
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 21 March 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1498965
 Lifeng Wang
Lifeng Wang Lijuan Xu*
Lijuan Xu*
Background: Studies have suggested that the administration of prebiotics, probiotics and synbiotics (pre-, pro-, and synbiotics) may potentially decrease the incidence of atopic dermatitis (AD) and alleviate its severity in children; however, recent studies have yielded inconclusive findings.
Objective: This umbrella meta-analysis aimed to comprehensively assess the effect of pre-, pro-, and synbiotics on AD among children.
Methods: A systematic search was carried out in the PubMed and Scopus databases up to April 2024 to identify relevant meta-analyses. Relative risks (RR) and weighted mean differences (WMD) along with their 95% confidence intervals (CI) were pooled using a random effects model to evaluate the impacts on both the incidence of AD and its severity, as assessed by the Scoring Atopic Dermatitis (SCORAD) index.
Results: This umbrella meta-analysis included 38 meta-analyses, with 127,150 participants. The analysis suggested that intervention with pre-, pro-, and synbiotics significantly reduced the incidence of AD (RR = 0.74, 95% CI: 0.70–0.79), which was confirmed by subgroup analyses. The treatment significantly reduced SCORAD score (WMD = −3.75, 95% CI: −5.08 to −2.42). In subgroup analysis, multi-strain probiotics, Lactobacillus, synbiotics, and pre-, pro-, and synbiotics mixtures were found to significantly decrease the SCORAD score, while, Bifidobacterium and prebiotics alone did not show a significant effect on the SCORAD score. The treatment resulted in a significant decrease in SCORAD score among children with moderate to severe AD, but not in subjects with mild AD.
Conclusions: Probiotics and synbiotics could be promising interventions to reduce the risk of developing AD and alleviate its severity in children.
Atopic dermatitis (AD) is a prevalent chronic inflammatory skin disease typically commencing during childhood, marked by itching and recurring eczematous lesions (1). The global prevalence of atopic AD has been on the rise in recent decades, impacting as many as 20% of children (2). Around 60% of individuals with AD experience onset before reaching 1 year of age, with 85% developing the condition before the age of 5; additionally, nearly a quarter of children diagnosed with AD may carry the condition into their young adult years (3). AD can substantially affect the children's quality of life and commonly heightens the susceptibility to asthma, allergies, and mental health complications (1, 4). The etiology of AD is multifaceted, arising from intricate interplays among skin barrier impairment, immunological responses, genetic predispositions, and environmental influences (5, 6). The primary treatment approach presently encompasses topical corticosteroids, antihistamines, and in some cases, antibiotics, although prolonged medication usage may result in undesired side effects (7). Nevertheless, these therapies frequently prove inadequate for addressing moderate to severe cases of AD, with symptoms prone to rapid recurrence following treatment cessation (3, 8). Considering the widespread occurrence of AD, its potential enduring health implications, and the safety issues related to current AD medications, the pursuit of novel therapies demonstrating both efficacy and safety for both preventive and therapeutic purposes holds considerable merit.
It has been recently identified that alterations in gut microbiome composition play a key role in the development of AD and other allergic diseases (9). Research indicates that an imbalance in these microbiomes can lead to altered immune responses, which are associated with inflammatory skin conditions such as AD, psoriasis, and acne (10). Specifically, gut dysbiosis may precede the onset of AD, suggesting a significant interplay between gut health and skin integrity. The gut microbiome influences skin health through mechanisms such as the production of short-chain fatty acids, which help regulate inflammation and maintain skin barrier function (11). Furthermore, both gut and skin microbiomes interact bidirectionally, meaning that disturbances in one can adversely affect the other, ultimately contributing to the pathogenesis of various skin disorders (12). Understanding these connections is essential for developing targeted therapeutic strategies that address both gut and skin dysbiosis in managing dermatological conditions.
A shift in gut bacterial diversity, characterized by an increase in Faecalibacterium prausnitzii, Clostridium, and Escherichia, alongside decreased bifidobacteria species observed in AD patients, could result in the release of molecules capable of harming the intestinal epithelium (12, 13). These alterations impact the skin condition via neuroendocrine, immunological, and metabolic pathways, hypothesized to contribute to the development of AD (14). Accordingly, prebiotics, probiotics, and synbiotics (pre-, pro-, and synbiotics) have emerged as potential interventions to prevent and treat AD in children by modulating the gut microbiome (15). Prebiotics are non-digestible food ingredients that stimulate the growth of beneficial gut bacteria, while probiotics are live microorganisms with health benefits. Synbiotics combine probiotics and prebiotics with the aim of synergistically improving gut microbial composition and function (16). Several meta-analyses have evaluated the effects of pre-, pro-, and synbiotics on AD in children. However, the results have been mixed, with some studies showing benefits (17, 18) and others finding no effect (19, 20). The heterogeneity in study designs, probiotic strains, duration of treatment, age of children, sample size, and outcome measures has made it difficult to draw definitive conclusions. This umbrella meta-analysis aimed to synthesize the evidence from existing meta-analyses to clarify the role of these interventions in preventing and treating AD in children.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to report this umbrella meta-analysis (21).
The PubMed/MEDLINE and Scopus, databases were systematically searched for relevant meta-analyses published up to April 2024 using the following text words and medical subject terms: (Probiotic* OR Prebiotic* OR Synbiotic* OR lactobacillus OR Bifidobacterium OR bifidobacteria OR lactobacilli OR saccharomyces) AND (eczema OR atopic eczema OR atopic dermatitis OR atopy OR sensitization OR allergic OR allergy OR allergies) AND (meta-analysis). The search was restricted to articles published in the English language. Additionally, we conducted manual searches of published reviews and their references to find any additional studies that align with the inclusion criteria.
All meta-analyses of randomized clinical trials (RCTs) assessing the impact of pre-, pro-, and synbiotics on AD, whether for prevention or treatment of the condition, were incorporated based on the following criteria: (1) target participants aged <18 years; (2) the intervention subjects (pregnant and/or nursing mothers to children) received pre-, pro-, or synbiotics orally; (3) placebo administered to the control group; (4) The outcomes included the risk of AD incidence and changes in the severity of the disease, as measured by the Scoring Atopic Dermatitis (SCORAD) index; (5) AD diagnosis aligned with standard criteria. Studies with irrelevant intervention, animal studies, letters, narrative reviews, protocols, comments, republished studies, and studies that were on the other allergic diseases were excluded. Two independent reviewers conducted eligibility assessments, resolving disagreements through author discussions.
Two reviewers independently extracted data from each study, utilizing a pre-designed datasheet, who then cross-checked each other's findings to prevent any mistake. The extracted data included, publication year, author name, sample size, number of analyzed studies, intervention details, risk of bias (ROB) assessment, the relative risk (RR) with 95% confidence interval (CI) for the incidence of AD, and weighted mean difference (WMD) with 95% CI for changes in SCORAD score after the treatment with pre-, pro-, and synbiotics, compared to the placebo. Moreover, the results of subgroup analyses based on the age of children, follow-up duration, type of pre-, pro-, and synbiotics, and supplemented subjects (children only, mothers and children, mothers only) were extracted.
The AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2) was used to evaluate the quality of the included meta-analyses (22). The AMSTAR 2 tool consists of 16 items that cover various aspects of the systematic review process, such as the research question, the inclusion and exclusion criteria, the literature search, the risk of bias assessment, the meta-analysis methods, and the interpretation of the results. Based on the AMSTAR 2, the quality of the studies were categorizes as critically low, low, moderate, and high.
For prevention studies, the RRs with 95% CIs were used as effect size to assess the effect of the intervention on the incidence of AD. For treatment studies, WMDs with their 95% CIs for SCORAD score in the treatment group, compared to the placebo, were applied to pool the data. Pooled estimates were obtained using a random-effects model according to the Der Simonian–Laird approach (23, 24). Heterogeneity across the studies was assessed using the χ2 test, with the degree of heterogeneity quantitatively evaluated through I2 (25). An I2 value exceeding 50% signified a notable level of heterogeneity. Subgroup analyses based on the quality of studies, treatment duration, children's age, type of intervention, and supplemented subjects (children only, mothers and children, mothers only) were carried out to identify the sources of the heterogeneity. Potential publication bias was evaluated using funnel plots and Egger's linear regression. If significant evidence of publication bias was identified, the pooled effect size was adjusted for the observed bias using the trim-and-fill method (26). Sensitivity analysis was conducted by systematically removing individual studies from the primary analyses to assess whether the pooled estimates were influenced by any particular study. The STATA version 14 (STATA Corporation, College Station, TX, USA) was applied to conduct all tests.
Initially, a total of 357 studies was collected, comprising 95 articles from PubMed and 362 from the Scopus database. After removing duplicate articles (73 studies) and excluding those that did not align with the research topic following title and abstract reviews (318 studies), an additional 28 irrelevant studies were excluded during full-text screening because they were qualitative reviews, research protocols, animal studies, case reports, studies on other allergic disease, or had an irrelevant intervention. Ultimately, this umbrella meta-analysis incorporated 38 articles (1–4, 8, 17–20, 27–55) published between 2007 and 2023, comprising a total of 127,150 participants. The literature screening process is illustrated in Figure 1. Twenty-five studies (113,083 participants) examined the risk of AD incidence (1, 3, 17–20, 29–33, 35–38, 41, 44–46, 48–53), while 15 studies (24,719 participants) assessed the severity of AD using the SCORAD index (2, 4, 8, 18, 27, 28, 34, 39, 40, 42, 43, 47, 52, 54, 55). The sample size of the included studies varied from 242 to 31,252 subjects. The risk of bias (ROB) in the primary studies was evaluated using different tools such as the Cochrane ROB tool, Jadad score, and PEDro tool. The percentage of primary studies with low ROB in each meta-analysis displayed significant variability, spanning from 0% to 100%. The majority of the included studies, performed subgroup analyses based on the type of intervention and supplemented population; for such studies, in addition to the main analysis, we used the results of the various subgroups to re-analyze the information by pooling the data from different studies. Data on the impact of multistrain probiotics were available in 30 studies, Lactobacillus in 20 studies, Bifidobacterium in 10 studies, and the combined effect of prebiotics and probiotics (synbiotics) in 5 studies among the analyzed studies. Concerning the supplemented population, the analysis involved 27 studies with children only as the intervention subjects [timing: started from birth to 13 years of age; median follow-up: 10 months (range: 4–54 months)], 20 studies with pregnant mothers and children as the intervention subjects [timing: started from 24 weeks before delivery (mother) to 12 months of age (infants); median follow-up: 3.5 years (range: 1–7 years)], 13 studies with pregnant mothers only [timing: started from 2 to 8 weeks before delivery until delivery; median follow-up: 4 years (range: 1–7 years)], 2 studies with pregnant mothers and breastfeeding mothers [Timing: started from the first trimester of pregnancy to the end of breastfeeding, median follow-up: 4 years (range: 2–6 years)], and 2 studies with pregnant mothers, breastfeeding mothers, and children as the intervention subjects [Timing: mothers started 4–8 weeks before delivery to the end of breastfeeding, infants started from birth to 12 months of age; median follow-up: 2.5 years (range: 1–5.5 years)]. The most commonly used genus of probiotics were Lactobacillus and Bifidobacterium. The most commonly used species and strains of probiotics were L. rhamnosus GG, Lactobacillus acidophilus AD031, L. reuteri ATCC 57730, Lactobacillus rhamnosus HN001, Lactobacillus plantarum CJLP133, Lactobacillus fermentum GM090, Lactobacillus salivarius LDR0723, Lactobacillus casei CECT 9104, Bifidobacterium lactis W52, Bifidobacterium animalis subsp. lactis Bb-12, Bifidobacterium longum BL999, Bifidobacterium bifidum BGN4, and Bifidobacterium breve Bb99.
Based on the AMSTAR2 criteria, the methodological quality of the included studies was high in 11 studies, moderate in 20 studies, and low in 7 studies (Supplementary Table S1). Table 1 presents the basic characteristics of the studies that were included in the analysis.
Employing the random-effects model, the combined effect sizes indicate that pre-, pro-, and synbiotics intervention has a significant impact on reducing the incidence of AD (RR = 0.74, 95% CI: 0.70–0.79). There was a notable heterogeneity across the analyzed studies (I2 = 80.8%, P = 0.001; Figure 2). This finding was consistently supported by various subgroups according to the quality of studies, type of intervention, supplemented population, children's age, and follow-up duration, with the exception of cases where the duration of supplementation exceeded 12 months (Table 2).
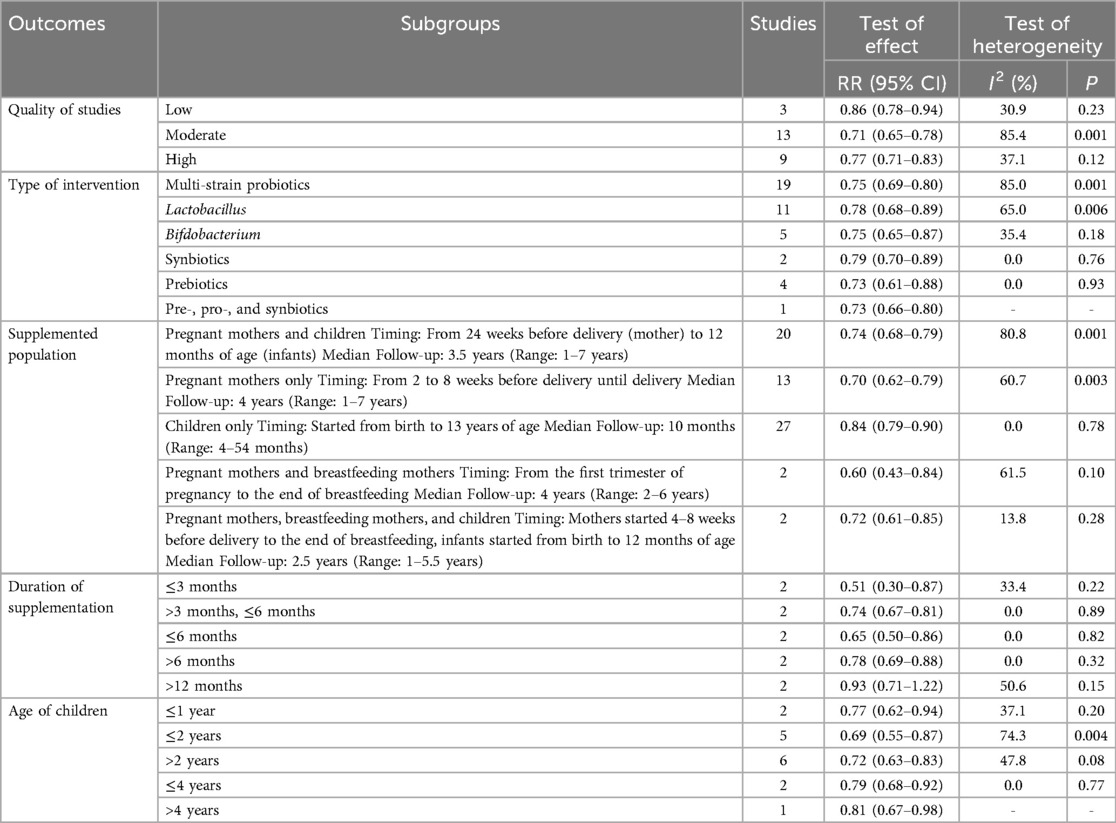
Table 2. Subgroup analyses for the effect of probiotics on the prevention of atopic dermatitis in children.
In the overall analysis, pooled effect size from available studies revealed a significant reduction in SCORAD score following the treatment with pre-, pro-, and synbiotics (WMD = −3.75, 95% CI: −5.08 to −2.42), with significant heterogeneity (I2 = 87.2%, P = 0.001; Figure 3). In the subgroup analysis by the type of intervention, treatment with multi-strain probiotics (WMD = −3.34, 95% CI: −4.69 to −1.99), Lactobacillus (WMD = −1.79, 95% CI: −3.47 to −0.11), synbiotics (WMD = −7.13, 95% CI: −9.83 to −4.43), and pre-, pro-, and synbiotics mixtures (WMD = −5.05, 95% CI: −8.53 to −1.53) significantly reduced SCORAD score, indicating that the addition of prebiotics to probiotics, yields a more substantial impact compared to the use of probiotics alone. However, no notable effect was observed for Bifidobacterium and Prebiotics alone (Table 3). In the subgroup analysis on the severity of disease, the intervention resulted in a significant decrease in SCORAD score among participants diagnosed with moderate to severe AD (WMD = −3.20, 95% CI: −3.55 to −2.84), but not in individuals with mild AD. The observed significant effects were not modified across subgroups based on the quality of studies, supplemented population, supplementation duration, and the age of children (Table 3).
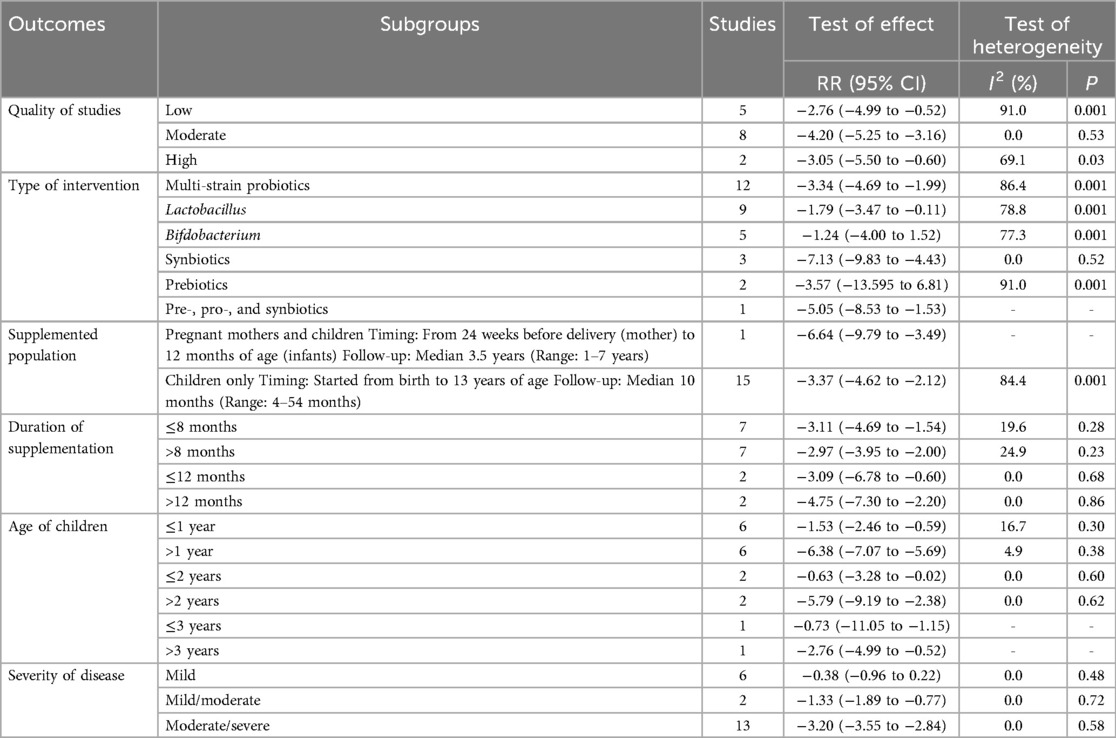
Table 3. Subgroup analyses for the effect of probiotics on the severity of atopic dermatitis (SCORAD) in children.
In the meta-regression analysis, the pooled effect sizes were not affected by sample size and the percentage of primary studies with low ROB in each meta-analysis. Sensitivity analysis demonstrated that individual studies did not impact the pooled effect sizes for outcomes. There was no publication bias detected in studies exploring the preventive impact of pre-, pro-, and synbiotics on AD incidence. However, significant evidence of publication bias was noted in studies focusing on the severity of AD (P = 0.002; Figure 4). When the pooled effect size was adjusted for the observed bias using the trim-and-fill analysis, the pooled estimate did not change significantly, showing the reliability of the findings. The strength of evidence was low for outcomes (Table 4).
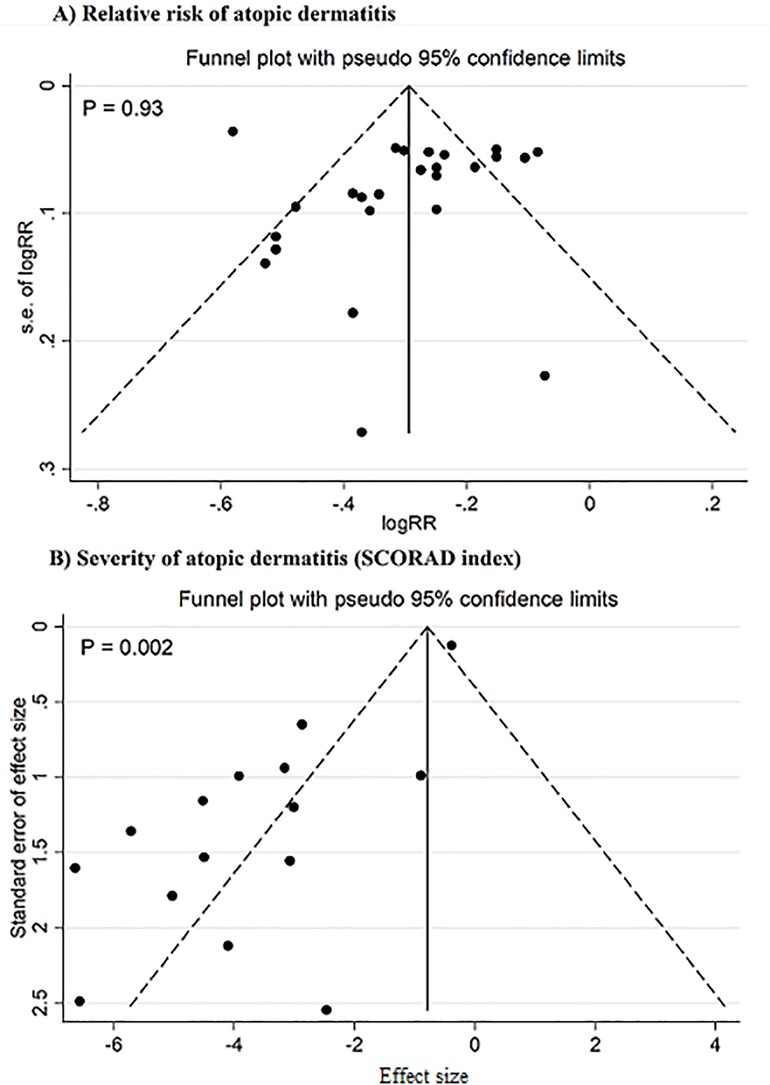
Figure 4. Funnel plot for publication bias. Studies on the relative risk of atopic dermatitis (A), studies on the severity of atopic dermatitis (SCORAD index) (B).
In this umbrella meta-analysis, we explored the preventive and therapeutic effects of pre-, pro-, and synbiotics on AD in children. The analysis unveiled that both the incidence and severity of AD could be notably diminished through the administration of the intervention to pregnant and/or nursing mothers or to children. Moreover, the analysis of the subgroups identified that probiotics mixtures, Lactobacillus, and synbiotics remarkably declined the severity of AD, however, no significant effect was observed for Bifidobacterium and prebiotics when administered alone, suggesting that the effects may be strain-specific. The reduction in AD severity was found for children with moderate to severe AD, whereas no such improvement was noted in patients with mild AD.
Recent studies indicated that probiotics influence the immune system's functioning and enhance the intestinal barrier, potentially aiding in the prevention and management of allergic conditions. Newborns are born with a sterile gastrointestinal tract, and the establishment of microflora during the initial postnatal phase plays a role in activating both adaptive and innate immune responses (56). A lack of sufficient microbial stimulation leads to an imbalanced gut microflora, promoting the dominance of a neonatal Th2-driven immune response, thereby contributing to the onset of atopic conditions (57). Research has shown the potential of probiotic supplementation in AD. Nevertheless, a consensus regarding the effectiveness of probiotics for the clinical prevention and treatment of AD is yet to be reached due to controversy in the results of the studies. These discrepancies could be elucidated by variations in children's ages, the timing of probiotic supplementation, intervention objects, duration of follow-up, disease severity, genetic background, and the specific probiotic strains utilized. The current umbrella analysis indicated that the beneficial effects of probiotic/synbiotic interventions in preventing and treating AD in children were comparable when provided during the prenatal and postpartum periods, whether given to mothers or children. Recent studies have demonstrated that the transmission of maternal microbes to offspring begins during pregnancy, creating an initial microbiome in the fetus (38). Microbial DNA has been identified in umbilical cord blood, fetal and placental membranes, amniotic fluid, and meconium (58). Consequently, the intimate immunological interplay between the mother and the fetus allows for the maternal microbiota to impact the immune development of the offspring, potentially influencing patterns of gut colonization in infants and their susceptibility to allergic diseases (59). Since children with AD exhibit distinct gut microbiota profiles in comparison to non-atopic individuals (12, 13), early probiotic supplementation could encourage a more favorable gut microbiota composition that, subsequently, reduces the risk of AD development.
We also revealed that interventions that included synbiotics were more effective in reducing the severity of AD, compared to probiotics alone. Synbiotics possess both probiotic and prebiotic effects, theoretically functioning more effectively than either component in isolation due to their synergetic effects in in modulating the gut microbiota and consequently the immune system (39). This finding is supported by some previous evidence, indicating the possible superior efficacy of synbiotics therapy over probiotics (34). Another result was that probiotic/synbiotic treatment lasting more than 12 months did not result in the prevention of AD. This contrasts with earlier research suggesting that prolonged probiotic use was advantageous in AD prevention (17). The discrepancy could be due to the natural course of AD, which may weaken the efficacy of any treatment over an extended period. However, this conclusion was based on the combined results of only 2 studies, indicating that caution is warranted in its interpretation due to the potential lack of statistical power to identify a distinction. Additionally, no advantage was observed in probiotic/synbiotic therapy for children with mild AD. This finding suggests that considering to the severity of AD is important when determining the need for probiotic/synbiotic supplementation in individuals with AD. Nonetheless, additional research is essential to validate the findings of the current study.
The preventive and therapeutic effects of probiotic/synbiotic supplementation on AD could be explained by several mechanisms, including modulation of the gut microbiome, anti-inflammatory effects, immunomodulation, competitive exclusion of pathogens, and improvement of skin barrier function (60). Probiotics and synbiotics can help restore a healthy gut microbiome by increasing the abundance of beneficial bacteria like Lactobacillus species (61). This can strengthen the gut epithelial barrier and reduce intestinal inflammation, which is linked to the development of AD (60). Probiotics and their metabolites, such as short-chain fatty acids (SCFAs), can downregulate pro-inflammatory cytokines and inhibit the production of reactive oxygen species (ROS). This can help attenuate the chronic systemic inflammation that plays a key role in AD pathogenesis (62). Probiotics can modulate the immune system by enhancing the production of regulatory T cells and reducing the activity of effector T cells (63). This can help restore the balance between Th1 and Th2 responses, which is often dysregulated in AD (64). Probiotics can competitively inhibit the adhesion and growth of pathogenic bacteria by producing antimicrobial substances and occupying binding sites on the intestinal epithelium. This can prevent the overgrowth of harmful microbes that may contribute to AD development (65). Probiotics and their metabolites can enhance the expression of tight junction proteins and increase the production of antimicrobial peptides in the skin (66). This can strengthen the skin barrier and reduce the penetration of allergens and irritants, which can trigger AD flare-ups. Different probiotic strains may have varying mechanisms of action. For example, Lactobacillus species have been shown to possess specific biological properties, such as the ability to prevent pathogen adhesion to the intestinal epithelium, which may be crucial for reducing bacterial translocation and modulating the inflammatory response (67). However, further research is needed to elucidate the specific mechanisms involved and to determine the optimal probiotic strains and dosages for AD management.
To our understanding, this study represents the first umbrella meta-analysis examining the impact of pre-, pro-, and synbiotics on AD in children. The strength of this analysis lies in the inclusion of a significant number of studies with a substantial sample size and comprehensive subgroup analyses coupled with meta-regression analyses to pinpoint potential sources of heterogeneity. Moreover, we utilized the GRADE assessment to clarify the quality of evidence, providing a transparent and systematic method for crafting evidence summaries and recommendations. This procedure enhances informed decision-making by reducing uncertainty and errors. Some limitations of this study need to be acknowledged. First, a significant evidence of heterogeneity was detected across the studies. Subgroup analysis revealed that the heterogeneity could be partially attributed to the differences in the quality of meta-analyses, type of intervention, and age of children. We applied a random effects analyses to reduce the effect of the heterogeneity on the outcomes. The Egger's test showed a significant evidence of publication bias in studies on SCORAD. We limited the search strategy to articles published in English languages, which could result in the exclusion of studies in other languages. This limitation could potentially affect the comprehensiveness and generalizability of our findings, as relevant research published in other languages may not have been included. However, the results were stable after adjusting the pooled estimates for the publication bias. Another limitation is that this analysis did not assess the long-term safety of the interventions due to lack of sufficient data in included studies, which is important to be evaluated in future studies. Nevertheless, evidence has shown that pre-, pro-, and synbiotics are generally safe in children (68).
In conclusion, our umbrella meta-analysis revealed that providing probiotics and synbiotics to both mothers and children can serve as effective measures in preventing and treating AD in children diagnosed with moderate to severe AD. However, the effectiveness of Bifidobacterium and prebiotics in addressing AD was not substantiated by our study. Future investigations should focus on pinpointing the optimal commencement time for probiotic supplementation, taking into account the pivotal role of prenatal provision, as well as determining the most effective dosage and duration of administration to establish an optimal preventive and therapeutic regimen.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
LW: Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. LX: Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1498965/full#supplementary-material
1. Jiang W, Ni B, Liu Z, Liu X, Xie W, Wu IX, et al. The role of probiotics in the prevention and treatment of atopic dermatitis in children: an updated systematic review and meta-analysis of randomized controlled trials. Paediatr Drugs. (2020) 22:535–49. doi: 10.1007/s40272-020-00410-6
2. Fijan S, Kolč N, Hrašovec M, Jamtvedt G, Pogačar MŠ, Mičetić Turk D, et al. Single-strain probiotic lactobacilli for the treatment of atopic dermatitis in children: a systematic review and meta-analysis. Pharmaceutics. (2023) 15(4):1256. doi: 10.3390/pharmaceutics15041256
3. Pan H, Su J. Association of probiotics with atopic dermatitis among infant: a meta-analysis of randomized controlled trials. Oxid Med Cell Longev. (2022) 2022:5080190. doi: 10.1155/2022/5080190
4. Kim K, Lee E, Kim M, Lee KS, Sol IS, Min TK, et al. Therapeutic effectiveness of probiotics for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials with subgroup analysis. Asian Pac J Allergy Immunol. (2023). doi: 10.12932/AP-280323-1576
5. Thomsen SF, Ulrik CS, Kyvik KO, Hjelmborg JB, Skadhauge LR, Steffensen I, et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy Asthma Proc. (2007) 28(5):535–9. doi: 10.2500/aap2007.28.3041
6. Mandlik DS, Mandlik SK. Atopic dermatitis: new insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol Immunotoxicol. (2021) 43(2):105–25. doi: 10.1080/08923973.2021.1889583
7. Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part II. J Eur Acad Dermatol Venereol. (2012) 26(9):1176–93. doi: 10.1111/j.1468-3083.2012.04636.x
8. Tanojo N, Citrashanty I, Utomo B, Listiawan Y, Ervianti E, Tinduh D. Oral postbiotics derived from Lactobacillus sp. in treatment of atopic dermatitis: a meta-analysis. Acta Dermatovenerol Alp Pannonica Adriat. (2023) 32(2):41–7. doi: 10.15570/actaapa.2023.9
9. Lee S-Y, Lee E, Park YM, Hong S-J. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. (2018) 10(4):354. doi: 10.4168/aair.2018.10.4.354
10. Polkowska-Pruszyńska B, Gerkowicz A, Krasowska D. The gut microbiome alterations in allergic and inflammatory skin diseases–an update. J Eur Acad Dermatol Venereol. (2020) 34(3):455–64. doi: 10.1111/jdv.15951
11. Widhiati S, Purnomosari D, Wibawa T, Soebono H. The role of gut microbiome in inflammatory skin disorders: a systematic review. Dermatol Reports. (2021) 14(1):9188. doi: 10.4081/dr.2022.9188
12. De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. (2021) 9(2):353. doi: 10.3390/microorganisms9020353
13. Song H, Yoo Y, Hwang J, Na Y-C, Kim HS. Faecalibacterium prausnitzii subspecies–level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. (2016) 137(3):852–60. doi: 10.1016/j.jaci.2015.08.021
14. Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. (2018) 9:382698. doi: 10.3389/fmicb.2018.01459
15. Van Der Aa LB, Heymans HS, Van Aalderen WM, Sprikkelman AB. Probiotics and prebiotics in atopic dermatitis: review of the theoretical background and clinical evidence. Pediatr Allergy Immunol. (2010) 21(2p2):e355–67. doi: 10.1111/j.1399-3038.2009.00915.x
16. Sekhon BS, Jairath S. Prebiotics, probiotics and synbiotics: an overview. J Pharm Educ Res. (2010) 1(2):13–36.
17. Cao L, Wang L, Yang L, Tao S, Xia R, Fan W. Long-term effect of early-life supplementation with probiotics on preventing atopic dermatitis: a meta-analysis. J Dermatolog Treat. (2015) 26(6):537–40. doi: 10.3109/09546634.2015.1027168
18. Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. (2008) 121(1):116–21.e11. doi: 10.1016/j.jaci.2007.10.043
19. Szajewska H, Horvath A. Lactobacillus rhamnosus GG in the primary prevention of eczema in children: a systematic review and meta-analysis. Nutrients. (2018) 10(9):1319. doi: 10.3390/nu10091319
20. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. (2007) (4):CD006474. doi: 10.1002/14651858.CD006474.pub2
21. Parums DV. Review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med Sci Monit. (2021) 27:e934475. doi: 10.12659/MSM.934475
22. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Br Med J. (2017) 358:j4008. doi: 10.1136/bmj.j4008
23. Rashidi K, Razi B, Darand M, Dehghani A, Janmohammadi P, Alizadeh S. Effect of probiotic fermented dairy products on incidence of respiratory tract infections: a systematic review and meta-analysis of randomized clinical trials. Nutr J. (2021) 20:1–12. doi: 10.1186/s12937-021-00718-0
24. Taheri A, Raeisi T, Darand M, Jafari A, Janmohammadi P, Razi B, et al. Effects of pre/probiotic supplementation on breast milk levels of TGF-b1, TGF-b2, and IgA: a systematic review and meta-analysis of randomized-controlled trial. Breastfeed Med. (2022) 17(1):22–32. doi: 10.1089/bfm.2021.0204
25. Rashidi K, Darand M, Garousi N, Dehghani A, Alizadeh S. Effect of infant formula supplemented with prebiotics and probiotics on incidence of respiratory tract infections: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. (2021) 63:102795. doi: 10.1016/j.ctim.2021.102795
26. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. (2000) 95(449):89–98. doi: 10.1080/01621459.2000.10473905
27. Michail SK, Stolfi A, Johnson T, Onady GM. Efficacy of probiotics in the treatment of pediatric atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. (2008) 101(5):508–16. doi: 10.1016/S1081-1206(10)60290-6
28. Boyle R, Bath-Hextall F, Leonardi-Bee J, Murrell D, Tang MLK. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. (2009) 39(8):1117–27. doi: 10.1111/j.1365-2222.2009.03305.x
29. Doege K, Grajecki D, Zyriax B-C, Detinkina E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood–a meta-analysis. Br J Nutr. (2012) 107(1):1–6. doi: 10.1017/S0007114511003400
30. Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach J-F, et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology. (2012) 23(3):402–14. doi: 10.1097/EDE.0b013e31824d5da2
31. Dang D, Zhou W, Lun ZJ, Mu X, Wang DX, Wu H. Meta-analysis of probiotics and/or prebiotics for the prevention of eczema. J Int Med Res. (2013) 41(5):1426–36. doi: 10.1177/0300060513493692
32. Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. (2013) 132(3):e666–76. doi: 10.1542/peds.2013-0246
33. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. (2013) 3:CD006474. doi: 10.1002/14651858.CD006474.pub3
34. Kim S-O, Ah Y-M, Yu YM, Choi KH, Shin W-G, Lee J-Y. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. (2014) 113(2):217–26. doi: 10.1016/j.anai.2014.05.021
35. Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. (2014) 179(6):580–92. doi: 10.7205/MILMED-D-13-00546
36. Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. (2015) 136(4):952–61. doi: 10.1016/j.jaci.2015.04.031
37. Panduru M, Panduru N, Sălăvăstru C, Tiplica GS. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. (2015) 29(2):232–42. doi: 10.1111/jdv.12496
38. Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. (2015) 70(11):1356–71. doi: 10.1111/all.12700
39. Chang Y-S, Trivedi MK, Jha A, Lin Y-F, Dimaano L, Garcia-Romero MT. Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr. (2016) 170(3):236–42. doi: 10.1001/jamapediatrics.2015.3943
40. Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. (2017) 7:392. doi: 10.3389/fcimb.2017.00392
41. Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, Robinson Z, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. (2018) 15(2):e1002507. doi: 10.1371/journal.pmed.1002507
42. Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, Murrell DF, Tang ML, Roberts A, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. (2018) 11(11):CD006135. doi: 10.1002/14651858.CD006135.pub3
43. Zhao M, Shen C, Ma L. Treatment efficacy of probiotics on atopic dermatitis, zooming in on infants: a systematic review and meta-analysis. Int J Dermatol. (2018) 57(6):635–41. doi: 10.1111/ijd.13873
44. Li L, Han Z, Niu X, Zhang G, Jia Y, Zhang S, et al. Probiotic supplementation for prevention of atopic dermatitis in infants and children: a systematic review and meta-analysis. Am J Clin Dermatol. (2019) 20:367–77. doi: 10.1007/s40257-018-0404-3
45. Amalia N, Orchard D, Francis KL, King E. Systematic review and meta-analysis on the use of probiotic supplementation in pregnant mother, breastfeeding mother and infant for the prevention of atopic dermatitis in children. Australas J Dermatol. (2020) 61(2):e158–73. doi: 10.1111/ajd.13186
46. Kuang L, Jiang Y. Effect of probiotic supplementation in pregnant women: a meta-analysis of randomised controlled trials. Br J Nutr. (2020) 123(8):870–80. doi: 10.1017/S0007114519003374
47. López AP, Portilla MT, Hernández FM-P, Palacios-Álvarez S. Probiotics to reduce the severity of atopic dermatitis in pediatric patients: a systematic review and meta-analysis. Actas Dermosifiliogr. (2021) 112(10):881–90. doi: 10.1016/j.adengl.2021.06.006
48. Sun M, Luo J, Liu H, Xi Y, Lin Q. Can mixed strains of lactobacillus and bifidobacterium reduce eczema in infants under three years of age? A meta-analysis. Nutrients. (2021) 13(5):1461. doi: 10.3390/nu13051461
49. Chen L, Ni Y, Wu X, Chen G. Probiotics for the prevention of atopic dermatitis in infants from different geographic regions: a systematic review and meta-analysis. J Dermatolog Treat. (2022) 33(7):2931–9. doi: 10.1080/09546634.2022.2091101
50. Sun S, Chang G, Zhang L. The prevention effect of probiotics against eczema in children: an update systematic review and meta-analysis. J Dermatolog Treat. (2022) 33(4):1844–54. doi: 10.1080/09546634.2021.1925077
51. Voigt J, Lele M. Lactobacillus rhamnosus used in the perinatal period for the prevention of atopic dermatitis in infants: a systematic review and meta-analysis of randomized trials. Am J Clin Dermatol. (2022) 23(6):801–11. doi: 10.1007/s40257-022-00723-x
52. Husein-ElAhmed H, Steinhoff M. Meta-analysis on preventive and therapeutic effects of probiotic supplementation in infant atopic dermatitis. J Dtsch Dermatol Ges. (2023) 21(8):833–43. doi: 10.1111/ddg.15120
53. Wang F, Wu F, Chen H, Tang B. The effect of probiotics in the prevention of atopic dermatitis in children: a systematic review and meta-analysis. Transl Pediatr. (2023) 12(4):731. doi: 10.21037/tp-23-200
54. Xue P, Qin H, Qin D, Liu H, Li J, Jin R, et al. The efficacy and safety of oral microecological agents as add-on therapy for atopic dermatitis: a systematic review and meta-analysis of randomized clinical trials. Clin Transl Allergy. (2023) 13(12):e12318. doi: 10.1002/clt2.12318
55. Xue X, Yang X, Shi X, Deng Z. Efficacy of probiotics in pediatric atopic dermatitis: a systematic review and meta-analysis. Clin Transl Allergy. (2023) 13(7):e12283. doi: 10.1002/clt2.12283
56. Zhao M, Shen C, Ma L. Treatment efficacy of probiotics on atopic dermatitis, zooming in on infants: a systematic review and meta-analysis. Int J Dermatol. (2018) 57(6):635–41. doi: 10.1111/ijd.13873
57. Rø A, Simpson MR, Rø TB, Storrø O, Johnsen R, Videm V, et al. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin Exp Allergy. (2017) 47(8):1014–21. doi: 10.1111/cea.12930
58. West CE, Jenmalm M, Prescott S. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. (2015) 45(1):43–53. doi: 10.1111/cea.12332
59. Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. (2012) 9(10):565–76. doi: 10.1038/nrgastro.2012.144
60. Fang Z, Li L, Zhang H, Zhao J, Lu W, Chen W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: a review. Front Immunol. (2021) 12:720393. doi: 10.3389/fimmu.2021.720393
61. Wang IJ, Wang JY. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin Exp Allergy. (2015) 45(4):779–87. doi: 10.1111/cea.12489
62. Kim H, Rutten N, Besseling-van Der Vaart I, Niers L, Choi Y, Rijkers G, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benefic Microbes. (2015) 6(6):783–90. doi: 10.3920/BM2015.0056
63. Kang H-J, Kim G-C, Lee C-G, Park S, Sharma G, Verma R, et al. Probiotics-derived metabolite ameliorates skin allergy by promoting differentiation of FOXP3+ regulatory T cells. J Allergy Clin Immunol. (2021) 147(4):1517–21. doi: 10.1016/j.jaci.2020.11.040
64. Zhang D-J, Hao F, Qian T, Cheng H-X. Expression of helper and regulatory T cells in atopic dermatitis: a meta-analysis. Front Pediatr. (2022) 10:777992. doi: 10.3389/fped.2022.777992
65. Silva DR, Sardi JCO, de Souza Pitangui N, Roque SM, da Silva ACB, Rosalen PL. Probiotics as an alternative antimicrobial therapy: current reality and future directions. J Funct Foods. (2020) 73:104080. doi: 10.1016/j.jff.2020.104080
66. Sharma G, Khanna G, Sharma P, Deol PK, Kaur IP. Mechanistic role of probiotics in improving skin health. In: Beri K, Deol PK, Sandhu SK, editors. Probiotic Research in Therapeutics. Singapore: Springer (2022). doi: 10.1007/978-981-16-5628-6_2
67. Yu Q, Yuan L, Deng J, Yang Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front Cell Infect Microbiol. (2015) 5:26. doi: 10.3389/fcimb.2015.00026
Keywords: probiotics, synbiotics, atopic dermatitis, children, umbrella-meta-analysis
Citation: Wang L and Xu L (2025) The impact of prebiotics, probiotics and synbiotics on the prevention and treatment of atopic dermatitis in children: an umbrella meta-analysis. Front. Pediatr. 13:1498965. doi: 10.3389/fped.2025.1498965
Received: 19 September 2024; Accepted: 7 March 2025;
Published: 21 March 2025.
Edited by:
Consolato M. Sergi, Children's Hospital of Eastern Ontario (CHEO), CanadaReviewed by:
Jiangxin Wang, Shenzhen University, ChinaCopyright: © 2025 Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Xu, MTM3MTc3MzU5NjlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.