
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 04 April 2025
Sec. Pediatric Infectious Diseases
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1485334
 Serkalem Aschalew Jima1
Serkalem Aschalew Jima1 Tamirat Beyene Gerete2*
Tamirat Beyene Gerete2* Fikadu Balcha Hailu3
Fikadu Balcha Hailu3 Girma Bacha Ayane3
Girma Bacha Ayane3 Merga Garoma Jatu4
Merga Garoma Jatu4 Temesgen Geta Hardido5
Temesgen Geta Hardido5 Elias kenea Tolosa6
Elias kenea Tolosa6
Background: Nosocomial infections are a significant public health problem worldwide, affecting hundreds of millions of patients annually. However, studies on nosocomial infections specifically focused on pediatric patients in Ethiopia are limited. Therefore, this study aimed to assess the prevalence of nosocomial infections and associated factors among children admitted at Jimma Medical Center, southwest Ethiopia.
Methods: An institution-based retrospective cross-sectional study design was conducted from June 1 to 30, 2023. Data were collected from the medical records of children. A systematic random sampling technique was employed to select a total of 417 medical records. Data were collected using structured checklists. The collected data were entered into Epi-data version 4.6, and Statistical Package for Social Science version 26.0 was used for analysis. The variables with a p-value less than 0.05 were considered as statistically significant.
Results: A total of 417 (92.87%) medical records of the pediatric patient the inclusion criteria. Of these, 99 (23.74%) of pediatric patients developed nosocomial infections. Malnutrition [AOR = 2.01; 95% CI: 1.18, 3.42], length of hospital stay [AOR = 3.19; 95% CI: 1.73, 5.90], antibiotics received at admission [AOR = 4.76; 95% CI: 1.86, 12.15], being on mechanical ventilation [AOR = 5.04; 95% CI: 2.44, 10.43], blood transfusion [AOR = 4.51; 95% CI: 2.43, 8.35], and urinary catheter [AOR = 3.26; 95% CI: 1.72, 6.18] were significantly associated.
Conclusion: The findings of this study indicated that nearly a quarter of children developed nosocomial infections. Malnutrition, length of hospital stay, antibiotics received at admission, being on mechanical ventilation, urinary catheter, and blood transfusion contributed to the development of nosocomial infections. Therefore, the concerned bodies should immediately prevent nosocomial infections and improve identified factors.
Nosocomial infections, also known as hospital-acquired infections, are infections that patients acquire while receiving healthcare in hospitals or other healthcare facilities (1). These infections typically manifest after 48 h of admission and are not present or incubating at admission (2). Various microorganisms contribute to nosocomial infections, with bacteria responsible for approximately 90% of cases, while viruses, fungi, and protozoa are less common culprits (3). The most prevalent types of nosocomial infections include bloodstream infections, urinary tract infections, surgical site infections, and pneumonia (4, 5).
The global impact of nosocomial infections is profound, affecting hundreds of millions of patients annually (6) and imposing significant economic burdens on healthcare systems (7). Incidence rates range from 5.7% to 19.1% in low- and middle-income countries and from 3.5% to 12% in developed nations (8).
Pediatric patients are particularly vulnerable due to factors such as immature immune systems, prolonged hospital stays, and nutritional challenges (9–11). The risk is exacerbated in developing countries, where inadequate infection prevention practices and increasing use of antimicrobial agents and medical devices heighten exposure (12, 13).
In high-income countries, nosocomial infections affect approximately 4%–8% of pediatric patients (14, 15). However, in African countries, the incidence is rising alarmingly due to negligence in adhering to infection prevention guidelines and insufficient healthcare funding (16). A study in Sub-Saharan Africa reported an incidence density of 26.7 infections per 1,000 pediatric patients per day (17).
Alarmingly, around 4.9 million children die from infectious diseases each year, with nosocomial infections contributing significantly to these fatalities (18). In neonatal populations, these infections account for between 4% and 56% of deaths among hospitalized babies (6). In Sub-Saharan Africa, 1.2 million newborns die before reaching 28 days of age, with a substantial portion of these deaths attributed to nosocomial infections (19).
The growing prevalence of nosocomial infections among pediatric patients raises concerns about increasing antibiotic resistance, prolonged hospital stays, and the financial burden on families and healthcare systems (18, 20–22). These infections are the most common complications faced by hospitalized children, often leading to emotional distress, functional disorders, and reduced quality of life (23).
While identifying the factors associated with nosocomial infections in pediatric patients is critical, there is a notable lack of studies focusing specifically on this demographic in Ethiopia, particularly at Jimma Medical Center. Key variables, such as immunization status and the variety of antibiotics used, have not been adequately investigated as predictors of nosocomial infections among pediatric patients. Therefore, this study aimed to assess the prevalence of nosocomial infections and their associated factors among children admitted to Jimma Medical Center in Southwest Ethiopia.
This research was carried out at Jimma Medical Center. Jimma Medical Center is the only tertiary hospital in the southwestern part of Ethiopia, located in Jimma town. 352 km from Addis Ababa, the capital city of Ethiopia. Being a well-established referral hospital, it provides for the diverse health needs of an estimated 20 million people living in that area. It provides services for approximately 15,000 inpatients and 160,000 outpatients. It can also serve 11,000 emergency cases and 4,500 deliveries per year. It has 800 beds and 1,600 staff members (24).
The Department of Pediatric and Child Health is one of the main units and contains 77 staff nurses, 1 pediatric oncologist, 2 senior surgeons, and 7 pediatricians. An average annual admission capacity of 3,564 patients, including the pediatric ward, pediatric oncology ward, PICU, and NICU. It contains 157 beds, including those in the pediatric intensive care unit, pediatric oncology ward, neonatal intensive care unit, and pediatric ward.
The study was conducted from June 1 to 30, 2023.
An institutional-based retrospective cross-sectional study design was employed.
All medical records of children who were admitted to Jimma Medical Center.
All selected medical records of children who were admitted to Jimma Medical Center from January 1, 2020, to December 31, 2022.
All medical records of children who were admitted to Jimma Medical Center and stayed for more than 48 h were included.
Records of children with incomplete medical records and those that have been reported.
A single population proportion formula, , was used to calculate the sample size for a specific objective. The margin of error (d) = 5%, 95% confidence level, and the proportion of nosocomial infection (p) = 21.4%, taken from a previous study (25). By considering 10% of lost medical records of paediatric patients, the first objective sample size was 284.
To determine the required sample size for the second specific objective of this study, taking into account various factors that are significantly associated with the outcome variables with a confidence level of 95%, a margin of error of 5%, a power of 80%, and a ratio of 1:1, and by using the Open Epi Info 7.2.5 StatCalc software program for the double population proportions formula. The sample size calculated for the second objective was greater than that of the first objective (Table 1).
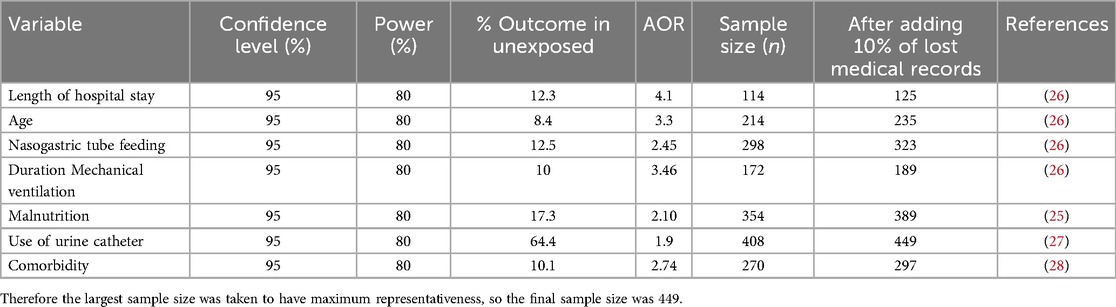
Table 1. Sample size determination using associated factors of nosocomial infection among children at Jimma Medical Center, Southwest Ethiopia, 2023.
To obtain the sample, medical record numbers were used as a sampling frame, sourced from the register logbook of pediatric wards, including the Pediatric Ward, Pediatric Intensive Care Unit (PICU), Neonatal Intensive Care Unit (NICU), and Pediatric Oncology Ward. A systematic random sampling technique was employed to select medical record numbers until the required sample size was achieved.
From January 1, 2020, to December 31, 2022, a total of 10,239 pediatric patients were admitted. The target sample size was determined to be 449. The sampling interval (K) was calculated by dividing the total admissions (10,239) by the target sample size (449), resulting in an interval of approximately 23. A random starting point between 1 and 23 was selected; for example, if the number 10 was chosen, the sample selection process began at this point. Subsequent medical record numbers were selected by advancing every 23rd unit until the required sample size was reached. The identified medical records were then retrieved from the medical record store for analysis.
Nosocomial infection was the outcome variable of this study. The socio-demographic characteristics (age and sex), clinical condition-related factors [malnutrition, comorbid condition, length of hospital stay, antibiotics received at admission, number of types of antibiotics received, place of admission (ward type), serostatus for HIV, anemia, immunization status, previous hospitalization], invasive medical devices, and other procedure-related factors [being on mechanical ventilation, duration of mechanical ventilation, urinary catheters, blood transfusions, surgical procedures, chest tubes, IV cannulas, and nasogastric tubes (NGT)] were independent variables.
An infection acquired by a patient during their stay in a hospital or other healthcare facility. This infection must not have been present or incubating at the time of admission and should occur after 48 h of admission (29, 30).
Refers to medical records that lack essential information, such as date of admission or discharge, or do not contain at least one independent variable.
An imbalance between the nutrients an individual's body needs and the nutrients they consume. This imbalance can be indicated by a weight-for-height ratio of fewer than −2 z-scores or a Middle Upper Arm Circumference (MUAC) measurement of less than 12.5 cm.
Any medical device that is introduced into the body through a break in the skin or another entry point, allowing it to penetrate the body.
The data collection tools were adapted after a review of different relevant literature (25–27, 29, 31–34). The tools were prepared in the English language. The checklist consists of three parts: sociodemographic characteristics, clinical condition-related factors, and invasive medical device procedure-related factors Supplementary File 1.
The data collection process was carried out by three Bachelor of Science nurses with prior experience in data collection who reviewed the medical records meeting the inclusion criteria and extracted the necessary information. The data collection process was supervised by a principal investigator.
Before data collection, training was given to data collectors and supervisors for one day on the purpose of data collection, the content of the checklist, where the medical records are located, and how to extract the necessary data from medical records. A pretest was conducted at Jimma Medical Center (from 2019 admission) by taking 5% (23) of the total sample size one week before the actual data collection. Based on the pretest results, necessary modifications were made, such as removing items that could not be found in the medical records. The supervisor monitored the data collection process daily. Additionally, the principal investigator and supervisor reviewed the complete checklists filled out by the data collectors to ensure the completeness and consistency of the collected information.
Before analysis, the data were cleaned, verified, and coded. The cleaned data were then imported into Epidata version 4.6 and exported to SPSS version 26 for analysis. Descriptive statistics were initially computed to summarize the data characteristics. To assess linear correlations between independent variables, inferential statistics were employed. Multicollinearity was evaluated using tolerance and variance inflation factors (VIF). Variables exhibiting a VIF greater than 10 or a tolerance less than 0.1 were excluded from further analysis. Specifically, the VIF values ranged from less than 2.825, and tolerance values were above 0.354. The study utilized binary logistic regression to explore the relationship between dependent and independent variables. Additionally, multivariable logistic regression analysis was conducted to identify factors associated with nosocomial infections in pediatric patients. The model's fit was assessed through the omnibus test (p = 0.00), and Hosmer and Lemeshow's test indicated a good fit (p = 0.770). To gauge the strength of correlations, odds ratios and 95% confidence intervals were calculated. The statistical significance threshold was set at a p-value of less than 0.05. The findings were presented using appropriate tables, charts, figures, graphs, and narrative prose to ensure clarity and comprehensibility.
This research was conducted in keeping with the Helsinki Declaration. Ethical approval for this study was obtained from the Ethical Review Committee of Jimma University (Ref No: JUIH 414/23). Subsequently, a letter was submitted to the administration of Jimma Medical Center (JMC) to obtain permission before the actual data collection. The information gathered from medical records was confidential and used only for research purposes.
Out of the reviewed 449 medical records, 417 met the inclusion criteria, with a response rate of 92.87%. Of the admitted pediatric patients, 148 (35.5%) were aged ≤28 days. 257 (61.6%) pediatric patients were male (Table 2).
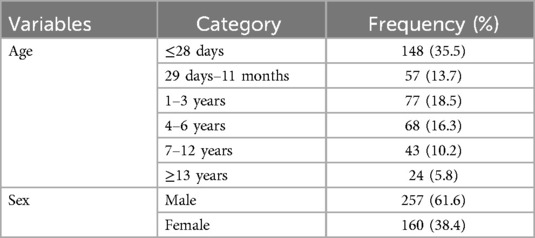
Table 2. Socio-demographic characteristics of children admitted at Jimma Medical Center, Southwest Ethiopia, 2023.
Out of a total of 417 samples, 236 (56.6%) were admitted to the pediatric ward. About 139 (33.3%) of pediatric patients had a history of previous hospitalizations. 388 (93.0%) were received antibiotics at admission. Of this, 339 (81.3%) had received less than or equal to three types of antibiotics. 124 (29.7%) were malnourished, 67 (16.1%) were diagnosed with anemia, 20 (4.8%) were HIV positive, and 88 (21.1%) had comorbid diseases. 260 (62.4%) of pediatric patients stayed in the hospital for more than seven days (Table 3).
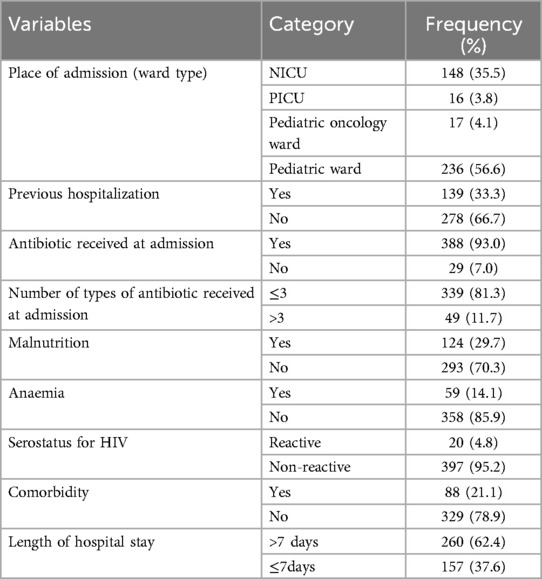
Table 3. Clinical characteristics of children admitted to Jimma Medical Center, Southwest Ethiopia, 2023.
The findings revealed that 148 (35.5%) were fully vaccinated. 201 (48.20%) were vaccinated according to their age. About 13 (3.10%) were partially vaccinated and 55 (13.20%) not vaccinated (Table 4).
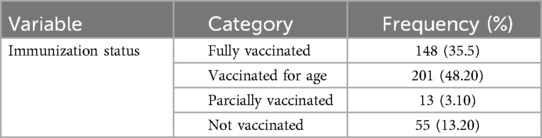
Table 4. Immunization status of children admitted to Jimma Medical Center, Southwest Ethiopia, 2023.
In a study of pediatric patients, 405 (97.1%) were found to have used invasive medical devices. Among these, 391 (93.8%) had a history of IV cannulas, 19 (4.6%) had chest tubes, and 279 (66.9%) had nasogastric tubes. Additionally, 45 (10.8%) of the pediatric patients had been on mechanical ventilation. Of these, 22 (5.3%) were on mechanical ventilation for seven days or more. Furthermore, 58 (13.9%) had a history of urinary catheters, and 106 (25.4%) underwent surgical procedures after admission. 67 (16.1%) had a history of blood transfusion (Table 5).
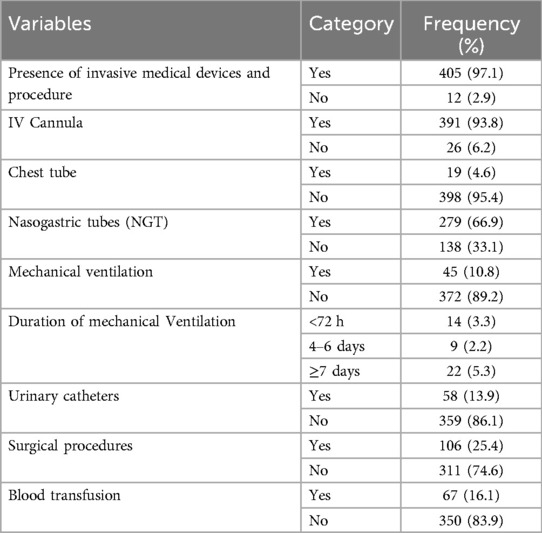
Table 5. Invasive medical device and other procedure procedures for children admitted to Jimma Medical Center, Southwest Ethiopia, 2023.
Cultures were performed for 97 (23.3%) pediatric patients who developed nosocomial infections, of which 91 (21.8%) had isolated organisms. The most commonly isolated organisms were Klebsiella pneumonia, 30 (7.2%), followed by Staphylococcus aureus, 22 (5.3%) (Table 6).
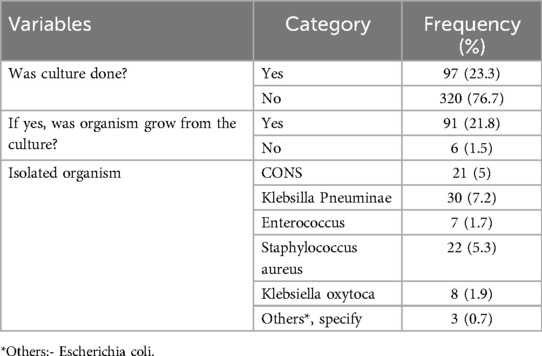
Table 6. Laboratory finding and bacteriological profile of children admitted to Jimma Medical Center, Southwest Ethiopia, 2023.
The overall prevalence of nosocomial infections among children in the current study was 23.74% (95% CI; 19.64, 27.84) (Figure 1).
The most common type of nosocomial infection among children observed in the current study was pneumonia, which accounts for about 38.4% (Figure 2).
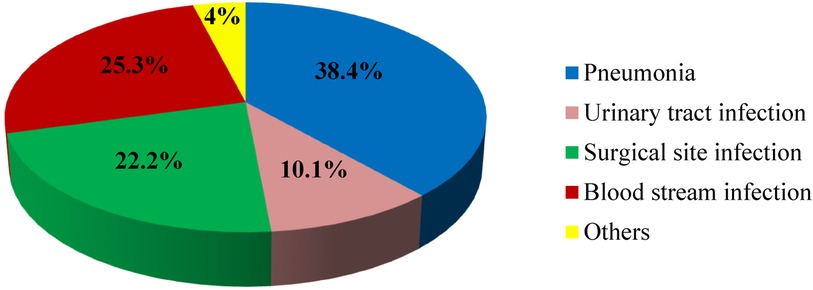
Figure 2. Types of nosocomial infections Among children at Jimma Medical Center, 2023. *Others: skin infection, meningitis, and gastrointestinal infection.
On multivariable logistic regression analysis, six variables were found to be statistically significant predictors of nosocomial infection among children, with a p-value of <0.05 and an AOR of 95% CI. Accordingly, malnutrition, length of hospital stay, antibiotic received at admission, being on mechanical ventilation, having a blood transfusion, and having a history of urinary catheters were the final independent predictors of nosocomial infections among children.
The odds of developing nosocomial infections among children who were malnourished were 2.01 times higher compared to those who were well-nourished [AOR = 2.01; 95% CI (1.18, 3.42); P = 0.010]. The odds of developing nosocomial infections among children who stay in the hospital for more than seven days were 3.19 times higher compared to those with a stay of seven days or less [AOR = 3.19; 95% CI (1.73, 5.90); P < 0.001]. The odds of developing nosocomial infections among children who received antibiotics at admission were 4.76 times higher compared to those who did not receive antibiotics at admission [AOR = 4.76; 95% CI (1.86, 12.15); P = 0.001].
The odds of developing nosocomial infections among children who had mechanical ventilation were 5.04 times higher compared to those without mechanical ventilation [AOR = 5.04; 95% CI (2.44, 10.43); P < 0.001]. The odds of developing nosocomial infections among children who received blood transfusions were 4.51 times higher compared to children who did not receive blood transfusions [AOR = 4.51; 95% CI (2.43, 8.35); P < 0.001]. The odds of developing nosocomial infections among children who had urinary catheters were 3.26 times higher compared to non-urinary catheterized children [AOR = 3.26; 95% CI (1.72, 6.18); P = 0.014] (Table 7).
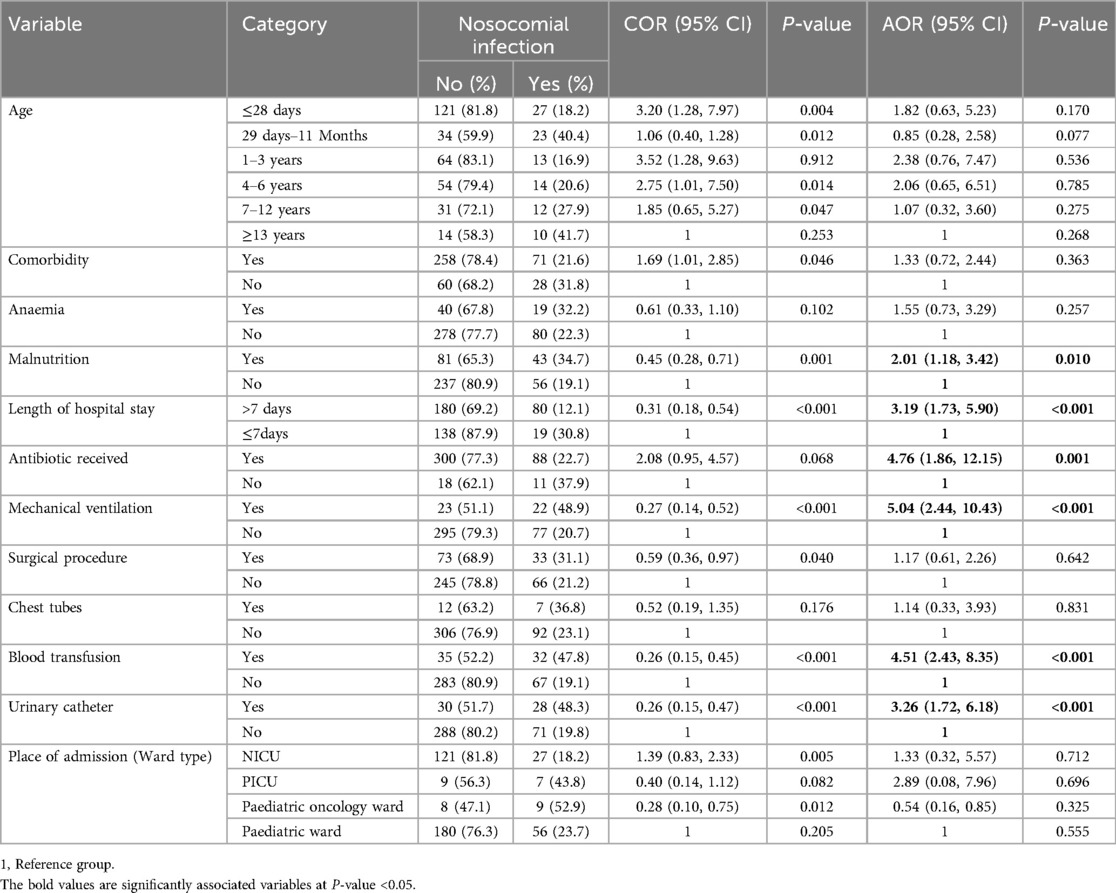
Table 7. The multivariable logistic regression factors associated with nosocomial infection among children admitted to Jimma Medical Center, Southwest Ethiopia, 2023.
This study, conducted at Jimma Medical Center in southwest Ethiopia, found that 23.74% (95% CI: 19.64, 27.84) of pediatric patients acquired nosocomial infections. Significant factors associated with these infections included malnutrition, prolonged hospital stays, antibiotics received at admission, mechanical ventilation, blood transfusions, and urinary catheterization.
The prevalence of nosocomial infections among children in the current study is consistent with previous studies conducted at Hawassa Comprehensive Specialized Hospital (21.4%) (25) and Ayder Hospital (20.2%) (26). However, it is higher when compared to previous studies conducted in Ecuador (13.5%) (35), Switzerland (6.7%) (28), the Philippines (11.37%) (36), the South of Iran (14.57%) (37), and Kenya (2.62%) (38). These discrepancies may stem from differences in inclusion criteria and study designs. For instance, studies in Ecuador and South Iran included patients older than one month, while our study encompassed patients from birth to 17 years. The Swiss study limited participants to those hospitalized for over 24 h, whereas our criteria were over 48 h. Additionally, we included patients from the pediatric oncology ward, unlike the studies from the Philippines and Kenya, which focused solely on pediatric wards, PICU, and NICU.
Conversely, our prevalence is lower than Turkey's report of 68.4% (39). likely due to their exclusive focus on bloodstream infections and a smaller sample size.
Malnutrition was shown to increase the odds of nosocomial infections by 2.01 times, consistent with findings from Ecuador, Indonesia, and Hawassa (25, 27, 35). This vulnerability can be attributed to compromised immune systems in malnourished children, reducing their ability to combat pathogens (40).
Pediatric patients with hospital stays exceeding seven days had 3.19 times higher odds of infection, supporting similar findings in Indonesia (27). Extended hospitalizations often indicate severe illness, increasing exposure to potential infections (41).
Antibiotics received at admission raised infection odds by 4.76 times, similar to results from Ayder Hospital (26). This association may relate to antibiotic overuse, contributing to resistance and subsequent infections (42).
Mechanical ventilation was associated with 5.04 times higher odds of nosocomial infections, consistent with findings from Turkey (43). The irritation and inflammation caused by endotracheal tubes may facilitate airway colonization, increasing infection risks (44).
Additionally, pediatric patients who received blood transfusions had 4.51 times higher odds of developing nosocomial infections compared to those who did not. This finding is supported by a study in South Africa (33). Blood transfusions may temporarily suppress the immune system, compromising the patient's ability to combat infections (45). Aseptic technique during the vein puncture process may also play a role.
Finally, patients with urinary catheters had 3.26 times higher odds of nosocomial infections compared to those without catheters, supported by studies in Switzerland and Indonesia (27, 28). This association may be due to the prolonged duration of catheterization and potential lapses in aseptic techniques (46).
The study included a new variable, immunization status, and the number of types of antibiotics, which had not been previously investigated in Ethiopia. However, since the study relied on secondary data, some crucial independent variables were missing. Furthermore, the findings are limited in their generalizability since the study was conducted solely at Jimma Medical Center.
The findings of this study revealed that nearly a quarter of children developed nosocomial infections. Factors such as malnutrition, length of hospital stay, antibiotics received at admission, being on mechanical ventilation, having a blood transfusion, and a history of urinary catheterization were identified as significant predictors of nosocomial infections among children. Hence, the concerned bodies should focus intervention strategies on contributing factors to nosocomial infections among children. Upcoming researchers should conduct a prospective cohort study to incorporate variables not addressed in a retrospective study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by This research was conducted in keeping with the Helsinki Declaration. Ethical approval for this study was obtained from the Ethical Review Committee of Jimma University (Ref No: JUIH 414/23). Subsequently, a letter was submitted to the administration of Jimma Medical Center (JMC) to obtain permission before the actual data collection. The information gathered from medical records was confidential and used only for research purposes. Written informed consent was obtained from the Ethics Committee of Jimma Medical Center before the initiation of the study. The information gathered from medical records was handled with strict confidentiality, securely stored, and used exclusively for research purposes. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because due to being of secondary data.
SJ: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. TB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FH: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing. GA: Data curation, Formal analysis, Resources, Software, Visualization, Writing – original draft. MJ: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. ET: Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. TGH: Investigation, Methodology, Project administration, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We thank Jimma University and Arsi University for their financial and technical support. We also extend our gratitude to Jimma's medical center management bodies, data collectors, supervisor, and medical record extractors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2025.1485334/full#supplementary-material
AOR, Adjusted Odds Ratio; CI, Confidence Interval; CONS, Coagulase-Negative Staphylococcus; ICU, Intensive Care Unit; IV, Intravenous; JMC, Jimma Medical Center; NGT, Naso Gastric Tube; Nis, Nosocomial Infections; NICU, Neonatal Intensive Care Unit; PICU, Pediatric Intensive Care Unit; WHO, World Health Organization.
2. Llanos-torres KH. Brief report nosocomial infections in emergency observation units and their association with overcrowding and ventilation. Rev Peru Med Exp Salud Publica. (2020) 37(4):721–5. doi: 10.17843/rpmesp.2020.374.5192
3. Horan TC, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2017) 36(5):18538699. doi: 10.1016/j.ajic.2008.03.002
4. CDC. Types of healthcare-associated infections. Healthcare Assoc Infect. (2016) 4(11):12. 10 August, 2016.
5. Mpinda-joseph P, Paramadhas BDA, Reyes G, Maruatona MB, Chise M, Monokwane- BB, et al. Healthcare-associated infections including neonatal bloodstream infections in a leading tertiary hospital in Botswana. Hosp Pract. (2019) 47(4):203–10. doi: 10.1080/21548331.2019.1650608
6. WHO. Healthcare-associated infections fact sheet. (2010). Available at: https://www.who.int/news-room/feature-stories/detail/the-burden-of-health-care-associated-infection-worldwide (Accessed April 29, 2010).
7. WHO. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. Geneva. (2011). Available at: https://www.who.int/publications/i/item/report-on-the-burden-of-endemic-health-care-associated-infection-worldwide (Accessed January 12, 2011).
8. WHO. Fact sheet healthcare-associated infections fact sheet healthcare-associated infections. Heal care-associated Infect FACT SHEET. (2019) 10:1–7.
9. Nozokomiyal Ç, İçin E, Faktörleri R, Çataklı T, Yöney A. Risk factors for nosocomial infections in children. Contemp Med. (2021) 11(5):622–6. doi: 10.16899/jcm.927301
10. Folgori L, Bernaschi P, Piga S, Carletti M, Cunha FP, Lara PHR, et al. Healthcare-associated infections in pediatric and neonatal intensive care units: impact of underlying risk factors and antimicrobial resistance on 30-day case-fatality in Italy and Brazil. Infect Control Hosp Epidemiol. (2016) 37(11):1302–9. doi: 10.1017/ice.2016.185
11. El-sahrigy SAF, Shouman MG, Ibrahim HM, Rahman AMOA, Habib SA. Prevalence and anti-microbial susceptibility of hospital acquired infections in two pediatric intensive care units in Egypt. Open Access Maced J Med Sci. (2019) 7(11):1744–9. doi: 10.3889/oamjms.2019.485
12. Ssekitoleko RT, Oshabaheebwa S, Munabi IG, Tusabe MS, Namayega C, Ngabirano BA, et al. The role of medical equipment in the spread of nosocomial infections : a cross- sectional study in four tertiary public health facilities in Uganda. BMC Public Health. (2020) 20(1):1561. doi: 10.1186/s12889-020-09662-w
13. Carcillo JA, Dean JM, Holubkov R, Berger J, Meert KL, Anand KJS, et al. Inherent risk factors for nosocomial infection in the long stay critically ill child without known baseline immunocompromise: a post –hoc analysis of the CRISIS trial. Pediatr Infect Dis. (2017) 35(412):1182–6.
14. Deptuła A, Trejnowska E, Ozorowski T, Hryniewicz W. Risk factors for healthcare-associated infection in light of two years of experience with the ECDC point prevalence survey of healthcare-associated infection and antimicrobial use in Poland. J Hosp Infect. (2015) 90(4):3010–315. doi: 10.1016/j.jhin.2015.03.005
15. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care–associated infections for the emerging infections program healthcare-associated infections and antimicrobial use prevalence survey team * centers for disease control and prevention. N Engl J Med. (2014) 370(13):1198–208. doi: 10.1056/NEJMoa1306801
16. Irek EO, Amupitan AA, Obadare TO, Aboderin AO. A systematic review of healthcare-associated infections in Africa: an antimicrobial resistance perspective. Afr J Lab Med. (2018) 7(2):796. doi: 10.4102/ajlm.v7i2.796
17. Mbim E, Mboto C, Agbo B. A review of nosocomial infections in sub-saharan Africa. Br Microbiol Res J. (2016) 15(1):1–11. doi: 10.9734/BMRJ/2016/25895
18. Rameshwarnath S, Naidoo S. Risk factors associated with nosocomial infections in the neonatal intensive care unit at mahatma gandhi memorial hospital between 2014 and 2015. S Afr J Infect Dis. (2018) 33(4):1–8.
19. WHO. Launches first ever global report on infection prevention and control. (2022). Available at: https://www.who.int/news/item/06-05-2022-who-launches-first (Accessed June 05, 2022).
20. Jia H, Li L, Li W, Hou T, Ma H, Yang Y, et al. Impact of healthcare-associated infections on length of stay : a study in 68 hospitals in China. Biomed Res Int. (2019) 3(10):31119159.
21. Shankar P. Book review: tackling drug-resistant infections globally. Arch Pharm Pract. (2016) 7(3):110. doi: 10.4103/2045-080X.186181
22. Tchouaket E, Beogo N, Sia D, Kilpatrick K, Séguin C, Baillot A, et al. Economic analysis of healthcare-associated infection prevention and control interventions in medical and surgical units: systematic review using a discounting approach. J Hosp Infect. (2020) 106(1):4–9.
23. Kozlowska K, Schollar-Root O, Savage B, Hawkes C, Chudleigh C, Raghunandan J, et al. Illness-promoting psychological processes in children and adolescents with functional neurological disorder. Children (Basel). (2023) 10(11):1724. doi: 10.3390/children10111724
24. Dechasa M, Chelkeba L, Jorise A, Sefera B, Melaku T. Antibiotics use evaluation among hospitalized adult patients at jimma medical center, southwestern Ethiopia : the way to pave for antimicrobial stewardship. J Pharm Policy Pract. (2022) 4:1–11.
25. Alemayehu T, Tadesse E, Ayalew S, Nigusse B, Yeshitila B, Amsalu A, et al. High burden of nosocomial infections caused by multi-drug Re-sistant pathogens in pediatric patients at hawassa university comprehensive specialized hospital. Ethiop Med J. (2019) 58:45–55.
26. Mohamed AA, Haftu H, Hadgu A, Seyoum D, Gebrekidan G, Ebrahim MM, et al. Prevalence, clinical profile and risk factors of nosocomial infection in ayder pediatric intensive care unit, Tigray, Ethiopia. Int J Gen Med. (2022) 15:7145–53. doi: 10.2147/IJGM.S384233
27. Murni IK, Duke T, Kinney S, Daley AJ, Wirawan MT, Soenarto Y. Risk factors for healthcare-associated infection among children in a low-and middle-income country. BMC Infect Dis. (2022) 22(1):1–7. doi: 10.1186/s12879-022-07387-2
28. Mühlemann K, Franzini C, Aebi C, Berger C, Nadal D, Stähelin J, et al. Prevalence of nosocomial infections in Swiss children’s hospitals. Infect Control Hosp Epidemiol. (2004) 25(9):765–71. doi: 10.1086/502474
29. Sahiledengle B, Seyoum F, Abebe D, Geleta EN, Negash G, Kalu A, et al. Incidence and risk factors for hospital-acquired infection among paediatric patients in a teaching hospital: a prospective study in southeast Ethiopia. BMJ Open. (2020) 10(12):1–10. doi: 10.1136/bmjopen-2020-037997
30. Fraser JL, Mwatondo A, Alimi YH, Varma JK, Del VJ, Vilas R. International journal of infectious diseases healthcare-associated outbreaks of bacterial infections in Africa, 2009–2018 : a review. Int J Infect Dis. (2021) 103:469–77. doi: 10.1016/j.ijid.2020.12.030
31. Comorbidities CDC, Parker J, Octaria R, Smith MD, Chao SJ, Davis M, et al. Characteristics, comorbidities, and data gaps for coronavirus disease deaths, Tennessee, USA. Emerg Infect Dis. (2021) 27(10):2521–8. doi: 10.3201/eid2710.211070
32. Ye D. Risk factors of healthcare-associated infections among pediatric hospitalized patients in Chinese general hospitals from 2001 to 2020 : a systematic review and meta-analysis. Res Sq. (2021) 1:850550.
33. Dramowski A, Whitelaw A, Cotton M. Burden, spectrum, and impact of healthcare-associated infection at a South African children’s hospital. Hosp Infec. (2016) 94(4):27717603. doi: 10.1016/j.jhin.2016.08.022
34. Reyna-figueroa J, Perezpeña-rosas D, Galindo-delgado P, Limon-rojas AE, Madrid-marina V. Association between an incomplete vaccination schedule and nosocomial sepsis among children with cancer. World J Vaccines. (2013) 2013(February):10–5. doi: 10.4236/wjv.2013.31002
35. Barzallo Ochoa P, Campoverde Espinoza CJ. Prevalence and associated factors for healthcare-associated infections in a pediatric setting and pediatric intensive care unit of the vicente cor ral moscoso hospital. Rev Ecuat Pediatr. (2021) 22(1):1–7.
36. Garcia EB, Makalinaw SR, Bernadeth ML, Manipon V. Prevalence of healthcare-associated infections among the pediatric patients admitted at philippine general hospital from the years 2011–2014. Pediatr Infect Dis Soc Philipp J. (2015) 16(1):12–20.
37. Saeed A. The prevalence of nosocomial infection in medical and surgical pediatric ICUs in a tertiary referral hospital in the South of Iran. Health Sci Surveill Syst. (2023) 11(2):364.
38. Patil RK, Kabera B, Muia CK, Ale BM. Hospital acquired infections in a private paediatric hospital in Kenya: a retrospective cross-sectional study. Pan Afr Med J. (2022) 41:28. doi: 10.11604/pamj.2022.41.28.25820
39. Bayhan I, Nur F, Metin Ö, Engül S, Özkan B. Clinical and microbiological features of resistant gram-negative bloodstream infections in children. Infect Public Health. (2017) 10:211–8. doi: 10.1016/j.jiph.2016.04.011
40. Gupta S, Lubree H, Sanghavi S. Compromised nutritional status as a risk factor for the incidence of nosocomial infections. Cureus. (2023) 15(10):e46502. doi: 10.7759/cureus.46502
41. Larson E. NIH public access. Infect Control Hosp Epidemiol. (2012) 33(12):1213–8. doi: 10.1086/668422
42. Nimer NA. Nosocomial infection and antibiotic-resistant threat in the Middle East. Infect Drug Resist. (2022) 10(February):15.
43. Aktar F, Tekin R, Güneş A, Ülgen C, Tan İ, Ertuğrul S, et al. Determining the independent risk factors and mortality rate of nosocomial infections in pediatric patients. Biomed Res Int. (2016) 2016:7240864. doi: 10.1155/2016/7240864
44. Khan FA, Qazi UM, Durrani SAJ, Saleem A, Masroor A, Abbas K. Outcomes of mechanically ventilated patients with nosocomial tracheobronchitis. Cureus. (2021) 13(12):13–7.
45. El Gendy FM. Transfusion of red blood cells as risk predictor for nosocomial infections in pediatric intensive care units. Med Res. (2017) 2(4):172–7.
Keywords: prevalence, nosocomial infection, children, Ethiopia, Jimma
Citation: Jima SA, Gerete TB, Hailu FB, Ayane GB, Jatu MG, Hardido TG and Tolosa Ek (2025) Prevalence and associated factors of nosocomial infection among children admitted at Jimma Medical Center, Southwest Ethiopia: a retrospective study. Front. Pediatr. 13:1485334. doi: 10.3389/fped.2025.1485334
Received: 23 August 2024; Accepted: 28 February 2025;
Published: 4 April 2025.
Edited by:
Yogendra Shrestha, Seven Hills College of Pharmacy, IndiaReviewed by:
Sunil Kumar Ellampati, Seven Hills College of Pharmacy, IndiaCopyright: © 2025 Jima, Gerete, Hailu, Ayane, Jatu, Hardido and Tolosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamirat Beyene Gerete, YmV5ZW5ldGFtZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.