
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 06 February 2025
Sec. Pediatric Immunology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1508613
Recurrent respiratory tract infections (RRTIs) are a common condition in pediatrics and significantly impact children's quality of life; however, their pathogenesis and contributing factors are not yet fully elucidated. Probiotics have recently emerged as promising agents for modulating intestinal microecology and have gained considerable attention in clinical research on preventing and treating RRTIs in children. This article provides an initial overview of the concept, classification, and mechanisms underlying probiotics. It emphasizes their beneficial effects on respiratory health by modulating intestinal microbial equilibrium, augmenting immune system functionality, and attenuating inflammatory responses. Subsequently, we examine existing research regarding the use of probiotics in pediatric RRTIs. Numerous clinical trials have unequivocally demonstrated that supplementing with probiotics can significantly reduce both the frequency and severity of RRTIs in children while also simultaneously decreasing antibiotic usage. However, there are ongoing controversies and challenges in current research concerning the influence of probiotic type, dosage, duration of use, and other factors on efficacy. Furthermore, variations have been observed across different studies. Additionally, it is crucial to further evaluate the safety and potential long-term side effects associated with probiotic use in children with RRTIs. In conclusion, we propose future research directions including conducting more high-quality randomized controlled trials to optimize application strategies for probiotics alongside other treatments while considering variations based on age and health conditions among pediatric populations. Finally, in summary although probiotics exhibit promising benefits in preventing and treating RRTIs in children; additional studies are necessary to refine their application strategies ensuring both safety and effectiveness.
RRTIs are highly prevalent respiratory diseases affecting pediatric populations worldwide, particularly young children. According to international research data, approximately 10%–15% of children experience RRTIs at some point, representing about one-third of cases seen in primary pediatric care settings and accounting for between 8% and 18% of acute hospitalizations related to respiratory infections. Preschool-aged children are especially susceptible, experiencing an average annual occurrence rate ranging from six to ten viral colds (1). Globally, these infections claim the lives of approximately four million individuals each year while remaining the leading cause of mortality among those under six years old (2). Following the onset of RRTIs, common symptoms include fever and cough. If left untreated, they can lead to a decline in lung function among affected children, significantly impacting their health as well as growth and development (3). During early childhood, RRTIs often result in frequent medical consultations and emergency room visits while exerting a substantial influence on both affected children's quality of life and that of their families. Additionally, it frequently disrupts schooling activities (4). Enhanced resistance levels and concerns over treatment effectiveness often arise from the heavy reliance on antibiotics and antiviral drugs in conventional treatments for RRTIs. The inappropriate use of antibiotics has led to a significant increase in rates of bacterial resistance, resulting not only in elevated patient mortality but also imposing substantial economic pressure on healthcare systems (5).
Probiotics are commonly defined as living microorganisms that confer positive health effects on the host when present in sufficient quantities (6). In recent years, public attention towards probiotics has continued to rise due to the increasing recognition of their various beneficial effects in the field of health (7). According to statistics, the global probiotic market is projected to exceed $90 billion by 2026. Probiotics have been extensively used as pharmaceuticals, food additives or nutritional supplements for preventing and treating pediatric diseases (8). By modulating the cellular and humoral immune mechanisms, probiotics can enhance their immune defense capability (9). The research findings of evidence-based medicine suggest that oral probiotics effectively prevent respiratory tract infections and reduce recurrence rates in healthy children, without any observed adverse side effects (10, 11). Probiotics are highly esteemed for their diverse effects, particularly their antiviral properties. Recently, probiotics with antiviral capabilities have been incorporated into dairy products and fermented foods to assist in preventing viral infections (12). Evidence suggests that the utilization of probiotics can diminish the susceptibility to upper respiratory tract infections and facilitate the establishment of a robust microbiota environment in the upper respiratory tract that exhibits resistance against viral intrusion (13, 14).
The human gut microbiome represents a complex community of microorganisms residing within the gastrointestinal tract, encompassing an estimated range of 500–1,000 distinct bacterial species (15). Dysbiosis within this microbial ecosystem weakens host defense mechanisms against drug resistance and pathogenic bacteria colonization, increasing susceptibility to pathogens (15). Studies on human microbiota have revealed that intestinal flora can affect immune responses in multiple mucosal systems and facilitate systemic immune maturation (16), playing a crucial role in maintaining physiological equilibrium and influencing human health. Multiple studies (17, 18) have demonstrated a direct correlation between intestinal dysbiosis and impairment of the intestinal mucosal barrier integrity, resulting in an increased susceptibility to pulmonary infections. Recent investigations have extensively elucidated the link between imbalanced gut microbiota composition and RRTIs. The etiology of RRTIs is multifaceted. However, probiotics are widely acknowledged for their capacity to modulate both microecology and immune responses (19). These microorganisms can stimulate antigen-presenting cells (APCs), regulate cytokine secretion, and immunoglobulin production levels, while also reinforcing the protective layer on intestinal mucosal cells. Moreover, they exert influence over mucus generation while conferring resistance against invading pathogens or viruses (20). The objective of this review is to provide a comprehensive overview of current research advancements on probiotics in preventing and treating RRTIs in children. Additionally, it proposes several innovative strategies to address the limitations associated with probiotic therapies for RRTIs. This involves delving deeper into the underlying mechanisms, summarizing findings from clinical trials, addressing existing challenges, and identifying key areas for future research. By synthesizing findings from multiple studies, this review seeks to offer clinicians, researchers, and policymakers a multifaceted perspective that promotes evidence-based utilization of probiotics in pediatric healthcare while also highlighting areas requiring further investigation.
Currently, there is no global consensus on the diagnostic criteria for RRTIs in children. Different countries have varying definitions for RRTIs; however, the methods employed to diagnose them are consistent and based on infection frequency (Figure 1). Most scholars tend to define childhood RRTIs as eight or more documented respiratory infections per year in children under 3 years old, and six or more episodes in children over 3 years old (21, 22). RRTIs encompass both recurrent upper respiratory tract infections and recurrent lower respiratory tract infections (23). While there is no consensus on the exact definition of relapse, certain specific respiratory diseases have precise and unambiguous criteria for relapse. For instance, infectious rhinitis occurring more than five times per year is considered a recurrence in cases of recurrent upper respiratory tract infections, while acute otitis media recurring three times within six months or four times within twelve months also qualifies as a relapse (22). Recurrent lower respiratory tract infections are defined as bronchitis, bronchiolitis, or pneumonia occurring three or more times per year (24). In healthy individuals, a symbiotic and mutually regulated dynamic equilibrium exists between the microorganisms in the respiratory tract and intestine and their hosts, which is crucial for maintaining normal physiological functions. However, when pathogenic microorganisms invade the human body, this delicate microbial balance can be disrupted, resulting in the development of pathological conditions (25). Due to their less responsive immature immune systems towards environmental pathogens (26), children often encounter respiratory tract infections. The intestine functions as a pivotal immunological organ crucial for the maturation and differentiation of immune cells (27). The intestinal microbiota can modulate the cells of the intestinal epithelium, facilitating immune tissue development associated with the intestinal mucosa and activating receptors on immune cells (28). It not only plays a local regulatory role in the mucosal immune system but also has a pivotal regulatory role in cell-mediated systemic immune response (29). In addition to aiding nutrient absorption, gut microbiota defends against infections by competing for resources with pathogens or secreting antibacterial substances that eradicate pathogens (30). The gut-lung axis refers to bidirectional communication established between intestinal microorganisms and their metabolites, which regulate or exchange information between intestinal and lung tissues via circulation through blood and lymphatic systems (31). Previous studies have demonstrated that this regulatory network influences respiratory diseases through its impact on gut microbiota (32). Additionally, histoembryological evidence confirms that both the intestine and respiratory tract originate from common embryonic tissue known as the foregut of the gastrum, with their mucosal inner walls being continuous (33). Due to the thinner intestinal wall, higher permeability, and weaker barrier function in children with RRTIs, allergens such as toxins and incompletely digested products may enter the body through the intestine and subsequently reach the lungs via blood circulation. Several studies have compared populations of Bifidobacterium and Lactobacillus in the gut of children with RRTIs to healthy children, revealing a significant reduction. This dysbiosis in intestinal microbiota leads to decreased levels of IgA-dominated immunoglobulins (34).
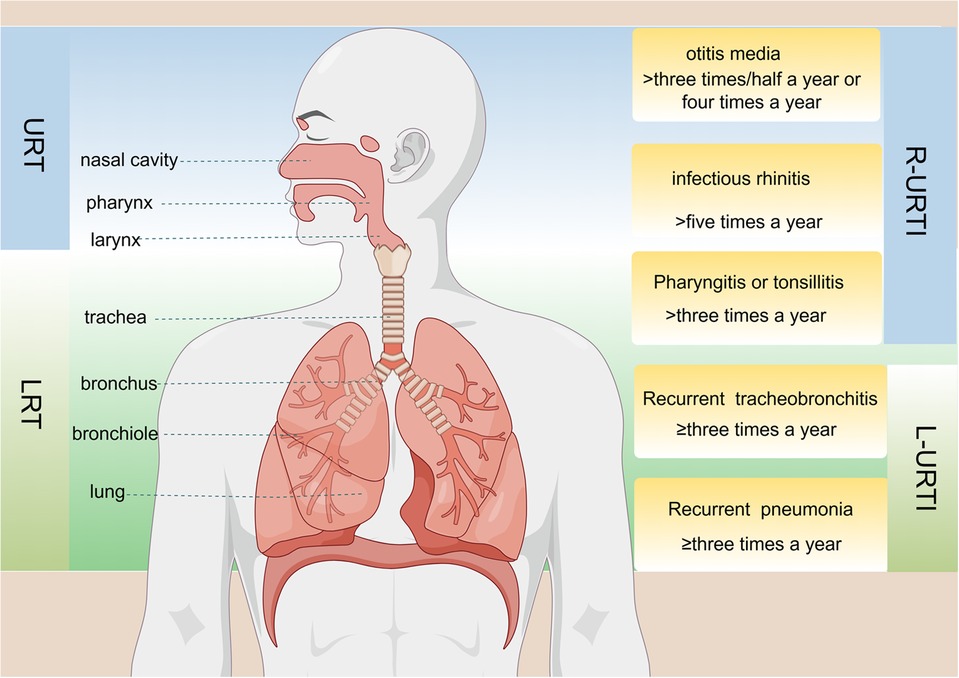
Figure 1. Anatomical structure of the respiratory tract and the definition of specific diseases associated with recurrent upper respiratory tract infections (R-URTIs) and recurrent lower respiratory tract infections (L-URTIs).
Probiotics are defined as “living microorganisms that confer health benefits to the host when administered in sufficient quantities” (35). Lactobacillus and Bifidobacteria, which are widely used in the field of probiotics (36), secrete various beneficial compounds like vitamins, short-chain fatty acids (SCFAs), bacteriocins, and exopolysaccharides (EPS) that contribute to their positive effects (37). Most children develop RRTIs due to their immature immune system during development, and typically there is no underlying disease present during this period (23). Certain probiotics strains play a crucial role in preventing and treating respiratory diseases by inhibiting pathogen growth and harmful bacteria, producing antimicrobial substances, enhancing the defense function of the epithelial cell barrier, and importantly regulating immune system activity (22), thereby impacting both innate and adaptive immunity in the host (Figure 2). Recent studies have unveiled a correlation between dysbiosis of intestinal flora and RRTIs. For instance, strains such as Lactobacillus rhamnosus and Escherichia coli Nissle 1917 can activate pattern recognition receptors (PRRs) on epithelial cells through microbially associated molecular patterns to regulate tight junctions and adhesion junctions, thereby preserving the integrity of the epithelial barrier (38). The probiotics inhibit the proliferation of pathogenic bacteria by competing for adhesion sites on epithelial cells or depleting their essential nutrients, while simultaneously secreting antibacterial substances to establish an unfavorable microenvironment for pathogen survival (39). Additionally, probiotic metabolites like SCFAs modulate the differentiation of immune cells and control excessive immune responses by interacting with G protein-coupled receptors (GPCRs) and inhibiting histone deacetylases (HDACs), thereby playing a crucial role in immune response, inflammation, and lung disease development (40). Moreover, probiotics can exhibit antiviral effects through direct interaction with viruses or the generation of antiviral metabolites while stimulating the host's immune system response (41). Gut-associated lymphoid tissue (GALTs) is a vital part of the peripheral immune system that houses around 80% of active immune cells. Probiotics have the potential to enhance systemic immunity by modulating GALTs' response, indirectly strengthening the respiratory tract's defense capabilities (42). Upon encountering intestinal microbiota, infectious pathogens, as well as ingested or inhaled antigens, GALTs facilitate frequent interactions between immune cells and these antigens, thus triggering an ongoing immune response that encompasses germ-center (GC) formation within intestinal microbiota and subsequent responses to pathogen invasion (43). The specific mechanism involves the encapsulation of GALTs by microfold (M) cells, facilitating their targeted delivery to dendritic cells (DCs) and subsequent antigen exposure, thereby initiating immune responses from T and B cells in the mucosa (44). Furthermore, probiotics mediate the innate immune response through PRRs, particularly toll-like receptors (TLRs), which recognize and bind to pathogen-associated molecular patterns (PAMPs) on the surface of pathogenic microorganisms. This interaction triggers the activation of signaling pathways such as nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) (45, 46). As a result, this process modulates the secretion of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), thereby regulating host-pathogen interactions and influencing the immune and inflammatory responses. Lactococcus lactis can initiate signal transduction in DCs through the TLR2, TLR3, and TLR9 pathways, thereby promoting DC maturation, enhancing Th1 cell differentiation, and upregulating the expression of IL-12, IFN-γ, and TNF-α (47). Additionally, probiotics have been shown to induce clonal expansion of IgA-producing B cells by elevating IL-6 levels in a TLR2-dependent manner (37). Therefore, they serve as a preventive and therapeutic strategy against respiratory infections.
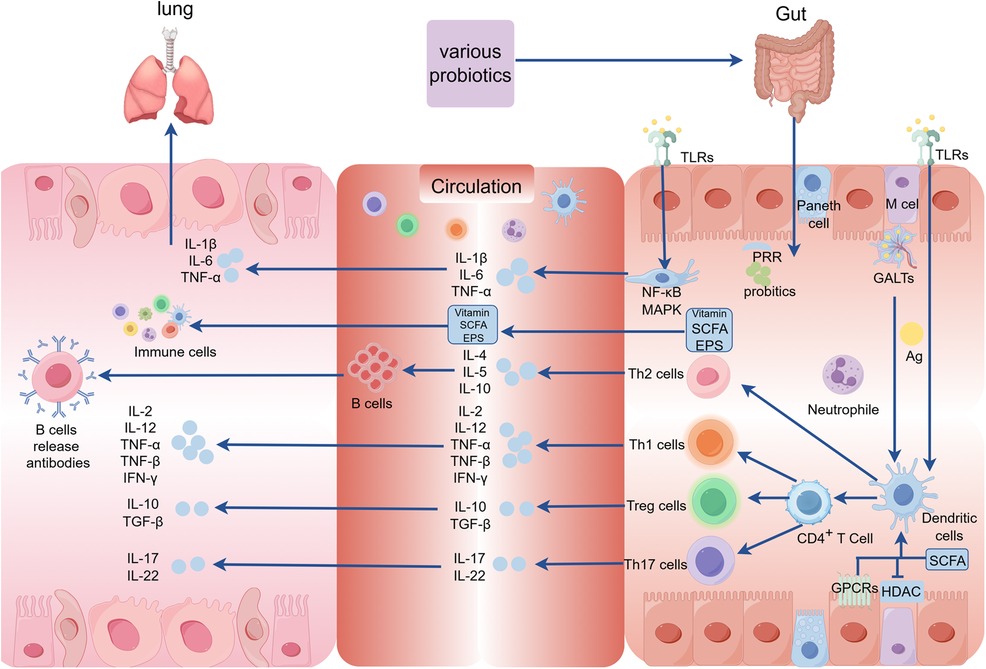
Figure 2. The mechanism underlying the intervention of probiotics on RRTIs in children. (GALTs, gut-associated lymphoid tissue; PRR, pattern recognition receptors; MAPK, mitogen-activated protein kinase; GPCRs, G protein-coupled receptors; HDAC, histone deacetylases; SCFA, short-chain fatty acids; TLRs, toll-like receptors; Ag, antige; EPS, exopolysaccharides).
Lactobacillus rhamnosus GG (LGG), isolated in 1983 by Gorbach and Goldin, is currently the most widely utilized probiotic due to its resistance to gastric acid and bile attack. As a probiotic strain, LGG confers gastrointestinal benefits (48) by regulating the microbial balance in the gut (49). It reduces harmful bacteria like Bacteroides and Proteus while increasing beneficial bacteria such as Lactobacillus, Bifidobacterium, and Butyricococcus (50–52). Additionally, LGG modulates the host's immune system with antiviral and antibacterial properties that help prevent and treat specific infections. Research has demonstrated that LGG safeguards intestinal epithelial cells from destruction by triggering an inflammatory response and activating macrophages (53, 54). Despite the manifold health benefits demonstrated by LGG, the findings exhibit some inconsistencies and potential interindividual variations in probiotic efficacy may exist.
The effectiveness of LGG has been proven in multiple studies for preventing and treating respiratory infections among children. In a randomized trial involving 281 children attending daycare centers, LGG significantly decreased upper respiratory tract infections and reduced the risk of respiratory tract infections lasting more than 3 days while also shortening the duration of respiratory symptoms. However, it is noteworthy that there was no significant effect observed on the risk of lower respiratory tract infections associated with LGG intake. These findings suggest that LGG may serve as an effective approach to mitigate upper respiratory tract infection risks among children attending daycare centers (55). Furthermore, based on four randomized controlled trials encompassing 1,805 participants' data analysis results indicate that LGG also diminishes acute otitis media (AOM) incidence rates along with antibiotic usage (56). Moreover, subgroup analysis focusing on children over 1 year old further confirms that LGG significantly decreases overall risks of respiratory infections (56). Another study showed that LGG significantly reduced the duration of respiratory tract infections (RTIs) (57). Additionally, LGG (ATCC 53103) can effectively decrease the frequency of rhinovirus-induced RTIs, highlighting its positive impact on reducing RTI incidence caused by rhinovirus infection in preterm infants during their first year of life (58). While LGG has demonstrated efficacy in ameliorating respiratory symptoms, there is insufficient statistical evidence supporting its antiviral activity and ability to mitigate associated manifestations. Rhinovirus, respiratory syncytial virus (RSV), and parainfluenza virus type 1 stand as the primary causative agents of pediatric respiratory ailments. In a 28-week randomized controlled trial with double-blinding and placebo control measures, LGG notably reduced monthly days with respiratory symptoms among children. However, it did not significantly influence the detection rate or severity of viral pathogens when present. While displaying potential for effectively alleviating respiratory distresses through symptomatic relief mechanisms alone without directly inhibiting viral infections (59). Overall, LGG may effectively reduce the risk of upper respiratory infections in children. Nevertheless, its effectiveness might exhibit interindividual variability. In a randomized study involving 619 participants aged 2–6 years and lasting for 16 weeks, it was observed that consumption of LGG DSM 33156 ameliorated symptoms during upper respiratory infections (URTIs) in children. But it is regrettable that the study failed to provide substantial evidence supporting a significant reduction in the incidence of URTIs (60).
Bifidobacterium is a group of beneficial bacteria commonly found in the human gut, especially in infant intestines. However, their abundance and species diversity tend to decline with age (61). The Gram-positive bacteria belonging to the family Bifidobacteriaceae are classified as members of Bifidobacteria. These bacteria are typically anaerobic but can occasionally tolerate oxygen. They lack spore-forming ability and exhibit various morphological forms (61). Due to its immunomodulatory function on the neonatal immune system, Bifidobacterium has garnered significant research attention (62–64). Studies have demonstrated that Bifidobacteria play a crucial preventive role in maintaining a healthy intestinal microbiota by regulating probiotic metabolism, promoting intestinal motility, adhering to and degrading harmful substances, as well as enhancing host immune function (65).
Bifidobacterium longum, a member of the Bifidobacterium genus, holds a dominant position in infants' intestinal microbiota and plays an indispensable role in their healthy growth and development. It can modulate the Th1/Th2 immune system balance to prevent associated diseases (66). A study conducted on Malaysian preschoolers demonstrated that Bifidobacterium elongatus BB536 can significantly enhance the abundance of anti-inflammatory and immunomodulatory fecal bacteria, potentially protecting against upper respiratory diseases by regulating gut microbiota (67).
Lactobacillus, a subspecies of Bifidobacterium animalis, has gained significant attention in the field of neonatal intestinal microbiota research due to its remarkable safety profile and multitude of positive effects on intestinal diseases (68, 69). In a double-blind study by Taipale et al., it was observed that early administration of BB-12 probiotics in infants reduced the risk of early infection and respiratory infections (70). However, other studies have indicated that Bifidobacterium animalis subsp. lactis may not exhibit efficacy in preventing common infections among hospitalized children, potentially attributed to lower-than-expected incidence rates of nosocomial infections (71).
Streptococcus salivarius, a constituent of the human oral microbiota, has been extensively investigated for its potential oral health benefits, particularly in the preventing and alleviating sore throats. The secretion of Lactobacillus acidophilus and salivary peroxidase by Streptococcus salivarius help maintain an optimal acid-base balance in the oral cavity and inhibits the proliferation of pathogenic bacteria, reducing bacterial load and minimizing sore throat occurrence. Research has demonstrated that Streptococcus salivarius exerts immune regulatory effects that restrict viral infectivity (72).
Streptococcus salivarius demonstrates exceptional oral adhesion capabilities while also producing bacteriocin-like inhibitors and possessing immunomodulatory properties. It has been employed for managing pharyngitis, tonsillitis, and otitis media (73). Studies have shown that administering prophylactic doses of S. salivarius K12 (Bactoblis®) to children who are prone to recurrent oral streptococcal infections can significantly reduce both bacterial/viral infection rates as well as the need for antibiotics or antipyretics (74). The clinical studies conducted by Wescombe, P.A., et al. have also shown the therapeutic potential of Streptococcus salivarius K12 strain in the treatment of streptococcal sore throat (75). Furthermore, S. salivarius, a non-pathogenic species and an integral member of the normal oral microbiome, has exhibited efficacy in reducing recurrent colonization by major pathogens in the URT. The effectiveness of intranasal administration of Streptococcus sialus 24SMB in reducing the incidence and frequency of antibiotic usage for recurrent acute otitis media in children aged 1–5 years was demonstrated through a prospective, randomized, double-blind, placebo-controlled design (76). Interestingly, clinical trials have indicated that S salivarius K12 does not exhibit a reduction in the occurrence of AOM in children (77).
Lactobacillus casei, a prevalent strain of the genus Lactobacillus, is commonly found in various natural sources including dairy products, vegetables, and meat. It exerts its immunomodulatory effects by enzymatically hydrolyzing casein into immunogenic peptides (78). In a 12-week clinical trial involving 1,003 Vietnamese children aged 3–5 years, daily consumption of fermented dairy products containing Lactobacillus casei strain Shirota (LcS) effectively reduced the incidence of constipation and acute respiratory infections (ARIs) among these children (79).
Bacillus coagulans is a highly stable probiotic strain with a long history of safe use in food and has been granted Generally Recognized as Safe (GRAS) status by the U.S. Food and Drug Administration, making it an ideal candidate for food and pharmaceutical preparations. It exhibits various beneficial roles such as regulating microbiome composition, immune response, and metabolism (80). GanedenBC30, scientifically known as Bacillus coagulans, is extensively used in health products and food items to promote gastrointestinal well-being and enhance immune system functionality. The study conducted by M.A. et al. demonstrated that the administration of probiotic GanedenBC30 (Bacillus coagulans GBI-30, 6,086 strain) had positive effects on the frequency, duration, and severity of symptoms associated with URTIs, as well as reducing the occurrence of distension events in school-age children (81).
Complex probiotics, also known as multi-bacterial probiotics, encompass preparations containing two or more distinct strains of probiotics. These formulations possess the ability to modulate the composition and diversity of intestinal flora, safeguard intestinal barrier function, and mitigate inflammation and intestinal damage. Using complex probiotics can address drug resistance arising from excessive use of a single strain. Previous studies have demonstrated that complex probiotics exhibit superior efficacy in maintaining intestinal health and regulating immune function compared to individual strains alone (82).
Complex probiotics have demonstrated potential positive effects in the prevention and treatment of RRTIs. Multiple studies indicated that specific combinations of probiotics can balance gut microbes and enhance immune system function, thereby reducing the frequency and severity of RRTIs. For example, probiotic formulations containing Lactobacillus BB-12 and Enterococcal Streptococcus faecalis L3 significantly increase salivary IgA levels and mitigate the risk of URTIs in healthy children (83). For children with recurrent respiratory infections, oral bifidobacteria quadruplex vaccine tablets containing Bifidobacteria infantis, Lactobacillus acidophilus, Enterococcus faecalis and Bacillus cereus not only enhanced the abundance of Bifidobacteria and Lactobacilli but also significantly reduced the average annual frequency of acute RTIs and antibiotic usage (34). Clinical trials show that probiotic sprays containing Streptococcus salivarius 24SMB and Streptococcus oralis 89a exhibited efficacy in preventing URTIs among pediatric patients (84). Additionally, they demonstrated a significant reduction in both the frequency of Group A beta-hemolytic Streptococcus (GABHS) infection episodes and antibiotic usage (84). Another study reported that Streptococcus salivarius 24SMB and Streptococcus oralis 89a effectively alleviated symptoms associated with recurrent respiratory infections in children (85). Furthermore, Campanella et al. provided further validation regarding the potential benefits and safety profile of probiotics for reducing the incidence of RTIs among pediatric populations (86). The combination of Lactobacillus reuteri ATCC PTA 5289 and Lactobacillus reuteri DSM 17938 has also demonstrated efficacy in alleviating symptoms associated with pharyngitis or tonsillitis in pediatric patients (87). In a clinical study, it was demonstrated that the preschool doses comprised Lactobacillus acidophilus CUL21 (NCIMB 30156), Lactobacillus acidophilus CUL60 (NCIMB 30157), Bifidobacterium bifidum CUL20 (NCIMB 30153), and Bifidobacterium animalis subsp. lactis CUL34 (NCIMB 30172) in Lab4 probiotic chewable tablets, along with 50 mg of vitamin C, which were effective in preventing URTIs and reducing the use of antibiotics (88). However, it should be noted that not all probiotics are equally effective against RTIs. The probiotic capsules containing Lactobacillus acidophilus CUL60, Lactobacillus acidophilus CUL21, Bifidobacterium bifidum CUL20, and Bifidobacterium animalis ssp. lactate were also found to have no significant impact on reducing the severity of symptoms associated with pharyngitis (89). Furthermore, a randomized controlled trial conducted by Santamaria et al. found no significant difference in respiratory tract infections or disease-free days when using Bifidobacterium mixtures (B. longum BB536, B. infantis M-63, B. breve M-16V) (90). In a Finnish double-blind, placebo-controlled trial, children aged 10 months to 6 years who were at high risk for OM received a daily probiotic capsule containing Lactobacillus rhamnosus GG, Bifidobacterium breve 99, and Propionibacterium freudenreichii JS. The study concluded that the probiotics did not significantly prevent AOM or reduce nasopharyngeal colonization by otitis media pathogens in these susceptible children (91).
In conclusion, although probiotics have demonstrated some efficacy in the prevention and treatment of RRTIs in children, their effectiveness may vary depending on the specific combination of strains and individual variations (Table 1). Therefore, meticulous selection of an appropriate probiotic product and customization of treatment for each individual is imperative to optimize efficacy.
Probiotics, which are a consortium of living bacteria, exert beneficial effects on the human body by modulating the gut microbiome, enhancing immune function, and improving digestive health. Although numerous probiotic products have demonstrated safety (92), their usage may also lead to certain adverse reactions. Currently, there is no globally unified standard for evaluating probiotics due to ongoing advancements in safety assessments across different countries. The utilization of probiotics may pose several potential risks and adverse effects. For example, some clinical reports have indicated that probiotics can lead to gastrointestinal side effects such as diarrhea and bloating (93). In addition, it is imperative to acknowledge the potential for horizontal gene transfer of antibiotic resistance genes from probiotic strains to other microorganisms. In 1998, viable probiotic bacteria were identified as donors in the conjugative transfer of antibiotic resistance (AR) genes, a phenomenon subsequently corroborated by additional studies confirming the intestinal transfer capability of AR genes (94). Moreover, AR gene transfer can also occur through transformation via free DNA or phage-mediated transduction (95). As highlighted in the preceding clinical application section, probiotics generally have exhibited a relatively favorable safety profile among healthy pediatric populations. However, it has been reported that certain probiotic strains may act as opportunistic pathogens in immunocompromised individuals, potentially leading to adverse effects such as life-threatening conditions like pneumonia (96), endocarditis (97), and sepsis (98). To ensure the safe application of probiotics, it is crucial to perform whole genome sequencing of probiotic strains. This method not only facilitates strain-level identification but also enables the detection of virulence factors, pathogenicity determinants, and AR genes, thereby enhancing the safety and efficacy of probiotic use (95).
The present review comprehensively analyzes recent advancements in using probiotics for preventing and treating RRTIs in pediatric patients. Multiple studies have demonstrated that probiotics exert a favorable influence on reducing the incidence of RRTIs and alleviating symptoms by modulating the gut microbiome, enhancing immune system functionality, attenuating inflammatory response, and other underlying mechanisms. In terms of prevention, multiple randomized controlled trials have consistently demonstrated the significant efficacy of specific strains of probiotic supplementation in reducing the incidence and duration of RRTIs in children. Moreover, probiotics have also been found to effectively decrease antibiotic usage frequency, thereby mitigating risks associated with adverse reactions and resistance. Regarding treatment, although available evidence is not as robust as that for prevention studies, preliminary research suggests that probiotics may expedite recovery from respiratory infections by enhancing host immune function. However, it should be noted that considerable variation exists in the efficacy of different strains and combinations necessitating further high-quality investigations to determine optimal treatment options. Despite showing promise in preventing and treating RRTIs in children, several challenges and unresolved issues still persist. For example, further investigation is required regarding aspects such as optimal dosage, duration, strain selection, and suitability for specific pediatric populations concerning probiotics. Moreover, significant gaps persist in understanding the long-term effects of probiotics on pediatric populations, particularly in children with chronic or recurrent conditions. When administering probiotics, it is imperative to conduct a comprehensive evaluation of individual differences, lifestyle factors, genetic backgrounds, potential risks, and long-term outcomes to ensure both the safety and efficacy of these interventions. The significance of whole-genome sequencing as an indispensable tool for evaluating the safety of probiotic strains cannot be overstated. It not only enhances strain identification for production tracing and infection investigation but also facilitates the classification and assessment of associated risks. Additionally, it enables the detection of potential virulence, pathogenicity, or antibiotic resistance genes within genomes. Probiotic formulations must ensure safety regarding purity, potency, and composition through rigorous testing protocols to prevent contamination, especially with live organisms. Stricter testing measures are imperative for products intended for vulnerable populations. In conclusion, while probiotics hold promising prospects in preventing and treating RTIs in children, their efficacy and safety necessitate verification through additional clinical trials. Future research ought to concentrate on identifying the optimal combination and usage strategies for probiotics, as well as exploring their integration with other interventions (e.g., vaccination, nutritional support) to provide more comprehensive approaches towards safeguarding children's health.
YZ: Conceptualization, Software, Visualization, Writing – original draft. YX: Writing – original draft. LH: Funding acquisition, Writing – review & editing, Supervision. XW: Writing – review & editing, Funding acquisition, Project administration, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Natural Science Foundation of Hubei (2023AFB1111), Open Foundation from Hubei Province Key Laboratory of Occupational Hazard Identification and Control (OHIC2021G01) and Research and Innovation Fund of Wuchang Hospital (WCYY2022G05).
We thank Figdraw for providing the drawing material.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pasternak G, Lewandowicz-Uszyńska A, Królak-Olejnik B. Recurrent respiratory tract infections in children. Pol Merkur Lekarski. (2020) 49(286):260–6.32827422
2. Okubo Y, Nochioka K, Testa MA. The impact of pediatric obesity on hospitalized children with lower respiratory tract infections in the United States. Clin Respir J. (2018) 12(4):1479–84. doi: 10.1111/crj.12694
3. Su P, Jiang C, Zhang Y. The implication of infection with respiratory syncytial virus in pediatric recurrent wheezing and asthma: knowledge expanded post-COVID-19 era. Eur J Clin Microbiol Infect Dis. (2024) 43(3):403–16. doi: 10.1007/s10096-023-04744-0
4. Corsello A, Milani GP, Picca M, Buzzetti R, Carrozzo R, Gambino M, et al. Recurrent upper respiratory tract infections in early childhood: a newly defined clinical condition. Ital J Pediatr. (2024) 50(1):30. doi: 10.1186/s13052-024-01600-5
5. Vaezi A, Healy T, Ebrahimi G, Rezvankhah S, Hashemi Shahraki A, Mirsaeidi M. Phage therapy: breathing new tactics into lower respiratory tract infection treatments. Eur Respir Rev. (2024) 33(172):240029. doi: 10.1183/16000617.0029-2024
6. Hong Y, Luo T. The potential protective effects of probiotics, prebiotics, or yogurt on chronic obstructive pulmonary disease: results from NHANES 2007–2012. Food Sci Nutr. (2024) 12(10):7233–41. doi: 10.1002/fsn3.4332
7. Zhang L, Zhang S, Jiang M, Ni X, Du M, Jiang H, et al. Limosilactobacillus reuteri alleviates anxiety-like behavior and intestinal symptoms in two stressed mouse models. Nutrients. (2024) 16(18):3209. doi: 10.3390/nu16183209
8. Depoorter L, Vandenplas Y. Probiotics in pediatrics. A review and practical guide. Nutrients. (2021) 13(7):2176. doi: 10.3390/nu13072176
9. Junaid M, Lu H, Li Y, Liu Y, Din AU, Qi Z, et al. Novel synergistic probiotic intervention: transcriptomic and metabolomic analysis reveals ameliorative effects on immunity, gut barrier, and metabolism of mice during Salmonella typhimurium infection. Genes (Basel). (2024) 15(4):435. doi: 10.3390/genes15040435
10. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. (2015) 15(1):9–20. doi: 10.1517/14712598.2015.980233
11. King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. (2014) 112(1):41–54. doi: 10.1017/S0007114514000075
12. Pradhan D, Biswasroy P, Kar B, Bhuyan SK, Ghosh G, Rath G. Clinical interventions and budding applications of probiotics in the treatment and prevention of viral infections. Arch Med Res. (2022) 53(2):122–30. doi: 10.1016/j.arcmed.2021.09.008
13. Wang Q, Lin X, Xiang X, Liu W, Fang Y, Chen H, et al. Oropharyngeal probiotic ENT-K12 prevents respiratory tract infections among frontline medical staff fighting against COVID-19: a pilot study. Front Bioeng Biotechnol. (2021) 9:646184. doi: 10.3389/fbioe.2021.646184
14. O'Donnell A, Murray A, Nguyen A, Salmon T, Taylor S, Morton JP, et al. Nutrition and golf performance: a systematic scoping review. Sports Med. (2024) 54(12):3081–95. doi: 10.1007/s40279-024-02095-0
15. Rahman MN, Barua N, Tin MCF, Dharmaratne P, Wong SH, Ip M. The use of probiotics and prebiotics in decolonizing pathogenic bacteria from the gut; a systematic review and meta-analysis of clinical outcomes. Gut Microbes. (2024) 16(1):2356279. doi: 10.1080/19490976.2024.2356279
16. Lehtoranta L, Latvala S, Lehtinen MJ. Role of probiotics in stimulating the immune system in viral respiratory tract infections: a narrative review. Nutrients. (2020) 12(10):3163. doi: 10.3390/nu12103163
17. Samuelson DR, Charles TP, de la Rua NM, Taylor CM, Blanchard EE, Luo M, et al. Analysis of the intestinal microbial community and inferred functional capacities during the host response to pneumocystis pneumonia. Exp Lung Res. (2016) 42(8–10):425–39. doi: 10.1080/01902148.2016.1258442
18. Hauptmann M, Schaible UE. Linking microbiota and respiratory disease. FEBS Lett. (2016) 590(21):3721–38. doi: 10.1002/1873-3468.12421
19. Dong Y, Li M, Yue X. Current research on probiotics and fermented products. Foods. (2024) 13(9):1406. doi: 10.3390/foods13091406
20. La Fata G, Weber P, Mohajeri MH. Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob Proteins. (2018) 10(1):11–21. doi: 10.1007/s12602-017-9322-6
21. Ciprandi G, La Mantia I, Damiani V, Passali D. Local Bacteriotherapy - a promising preventive tool in recurrent respiratory infections. Expert Rev Clin Immunol. (2020) 16(11):1047–52. doi: 10.1080/1744666X.2021.1833720
22. Chiappini E, Santamaria F, Marseglia GL, Marchisio P, Galli L, Cutrera R, et al. Prevention of recurrent respiratory infections: inter-society consensus. Ital J Pediatr. (2021) 47(1):211. doi: 10.1186/s13052-021-01150-0
23. Zhang X, Dai X, Li X, Xie X, Chen Y, Chen Y, et al. Recurrent respiratory tract infections in children might be associated with vitamin A status: a case-control study. Front Pediatr. (2023) 11:1165037. doi: 10.3389/fped.2023.1165037
24. de Benedictis FM, Bush A. Recurrent lower respiratory tract infections in children. Br Med J. (2018) 362:k2698. doi: 10.1136/bmj.k2698
25. Stavropoulou E, Kantartzi K, Tsigalou C, Konstantinidis T, Voidarou C, Konstantinidis T, et al. Unraveling the interconnection patterns across lung microbiome, respiratory diseases, and COVID-19. Front Cell Infect Microbiol. (2020) 10:619075. doi: 10.3389/fcimb.2020.619075
26. Pasinato A, Fama M, Tripepi G, Egan CG, Baraldi E, LIRAR Study Group. Lactoferrin in the prevention of recurrent respiratory infections in preschool children: a prospective randomized study. Children (Basel). (2024) 11(2):249. doi: 10.3390/children11020249
27. Zhou J, Wang T, Fan L, Xiao H, Ji H, Zhou N, et al. Enterococcus faecium HDRsEf1 promotes systemic Th1 responses and enhances resistance to Salmonella typhimurium infection. Nutrients. (2023) 15(19):4241. doi: 10.3390/nu15194241
28. Selma-Royo M, Calatayud Arroyo M, García-Mantrana I, Parra-Llorca A, Escuriet R, Martínez-Costa C, et al. Perinatal environment shapes microbiota colonization and infant growth: impact on host response and intestinal function. Microbiome. (2020) 8(1):167. doi: 10.1186/s40168-020-00940-8
29. Negi S, Pahari S, Bashir H, Agrewala JN. Gut microbiota regulates mincle mediated activation of lung dendritic cells to protect against Mycobacterium tuberculosis. Front Immunol. (2019) 10:1142. doi: 10.3389/fimmu.2019.01142
30. Browne HP, Iqbal NT, Osman M, Tigoi C, Lawley TD, Gordon JI, et al. Boosting microbiome science worldwide could save millions of children’s lives. Nature. (2024) 625(7994):237–40. doi: 10.1038/d41586-024-00017-8
31. Wu Y, Li Y, Luo Y, Zhou Y, Wen J, Chen L, et al. Gut microbiome and metabolites: the potential key roles in pulmonary fibrosis. Front Microbiol. (2022) 13:943791. doi: 10.3389/fmicb.2022.943791
32. Narayana JK, Aliberti S, Mac Aogáin M, Jaggi TK, Ali NABM, Ivan FX, et al. Microbial dysregulation of the gut-lung axis in bronchiectasis. Am J Respir Crit Care Med. (2023) 207(7):908–20. doi: 10.1164/rccm.202205-0893OC
33. He Y, Li C, Wang Z, Yang Z, Wei J, Ren L, et al. C-fiber degeneration enhances alveolar macrophage-mediated IFN-α/β response to respiratory syncytial virus. Microbiol Spectr. (2022) 10(6):e0241022. doi: 10.1128/spectrum.02410-22
34. Li KL, Wang BZ, Li ZP, Li YL, Liang JJ. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J Pediatr. (2019) 15(3):255–61. doi: 10.1007/s12519-019-00248-0
35. Marco ML, Sanders ME, Gänzle M, Arrieta MC, Cotter PD, De Vuyst L, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. (2021) 18(3):196–208. doi: 10.1038/s41575-020-00390-5
36. Suissa R, Oved R, Jankelowitz G, Turjeman S, Koren O, Kolodkin-Gal I. Molecular genetics for probiotic engineering: dissecting lactic acid bacteria. Trends Microbiol. (2022) 30(3):293–306. doi: 10.1016/j.tim.2021.07.007
37. Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. (2023) 12(1):184. doi: 10.3390/cells12010184
38. Yamanishi S, Pawankar R. Current advances on the microbiome and role of probiotics in upper airways disease. Curr Opin Allergy Clin Immunol. (2020) 20(1):30–5. doi: 10.1097/ACI.0000000000000604
39. Lopes SA, Roque-Borda CA, Duarte JL, Di Filippo LD, Borges Cardoso VM, Pavan FR, et al. Delivery strategies of probiotics from nano- and microparticles: trends in the treatment of inflammatory bowel disease-an overview. Pharmaceutics. (2023) 15(11):2600. doi: 10.3390/pharmaceutics15112600
40. Sun W, Zhou T, Ding P, Guo L, Zhou X, Long K. Bibliometric analysis of intestinal microbiota and lung diseases. Front Cell Infect Microbiol. (2024) 14:1347110. doi: 10.3389/fcimb.2024.1347110
41. Sundararaman A, Ray M, Ravindra PV, Halami PM. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl Microbiol Biotechnol. (2020) 104(19):8089–104. doi: 10.1007/s00253-020-10832-4
42. Chakradhar S. A curious connection: teasing apart the link between gut microbes and lung disease. Nat Med. (2017) 23(4):402–4. doi: 10.1038/nm0417-402
43. Bemark M, Pitcher MJ, Dionisi C, Spencer J. Gut-associated lymphoid tissue: a microbiota-driven hub of B cell immunity. Trends Immunol. (2024) 45(3):211–23. doi: 10.1016/j.it.2024.01.006
44. Rastogi S, Mohanty S, Sharma S, Tripathi P. Possible role of gut microbes and host’s immune response in gut-lung homeostasis. Front Immunol. (2022) 13:954339. doi: 10.3389/fimmu.2022.954339
45. Han X, Hu X, Jin W, Liu G. Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets. Anim Nutr. (2024) 17:188–207. doi: 10.1016/j.aninu.2023.12.010
46. De Jesus LCL, Aburjaile FF, Sousa TJ, Felice AG, Soares SC, Alcantara LCJ, et al. Genomic characterization of Lactobacillus delbrueckiistrains with probiotics properties. Front Bioinform. (2022) 2:912795. doi: 10.3389/fbinf.2022.912795
47. Zhang T, Wei X, Li Y, Huang S, Wu Y, Cai S, et al. Dendritic cell-based vaccine prepared with recombinant Lactococcus lactis enhances antigen cross-presentation and antitumor efficacy through ROS production. Front Immunol. (2023) 14:1208349. doi: 10.3389/fimmu.2023.1208349
48. Petrova MI, Reid G, Ter Haar JA. Lacticaseibacillus rhamnosus GR-1, a.k.a. Lactobacillus rhamnosus GR-1: past and future perspectives. Trends Microbiol. (2021) 29(8):747–61. doi: 10.1016/j.tim.2021.03.010
49. Ni C, Li X, Wang L, Li X, Zhao J, Zhang H, et al. Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activity via a short-chain fatty acid-dependent mechanism. Food Funct. (2021) 12(15):7054–67. doi: 10.1039/D1FO00198A
50. Zhao H, Chen X, Zhang L, Meng F, Zhou L, Pang X, et al. Lacticaseibacillus rhamnosus Fmb14 prevents purine induced hyperuricemia and alleviate renal fibrosis through gut-kidney axis. Pharmacol Res. (2022) 182:106350. doi: 10.1016/j.phrs.2022.106350
51. Li Y, Zhu J, Lin G, Gao K, Yu Y, Chen S, et al. Probiotic effects of Lacticaseibacillus rhamnosus 1155 and Limosilactobacillus fermentum 2644 on hyperuricemic rats. Front Nutr. (2022) 9:993951. doi: 10.3389/fnut.2022.993951
52. Li X, Hu S, Yin J, Peng X, King L, Li L, et al. Effect of synbiotic supplementation on immune parameters and gut microbiota in healthy adults: a double-blind randomized controlled trial. Gut Microbes. (2023) 15(2):2247025. doi: 10.1080/19490976.2023.2247025
53. Ren D, Ding M, Su J, Ye J, He X, Zhang Y, et al. Stachyose in combination with L. rhamnosus GG ameliorates acute hypobaric hypoxia-induced intestinal barrier dysfunction through alleviating inflammatory response and oxidative stress. Free Radic Biol Med. (2024) 212:505–19. doi: 10.1016/j.freeradbiomed.2024.01.009
54. Tripathy A, Swain N, Padhan P, Raghav SK, Gupta B. Lactobacillus rhamnosus reduces CD8+ T cell mediated inflammation in patients with rheumatoid arthritis. Immunobiology. (2023) 228(4):152415. doi: 10.1016/j.imbio.2023.152415
55. Hojsak I, Snovak N, Abdović S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. (2010) 29(3):312–6. doi: 10.1016/j.clnu.2009.09.008
56. Liu S, Hu P, Du X, Zhou T, Pei X. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. (2013) 50(4):377–81. doi: 10.1007/s13312-013-0123-z
57. Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers-a systematic review. Eur J Pediatr. (2018) 177(7):979–94. doi: 10.1007/s00431-018-3167-1
58. Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. (2014) 133(2):405–13. doi: 10.1016/j.jaci.2013.08.020
59. Kumpu M, Lehtoranta L, Roivainen M, Rönkkö E, Ziegler T, Söderlund-Venermo M, et al. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J Med Virol. (2013) 85(9):1632–8. doi: 10.1002/jmv.23623
60. Damholt A, Keller MK, Baranowski K, Brown B, Wichmann A, Melsaether C, et al. Lacticaseibacillus rhamnosus GG DSM 33156 effects on pathogen defence in the upper respiratory tract: a randomised, double-blind, placebo-controlled paediatric trial. Benef Microbes. (2022) 13(1):13–23. doi: 10.3920/BM2021.0065
61. Sibanda T, Marole TA, Thomashoff UL, Thantsha MS, Buys EM. Bifidobacterium species viability in dairy-based probiotic foods: challenges and innovative approaches for accurate viability determination and monitoring of probiotic functionality. Front Microbiol. (2024) 15:1327010. doi: 10.3389/fmicb.2024.1327010
62. Laursen MF, Sakanaka M, von Burg N, Mörbe U, Andersen D, Moll JM, et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol. (2021) 6(11):1367–82. doi: 10.1038/s41564-021-00970-4
63. Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. (2021) 184(15):3884–98.e11. doi: 10.1016/j.cell.2021.05.030
64. Samara J, Moossavi S, Alshaikh B, Ortega VA, Pettersen VK, Ferdous T, et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe. (2022) 30(5):696–711.e5. doi: 10.1016/j.chom.2022.04.005
65. Li M, Ding J, Stanton C, Ross RP, Zhao J, Yang B, et al. Bifidobacterium longum subsp. infantis FJSYZ1M3 ameliorates DSS-induced colitis by maintaining the intestinal barrier, regulating inflammatorycytokines, and modifying gut microbiota. Food Funct. (2023) 14(1):354–68. doi: 10.1039/D2FO03263E
66. Ding M, Li B, Chen H, Ross RP, Stanton C, Jiang S, et al. Bifidobacterium longum subsp. infantis regulates Th1/Th2 balance through the JAK-STAT pathway in growing mice. Microbiome Res Rep. (2024) 3(2):16. doi: 10.20517/mrr.2023.64
67. Lau AS, Yanagisawa N, Hor YY, Lew LC, Ong JS, Chuah LO, et al. Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Benef Microbes. (2018) 9(1):61–70. doi: 10.3920/BM2017.0063
68. Nocerino R, De Filippis F, Cecere G, Marino A, Micillo M, Di Scala C, et al. The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12(®) in infant colic: a randomised, double blind, placebo-controlled trial. Aliment Pharmacol Ther. (2020) 51(1):110–20. doi: 10.1111/apt.15561
69. Wong CB, Odamaki T, Xiao JZ. Insights into the reason of human-residential bifidobacteria (HRB) being the natural inhabitants of the human gut and their potential health-promoting benefits. FEMS Microbiol Rev. (2020) 44(3):369–85. doi: 10.1093/femsre/fuaa010
70. Taipale TJ, Pienihäkkinen K, Isolauri E, Jokela JT, Söderling EM. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr Res. (2016) 79(1-1):65–9. doi: 10.1038/pr.2015.174
71. Hojsak I, Tokić Pivac V, Močić Pavić A, Pasini AM, Kolaček S. Bifidobacterium animalis subsp. lactis fails to prevent common infections in hospitalized children: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. (2015) 101(3):680–4. doi: 10.3945/ajcn.114.102004
72. Laws GA, Harold LK, Tagg JR, Hale JDF. Interferon gamma response in human saliva following exposure to the oral probiotic Streptococcus salivarius BLIS K12. Probiotics Antimicrob Proteins. (2024) 16(1):93–8. doi: 10.1007/s12602-022-10010-0
73. Peng X, Li Z, Pei Y, Zheng S, Liu J, Wang J, et al. Streptococcus salivarius K12 alleviates oral mucositis in patients undergoing radiotherapy for malignant head and neck tumors: a randomized controlled trial. J Clin Oncol. (2024) 42(12):1426–35. doi: 10.1200/JCO.23.00837
74. Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf. (2014) 6:15–20. doi: 10.2147/DHPS.S59665
75. Wescombe PA, Hale JD, Heng NC, Tagg JR. Developing oral probiotics from Streptococcus salivarius. Future Microbiol. (2012) 7(12):1355–71. doi: 10.2217/fmb.12.113
76. Marchisio P, Santagati M, Scillato M, Baggi E, Fattizzo M, Rosazza C, et al. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur J Clin Microbiol Infect Dis. (2015) 34(12):2377–83. doi: 10.1007/s10096-015-2491-x
77. Sarlin S, Koskela U, Honkila M, Tähtinen PA, Pokka T, Renko M, et al. Streptococcus salivarius probiotics to prevent acute otitis media in children: a randomized clinical trial. JAMA Netw Open. (2023) 6(11):e2340608. doi: 10.1001/jamanetworkopen.2023.40608
78. Maiga MA, Morin S, Bernard H, Rabot S, Adel-Patient K, Hazebrouck S. Neonatal mono-colonization of germ-free mice with Lactobacillus casei enhances casein immunogenicity after oral sensitization to cow’s milk. Mol Nutr Food Res. (2017) 61(9):1600862. doi: 10.1002/mnfr.201600862
79. Mai TT, Thi Thu P, Thi Hang H, Trang TTT, Yui S, Shigehisa A, et al. Efficacy of probiotics on digestive disorders and acute respiratory infections: a controlled clinical trial in young Vietnamese children. Eur J Clin Nutr. (2021) 75(3):513–20. doi: 10.1038/s41430-020-00754-9
80. Mu Y, Cong Y. Bacillus coagulans and its applications in medicine. Benef Microbes. (2019) 10(6):679–88. doi: 10.3920/BM2019.0016
81. Anaya-Loyola MA, Enciso-Moreno JA, López-Ramos JE, García-Marín G, Orozco Álvarez MY, Vega-García AM, et al. Bacillus coagulans GBI-30, 6068 decreases upper respiratory and gastrointestinal tract symptoms in healthy Mexican scholar-aged children by modulating immune-related proteins. Food Res Int. (2019) 125:108567. doi: 10.1016/j.foodres.2019.108567
82. Li W, Zhang S, Wang Y, Bian H, Yu S, Huang L, et al. Complex probiotics alleviate ampicillin-induced antibioticassociated diarrhea in mice. Front Microbiol. (2023) 14:1156058. doi: 10.3389/fmicb.2023.1156058
83. Di Pierro F, Lo Russo P, Danza ML, Basile I, Soardo S, Capocasale G, et al. Use of a probiotic mixture containing Bifidobacterium animalis subsp. lactis BB-12 and Enterococcus faecium L3 as prophylaxis to reduce the incidence of acute gastroenteritis and upper respiratory tract infections in children. Minerva Pediatr (Torino). (2021) 73(3):222–9. doi: 10.23736/S2724-5276.20.05925-3
84. Andaloro C, Santagati M, Stefani S, La Mantia I. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a oral spray for children with recurrent streptococcal pharyngotonsillitis: a randomized placebo-controlled clinical study. Eur Arch Otorhinolaryngol. (2019) 276(3):879–87. doi: 10.1007/s00405-019-05346-3
85. Manti S, Parisi GF, Papale M, Licari A, Salpietro C, Miraglia Del Giudice M, et al. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for treatment of upper respiratory tract infections in children: a pilot study on short-term efficacy. Ital J Pediatr. (2020) 46(1):42. doi: 10.1186/s13052-020-0798-4
86. Campanella V, Syed J, Santacroce L, Saini R, Ballini A, Inchingolo F. Oral probiotics influence oral and respiratory tract infections in pediatric population: a randomized double-blinded placebo-controlled pilot study. Eur Rev Med Pharmacol Sci. (2018) 22(22):8034–41. doi: 10.26355/eurrev_201811_16433
87. Maya-Barrios A, Lira-Hernandez K, Jiménez-Escobar I, Hernández L, Ortiz-Hernandez A, Jiménez-Gutiérrez C, et al. Limosilactobacillus reuteri ATCC PTA 5289 and DSM 17938 as adjuvants to improve evolution of pharyngitis/tonsillitis in children: randomised controlled trial. Benef Microbes. (2021) 12(2):137–45. doi: 10.3920/BM2020.0171
88. Paduchová Z, Nagyová Z, Wang D, Muchová J. The impact of probiotics and vitamin C on the prevention of upper respiratory tract symptoms in two preschool children cohorts. Nutr Res Pract. (2024) 18(1):98–109. doi: 10.4162/nrp.2024.18.1.98
89. Little P, Stuart B, Wingrove Z, Mullee M, Thomas T, Johnson S, et al. Probiotic capsules and xylitol chewing gum to manage symptoms of pharyngitis: a randomized controlled factorial trial. Cmaj. (2017) 189(50):E1543–50. doi: 10.1503/cmaj.170599
90. Santamaria F, Montella S, Stocchero M, Pirillo P, Bozzetto S, Giordano G, et al. Effects of pidotimod and bifidobacteria mixture on clinical symptoms and urinary metabolomic profile of children with recurrent respiratory infections: a randomized placebo-controlled trial. Pulm Pharmacol Ther. (2019) 58:101818. doi: 10.1016/j.pupt.2019.101818
91. Marsh RL, Aho C, Beissbarth J, Bialasiewicz S, Binks M, Cervin A, et al. Panel 4: recent advances in understanding the natural history of the otitis media microbiome and its response to environmental pressures. Int J Pediatr Otorhinolaryngol. (2020) 130(Suppl 1):109836. doi: 10.1016/j.ijporl.2019.109836
92. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. (2019) 10(suppl_1):S49–66. doi: 10.1093/advances/nmy063
93. Liu R, Sun B. Lactic acid bacteria and aging: unraveling the interplay for healthy longevity. Aging Dis. (2023) 15(4):1487–98. doi: 10.14336/AD.2023.0926
94. Crits-Christoph A, Hallowell HA, Koutouvalis K, Suez J. Good microbes, bad genes? The dissemination of antimicrobial resistance in the human microbiome. Gut Microbes. (2022) 14(1):2055944. doi: 10.1080/19490976.2022.2055944
95. Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA, et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. (2023) 15(1):2185034. doi: 10.1080/19490976.2023.2185034
96. Doern CD, Nguyen ST, Afolabi F, Burnham CA. Probiotic-associated aspiration pneumonia due to Lactobacillus rhamnosus. J Clin Microbiol. (2014) 52(8):3124–6. doi: 10.1128/JCM.01065-14
97. Boumis E, Capone A, Galati V, Venditti C, Petrosillo N. Probiotics and infective endocarditis in patients with hereditary hemorrhagic telangiectasia: a clinical case and a review of the literature. BMC Infect Dis. (2018) 18(1):65. doi: 10.1186/s12879-018-2956-5
Keywords: recurrent respiratory tract infections, probiotics, children, prevention, health
Citation: Zhang Y, Xu Y, Hu L and Wang X (2025) Advancements related to probiotics for preventing and treating recurrent respiratory tract infections in children. Front. Pediatr. 13:1508613. doi: 10.3389/fped.2025.1508613
Received: 9 October 2024; Accepted: 17 January 2025;
Published: 6 February 2025.
Edited by:
Cinzia Milito, Sapienza University of Rome, ItalyReviewed by:
Simone Foti Randazzese, University of Messina, ItalyCopyright: © 2025 Zhang, Xu, Hu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Hu, bGluZ2h1QHd1c3QuZWR1LmNu; Xiaomei Wang, V2FuZ3hpYW9tZWlAd3VzdC5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.