webster, PhD (University of Ottawa), who assisted with study data management. These individuals were compensated for their roles in the study through funding from the Public Health Agency of Canada.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1524617/full#supplementary-material
CI, confidence interval; ED, emergency department; ISARIC, International Severe Acute Respiratory and Emerging Infection Consortium; IQR, inter-quartile range; US, United States; WHO, World Health Organization.
1. El Omrani O, Carmen VA, Bionat JF, Ghebreyesus TA, Fore H, Wickramanayake J. COVID-19, mental health, and young people’s engagement. J Adolesc Health. (2023) 72(1s):S18–s19. doi: 10.1016/j.jadohealth.2021.03.020
2. Loades ME, Chatburn E, Higson-Sweeney N, Reynolds S, Shafran R, Brigden A, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. (2020) 59(11):1218–39.e3. doi: 10.1016/j.jaac.2020.05.009
3. Jones SE, Ethier KA, Hertz M, DeGue S, Le VD, Thornton J, et al. Mental health, suicidality, and connectedness among high school students during the COVID-19 pandemic—adolescent behaviors and experiences survey, United States, January–June 2021. MMWR Suppl. (2022) 71(3):16–21. doi: 10.15585/mmwr.su7103a3
4. Rao S, Gross RS, Mohandas S, Stein CR, Case A, Dreyer B, et al. Postacute sequelae of SARS-CoV-2 in children. Pediatrics. (2024) 153(3):e2023062570. doi: 10.1542/peds.2023-062570
5. Hossain MM, Tasnim S, Sultana A, Faizah F, Mazumder H, Zou L, et al. Epidemiology of mental health problems in COVID-19: a review. F1000Res. (2020) 9:636. doi: 10.12688/f1000research.24457.1
6. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. (2020) 395(10227):912–20. doi: 10.1016/S0140-6736(20)30460-8
7. Hamatani S, Hiraoka D, Makita K, Tomoda A, Mizuno Y. Longitudinal impact of COVID-19 pandemic on mental health of children in the ABCD study cohort. Sci Rep. (2022) 12(1):19601. doi: 10.1038/s41598-022-22694-z
8. Penninx BWJH, Benros ME, Klein RS, Vinkers CH. How COVID-19 shaped mental health: from infection to pandemic effects. Nat Med. (2022) 28(10):2027–37. doi: 10.1038/s41591-022-02028-2
9. Goldman RD. Long COVID in children. Can Fam Physician. (2022) 68(4):263–5. doi: 10.46747/cfp.6804263
10. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8(5):416–27. doi: 10.1016/S2215-0366(21)00084-5
11. Hampshire A, Azor A, Atchison C, Trender W, Hellyer PJ, Giunchiglia V, et al. Cognition and memory after COVID-19 in a large community sample. N Engl J Med. (2024) 390(9):806–18. doi: 10.1056/NEJMoa2311330
12. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. (2021) 594(7862):259–64. doi: 10.1038/s41586-021-03553-9
13. Ali MM, Schreier A, West KD, Plourde E. Mental health conditions among children and adolescents with a COVID-19 diagnosis. Psychiatr Serv. (2022) 73(12):1412–3. doi: 10.1176/appi.ps.202100646
14. Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Petersen MNS, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. (2022) 6(9):614–23. doi: 10.1016/S2352-4642(22)00154-7
15. Hascup ER, Hascup KN. Does SARS-CoV-2 infection cause chronic neurological complications? Geroscience. (2020) 42(4):1083–7. doi: 10.1007/s11357-020-00207-y
16. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. (2020) 89:594–600. doi: 10.1016/j.bbi.2020.07.037
17. Benedetti F, Mazza M, Cavalli G, Ciceri F, Dagna L, Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J Neuroimmune Pharmacol. (2021) 16(1):1–3. doi: 10.1007/s11481-020-09966-z
18. Lorkiewicz P, Waszkiewicz N. Biomarkers of post-COVID depression. J Clin Med. (2021) 10(18):4142. doi: 10.3390/jcm10184142
19. Trivedi MH. The link between depression and physical symptoms. Prim Care Companion J Clin Psychiatry. (2004) 6(Suppl 1):12–6.16001092
20. Bontempo AC. The effect of personalized invalidation of symptoms by healthcare providers on patient depression: the mediating role of self-esteem. Patient Educ Couns. (2022) 105(6):1598–605. doi: 10.1016/j.pec.2021.09.034
21. Matthews T, Danese A, Wertz J, Odgers CL, Ambler A, Moffitt TE, et al. Social isolation, loneliness and depression in young adulthood: a behavioural genetic analysis. Soc Psychiatry Psychiatr Epidemiol. (2016) 51(3):339–48. doi: 10.1007/s00127-016-1178-7
22. Askeland KG, Bøe T, Lundervold AJ, Stormark KM, Hysing M. The association between symptoms of depression and school absence in a population-based study of late adolescents. Front Psychol. (2020) 11:1268. doi: 10.3389/fpsyg.2020.01268
23. Rubin R. From “immunity debt” to “immunity theft”-how COVID-19 might be tied to recent respiratory disease surges. JAMA. (2024) 331(5):378–81. doi: 10.1001/jama.2023.26608
24. Wang Y, Su B, Xie J, Garcia-Rizo C, Prieto-Alhambra D. Long-term risk of psychiatric disorder and psychotropic prescription after SARS-CoV-2 infection among UK general population. Nat Human Behav. (2024) 8(6):1076–87. doi: 10.1038/s41562-024-01853-4
25. Bialy L, Plint A, Zemek R, Johnson D, Klassen T, Osmond M, et al. Pediatric emergency research Canada: origins and evolution. Pediatr Emerg Care. (2018) 34(2):138–44. doi: 10.1097/PEC.0000000000001360
26. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. (2007) 4(10):e297. doi: 10.1371/journal.pmed.0040297
27. Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. (2019) 206:256–67.e3. doi: 10.1016/j.jpeds.2018.09.021
28. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21(3):133–46. doi: 10.1038/s41579-022-00846-2
29. Gupta RK, Harrison EM, Ho A, Docherty AB, Knight SR, van Smeden M, et al. Development and validation of the ISARIC 4C deterioration model for adults hospitalised with COVID-19: a prospective cohort study. Lancet Respir Med. (2021) 9(4):349–59. doi: 10.1016/S2213-2600(20)30559-2
30. World Health Organization. A Clinical Case Definition for Post-COVID-19 Condition in Children and Adolescents by Expert Consensus (2023). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1 (accessed March 7, 2023)
31. Funk AL, Florin TA, Kuppermann N, Tancredi DJ, Xie J, Kim K, et al. Outcomes of SARS-CoV-2–positive youths tested in emergency departments. JAMA Netw Open. (2022) 5(1):e2142322. doi: 10.1001/jamanetworkopen.2021.42322
32. Sumner MW, Xie J, Zemek R, Winston K, Freire G, Burstein B, et al. Comparison of symptoms associated with SARS-CoV-2 variants among children in Canada. JAMA Netw Open. (2023) 6(3):e232328. doi: 10.1001/jamanetworkopen.2023.2328
33. Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19. JAMA Pediatr. (2021) 175(11):1142–50. doi: 10.1001/jamapediatrics.2021.2482
34. Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. Mental health surveillance among children—United States, 2013–2019. MMWR Suppl. (2022) 71(2):1–42. doi: 10.15585/mmwr.su7102a1
35. Agresti A, Caffo B. Simple and effective confidence intervals for proportions and differences of proportions result from adding two successes and two failures. Am Stat. (2000) 54(4):280–8. doi: 10.1080/00031305.2000.10474560
36. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Royal Stats Soc. (1995) 57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
37. American Academy of Pediatrics. AAP-AACAP-CHA Declaration of a National Emergency in Child and Adolescent Mental Health. American Academy of Pediatrics (2021). Available online at: https://www.aap.org/en/advocacy/child-and-adolescent-healthy-mentaldevelopment/aap-aacap-cha-declaration-of-anational-emergency-in-child-and-adolescentmental-health/ (accessed January 21, 2025)
38. US Department of Health and Human Services Office of the Surgeon General. Protecting Youth Mental Health: The US Surgeon General’s Advisory. (2021). Available online at: https://www.hhs.gov/sites/default/files/surgeon-generalyouth-mental-health-advisory.pdf (accessed January 25, 2025)
39. Centers for Disease Control and Prevention - National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Division of Adolescent and School Health. Youth Risk Behavior Survey: Data Summary & Trends Report 2009–2019. (2020). Available online at: https://stacks.cdc.gov/view/cdc/115813 (accessed January 25, 2025)
40. Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, et al. Mental health surveillance among children–United States, 2005–2011. MMWR Suppl. (2013) 62(2):1–35.23677130
41. Daskalakis NP, Rijal CM, King C, Huckins LM, Ressler KJ. Recent genetics and epigenetics approaches to PTSD. Curr Psychiatry Rep. (2018) 20(5):30. doi: 10.1007/s11920-018-0898-7
42. Casagrande M, Favieri F, Tambelli R, Forte G. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. (2020) 75:12–20. doi: 10.1016/j.sleep.2020.05.011
43. Qu Z, Wang C-W, Zhang X, Ho AHY, Wang X, Chan CLW. Prevalence and determinants of depression among survivors 8 months after the Wenchuan earthquake. J Nerv Ment Dis. (2014) 202(4):275–9. doi: 10.1097/NMD.0000000000000118
44. Xie X, Xue Q, Zhou Y, Zhu K, Liu Q, Zhang J, et al. Mental health status among children in home confinement during the coronavirus disease 2019 outbreak in Hubei province, China. JAMA Pediatr. (2020) 174(9):898–900. doi: 10.1001/jamapediatrics.2020.1619
45. Hu T, Wang Y, Lin L, Tang W. The mediating role of daytime sleepiness between problematic smartphone use and post-traumatic symptoms in COVID-19 home-refined adolescents. Child Youth Serv Rev. (2021) 126:106012. doi: 10.1016/j.childyouth.2021.106012
46. Yue J, Zang X, Le Y, Anxiety AY. Depression and PTSD among children and their parent during 2019 novel coronavirus disease (COVID-19) outbreak in China. Curr Psychol. (2022) 41(8):5723–30. doi: 10.1007/s12144-020-01191-4
47. Gupte A, Sriram S, Gunasekaran V, Chaudhari K, Kamat D. The triad of COVID-19 in children: acute COVID-19, multisystem inflammatory syndrome, and long COVID-part II. Pediatr Ann. (2025) 54(1):e40–4. doi: 10.3928/19382359-20241106-01
48. Lewis SJ, Arseneault L, Caspi A, Fisher HL, Matthews T, Moffitt TE, et al. The epidemiology of trauma and post-traumatic stress disorder in a representative cohort of young people in England and Wales. Lancet Psychiatry. (2019) 6(3):247–56. doi: 10.1016/S2215-0366(19)30031-8
49. Cohen ZP, Cosgrove KT, DeVille DC, Akeman E, Singh MK, White E, et al. The impact of COVID-19 on adolescent mental health: preliminary findings from a longitudinal sample of healthy and at-risk adolescents. Front Pediatr. (2021) 9:622608. doi: 10.3389/fped.2021.622608
50. Ramos-Casals M, Brito-Zeron P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. (2021) 17(6):315–32. doi: 10.1038/s41584-021-00608-z
51. Rao S, Lee GM, Razzaghi H, Lorman V, Mejias A, Pajor NM, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. (2022) 176(10):1000–9. doi: 10.1001/jamapediatrics.2022.2800
52. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children—a nationwide cohort study. Eur J Pediatr. (2022) 181(4):1597–607. doi: 10.1007/s00431-021-04345-z
53. Dun-Dery F, Xie J, Winston K, Burstein B, Gravel J, Emsley J, et al. Post-COVID-19 condition in children 6 and 12 months after infection. JAMA Network Open. (2023) 6(12):e2349613. doi: 10.1001/jamanetworkopen.2023.49613
54. Funk AL, Kuppermann N, Florin TA, Tancredi DJ, Xie J, Kim K, et al. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open. (2022) 5(7):e2223253. doi: 10.1001/jamanetworkopen.2022.23253
55. Moss SJ, Stelfox M, McArthur E, Sriskandarajah C, Ahmed SB, Birnie K, et al. Social factors associated with self-reported changes in mental health symptoms among youth in the COVID-19 pandemic: a cross-sectional survey. BMC Public Health. (2024) 24(1):631. doi: 10.1186/s12889-024-18087-8
56. Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. (2015) 10(5):e0126982. doi: 10.1371/journal.pone.0126982
57. Keller AS, Leikauf JE, Holt-Gosselin B, Staveland BR, Williams LM. Paying attention to attention in depression. Transl Psychiatry. (2019) 9(1):279. doi: 10.1038/s41398-019-0616-1
58. Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. (2008) 10(3):329–36. doi: 10.31887/DCNS.2008.10.3/dnutt
59. Plummer AM, Matos YL, Lin HC, Ryman SG, Birg A, Quinn DK, et al. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front Neurosci. (2023) 17:1232480. doi: 10.3389/fnins.2023.1232480
60. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood-brain barrier. Neurobiol Dis. (2020) 146:105131. doi: 10.1016/j.nbd.2020.105131
61. Cardinale V, Capurso G, Ianiro G, Gasbarrini A, Arcidiacono PG, Alvaro D. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: a working hypothesis. Dig Liver Dis. (2020) 52(12):1383–9. doi: 10.1016/j.dld.2020.09.009
62. Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. (2020) 53:13–24. doi: 10.1016/j.cytogfr.2020.05.009
63. Bernard-Raichon L, Venzon M, Klein J, Axelrad JE, Zhang C, Sullivan AP, et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat Commun. (2022) 13(1):5926. doi: 10.1038/s41467-022-33395-6
64. Khan YS, Khan AW, Noureldin Ahmed IA, Hammoudeh S, Salim H, AbuKhattab M, et al. Prevalence of elevated anxiety symptoms among children in quarantine with COVID-19 infection in the state of Qatar: a cross-sectional study. Scand J Child Adolesc Psychiatr Psychol. (2021) 9:187–95. doi: 10.21307/sjcapp-2021-021
65. Matsumoto N, Kadowaki T, Takanaga S, Shigeyasu Y, Okada A, Yorifuji T. Longitudinal impact of the COVID-19 pandemic on the development of mental disorders in preadolescents and adolescents. BMC Public Health. (2023) 23(1):1308. doi: 10.1186/s12889-023-16228-z
66. Nearchou F, Flinn C, Niland R, Subramaniam SS, Hennessy E. Exploring the impact of COVID-19 on mental health outcomes in children and adolescents: a systematic review. Int J Environ Res Public Health. (2020) 17(22):8479. doi: 10.3390/ijerph17228479
67. Guessoum SB, Lachal J, Radjack R, Carretier E., Minassian S, Benoit L, et al. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatry Res. (2020) 291:113264. doi: 10.1016/j.psychres.2020.113264
68. Whalen DJ, Sylvester CM, Luby JL. Depression and anxiety in preschoolers: a review of the past 7 years. Child Adolesc Psychiatr Clin N Am. (2017) 26(3):503–22. doi: 10.1016/j.chc.2017.02.006
69. von Gontard A, Moritz AM, Thome-Granz S, Equit M. Abdominal pain symptoms are associated with anxiety and depression in young children. Acta Paediatr. (2015) 104(11):1156–63. doi: 10.1111/apa.13134
70. Silverman WK, Eisen AR. Age differences in the reliability of parent and child reports of child anxious symptomatology using a structured interview. J Am Acad Child Adolesc Psychiatry. (1992) 31(1):117–24. doi: 10.1097/00004583-199201000-00018
71. Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull. (1987) 101(2):213–32. doi: 10.1037/0033-2909.101.2.213
72. Wang J, Liu L, Wu H, Yang X, Wang Y, Wang L. Agreement between parents and adolescents on emotional and behavioral problems and its associated factors among Chinese school adolescents: a cross-sectional study. BMC Psychiatry. (2014) 14:114. doi: 10.1186/1471-244X-14-114
73. Waters E, Stewart-Brown S, Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child Care Health Dev. (2003) 29(6):501–9. doi: 10.1046/j.1365-2214.2003.00370.x
74. Coughlin SS. Anxiety and depression: linkages with viral diseases. Public Health Rev. (2012) 34(2):7. doi: 10.1007/BF03391675
75. Sumner MW, Kanngiesser A, Lotfali-Khani K, Lodha N, Lorenzetti D, Funk AL, et al. Severe outcomes associated with SARS-CoV-2 infection in children: a systematic review and meta-analysis. Front Pediatr. (2022) 10:916655. doi: 10.3389/fped.2022.916655
76. Rayner DG, Wang E, Su C, Patel OD, Aleluya S, Giglia A, et al. Risk factors for long COVID in children and adolescents: a systematic review and meta-analysis. World J Pediatr. (2024) 20(2):133–42. doi: 10.1007/s12519-023-00765-z
Keywords: SARS-CoV-2, COVID, anxiety, depression, children, emergency department (ED)
Citation: Dun-Dery F, Xie J, Zemek R, Winston K, Burstein B, Sabhaney V, Emsley J, Gravel J, Kam A, Mater A, Beer D, Porter R, Freire G, Poonai N, Moffatt A, Berthelot S, Salvadori MI, Reddy D, Wright B and Freedman SB (2025) Pediatric SARS-CoV-2 infection and development of anxiety and depression. Front. Pediatr. 13:1524617. doi: 10.3389/fped.2025.1524617
Received: 7 November 2024; Accepted: 11 February 2025;
Published: 17 March 2025.
Edited by:
Rowena Ng, Kennedy Krieger Institute, United StatesReviewed by:
Angelo Mazza, Papa Giovanni XXIII Hospital, ItalyCopyright: © 2025 Dun-Dery, Xie, Zemek, Winston, Burstein, Sabhaney, Emsley, Gravel, Kam, Mater, Beer, Porter, Freire, Poonai, Moffatt, Berthelot, Salvadori, Reddy, Wright and Freedman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen B. Freedman, c3RlcGhlbi5mcmVlZG1hbkBhaHMuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.