
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 03 March 2025
Sec. Pediatric Immunology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1518881
This article is part of the Research TopicAdvances in the diagnosis, management and outcomes of Immune-Mediated Skin Diseases in childrenView all 5 articles
Neonatal lupus erythematosus (NLE) is a rare autoimmune disorder characterized by cutaneous and/or cardiac manifestations resulting from the transplacental passage of maternal antibodies, including anti-SSA/Ro, anti-SSB/La, and occasionally anti-U1RNP. This report describes two siblings with distinct NLE presentations, emphasizing the importance of early diagnosis and management, particularly in light of the rising rates of multiple births. A 15-day-old girl (Case 1) presented with classic annular skin lesions and strongly positive SSA and SSB antibodies. Six years later, her brother (Case 2) developed atypical red papules with similar serologic findings. Their mother, diagnosed with Sjögren's syndrome after the first child's (Case 1) presentation, demonstrated suboptimal treatment adherence, which may have contributed to the occurrence of NLE in her second child (Case 2). Neither sibling exhibited systemic involvement, including cardiac manifestations; however, regular monitoring remains essential. These cases highlight the variable NLE phenotype, even within families. In pregnancies with SSA/SSB antibody positivity, close monitoring of antibody titers, electrocardiograms (ECGs), and echocardiograms is paramount for early NLE detection and optimal management, especially given inconsistent maternal treatment. These cases underscore the need for heightened vigilance and proactive strategies in high-risk pregnancies.
Neonatal lupus erythematosus (NLE), first described by Bridge and Foley in 1954, is an autoimmune syndrome caused by the transplacental transmission of maternal autoantibodies, primarily anti-SSA/Ro and anti-SSB/La, and occasionally anti-U1RNP, which bind to fetal tissues (1–3). The incidence of NLE is approximately 1 in 12,500–20,000 live births, with a 2% occurrence in children of mothers carrying these antibodies and an 18%–20% recurrence risk in subsequent pregnancies (3, 4).
Clinically, NLE presents with various manifestations, including the characteristic periorbital annular skin rash (often referred to as an “eye mask” or “raccoon-like” appearance), cytopenia, hepatitis, and, most severely, congenital heart block (CHB) (3, 5).
In NLE, autoantibodies are prevalent in both children and their mothers. Children commonly test positive for anti-SSA (87.5%), anti-SSB (50%), or both (55%), and occasionally for anti-U1RNP (6, 7), while mothers exhibit similar rates: 84.6% for anti-SSA, 58.9% for anti-SSB, and 35.5% for both (8). Additionally, mothers may exhibit other autoantibodies depending on their underlying autoimmune diseases, such as systemic lupus erythematosus (SLE) or Sjögren's syndrome (3, 6).
Diagnosis relies on clinical presentation and detection of anti-SSA/Ro and/or anti-SSB/La in both the infant and mother; skin biopsy is optional (9). Differential diagnoses include congenital syphilis, tinea corporis, sarcoidosis, granuloma annulare, Langerhans histiocytosis, Sweet syndrome, and urticaria (3).
A significant proportion (25%–60%) of mothers are asymptomatic, which poses diagnostic challenges and increases the risk for their offspring (5, 10). Infants may develop other autoimmune diseases later, highlighting the importance of screening women of reproductive age for autoantibodies, even those with mild symptoms (5, 11, 12).
While the global incidence of NLE is a concern, the true incidence is likely underestimated (4). In China, the combination of a large population exceeding 1.4 billion (13) and a high prevalence of autoimmune diseases, such as Sjögren's syndrome (0.33%–0.77%) (14) and SLE (14.09 per 100,000 person-years in 2017) (15), suggests a considerable potential impact of NLE in the country. Furthermore, the implementation of the two-child policy in 2016 may lead to an increase in the incidence of CHB in NLE infants and a rise in overall NLE cases among siblings due to higher numbers of second births (12, 16). These factors collectively highlight the significant public health implications of NLE in China.
Management includes serial fetal echocardiograms biweekly between 16 and 28 weeks' gestation (3, 5), sun protection for cutaneous manifestations (5), and hydroxychloroquine to potentially reduce the recurrence of CHB (17). The role of intravenous immunoglobulin (IVIG) is under investigation (18). The prognosis of NLE varies. Cutaneous, hematologic, and hepatic abnormalities are typically transient. However, CHB can be potentially permanent, particularly third-degree CHB, which is more severe and frequently requires pacemaker implantation due to its significant mortality (20%–30%) and morbidity (19, 20).
These factors underscore the critical need for further research and enhanced surveillance, particularly in countries like China, to better understand and mitigate the impact of NLE.
In October 2015, a 15-day-old girl (patient 1) presented with erythema on her body. Born via vaginal delivery at 40 weeks to a 29-year-old mother, she exhibited targetoid erythematous plaques with central atrophy and raised margins on her face (Figure 1a), trunk, extremities, palms, and soles (Figure 1b). Laboratory tests indicated that the liver and kidney functions and the complete blood count were normal. Serologic tests revealed strongly positive SSA (Ro) and SSB (La) antibodies, a reactive ANA titer of 1:1000 with a speckled nuclear pattern by indirect immunofluorescence (IIF), and a negative syphilis test. Echocardiogram and electrocardiogram (ECG) results were normal. NLE was diagnosed based on the clinical presentation and serologic findings.
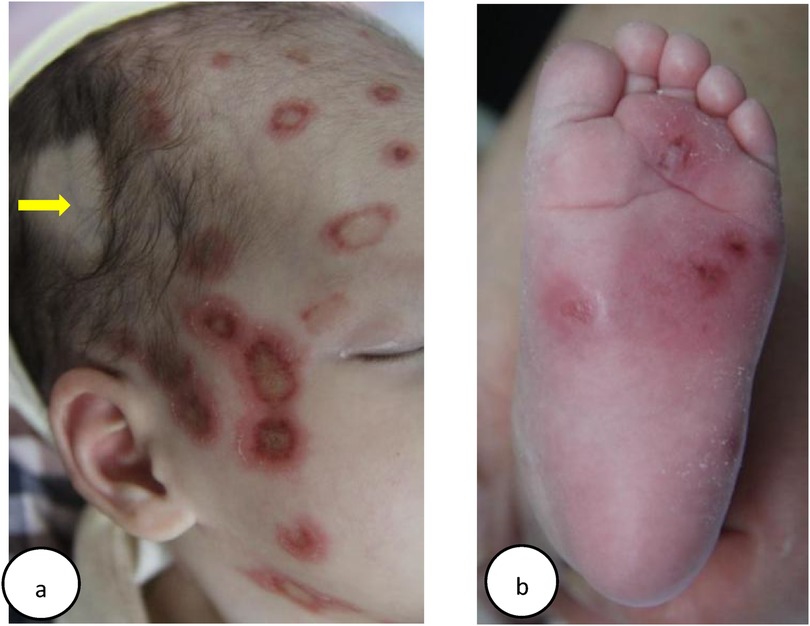
Figure 1. (a) Case 1: clinical appearances of head lesions: inflammatory annular plaques, with hyperkeratotic borders and atrophic centers. The alopecia spot on the right side of the head was due to shave by healthcare workers, which could expose the superficial vein of the scalp (yellow arrow), for the purpose of taking blood tests. (b) Case 1: multiple discoid skin lesions on the left plantar. Despite their rarity in NLE, plantar lesions are significant indicators of potential systemic involvement, requiring comprehensive evaluations and appropriate management for a favorable prognosis (21).
Following the infant's positive autoantibody tests, the mother was referred to a rheumatologist. She reported a 2-year history of dry mouth and tested strongly positive for SSA and SSB antibodies, with an ANA titer of >1:1000 and a speckled nuclear pattern by IIF. Schirmer's test indicated reduced tear production in both eyes (the right eye at <5 mm/5 min and the left eye at 5 mm/5 min), and her labial salivary gland biopsy showed focal lymphocytic sialadenitis, characterized by preserved lobular architecture, ductal dilation, and at least three lymphocytic foci per 4 mm2. These findings confirmed a diagnosis of Sjögren's syndrome.
The mother was treated with prednisone (20 mg/day for 3 years, then 10 mg/day maintenance) and hydroxychloroquine (400 mg/day), but she showed poor adherence and irregular monitoring of antibody levels.
The infant's rash resolved spontaneously within 6 months with sun protection, and serologic tests for ANA, SSA, and SSB were negative at the age of 1 year. However, residual telangiectasias and hyperpigmentation were observed 8 years later. Notably, the mother's Sjögren's syndrome diagnosis was made after the infant's NLE presentation.
Six years later, the mother became pregnant again. At 4 weeks of gestation, serologic tests revealed strongly positive SSA and SSB antibodies, a reactive ANA (>1:1000) with a speckled nuclear pattern (as determined by IIF), consistent with findings from 6 years prior, and an elevated erythrocyte sedimentation rate (ESR) of 90 mm/h (normal <10 mm/h). The mother was prescribed prednisone (10 mg/day), hydroxychloroquine (400 mg/day), and calcium carbonate (600 mg/day) due to her active condition. However, she poorly adhered to the regimen, stopping medications at 20 and 35 weeks of pregnancy due to a cold. Missed doses limited medication use during pregnancy to <20 weeks. At the 32nd week of gestation, her oral glucose tolerance test (OGTT) was normal, but recommended prenatal screenings, such as fetal echocardiograms, were inconsistently performed due to non-adherence.
A male infant (patient 2) was delivered via cesarean section at 39 weeks' gestation due to fetal macrosomia, with a birth weight of 4,500 g, and normal fasting blood glucose (5.1 mmol/L) in the newborn. The ECG result of patient 2 at birth indicated sinus tachycardia, with atrial and ventricular rates of 166 bpm, a P-R interval of 100 ms, and a QRS duration of 77 ms. Although this heart rate was slightly above the normal range for newborns (120–160 bpm) (22), subsequent monitoring revealed stabilization at 140 bpm without the development of CHB.
At 1 week after birth, he gradually developed a localized rash on the scalp and face, characterized by erythema and papules (Figure 2a), distinct from his sister's (Case1) widespread targetoid plaques. Laboratory tests revealed positive SSA, SSB, and ANA (1:100) with a speckled nuclear pattern, as determined by IIF. Skin biopsy revealed a vacuolar change of epidermal basal cells and perivascular and periadnexal mononuclear infiltrate in the dermis (Figure 2b). A diagnosis of NLE for Case 2 was made.
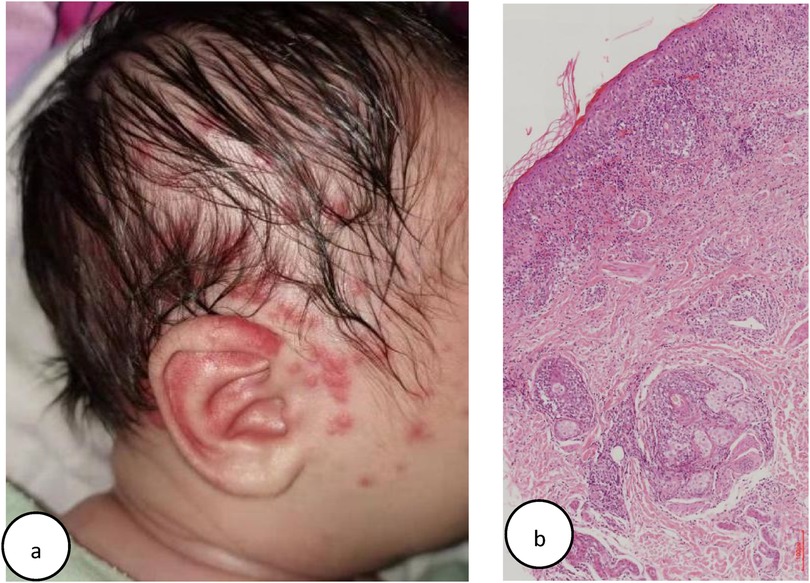
Figure 2. (a) Case 2: multiple erythema and papules on one face. (b) Case 2: histology of lesional skin: slight epidermal atrophy and hyperkeratosis, vacuolar degeneration at the dermal-epidermal junction, perivascular and periadnexal mononuclear infiltrate in the dermis (Hematoxylin–eosin stain, original magnifification, ×100).
In Case 2, the rash resolved by 5.5 months without treatment, with no residual pigmentation or telangiectasia. At 8 months, ANA and SSB were negative, with transient Anti-dsDNA elevation (161 IU/ml), which later turned negative after 2 years. At present, both siblings remain free of skin lesions and autoimmune diseases, under ongoing follow-up.
Consent for publication of this case report and accompanying images was obtained from the patient's parents after they were fully informed and had provided written authorization.
The main results of this study highlight the phenotypic diversity of NLE, as evidenced by the varied dermatological manifestations in siblings, including typical annular plaques and atypical red papules. The study's strengths lie in its comprehensive clinical documentation and extended follow-up, providing valuable insights into the natural history and treatment approaches for NLE in sibling pairs. These findings underscore the critical importance of early diagnosis and treatment.
Comparison with the literature reveals that cases of NLE among siblings have been reported, highlighting the variable clinical presentations of NLE within families. This emphasizes the importance of monitoring and preventive measures for mothers with a history of autoimmune diseases and their offspring. Zuppa et al. (23) discuss the differing manifestations of NLE among siblings, while Lee et al. (24) focus on the increased risk of NLE in successive pregnancies for mothers who have previously given birth to a child with NLE. Collectively, these studies underscore the need for vigilant prenatal care and counseling for families with a history of NLE.
The morphology of the NLE rash was heterogeneous, with typical cutaneous lesions resembling those seen in subacute cutaneous lupus erythematosus (SCLE) (3). A retrospective cohort study (25) revealed that annular (70.7%) and papulosquamous (70.7%) rashes were common, while pink/red macules were less frequent (11.1%).
Our findings align with the literature. Case 1 showed typical annular scaly erythema similar to SCLE, while Case 2 had less common red papules and macules, potentially due to varying specificity of anti-Ro autoantibodies or other fetal-maternal factors, including environmental, intrauterine, or genetic influences (23, 26).
The association between maternal treatment adherence and NLE recurrence requires further empirical evidence. However, it is well-recognized that pregnant women testing positive for autoantibodies to SSA/Ro or SSB/La, particularly those with a prior history of NLE, are at increased risk of delivering a child with NLE (4, 6). Furthermore, recent research has highlighted the notable possibility that normal sinus rhythm can progress to third-degree heart block within just 7 days (19). Given these risks, it is essential to monitor the fetal PR interval through echocardiogram during gestation. The absence of such monitoring is deemed potentially hazardous, underscoring the necessity for comprehensive counseling, fetal and maternal screening, and the implementation of preventive or management strategies for heart disease (6, 27). Our findings align with these observations, suggesting that suboptimal adherence to treatment, which may lead to sustained high levels of autoantibodies such as SSA, SSB, and ANA, could contribute to the recurrence of NLE in subsequent pregnancies. This underscores the crucial importance of strict adherence to treatment protocols to minimize recurrence risk and enhance patient outcomes (19).
Preventing NLE in siblings is challenging due to its genetic and autoimmune etiologies. Key prevention strategies include maternal education on autoimmune disease risks and genetic counseling. In our case, the mother, who had experienced xerostomia for over 2 years, only sought medical attention after her first child was diagnosed with NLE. Despite being diagnosed with Sjögren's syndrome and prescribed treatments, her suboptimal adherence to medication may have contributed to the recurrence of NLE in her second child. This underscores the importance of educating mothers about autoimmune disease risks during pregnancy and the necessity of regular prenatal care and autoantibody monitoring (23, 26). Genetic counseling is recommended for families with a history of NLE to discuss the implications of maternal autoimmune diseases and the associated recurrence risks (26). These measures are crucial for informing clinical practice and research, thereby improving outcomes for families affected by NLE (23).
The study is limited by its small sample size, which may restrict the generalizability of the findings. Additionally, the reliance on parental adherence to treatment introduces variability in disease outcomes, as evidenced by the differing levels of maternal treatment adherence in our two cases. Future studies with larger cohorts and standardized treatment protocols are necessary to address these limitations and provide more robust data on NLE management and outcomes (8).
A retrospective cohort study (25) reported cutaneous sequelae in 34% of 106 patients at a mean follow-up duration of 4 years (range 0.5–18.7 years), including 13% telangiectasia, 17% dyspigmentation, and 9% atrophic scarring. Our findings are consistent with these observations: Case 1 exhibited telangiectasia and hyperpigmentation following rash regression, while Case 2 showed no sequelae. The variability in follow-up durations underscores the importance of long-term monitoring to better understand the disease's natural history and long-term outcomes (27).
These sibling cases of NLE with distinct cutaneous manifestations highlight the heterogeneous nature of the disease and underscore the importance of early diagnosis and management. Maternal treatment adherence is crucial for preventing recurrence. Long-term follow-up, including cardiac monitoring, is essential even in the absence of initial cardiac manifestations. These findings emphasize the need for increased awareness among healthcare professionals and expectant mothers about NLE, its potential complications, and the importance of preventive strategies. Further research is needed to explore the underlying mechanisms of NLE and to develop more effective prevention and treatment strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the medical ethics committee of Shenzhen Children's Hospital, Shenzhen Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
PT: Writing – original draft, Funding acquisition. HZ: Writing – review & editing, Data curation, Resources. PL: Writing – review & editing, Validation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Guangdong High-level Hospital Construction Fund Clinical Research Project of Shenzhen Children's Hospital (No. LCYJ2022061). This funding covered expert consultation fees, compensation for data collection and organization by graduate students, and the article processing charges.
We are grateful to Xingliang Zhang (Institute of Pediatrics, Shenzhen Children's Hospital) and Bin Zhang (Department of Dermatology, Beijing Children's Hospital) for polishing this manuscript, and we appreciate the support of the patients and their parents for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bridge RG, Foley FE. Placental transmission of the lupus erythematosus factor. Am J Med Sci. (1954) 227(1):1–8. doi: 10.1097/00000441-195401000-00001
2. Heelan K, Watson R, Collins SM. Neonatal lupus syndrome associated with ribonucleoprotein antibodies. Pediatr Dermatol. (2013) 30(4):416–23. doi: 10.1111/pde.12088
3. Derdulska JM, Rudnicka L, Szykut-Badaczewska A, Mehrholz D, Nowicki RJ, Barańska-Rybak W, et al. Neonatal lupus erythematosus - practical guidelines. J Perinat Med. (2021) 49(5):529–38. doi: 10.1515/jpm-2020-0543
4. Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. (2003) 142(5):678–83. doi: 10.1067/mpd.2003.233
5. Erden A, Fanouriakis A, Kılıç L, Sari A, Armağan B, Bilgin E, et al. Geoepidemiology and clinical characteristics of neonatal lupus erythematosus: a systematic literature review of individual patients’ data. Turk J Med Sci. (2020) 50(3):281–90. doi: 10.3906/sag-1910-39
6. Brito-Zerón P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol. (2015) 11(5):301–12. doi: 10.1038/nrrheum.2015.29
7. Wisuthsarewong W, Soongswang J, Chantorn R. Neonatal lupus erythematosus: clinical character, investigation, and outcome. Pediatr Dermatol. (2011) 28(2):115–21. doi: 10.1111/j.1525-1470.2011.01300.x
8. Liszewska A, Woźniacka A. Neonatal lupus erythematosus - prevention is better than cure. Postepy Dermatol Alergol. (2022) 39(6):1021–6. doi: 10.5114/ada.2022.122601
9. Neiman AR, Lee LA, Weston WL, Buyon JP. Cutaneous manifestations of neonatal lupus without heart block: characteristics of mothers and children enrolled in a national registry. J Pediatr. (2000) 137(5):674–80. doi: 10.1067/mpd.2000.109108
10. Ma J, Li Z, Song H, Zhang L. High-risk groups of neonatal lupus erythematosus in term infants: a birth cohort study. Eur J Pediatr. (2023) 183(3):149–55. doi: 10.1007/s00431-023-05283-8
11. Lee LA. Transient autoimmunity related to maternal autoantibodies: neonatal lupus. Autoimmun Rev. (2005) 4(4):207–13. doi: 10.1016/j.autrev.2004.11.003
12. Yu Y, Du L, Pan J, Zheng J, Chen A, Chen L. A 10-year retrospective study of neonatal lupus erythematosus in China. Asian Pac J Allergy Immunol. (2016) 34(2):174–8. doi: 10.12932/AP0671.34.2.2016
13. Peng D. Negative population growth and population ageing in China. China Popul Dev Stud. (2023) 7:95–103. doi: 10.1007/s42379-023-00138-z
14. Zhang NZ, Shi CS, Yao QP, Pan GX, Wang LL, Wen ZX, et al. Prevalence of primary Sjögren’s syndrome in China. J Rheumatol. (1995) 22(4):659–61.7791159
15. Li M, Li C, Cao M, Lu K, Wu C, Wang J, et al. Incidence and prevalence of systemic lupus erythematosus in urban China, 2013-2017: a nationwide population-based study. Sci Bull. (2024) 69(19):3089–97. doi: 10.1016/j.scib.2024.04.075
16. Feng W, Gu B, Cai Y. The end of China’s one-child policy. Stud Fam Plann. (2016) 47(1):83–6. doi: 10.1111/j.1728-4465.2016.00052.x
17. Izmirly P, Kim M, Friedman DM, Costedoat-Chalumeau N, Clancy R, Copel JA, et al. Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/Ro-positive mothers. J Am Coll Cardiol. (2020) 76(3):292–302. doi: 10.1016/j.jacc.2020.05.045
18. Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. (2010) 62(4):1138–46. doi: 10.1002/art.39696
19. Buyon JP, Clancy RM, Friedman DM. Autoimmune associated congenital heart block: integration of clinical and research clues in the management of the maternal/fetal dyad at risk. J Intern Med. (2009) 265(6):653–62. doi: 10.1111/j.1365-2796.2009.02086.x
20. Pilania RK, Thangaraj A, Sil A, Dhaliwal M, Sharma S, Jindal AK, et al. Neonatal lupus erythematosus: 24 years of experience from a tertiary centre at Chandigarh, north India. Lupus. (2024) 34(1):102–7. doi: 10.1177/09612033241308115
21. Admani S, Krakowski AC. Neonatal lupus erythematosus presenting as atypical targetoid-like lesions involving genitals and soles of feet following brief sun exposure. J Clin Aesthet Dermatol. (2013) 6(5):19–23.23710267
22. Tveiten L, Diep LM, Halvorsen T, Markestad T. Heart rate during the first 24h in term-born infants. Arch Dis Child Fetal Neonatal Ed. (2021) 106(5):489–93. doi: 10.1136/archdischild-2020-320761
23. Zuppa AA, Delogu AB, De Rosa G, De Luca D, Visintini F, Cota F, et al. Lupus néonatal: une fratrie de nouveau-nés avec différentes expressions cliniques [Neonatal lupus: different clinical neonatal expression in siblings]. Arch Pediatr. (2004) 11(8):936–9. doi: 10.1016/j.arcped.2004.05.009
24. Lee LA, Lillis PJ, Fritz KA, Huff JC, Norris DA, Weston WL. Neonatal lupus syndrome in successive pregnancies. J Am Acad Dermatol. (1983) 9(3):401–6. doi: 10.1016/s0190-9622(83)70149-0
25. Levy R, Briggs L, Silverman E, Pope E, Lara-Corrales I. Cutaneous sequelae in neonatal lupus: a retrospective cohort study. J Am Acad Dermatol. (2019) 83(2):440–6. doi: 10.1016/j.jaad.2019.09.083
26. Killen SA, Buyon JP, Friedman DM. Discordant spectrum of cardiac manifestations of neonatal lupus in twins. Lupus. (2012) 21(5):559–62. doi: 10.1177/0961203311430512
Keywords: neonatal lupus erythematosus, cutaneous manifestations, siblings, infant, Sjögren's syndrome
Citation: Tang P, Zhang H and Li P (2025) Case Report: Siblings with neonatal lupus erythematosus. Front. Pediatr. 13:1518881. doi: 10.3389/fped.2025.1518881
Received: 29 October 2024; Accepted: 17 February 2025;
Published: 3 March 2025.
Edited by:
Mao Lin, Sichuan University, ChinaReviewed by:
Fatma Gül Demirkan, Istanbul University, TürkiyeCopyright: © 2025 Tang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bGlwaW5nMjAwODExMTBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.