
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 21 February 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1514323
 Yulu Lai1,†
Yulu Lai1,† Jieting Lu1,†
Jieting Lu1,† Yanqing Liu2,†
Yanqing Liu2,† Jixiao Zeng2
Jixiao Zeng2 Shenwei Huang2
Shenwei Huang2 Lin Li2
Lin Li2 Bingtong Wang3
Bingtong Wang3 Pengfei Wei2
Pengfei Wei2 Yu Ouyang4
Yu Ouyang4 Junjian Lv2
Junjian Lv2 Wei Zhong2
Wei Zhong2 Chaoting Lan2*
Chaoting Lan2* Huimin Xia1,2*
Huimin Xia1,2* Qiuming He2,4*
Qiuming He2,4*
Introduction: Hirschsprung Disease (HSCR) is a common congenital intestinal disease in pediatrics. Early diagnosis and treatment after birth alleviate the occurrence of complications. Consequently, we aim to identifiy a biomarker with ease of use, non-invasiveness, and highly accurate for diagnosis.
Methods: Plasma samples were collected from HSCR group, other intestinal disease controls (DC) and healthy controls (HC), while colon samples were collected from HSCR and DC groups. We conducted human neural autoantibody microarray analyses on plasma. The candidate biomarker was further validated using enzyme-linked immunosorbent assay (ELISA) in colon tissue and plasma. The receiver operating characteristic curve (ROC) was used to assess the diagnostic performance of the plasma biomarker.
Results: Microarray analysis revealed that the level of plasma N-methyl-D-Aspartate receptor (NMDAR) autoantibody in HSCR group was significantly higher than those in the HC group (p = 0.008). In plasma analyzed cohort, the level of NMDAR autoantibodies in HSCR group (n = 38) were significantly elevated compared to both the HC (n = 31, p < 0.0001) and the DC (n = 20, p < 0.0001). We further validated the diagnostic efficacy of plasma NMDAR autoantibody, it demonstrated AUCs of 0.96 and 0.81 for diagnosing HSCR when compared to HC and DC.
Conclusions: Plasma NMDAR autoantibody might be served as an efficient, non-invasive biomarker for diagnosing HSCR.
Hirschsprung Disease (HSCR) is a congenital disorder due to lack of enteric neurons in the distal colon (1–3). HSCR primarily necessitates surgical intervention due to its life-threatening complications and persistently high mortality rates (4–6). HSCR diagnosis and management, particularly the preoperative assessments, continue to be complex and challenging.
At present, HSCR presents with nonspecific clinical manifestations, and intraoperative diagnosis relies heavily on histopathological confirmation of aganglionosis in the distal colon. Common preoperative diagnostic adjuncts include barium enema (BE), anorectal manometry (ARM), and rectal biopsy, all of which are invasive or associated with significant discomfort (7–9). Emerging immunostaining markers, such as acetylcholinesterase (AChE) and calretinin, lack large-scale comparative studies and present challenges in preoperative application (8, 10). Consequently, the investigation of non-invasive, high-precision biomarkers holds significant value for the diagnosis of HSCR.
Recent studies have found that neural autoantibodies cause developmental abnormalities and neuronal damage by binding to neuronal surface antigens (11–13), a mechanism similar to the enteric neuronal developmental disorders in HSCR (14, 15). However, it is unclear whether neural autoantibodies that cause neuronal development and damage are involved in the occurrence and development of HSCR.
Here, we conducted plasma human neural autoantibody microarray analysis in HSCR, and this study aims to further explore biomarkers of diagnostic value.
Our study included three groups: HSCR group, DC group diagnosed with anal atresia or intestinal stenosis, and HC group comprising children undergoing routine physical examinations. The inclusion and exclusion criteria for the three groups are as follows.
Inclusion and Exclusion Criteria for HSCR group:
1. Patients who underwent surgical resection of the affected intestinal tissue with histopathological biopsies showing no aganglionosis (lack of ganglion cells).
2. No other congenital anomalies or syndromes present.
3. Not during an acute enteritis phase.
Inclusion and Exclusion Criteria for Group DC group:
1. Patients clinically diagnosed with congenital anal atresia or intestinal stenosis.
2. After surgical resection of the colonic tissue, histopathological biopsies show normal development of ganglion cells in the colonic tissue.
3. Not during an acute enteritis phase.
Inclusion and Exclusion Criteria for HC group:
1. Children diagnosed as healthy during routine physical examinations.
2. No congenital developmental abnormalities.
In this study, the cohort subjected to Human neural autoantibody microarray analysis of plasma is termed the discovery cohort. For the validation of NMDAR autoantibodies in plasma and colon tissue using ELISA, two additional cohorts were independently recruited at different time periods and are defined as independent cohorts. Recruitment for all three cohorts was strictly in accordance with the aforementioned inclusion criteria, with matching of age and gender among the study subjects across different groups.
Discovery cohort: HSCR (n = 5), DC (n = 5) and HC (n = 5) were tested on the Guangzhou AiGene Biotechnology's human neuroautoantibody microarray platform (Supplementary Table S1).
An independent cohort for ELISA validation of colon tissue: HSCR (n = 36), DC (n = 11) (Supplementary Table S2).
An additional independent cohort for ELISA validation of plasma: HSCR (n = 38), DC (n = 20) and HC (n = 31) (Supplementary Table S3).
All samples were collected from the Guangzhou Women and Children's Medical Center Clinical Sample Repository.
Plasma samples were mixed with human neuroautoantibody microarray that composed of over 100 human neural autoantibody probes, and incubated on a shaker at room temperature for 60 min. After washing, the anti-human IgG and anti-human IgM fluorescent secondary antibodies were diluted 1:1000 and incubated. The microarray was imaged by LuxScan 10K-B scanner and the raw data was read using LuxScan 3.0 software.
Colon tissues with and without ganglia from the HSCR group were collected during radical surgery, while those from DC group were harvested from the margins of excision in cases of anal atresia and intestinal stricture. After collection, samples were immediately placed in pre-chilled isotonic saline to prevent tissue degradation and swiftly transferred to the laboratory for further processing. In order to preserve the activity of neural autoantibodies to the greatest extent, the samples were quickly frozen in liquid nitrogen post-collection. Frozen samples were stored in an ultra-low temperature freezer at −80°C.
Blood was collected from HSCR group and the DC group during surgery. Plasma for the HC group was obtained from the residual blood of healthy children after physical examinations. 3 ml of fasting peripheral blood samples from participants were collected using EDTA vacuum tubes and immediately centrifuged at 4°C (1,500 × rpm, 20 min) to separate plasma and blood cells. Following an additional centrifugation at 4°C (3,000 × rpm, 15 min), the plasma samples were promptly frozen and stored at −80°C.
We further used the NMDAR antibody ELISA kit (Shanghai Zhenke Biotechnology Co., Ltd. ZK-4316) to measure NMDAR autoantibody levels in the plasma and colon tissue via ELISA. Each sample underwent three tests, and the average was calculated. The testers were completely unaware of the test results and sample grouping.
For microarray analysis, we used the Robust Linear Model method for normalization, followed by cluster analysis after M-statistic screening. The data obtained from ELISA testing were compared between groups using unpaired t-tests. Linear correlation analysis was used to evaluate the correlation between NMDAR autoantibody expression level in colon tissue with and without ganglionic segments in HSCR. We assessed the diagnostic efficacy of NMDAR autoantibody using the area under the Receiver Operating Characteristic curve (AUC). The statistical software used was R software (version 4.2.3) and GraphPad Prism 9.5 (GraphPad Software Inc., CA, USA). All p-values were two-sided, with p < 0.05 deemed to have statistical significance.
This project was approved by the review committee of Institution Guangzhou Women and Children's Medical Center (No. 2016042036), and the use of clinical data and samples has been consented to in writing by the parents or legal guardians of the participants.
Human neural autoantibody microarray cluster analysis (Figure 1A) showed that the expression level of plasma neural autoantibodies significant differences in HSCR, DC and HC group. Compared with the HC group, the HSCR group exhibited significantly higher levels of NMDAR autoantibody (p = 0.008). Additionally, HSCR group also showed an upward trend compared with the DC group (Figure 1B).
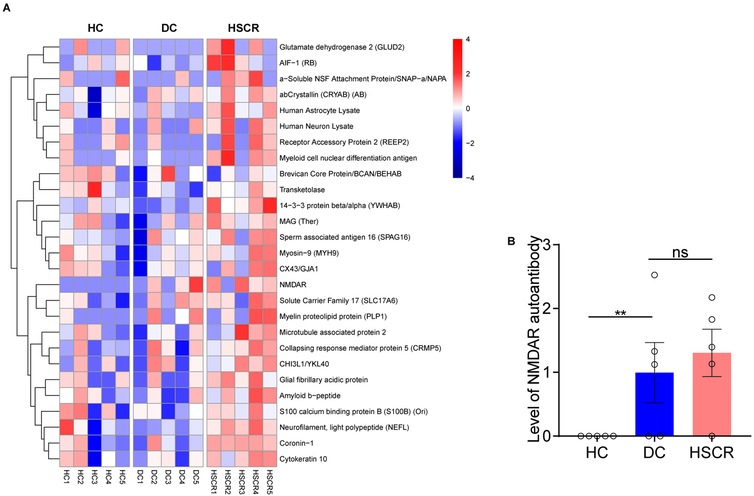
Figure 1. HSCR group had higher plasma NMDAR autoantibody levels than the other control groups. (A) The heatmap showed the expression of neural autoantibodies in three groups, respectively [from red (high) to blue (low)]. (B) Compare the NMDAR autoantibody level among HSCR, DC, and HC groups based on microarray data. Hirschsprung's disease group (HSCR), other intestinal disease controls (DC) of anal atresia and intestinal stenosis, healthy controls (HC).
To explore if there is an increased expression of NMDAR autoantibody in colon tissue, we validated using ELISA testing in independent cohort. The results showed that the expression level of NMDAR autoantibody in HSCR group were higher than those in the DC group, regardless of whether in the HSCR Aganglion (HA, p < 0.0001) or HSCR Ganglion (HG, p < 0.0001) (Figure 2A). Moreover, when the expression level of NMDAR autoantibody increases in HA, it also shows a similar trend of increase in HG, revealing a highly positive linear correlation (r = 0.9762) (Figure 2B).
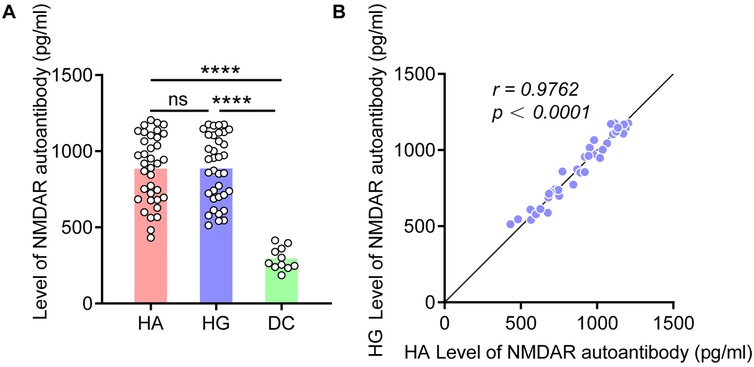
Figure 2. NMDAR autoantibody levels increased in HSCR colon tissue. (A) Compare the NMDAR autoantibody level in the colon tissue of HA, HG, and DC. (B) Correlation analysis of the expression level of NMDAR autoantibody in the HA and HG of colon tissue. Hirschsprung Disease group (HSCR), HSCR Aganglion (HA), HSCR Ganglion (HG), other intestinal disease controls (DC) of anal atresia and intestinal stenosis. Correlation coefficient (r), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In another independent cohort, plasma levels of NMDAR autoantibodies were validated using ELISA, showing significant differences in the HSCR group compared to both the HC (p < 0.0001) and DC (p < 0.0001) (Figure 3A). Additionally, we assessed the diagnostic performance of the candidate biomarker (Figure 3B). Compared with HC, the plasma NMDAR autoantibody has an AUC of 0.96 in the diagnosis of HSCR (sensitivity 87.1%, specificity 94.74%). While compared with DC, the plasma NMDAR autoantibody has an AUC of 0.81 (sensitivity 70%, specificity 73.68%). These findings further indicate that plasma NMDAR autoantibody can act as a plasma biomarker in the diagnosis of HSCR.
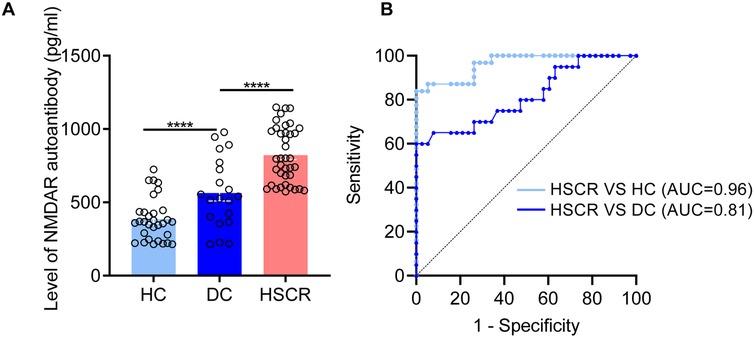
Figure 3. NMDAR autoantibody was identified as a biomarkers for the diagnosis of HSCR. (A) Quantitative comparison of plasma NMDAR autoantibody between HSCR group and different control groups (DC, HC). (B) The receiver operating characteristic curve is utilized to statistically validate the diagnostic performance of NMDAR autoantibody. Hirschsprung Disease group (HSCR), other intestinal disease controls (DC) of anal atresia and intestinal stenosis, healthy controls (HC). The area under the Receiver Operating Characteristic curve (AUC). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
HSCR is a complex intestinal developmental disorder with diverse etiologies. Recent research indicates that autoimmune activation is involved in the progression of HSCR (16). In this study, we utilized a human neuroautoantibody microarray and observed a significant increase in neuronal autoantibodies in the plasma of HSCR patients. Furthermore, we validated that the NMDAR autoantibody in plasma can serve as an objective biomarker for HSCR diagnosis.
BE is one of the most commonly used screening methods for HSCR, with a sensitivity of 70% (64%–76%) and a specificity of 83% (74%–90%) (17). ARM is an effective screening method for HSCR, with a sensitivity of 91% (85%–95%) and a specificity of 94% (89%–97%) in children over six months of age (18). However, it is challenging to achieve the absolute sedation required for neonates (7). Rectal suction biopsy is a diagnostic method for HSCR, requiring the submucosal layer to constitute at least one-third of the sampled tissue. Approximately 9% to 30% of pediatric patients may necessitate repeat biopsies due to insufficient specimens (19). Our study results indicate that plasma NMDAR autoantibody exhibits extremely high accuracy in ruling out healthy individuals, providing a reliable basis for clinical preliminary screening. This can effectively reduce the risk of missed diagnosis, ensuring that patients receive timely attention and further diagnosis. In the disease control group, the expression levels and distribution characteristics of NMDAR autoantibody may change due to the presence of multiple complications or pathological conditions in patients, thereby affecting diagnostic performance. Overall, this diagnostic approach offers a rapid, non-invasive, and efficient alternative for HSCR diagnosis, circumventing the need for pediatric sedation.
NMDAR, a member of the glutamate receptor family, widely expressed in the central nervous system, playing a crucial role in neuronal development and function (20). Studies have shown that NMDAR activation not only stimulates the migration of embryonic cortical neurons (21, 22) but also promotes the proliferation (23–25) and differentiation of neural progenitor cells (26, 27). These processes can be inhibited by NMDAR antagonists. Activation of NMDAR prevents neuronal damage, while NMDAR autoantibodies can downregulate energy metabolism in cultured neurons, leading to neuronal death (28–30). HSCR is caused by migration, proliferation, and differentiation disorders of enteric neural crest cells, with a pathogenesis similar to that of NMDAR autoantibodies-induced neurodevelopmental disorders (31). We found that NMDAR autoantibodies were significantly elevated in the plasma and tissues of the HSCR group, with the highest levels observed in a patient with total colonic HSCR who had a postoperative relapse. Therefore, we speculate whether NMDAR autoantibody is involved in the occurrence of HSCR by inhibiting neuronal migration proliferation, and differentiation, or by inducing neuronal death, and the level of NMDAR autoantibody may be related to the severity and recurrence of HSCR. Interestingly, we observed nearly identical NMDAR autoantibody levels in the HSCR HA and HG. This finding may suggest that the NMDAR autoantibody response is not solely dependent on the presence or absence of ganglia in the affected tissue. One possible explanation could be the presence of upstream regulatory mechanisms that influence NMDAR autoantibody production in both aganglionic and ganglionic segments of the bowel. Additionally, this similarity in autoantibody levels might reflect the systemic nature of the immune response in HSCR patients, which is not limited to the aganglionic segment alone. Further studies are warranted to explore these hypotheses and to elucidate the precise role of NMDAR autoantibody in the pathogenesis of HSCR.
This study has several limitations. Firstly, differences in neonatal history, sample size, methods of sample collection, and environmental factors between cases and controls may potentially affect the levels of NMDAR autoantibody, which is indeed a significant factor to be considered in study design. To minimize the potential confounding factors, it is necessary to strictly control these variables in the experimental design and conduct prospective studies for further validation.
Secondly, this study was conducted at a single center. Although NMDAR autoantibody demonstrated high diagnostic accuracy in distinguishing the HSCR group from the HC group, their diagnostic efficacy was moderate when comparing the HSCR group with the DC group. To assess the diagnostic accuracy of NMDAR autoantibody and to exclude potential selection bias, future studies should involve multicenter, large-sample clinical cohorts.
Ultimately, we have not further elucidated whether NMDAR autoantibody participates in the occurrence and development of HSCR, or if targeting these autoantibodies could serve as a treatment for HSCR, which will be the focus of future investigations.
In summary, our research has identified and provided preliminary evidence for that NMDAR autoantibody is a highly effective plasma biomarker for diagnosing HSCR. Furthermore, it may be implicated in the pathogenesis of HSCR.
All relevant data is contained within the article: The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the review committee of Institution Guangzhou Women and Children's Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. The manuscript presents research on animals that do not require ethical approval for their study.
YL: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Data curation, Formal Analysis, Software, Writing – review & editing. YL: Data curation, Formal Analysis, Software, Writing – review & editing. JZ: Funding acquisition, Resources, Writing – review & editing. SH: Resources, Writing – review & editing. LL: Resources, Writing – review & editing. BW: Resources, Writing – review & editing. PW: Resources, Writing – review & editing. YO: Resources, Writing – review & editing. JL: Resources, Writing – review & editing. WZ: Software, Writing – review & editing, Funding acquisition. CL: Funding acquisition, Resources, Writing – review & editing. HX: Funding acquisition, Resources, Supervision, Writing – review & editing. QH: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the Science and Technology Project of Guangzhou (Grant No. 2024A03J1238), (Grant No. 202201020612), and (Grant No. 2025A03J4456). This study was funded by grants from the National Natural Science Foundation of China (Grant No. 82301955), (Grant No. 82370526), and (Grant No. 82170528). This study was funded by grants from the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2024A1515013190) and (Grant No. 2022A1515012254). This study was funded by grants from the Guangxi Basic and Applied Basic Research Foundation (Grant No. 2024JJB140724).
We thank the Clinical Biological Resource Bank of Guangzhou women and children's medical center for providing the clinical samples. All steps in the study involving human participants were according to the ethical standards of the Institutional Review Board (No. 2016042036) of Guangzhou Women's and Children's Medical Centre and informed consent was obtained from the parents or legal guardians of the participants. The study was conducted with the Declaration of Helsinki (revised 2013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1514323/full#supplementary-material
1. Best KE, Addor MC, Arriola L, Balku E, Barisic I, Bianchi F, et al. Hirschsprung’s disease prevalence in Europe: a register based study. Birth Defects Res A Clin Mol Teratol. (2014) 100(9):695–702. doi: 10.1002/bdra.23269
2. Liu JA, Lai FP, Gui HS, Sham MH, Tam PK, Garcia-Barcelo MM, et al. Identification of GLI mutations in patients with Hirschsprung disease that disrupt enteric nervous system development in mice. Gastroenterology. (2015) 149(7):1837–48. doi: 10.1053/j.gastro.2015.07.060
3. Li Y, Liu H, Dong Y. Significance of neurexin and neuroligin polymorphisms in regulating risk of Hirschsprung’s disease. J Investig Med. (2018) 66(5):1–8. doi: 10.1136/jim-2017-000623
4. Tam P, Chung P, St Peter S, Gayer CP, Ford HR, Tam G, et al. Advances in paediatric gastroenterology. Lancet. (2017) 390(10099):1072–82. doi: 10.1016/S0140-6736(17)32284-5
5. Nakamura H, Tomuschat C, Coyle D, O'Donnel AM, Lim T, Puri P. Altered goblet cell function in Hirschsprung’s disease. Pediatr Surg Int. (2018) 34(2):121–8. doi: 10.1007/s00383-017-4178-0
6. Harooni S, Prasad GR, Danda GR, Naureen M. Mortality prediction score for Hirschsprung’s disease-associated enterocolitis: a novel mortality prediction model. J Indian Assoc Pediatr Surg. (2022) 27(5):594–9. doi: 10.4103/jiaps.jiaps_243_21
7. Schäppi MG, Staiano A, Milla PJ, Smith VV, Dias JA, Heuschkel R, et al. A practical guide for the diagnosis of primary enteric nervous system disorders. J Pediatr Gastroenterol Nutr. (2013) 57(5):677–86. doi: 10.1097/MPG.0b013e3182a8bb50
8. Sergi CM, Caluseriu O, McColl H, Eisenstat DD. Hirschsprung’s disease: clinical dysmorphology, genes, micro-RNAs, and future perspectives. Pediatr Res. (2017) 81(1-2):177–91. doi: 10.1038/pr.2016.202
9. Chen X, Xiaojuan W, Zhang H, Jiao C, Yu K, Zhu T, et al. Diagnostic value of the preoperatively detected radiological transition zone in Hirschsprung’s disease. Pediatr Surg Int. (2017) 33(5):581–6. doi: 10.1007/s00383-017-4064-9
10. Pan W, Goldstein AM, Hotta R. Opportunities for novel diagnostic and cell-based therapies for Hirschsprung disease. J Pediatr Surg. (2022) 57(9):61–8. doi: 10.1016/j.jpedsurg.2021.10.049
11. Bien CG, Vincent A, Barnett MH, Becker AJ, Blumcke I, Graus F, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. (2012) 135(Pt 5):1622–38. doi: 10.1093/brain/aws082
12. Zong S, Correia-Hoffmann C, Mané-Damas M, Kappelmann N, Molenaar PC, van Grootheest G, et al. Novel neuronal surface autoantibodies in plasma of patients with depression and anxiety. Transl Psychiatry. (2020) 10(1):404. doi: 10.1038/s41398-020-01083-y
13. Ramanathan S, Brilot F, Irani SR, Dale RC. Origins and immunopathogenesis of autoimmune central nervous system disorders. Nat Rev Neurol. (2023) 19(3):172–90. doi: 10.1038/s41582-023-00776-4
14. McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. (2013) 2(1):113–29. doi: 10.1002/wdev.57
15. Ji Y, Tam PK, Tang CS. Roles of enteric neural stem cell niche and enteric nervous system development in Hirschsprung disease. Int J Mol Sci. (2021) 22(18):9659. doi: 10.3390/ijms22189659
16. Zhong W, Lan C, Chen Y, Song K, Ma Z, Zeng J, et al. Virus-triggered autoimmunity was associated with Hirschsprung’s disease through activation of innate immunity. J Immunol Res. (2024) 2024:4838514. doi: 10.1155/2024/4838514
17. de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. (2006) 42(5):496–505. doi: 10.1097/01.mpg.0000214164.90939.92
18. Tang YF, Chen JG, An HJ, Jin P, Yang L, Dai ZF, et al. High-resolution anorectal manometry in newborns: normative values and diagnostic utility in Hirschsprung disease. Neurogastroenterol Motil. (2014) 26(11):1565–72. doi: 10.1111/nmo.12423
19. Hayes CE, Kawatu D, Mangray S, LeLeiko NS. Rectal suction biopsy to exclude the diagnosis of Hirschsprung disease. J Pediatr Gastroenterol Nutr. (2012) 55(3):268–71. doi: 10.1097/MPG.0b013e31824c0acc
20. Dong YN, Hsu FC, Koziol-White CJ, Stepanova V, Jude J, Gritsiuta A, et al. Functional NMDA receptors are expressed by human pulmonary artery smooth muscle cells. Sci Rep. (2021) 11(1):8205. doi: 10.1038/s41598-021-87667-0
21. Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, et al. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci. (1999) 19(11):4449–61. doi: 10.1523/JNEUROSCI.19-11-04449.1999
22. Shrestha A, Sultana R, Adeniyi PA, Lee CC, Ogundele OM. Positive modulation of SK channel impedes neuron-specific cytoskeletal organization and maturation. Dev Neurosci. (2020) 42(1):59–71. doi: 10.1159/000507989
23. Mochizuki N, Takagi N, Kurokawa K, Kawai T, Besshoh S, Tanonaka K, et al. Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett. (2007) 417(2):143–8. doi: 10.1016/j.neulet.2007.02.066
24. Fan H, Gao J, Wang W, Li X, Xu T, Yin X. Expression of NMDA receptor and its effect on cell proliferation in the subventricular zone of neonatal rat brain. Cell Biochem Biophys. (2012) 62(2):305–16. doi: 10.1007/s12013-011-9302-5
25. Lai Q, Hu P, Li Q, Li X, Yuan R, Tang X, et al. NMDA receptors promote neurogenesis in the neonatal rat subventricular zone following hypoxic-ischemic injury. Mol Med Rep. (2016) 13(1):206–12. doi: 10.3892/mmr.2015.4501
26. Kitayama T, Yoneyama M, Tamaki K, Yoneda Y. Regulation of neuronal differentiation by n-methyl-d-aspartate receptors expressed in neural progenitor cells isolated from adult mouse hippocampus. J Neurosci Res. (2004) 76(5):599–612. doi: 10.1002/jnr.20095
27. Yoneyama M, Nakamichi N, Fukui M, Kitayama T, Georgiev DD, Makanga JO, et al. Promotion of neuronal differentiation through activation of n-methyl-d-aspartate receptors transiently expressed by undifferentiated neural progenitor cells in fetal rat neocortex. J Neurosci Res. (2008) 86(11):2392–402. doi: 10.1002/jnr.21696
28. Ogita K, Okuda H, Yamamoto Y, Nishiyama N, Yoneda Y. In vivo neuroprotective role of NMDA receptors against kainate-induced excitotoxicity in murine hippocampal pyramidal neurons. J Neurochem. (2003) 85(5):1336–46. doi: 10.1046/j.1471-4159.2003.01778.x
29. Lauvsnes MB, Beyer MK, Kvaloy JT, Greve OJ, Appenzeller S, Kvivik I, et al. Association of hippocampal atrophy with cerebrospinal fluid antibodies against the nr2 subtype of the n-methyl-d-aspartate receptor in patients with systemic lupus erythematosus and patients with primary Sjogren’s syndrome. Arthritis Rheumatol. (2014) 66(12):3387–94. doi: 10.1002/art.38852
30. Chang EH, Volpe BT, Mackay M, Aranow C, Watson P, Kowal C, et al. Selective impairment of spatial cognition caused by autoantibodies to the n-methyl-d-aspartate receptor. Ebiomedicine. (2015) 2(7):755–64. doi: 10.1016/j.ebiom.2015.05.027
Keywords: biomarker, diagnosis, Hirschsprung disease, NMDAR autoantibody, enteric nervous system
Citation: Lai Y, Lu J, Liu Y, Zeng J, Huang S, Li L, Wang B, Wei P, Ouyang Y, Lv J, Zhong W, Lan C, Xia H and He Q (2025) Plasma NMDAR autoantibody: a new biomarker for the diagnosis of Hirschsprung disease. Front. Pediatr. 13:1514323. doi: 10.3389/fped.2025.1514323
Received: 20 October 2024; Accepted: 3 February 2025;
Published: 21 February 2025.
Edited by:
Thomai Karagiozoglou- Lampoudi, International Hellenic University, GreeceCopyright: © 2025 Lai, Lu, Liu, Zeng, Huang, Li, Wang, Wei, Ouyang, Lv, Zhong, Lan, Xia and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaoting Lan, bGFuY3RseXFAZm94bWFpbC5jb20=; Huimin Xia, eGlhLWh1aW1pbkBmb3htYWlsLmNvbQ==; Qiuming He, cWl1bWluZ2hlQGZveG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.