- 1Department of Ultrasound Medicine, The First Affiliated Hospital of Shihezi University, Shihezi, Xinjiang, China
- 2Department of Research, The First Affiliated Hospital of Shihezi University, Shihezi, Xinjiang, China
- 3Department of Pediatrics, The First Affiliated Hospital of Shihezi University, Shihezi, Xinjiang, China
Objective: To evaluate the diagnostic efficacy and determine the optimal cut-off values of lung ultrasound score for diagnosing neonatal respiratory distress syndrome and its accuracy in assessing the efficacy of neonatal respiratory distress syndrome.
Method: This prospective study included 100 neonates with suspected neonatal respiratory distress syndrome. Each patient underwent both the 14-zone and 12-zone lung ultrasound methods, as well as a chest x-ray, performed after birth and before initiating drug treatment. Surfactant replacement therapy was administered to patients who were diagnosed with neonatal respiratory distress syndrome and met the criteria for medication. Lung ultrasound was conducted and recorded at the 24th hour, the 48th hour, the 72nd hour, and the 7th day after drug administration. ROC curve analysis, Kappa statistics, and ANOVA were utilized to identify the optimal cut-off values for the lung ultrasound scores in diagnosing neonatal respiratory distress syndrome.
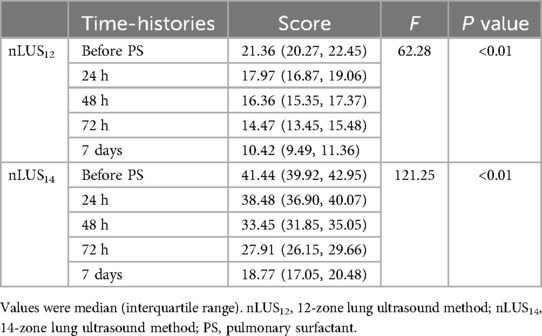
Results: 89 neonates were diagnosed with respiratory distress syndrome, of whom 64 received surfactant replacement therapy. The mean scores of 12-zone lung ultrasound score, 14-zone lung ultrasound score, and chest x-ray score are 18.22 ± 7.15, 38.92 ± 9.69, and 2.15 ± 0.97, respectively. The diagnostic AUC for the 12-zone lung ultrasound score is 0.84 (95% CI: 0.73–0.95), with an optimal cut-off value of 13.5 for diseased vs. not diseased, while the AUC for the 14-zone lung ultrasound score is 0.88 (95% CI: 0.76–0.99), with an optimal cut-off value of 34 for diseased vs. not diseased. There is significant concordance between the neonatal lung ultrasonography scores and the chest x-ray score for diagnosis respiratory distress syndrome (P < 0.01). The optimal cut-off values for the grading diagnosis of neonatal respiratory distress syndrome using the 14-zone lung ultrasound score are identified as 36.5, 40.5, and 44.5. The 12-zone lung ultrasound score does not have a significant difference between the 12th hour after receiving surfactant replacement therapy and the 48th hour after treatment (P = 0.08). All other comparisons demonstrated significant differences.
Conclusion: The 14-zone lung ultrasound score demonstrates higher diagnostic efficacy in diagnosing neonatal respiratory distress syndrome and can accurately evaluate the early efficacy of surfactant replacement therapy in neonates.
Introduction
Neonatal respiratory distress syndrome (NRDS) is a group of disorders caused by immature lung development or a lack of pulmonary surfactant (PS), resulting in the formation of eosinophilic transparent membranes and diffuse lung damage in the respiratory bronchioles and below (1–3). Neonates often present with cyanosis, progressive dyspnoea, and even respiratory failure, accompanied by intercostal and subcostal retractions and expiratory moaning (4). The incidence of NRDS ranges from 1.72% to 8.2%, and it is one of the leading causes of early mortality among neonatal respiratory diseases worldwide (5, 6). Early diagnosis and timely treatment of NRDS play a key role in reducing complications and improving prognosis.
NRDS is primarily diagnosed through a combination of clinical manifestations, radiologic findings, and arterial blood gas analysis. Chest x-ray (CXR) remains the most commonly used imaging tool to diagnose NRDS. However, CXR imaging of NRDS lacks specificity, and various lung diseases may present similar reticular shadows or “white lung” phenomena, which may interfere with the diagnosis of NRDS (7). Neonates are at the peak of growth and development, which makes them more sensitive to ionizing radiation and radiation damage, limiting the use of CXR for repeated examinations and dynamic monitoring of the course of RDS in neonates (8, 9). Additionally, cumulative radiation exposure may elevate the risk of genetic mutation, gonadal damage, and thyroid cancer in neonates (10, 11). Lung ultrasound (LUS) has been gradually involved in the diagnosing and grading of NRDS due to its advantages of being accurate, rapid, radiation-free, and repeatable recently (12, 13). Relevant guidelines have recognized LUS as an important diagnostic imaging method for neonatal lung disease, and higher sensitivity and specificity have been shown in some studies. Its diagnostic value may surpass that of the traditional CXR examinations (14–16).
Replacement therapy with exogenous PS remains an important treatment for NRDS. Early administration of PS reduces the requirement for mechanical ventilation, pneumothorax occurrence, and mortality in neonates (17). The neonatal lung ultrasonography score (nLUS) is strongly associated with neonatal oxygenation status and can accurately determine whether neonates with NRDS under continuous positive airway pressure ventilation require PS replacement therapy (18, 19). Studies advocate for nLUS to be used for semi-quantitative evaluation of lung ventilation to guide the diagnosis of respiratory diseases. Current research evaluating NRDS primarily utilizes the six-zone, ten-zone, and twelve-zone lung ultrasound methods. Studies related to the 14-zone lung ultrasound score (nLUS14) is limited, and there is relatively little comparative research on the diagnosis and grading of NRDS between the 12-zone lung ultrasound score (nLUS12) and nLUS14.
This study compared the diagnostic accuracy of nLUS12, nLUS14, and CXR in neonates with suspected NRDS. The function of LUS in diagnosing and grading NRDS was investigated, as well as the value of nLUS12 and nLUS14 in PS replacement therapy.
Materials and methods
One hundred neonates with highly suspected NRDS in the NICU of the First Affiliated Hospital of Shihezi University from January 2022 to April 2023 were prospectively and consecutively included in this study. All participants underwent LUS and CXR examinations, and of the diagnosed patients, 64 neonates underwent PS replacement therapy for respiratory distress. Inclusion criteria comprised neonates with a gestational age of 24–36 weeks who were treated with nasal continuous positive airway pressure for respiratory distress. The diagnostic criteria for NRDS used in this study were: (1) clinical manifestation: progressive tachypnea, expiratory grunting, subcostal retractions, and cyanosis; (2) chest x-ray abnormalities: air bronchogram signs, dense B-line, ground-glass opacities, or “white lungs”; (3) arterial blood gas analysis: hypoxia, hypercapnia, and oxygen tension/fraction of inspired oxygen ratio PaO2/FiO2 ≤ 26.7 kPa (17, 20, 21). Exclusion criteria comprised intubation or receiving PS treatment before imaging, neonatal air leak syndrome, chromosomal abnormalities, sepsis, and congenital lung diseases. The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Shihezi University (No. KJ2022-317-01), and the guardians of the neonates signed a written informed consent. This study strictly adhered to the principles outlined in the Declaration of Helsinki.
Ultrasound detection methods and nLUS
This study adhered to published guidelines and relevant normative requirements for simultaneous nLUS12 and nLUS14 in neonates (22, 23). Bedside LUS was performed using a Samsung HM70 portable color Doppler ultrasound device equipped with a high-frequency (10–15 MHz) transducer. The examinations were conducted by two sonographers with over 10 years of qualification and rich experience in pediatric LUS diagnosis. They independently performed the ultrasound sweeps and image analysis simultaneously while blinded to the clinical data of the neonates. Mean B-lines were calculated if the ultrasound window covered more than one intercostal space. The most severe ultrasound manifestations in a given area were scored. Considering that the US inspection results are closely related to the operator's experience and level. When the difference in scores between two doctors does not exceed 5 points, take the average of the rating results of two sonographers. If the difference in scores between two sonographers is significant or even exceeds 5 points, a senior pediatric ultrasound specialist with more than 15 years of experience performed an additional blinded ultrasound examination to provide the nLUS. This study utilized the intraclass correlation coefficients (ICC) to determine the inter-operator variability of ultrasound diagnostic results between two sonographers.
Neonates were scanned in supine, lateral, and prone positions while at rest. Lungs were divided into six regions: anterior-superior, anterior-inferior, axillary-superior, axillary-inferior, posterior-superior, and posterior-inferior, resulting in a total of twelve regions bilaterally. The LUS14 was based on the LUS12 by adding bilateral lung base regions to be swept with the bilateral rib arch as the boundary. When measuring each zone in supine or lateral position, starting from the zone centerline, the probe takes a longitudinal section and slides outward to the boundary line, returns to the centerline, slides inward to the boundary line, and then returns to the centerline. For the scanning of the four zones of the chest after the prone position, the main approach is to scan from the scapular line to the posterior axillary line.
nLUS12 assigns a maximum score of three points and a minimum score of zero points for each region, with 12 regions and a total score totaling thirty-six points. The specific scoring criteria are as follows: (1) Zero points: the presence of A-lines only; (2) One point: the presence of A-lines in the upper part of the lungs, presence of fused B-lines or at least three B-lines in the lower part of the lungs; (3) Two points: the presence of fused B-lines; and (4) Three points: abnormal pleural lines and presence of solid lung lesions (18, 24).
nLUS14 assigns a maximum score of five points and a minimum score of zero points for each region, with 14 regions and a total score totaling seventy points. The specific scoring criteria are as follows: (1) Zero point: predominantly A-lines, with scattered (<3) B-lines; (2) One point: scattered B-lines (≥3), no fused B-lines; (3) Two points: dense B-lines, with partially fused B-lines; (4) Three points: fused B-lines; (5) Four points: abnormal pleural lines, with subpleural lung solid lesions; and (6) Five points: abnormal pleural lines, with extensive lung solid lesions (25).
In this study, CXR was performed and scored after LUS examination and prior to PS replacement therapy. A pediatric radiologist with more than 10 years of experience performed CXR photography by using a transportable x-ray machine, GE TMX+ (General Electric, Boston, MA, USA), and Agfa CR 30-X computed radiography imaging system (Agfa-Gevaert, Mortsel, Belgium). Image quality was improved and CXR scoring was performed by pre-processing work such as noise suppression and contrast enhancement.
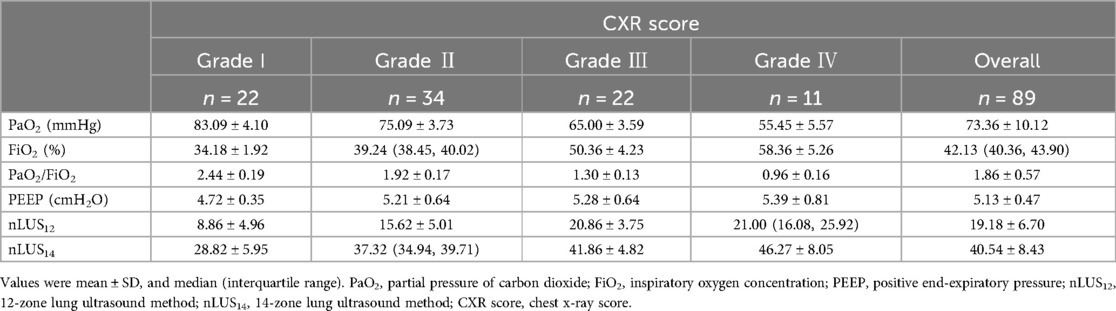
The CXR classified each region into four grades: (1) Grade I: both lungs were slightly less inflated, with reduced translucency, and fine granular densification was seen in the lung fields. (2) Grade II: the translucency of both lungs is further reduced, and there is ground-glass-like change, with evenly distributed fine granular densification and bronchial insufflation in the lung fields, while the diaphragmatic surface of the heart margin is still clear. (3) Grade III: the fine granular hyperintense shadows in the lungs are fused and enlarged, with blurred edges, increased density in the lung fields, markedly reduced translucency, blurred cardiac margins, and bronchial congestion signs are more pronounced. (4) Grade IV: The uniformly increased density of both lung fields is “white lung”, and the heart edge of the diaphragm disappears completely (26).
Pulmonary surfactant replacement therapy and examination
This study performed PS replacement therapy on some neonates diagnosed with NRDS. This study administered poractant-α to neonates with gestational age less than 26 weeks and respiratory oxygen concentration (FiO2) greater than 30% or to those with a gestational age greater than 26 weeks and FiO2 greater than 40%. Positive end-expiratory pressure (PEEP) greater than or equal to 5.0 cmH2O is also one of the criterion for exogenous PS replacement therapy (27). All neonates who require PS replacement therapy received surfactants through the endotracheal intubation technique. The initial dose administered was 200 mg/kg poractant-α. At the 24th hour, the 48th hour, the 72nd hour, and the 7th day after PS administration, nLUS12 and nLUS14 were performed simultaneously on the patient. To minimize the risk of cancer in neonates and adhere to ethical requirements, this study only conducted one CXR examination after birth and prior to PS replacement therapy. CXR examinations were not performed dynamically after PS treatment.
Statistical analysis
Statistical analyses were conducted using SPSS version 26.0. Quantitative data that conformed to normal distribution were expressed as mean plus or minus standard deviation , and non-normally distributed data were expressed as median and quartiles. Clinical baseline data between NRDS and non-NRDS groups were analyzed using an unpaired t-test. Correlation analyses were conducted using Spearman's test. The accuracy of nLUS and CXR scores for the diagnosis and grading scores of NRDS was compared by plotting the Receiver operating characteristic (ROC) curve and calculating the corresponding areas under the ROC curve (AUC). The optimal cut-off point was searched to determine the best threshold for NRDS diagnosis and PS administration or not. The diagnostic consistency of nLUS and CXR was evaluated by the Kappa test to predict the diagnostic efficacy of nLUS in diagnosing NRDS. Differences in nLUS at different time points after PS treatment were evaluated using analysis of variance (ANOVA). Kappa values > 0.75 were considered indicative of good consistency, and P < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
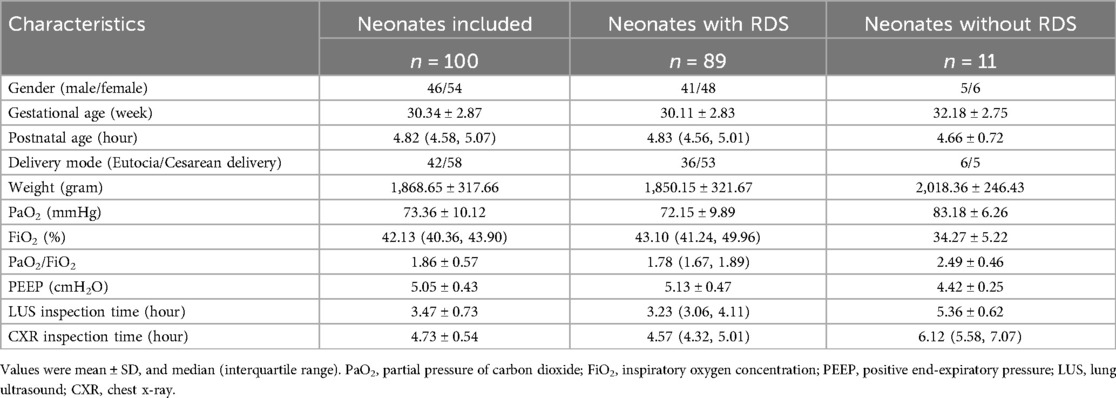
One hundred neonates with suspected NRDS were included in this study, of whom 89 were diagnosed with NRDS. The clinical baseline characteristics of the included neonates are presented in Table 1. There are 46 males and 54 females, with a mean gestational age of 30.34 ± 2.89 weeks, a median postnatal age of 4.82 (interquartile range: 4.58–5.07) hours, and a mean birth weight of 1,868.65 ± 317.66 g. There are no significant differences in sex, gestational age, postnatal age, and birth weight between the NRDS and non-NRDS groups (P > 0.05).
Diagnostic accuracy analysis of nLUS and CXR scores
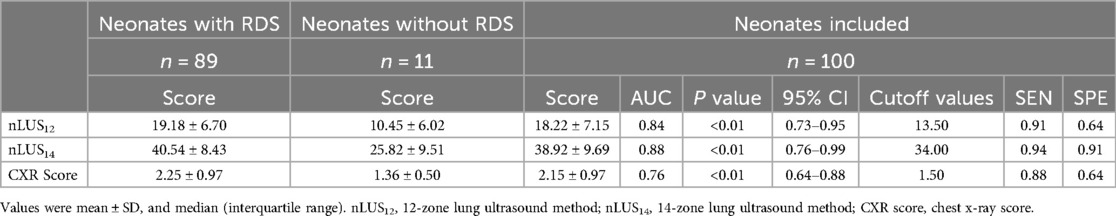
The LUS and CXR scores performed by the subjects within 9 h after birth and prior to PS replacement therapy are shown in Table 2 and Figure 1A. The ICC between the two sonographers was 0.87 (95% CI: 0.84–0.90). The mean scores for nLUS12, nLUS14, and CXR score are 18.22 ± 7.15, 38.92 ± 9.69, and 2.15 ± 0.97, respectively. No significant differences are observed among the three groups (P > 0.05). Spearman's correlation analysis reveals that CXR score is positively correlated with nLUS12 and nLUS14 (r = 0.75, P < 0.01. r = 0.71, P < 0.01). CXR score is significantly negatively correlated with the partial pressure of carbon dioxide (PaO2) and PaO2/FiO2 ratio (r = −0.91, P < 0.01. r = −0.94, P < 0.01). CXR score has a significant positive correlation with FiO2 (r = 0.93, P < 0.01).

Figure 1. Nlus and CXR score predict the diagnostic value of NRDS and whether PS replacement therapy is needed. (A) Diagnostic accuracy analysis of nLUS and CXR score. (B) nLUS and CXR scores for assessing the need for PS treatment.
The diagnostic sensitivity (SEN) of nLUS12 is 0.91, specificity (SPE) is 0.64, accuracy (ACC) is 0.88, positive predictive value (PPV) is 0.96, and negative predictive value (NPV) is 0.44. The ROC curve is plotted, the AUC of nLUS12 is 0.84 (95% CI: 0.73–0.95), and the optimal cut-off value identifies as 13.5. The diagnostic SEN for nLUS14 is 0.94, SPE is 0.91, ACC is 0.94, PPV is 0.99, and NPV is 0.67. Plotting the ROC curve, the AUC for nLUS14 is 0.88 (95% CI: 0.76–0.99), and the optimal cut-off value identifies as 34. The diagnostic SEN for the CXR score is 0.88, SPE is 0.64, ACC is 0.85, PPV is 0.96, and NPV is 0.37. Plotting the ROC curve, the AUC for the CXR score is 0.76 (95% CI: 0.64–0.88) (see Table 2). There is a significant difference in diagnostic consistency among nLUS12, nLUS14, and the CXR score. There is good consistency between nLUS12 and the CXR score (Kappa = 0.78, P < 0.01) There is moderate consistency between nLUS14 and the CXR score (Kappa = 0.53, P < 0.01).
nLUS judgment of CXR grading diagnostic score thresholds
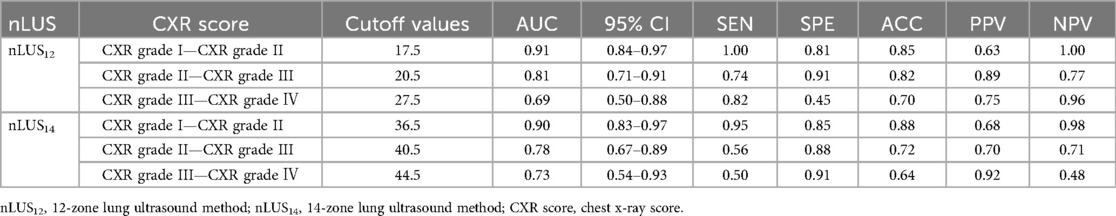
The relevant data for nLUS12, LUS14, and the CXR score are presented in Table 3. In nLUS12, the optimal cut-off value between CXR grade I and CXR grade II is 17.5, with AUC is 0.91. The optimal cut-off value between CXR grade II and CXR grade III is 20.5, with AUC is 0.81. The optimal cut-off value between CXR grade III and CXR grade IV is 27.5, with AUC is 0.69 (see Table 4, Figure 2A).
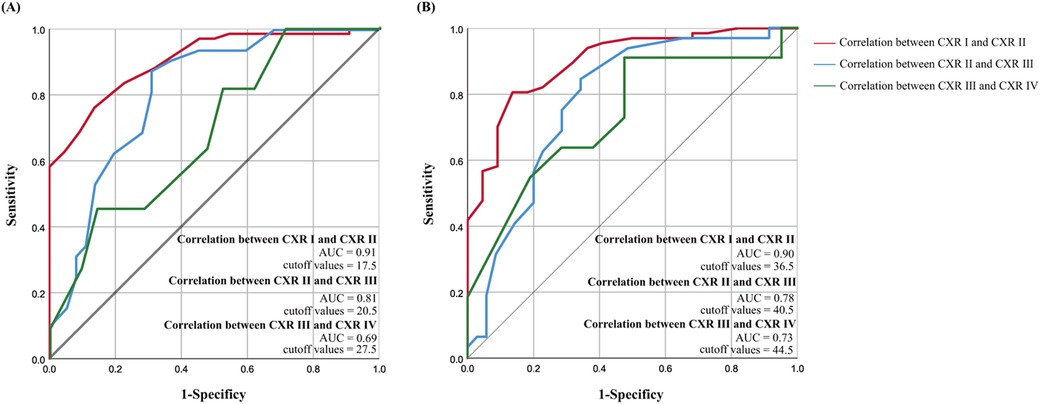
Figure 2. Nlus determines the threshold for CXR grading diagnosis score. (A) nLUS12 determines the threshold for CXR grading diagnosis score. (B) nLUS14 determines the threshold for CXR grading diagnosis score.
In nLUS14, the optimal cut-off value between CXR grade I and CXR grade II is 36.5, with AUC is 0.90. The optimal cut-off value between CXR grade II and CXR grade III is 40.5, with AUC is 0.78. The optimal cut-off value between CXR grade III and CXR grade IV is 44.5, with AUC is 0.73 (see Table 4, Figure 2B).
nLUS and CXR score to assess the need for PS treatment
Sixty-four neonates with confirmed NRDS received PS replacement therapy. The mean scores for nLUS12, nLUS14, and CXR score of all neonates treated with PS are 19.18 ± 6.70, 40.54 ± 8.43, and 2.25 ± 0.97, respectively. The accuracy of nLUS12 and nLUS14 are significantly higher than that of the CXR score (P < 0.01), whereas no significant difference is observed between the nLUS12 and nLUS14 (P = 0.52). Plotting the ROC curve, the AUC of nLUS12 is 0.97 (95% CI: 0.93–1.00), and the optimal cut-off value for acceptance or non-acceptance of PS treatment is 15.5. The AUC of nLUS14 is 0.99 (95% CI: 0.97–1.00), and the optimal cut-off value for acceptance or non-acceptance of PS treatment is 36.5. The best cut-off value is 36.5. The AUC of the CXR score is 0.83 (95% CI: 0.74–0.92) (see Table 5, Figure 1B).
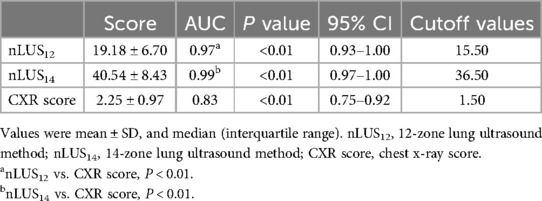
Table 5. Assessment of whether NRDS patients should receive PS replacement therapy in pairs nLUS and CXR score.
nLUS evaluation of PS treatment efficacy
The nLUS results for NRDS neonates who received PS replacement therapy at the 24th hour, the 48th hour, the 72nd hour, and the 7th day are presented in Table 6. After PS treatment, nLUS gradually decreased. The progression of lung lesion improvement after PS replacement therapy followed a front-to-back and top-to-bottom pattern. The patients first experiences a gradual reduction or disappearance of subpleural consolidation, followed by a decrease in B-lines and a gradual appearance of A-lines (see Figures 3, 4). Clinical symptoms improve, including hypoxia and cyanosis. The results of the analysis of variance showed that there was no significant difference (P = 0.78) between the 24th hours after receiving PS treatment and the 48th hours after receiving PS treatment using the lung twelve zone method, while there were significant differences (P < 0.05) in other nLUS. nLUS can better measure the efficacy of PS treatment, among which the pulmonary fourteen zone method can better reflect the early effects of PS replacement therapy.

Figure 3. LUS of neonates before and 24 h after PS replacement therapy. (A) LUS before the neonates receives PS treatment. The continuity of the pleural line is interrupted, and large areas of atelectasis echo can be seen below the pleural line, with bronchial inflation sign visible inside. (B) LUS of the neonates 24 h after receiving PS treatment. Loss of atelectasis and appearance of dense B-line in lung tissue.
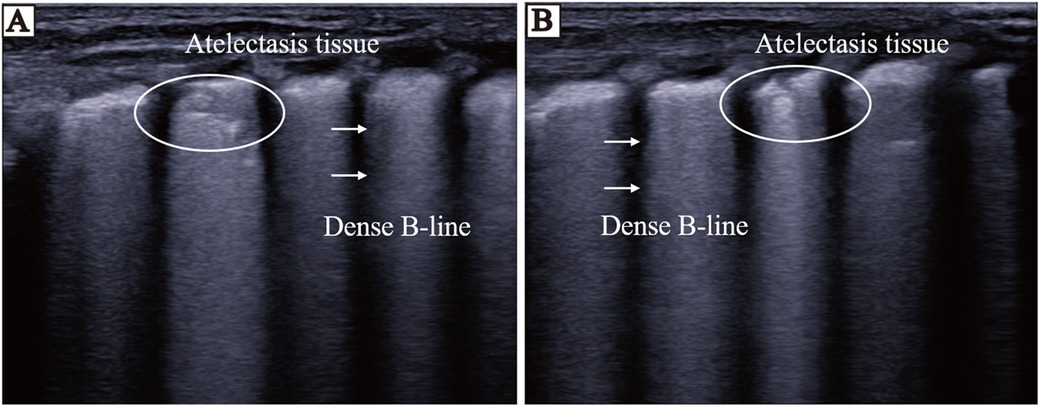
Figure 4. LUS of neonates before and 72 h after PS replacement therapy. (A) LUS before the neonates receives PS treatment. Thickening of pleural line, disappearance of A-line, visible dense B-line, echoes of atelectasis in some areas below pleural line, and bronchial inflation sign visible inside. (B) LUS of the neonates 72 h after receiving PS treatment. The area below the pleural line shows atelectasis, and the echogenicity of lung tissue is significantly reduced after treatment.
Discussion
This study evaluated the diagnostic accuracy of nLUS and CXR score in neonates with RDS and related trends of dynamic efficacy evaluation after PS replacement therapy and introduced the nLUS14 for comparative analysis. The results indicated that nLUS exhibits high accuracy in diagnosing NRDS, thereby enhancing the diagnosis value of LUS as a new non-invasive tool in NRDS (28–31). This study was strictly conducted by two sonographers with over 10 years of experience to ensure the accuracy and analyzability of the research results. The study found that nLUS and CXR scoring results demonstrate good consistency in the diagnosis of NRDS. Ultrasound can significantly reduce the possibility of malignant consequences caused by potential factors (e.g., long-term multiple movements, stress, etc.) during the examination process in neonates. Its technical requirements and experience reserves for operating physicians are not strict, which is conducive to the implementation of LUS in routine clinical practice at different levels of hospitals and improves the rapid diagnosis and treatment efficiency of NRDS.
Zhu et al. (32) found that nLUS positively correlates with the volume of pulmonary edema and the pathological severity of lung tissue. Blank et al. (33) demonstrated that complete airway fluid clearance cannot be accomplished in the first 4 h after delivery, healthy neonates being able to accomplish lung ventilation and alveolar fluid clearance in the first minute after birth. When using nLUS12 with an optimal diagnostic threshold of 13.5, the SEN, SPE, and ACC values are 0.94, 0.91, and 0.94, respectively. Pang et al. (34) used 21.5 as the diagnostic threshold, with a SEN of 0.80, SPE of 1.00, and ACC of 0.90. The optimal cut-off value of nLUS14 was 34.0 in this study, whereas the diagnostic threshold of nLUS14 in the experiment of Jiang et al. (33) was 41.0 points. This study differs slightly from other researchers' research, possibly because it delay LUS examination for some patients whose clinical manifestations are not critical. The alveoli are fully dilated and ventilated, and physiological alveolar and interstitial fluids are maximally cleared, reducing the interference of physiological tiny B-lines extending from the pleura to the deep lungs during ultrasound characterization.
Pang et al. (35) in their study of NRDS neonates with transient neonatal shortness of breath found that the LUS score had the best sensitivity and specificity for severe vs. mild/moderate NRDS using a cut-off value of 25.5, and Hua et al. (1) in a related study defined the optimal cut-off value for non-severe NRDS vs. severe NRDS to be 26.5. In the present study, we found that the optimal cut-off value for CXR grade III and CXR grade IV was 27.5, and when the cut-off value was 27.5, the LUS score SEN was 0.82, SPE was 0.45, ACC was 0.70, and AUC was 0.69 (0.50, 0.88). The relatively consistent cut-off values may indicate that CXR III and IV classification can be used as one of the criteria to judge severe vs. mild/moderate NRDS, and the fluctuation in the range of cut-off values may demonstrate the intersectionality of LUS in severe vs. mild/moderate imaging manifestations of NRDS. Among the misdiagnosed cases in this study, patients with pleural effusion accounted for a large proportion. In neonates lying supine in the incubator for a long period, a small amount of pleural fluid accumulates in the alveoli and interstitium in the posterior thorax and lung base due to gravity, and it is difficult for CXR to differentiate the small amount of fluid in the lung tissue in the surrounding area. The misdiagnosis of NRDS can be significantly reduced by using LUS, which has a very high sensitivity to aqueous density, for zonal scanning of the lungs, and in particular the nLUS14 for scanning the lung base alone.
LUS can assist in determining the nature of the pleural effusion which can be used to provide adjunctive support to the clinical trial data. The site of improvement of lung lesions after receiving PS replacement therapy shows an anterior-to-posterior, top-to-bottom order also correlates with gravitational deposition of effusion in the lungs (33). LUS can determine whether a child needs treatment in the neonatal intensive care unit and predict whether the child requires ventilator assistance and support (19). Exogenous PS replacement therapy via nasal continuous positive airway pressure conditions is an important treatment for NRDS. Surfactant entry into the alveoli results in a rapid reduction in alveolar surface tension, reconstruction of collapsed and atrophied alveoli, and gradual recovery of lung ventilation. In this study, we found that nLUS relative to the CXR score more accurately predicted the need for PS replacement therapy in neonates. Previous studies have suggested that the sensitivity and specificity of nLUS12 for pulmonary solid lesions are 0.90 and 0.98, respectively (3, 36, 37). In the early stages of PS treatment, the primary indication that patients with severe NRDS are gradually beginning to improve is the gradual reduction but not disappearance of the extent of pulmonary solid lesions. nLUS14 is more helpful for the control of severe NRDS patients' conditions, as it is refined into subpleural lung solid lesions and extensive lung solid lesions at a depth of one centimeter based on nLUS12 for the legal determination of the presence or absence of lung solid lesions (see Figures 3, 4). Although the dynamic observation of CXR was not performed in this study, it was confirmed that nLUS14 could better reflect the early effect after drug administration than nLUS12, and this result was consistent with the findings of Jiang et al. (33). Previous studies have confirmed the use of nLUS in identifying the cause of respiratory distress, the administration of surfactant or not, the time of drug withdrawal, the administration of mechanical ventilation, and the timing of withdrawal in neonates with NRDS (18, 19, 26, 38, 39). The present study refined some of the previous ideas and confirmed the value of LUS, especially nLUS14, in the diagnosis and treatment of patients with NRDS. However, there are still obvious limitations: firstly, the number of study cases included in this study was relatively small, and the reliability of the data needs to be verified by large-scale multicentre trials. This limits the validity and generalisability of the findings. Second, in view of subject health and ethical safety considerations, this study did not perform CXR examination during surfactant replacement sessions and did not compare the evaluation of the dynamic efficacy of nLUS and CXR on PS treatment. Given the above, our team will continue to carry out relevant studies to supplement the advancement of the study results at a later stage.
Conclusion
LUS accurately detects NRDS in newborns presenting with rapid breathing. nLUS, particularly nLUS14, is reliable for determining whether NRDS patients need PS replacement therapy. Moreover, nLUS14 effectively assesses the early efficacy of PS replacement therapy, facilitating timely evaluation of treatment outcomes in infants with NRDS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the First Affiliated Hospital of Shihezi University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JD: Project administration, Writing – original draft. YD: Conceptualization, Methodology, Writing – review & editing. JT: Data curation, Formal Analysis, Project administration, Software, Supervision, Writing – review & editing. TD: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. WL: Conceptualization, Validation, Visualization, Writing – review & editing. YG: Conceptualization, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research Project of Shihezi University (ZZZC202199).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xin H, Wang L, Hao W, Hu H, Li H, Liu B. Lung ultrasound in the evaluation of neonatal respiratory distress syndrome. J Ultrasound Med. (2023) 42(3):713–21. doi: 10.1002/jum.16097
2. Yehya N, Smith L, Thomas NJ, Steffen KM, Zimmerman J, Lee JH, et al. Definition, incidence, and epidemiology of pediatric acute respiratory distress syndrome: from the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med. (2023) 24(12 Suppl 2):S87–98. doi: 10.1097/PCC.0000000000003161
3. Khemani RG, Smith LS, Zimmerman JJ, Erickson S. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. (2015) 16(5 Suppl 1):S23–40. doi: 10.1097/PCC.0000000000000432
4. Raimondi F, Migliaro F, Corsini I, Meneghin F, Pierri L, Salomè S, et al. Neonatal lung ultrasound and surfactant administration: a pragmatic, multicenter study. Chest. (2021) 160(6):2178–86. doi: 10.1016/j.chest.2021.06.076
5. Liu J, Shi Y, Dong JY, Zheng T, Li JY, Lu LL, et al. Clinical characteristics, diagnosis and management of respiratory distress syndrome in full-term neonates. Chin Med J. (2010) 123(19):2640–4. doi: 10.3760/cma.j.issn.0366-6999.2010.19.004
6. Alfarwati TW, Alamri AA, Alshahrani MA, Al-Wassia H. Incidence, risk factors and outcome of respiratory distress syndrome in term infants at Academic Centre, Jeddah, Saudi Arabia. Med Arch. (2019) 73(3):183–6. doi: 10.5455/medarh.2019.73.183-186
7. Rocha G, Rodrigues M, Guimarães H. Respiratory distress syndrome of the preterm neonate–placenta and necropsy as witnesses. J Matern Fetal Neonatal Med. (2011) 24(1):148–51. doi: 10.3109/14767058.2010.482613
8. Bahreyni Toossi MT, Malekzadeh M. Radiation dose to newborns in neonatal intensive care units. Iran J Radiol. (2012) 9(3):145–9. doi: 10.5812/iranjradiol.8065
9. Linet MS, Kim KP, Rajaraman P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol. (2009) 39(Suppl 1):S4–26. doi: 10.1007/s00247-008-1026-3
10. Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. (2010) 96(4):269–74. doi: 10.1136/hrt.2008.160309
11. Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. (2006) 36(Suppl 2):121–5. doi: 10.1007/s00247-006-0191-5
12. Cattarossi L, Copetti R, Poskurica B. Radiation exposure early in life can be reduced by lung ultrasound. Chest. (2011) 139(3):730–1. doi: 10.1378/chest.10-2338
13. Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. (2018) 21(3):183–95. doi: 10.1007/s40477-018-0306-5
14. Zhan C, Grundtvig N, Klug BH. Performance of bedside lung ultrasound by a pediatric resident: a useful diagnostic tool in children with suspected pneumonia. Pediatr Emerg Care. (2018) 34(9):618–22. doi: 10.1097/PEC.0000000000000888
15. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. (2012) 38(4):577–91. doi: 10.1007/s00134-012-2513-4
16. Zhu Z, Lian X, Zeng Y, Wu W, Xu Z, Chen Y, et al. Point-of-care ultrasound-a new option for early quantitative assessment of pulmonary edema. Ultrasound Med Biol. (2020) 46(1):1–10. doi: 10.1016/j.ultrasmedbio.2019.08.008
17. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. (2019) 115(4):432–50. doi: 10.1159/000499361
18. Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. (2015) 169(8):e151797. doi: 10.1001/jamapediatrics.2015.1797
19. Perri A, Riccardi R, Iannotta R, Di Molfetta DV, Arena R, Vento G, et al. Lung ultrasonography score versus chest x-ray score to predict surfactant administration in newborns with respiratory distress syndrome. Pediatr Pulmonol. (2018) 53(9):1231–6. doi: 10.1002/ppul.24076
20. Hiles M, Culpan AM, Watts C, Munyombwe T, Wolstenhulme S. Neonatal respiratory distress syndrome: chest x-ray or lung ultrasound? A systematic review. Ultrasound. (2017) 25(2):80–91. doi: 10.1177/1742271X16689374
21. Mohy Eldeen S, Ali S, Salama H. Clinical characteristics, diagnosis, and management outcome of surfactant deficiency respiratory distress syndrome in term and near-term neonates. A retrospective observational study. Acta Biomed. (2022) 93(6):e2022337. doi: 10.23750/abm.v93i6.13794
22. Liu J, Copetti R, Sorantin E, Lovrenski J, Rodriguez-Fanjul J, Kurepa D, et al. Protocol and guidelines for point-of-care lung ultrasound in diagnosing neonatal pulmonary diseases based on international expert consensus. J Vis Exp. (2019) 145:e58990. doi: 10.3791/58990
23. Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, et al. International evidence-based guidelines on point of care ultrasound (POCUS) for critically ill neonates and children issued by the POCUS working group of the European society of paediatric and neonatal intensive care (ESPNIC). Crit Care. (2020) 24(1):65. doi: 10.1186/s13054-020-2787-9
24. Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. (2010) 38(1):84–92. doi: 10.1097/CCM.0b013e3181b08cdb
25. Jiang QX, Shi LJ, Shen LY, Li XQ, Huang RS, Chen LJ, et al. Application value of a new lung ultrasound scoring method in neonatal respiratory distress syndrome treatment. Ultrasound Med Biol. (2022) 48(2):275–82. doi: 10.1016/j.ultrasmedbio.2021.10.009
26. Sefic Pasic I, Riera Soler L, Vazquez Mendez E, Castillo Salinas F. Comparison between lung ultrasonography and chest x-ray in the evaluation of neonatal respiratory distress syndrome. J Ultrasound. (2023) 26(2):435–48. doi: 10.1007/s40477-022-00728-6
27. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome - 2016 update. Neonatology. (2017) 111(2):107–25. doi: 10.1159/000448985
28. Vergine M, Copetti R, Brusa G, Cattarossi L. Lung ultrasound accuracy in respiratory distress syndrome and transient tachypnea of the newborn. Neonatology. (2014) 106(2):87–93. doi: 10.1159/000358227
29. Copetti R, Cattarossi L, Macagno F, Violino M, Furlan R. Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis. Neonatology. (2008) 94(1):52–9. doi: 10.1159/000113059
30. Liu J, Liu F, Liu Y, Wang HW, Feng ZC. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. (2014) 146(2):383–8. doi: 10.1378/chest.13-2852
31. Volpicelli G, Silva F, Radeos M. Real-time lung ultrasound for the diagnosis of alveolar consolidation and interstitial syndrome in the emergency department. Eur J Emerg Med. (2010) 17(2):63–72. doi: 10.1097/MEJ.0b013e3283101685
32. Corsini I, Parri N, Gozzini E, Coviello C, Leonardi V, Poggi C, et al. Lung ultrasound for the differential diagnosis of respiratory distress in neonates. Neonatology. (2019) 115(1):77–84. doi: 10.1159/000493001
33. Blank DA, Kamlin COF, Rogerson SR, Fox LM, Lorenz L, Kane SC, et al. Lung ultrasound immediately after birth to describe normal neonatal transition: an observational study. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F157–62. doi: 10.1136/archdischild-2017-312818
34. Pang H, Zhang B, Shi J, Zang J, Qiu L. Diagnostic value of lung ultrasound in evaluating the severity of neonatal respiratory distress syndrome. Eur J Radiol. (2019) 116:186–91. doi: 10.1016/j.ejrad.2019.05.004
35. Sahin O, Colak D, Tasar S, Yavanoglu Atay F, Guran O, Mungan Akin I. Point-of-care ultrasound versus chest x-ray for determining lung expansion based on rib count in high-frequency oscillatory ventilation. Neonatology. (2023) 120(6):736–40. doi: 10.1159/000533318
36. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. (2009) 135(6):1421–5. doi: 10.1378/chest.08-2281
37. Lichtenstein DA, Lascols N, Mezière G, Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. (2004) 30(2):276–81. doi: 10.1007/s00134-003-2075-6
38. Raimondi F, Migliaro F, Sodano A, Ferrara T, Lama S, Vallone G, et al. Use of neonatal chest ultrasound to predict noninvasive ventilation failure. Pediatrics. (2014) 134(4):e1089–94. doi: 10.1542/peds.2013-3924
Keywords: neonatal respiratory distress syndrome, pulmonary surfactant, neonatal lung ultrasonography score, chest x-ray, grade diagnoses
Citation: Dong J, Deng Y, Tong J, Du T, Liu W and Guo Y (2025) The diagnostic value and efficacy evaluation of lung ultrasound score in neonatal respiratory distress syndrome: a prospective observational study. Front. Pediatr. 13:1500500. doi: 10.3389/fped.2025.1500500
Received: 23 September 2024; Accepted: 16 January 2025;
Published: 30 January 2025.
Edited by:
Michael Hermon, Medical University of Vienna, AustriaReviewed by:
Erik S. Küng, Medical University of Vienna, AustriaDan Waisman, Technion Israel Institute of Technology, Israel
Copyright: © 2025 Dong, Deng, Tong, Du, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Dong, ZG9uZ2ppYW4wNTExQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jian Dong
Jian Dong Yuhong Deng
Yuhong Deng Jin Tong
Jin Tong Tingting Du
Tingting Du Wenguang Liu
Wenguang Liu Yan Guo
Yan Guo