
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 07 March 2025
Sec. Neonatology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1483522
This article is part of the Research TopicMethods In Pediatric Infectious Diseases 2024View all 8 articles
Purpose: The purpose of this study is to investigate the early diagnostic value of the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and C-reactive protein (CRP) in neonatal late-onset sepsis (LOS), as well as to evaluate the combined diagnostic utility of these markers for the early detection of neonatal LOS.
Methods: The late-onset sepsis of newborns admitted to the neonatal intensive care unit of our hospital were retrospectively collected. 142 children with Late-Onset Sepsis (LOS) were selected as the LOS group, 50 neonates with systemic infection were selected as the systemic infection group, 50 neonates who underwent physical examination were selected as the non-systemic infection group. The differences of NLR, PLR, platelet-to-neutrophil ratio (PNR), and C-reactive protein (CRP), Procalcitonin among the three groups were compared.
Results: The levels of NLR and PLR in LOS group were significantly higher than those in systemic infection group and non-systemic infection group. The Receiver Operating Characteristic (ROC) curve result revealed that the area under ROC (AUC, Area Under Curve) of NLR for the diagnosis of LOS was 0.903. When the optimal cut-off value was 1.30, the sensitivity and specificity were 89.4% and 81.0%. The AUC of PLR for the diagnosis of LOS was 0.833. When the optimal truncation value was 57.86, the sensitivity and specificity were 92.3% and 68.0%. The AUC of CRP for the diagnosis of LOS was 0.876, and the sensitivity and specificity were 76.8% and 87.0% when the optimal cut-off value was 10.21 mg/dl. When NLR, PLR, and CRP were combined to diagnosis LOS, The AUC was 0.942, the sensitivity and specificity were 90.8% and 86.0%.
Conclusions: The levels of NLR and PLR in the LOS were higher, which have certain value in the early diagnosis of LOS, and combined with CRP can improve the diagnostic efficiency.
Neonatal sepsis (NS) is a systemic infection in newborns caused by pathogenic microorganisms entering the bloodstream through various routes (1, 2). When it occurs after 72 h of birth, it is classified as late-onset sepsis (LOS). NS is common in neonatal intensive care units (NICUs) and is a leading contributor to neonatal mortality. A 2018 epidemiological study across 12 countries reported an incidence of 2.2%, with a mortality rate ranging from 11% to 19% (3, 4). This highlights NS as a critical global public health concern.
Neonatal sepsis (NS) can affect multiple organ systems, including the digestive, respiratory, circulatory, and hematologic systems, often presenting with nonspecific symptoms. This complexity makes early identification based on clinical signs challenging. Moreover, neonates’ underdeveloped immune systems and immature organ structures contribute to their heightened susceptibility to infections. Without timely intervention, the infection can rapidly disseminate, progressing from asymptomatic to septic shock, disseminated intravascular coagulation, or even death. Therefore, early detection, accurate diagnosis, and prompt treatment are critical to reducing NS-related mortality. Although blood cultures are essential for detecting infection, sepsis is primarily a clinical diagnosis based on a life-threatening response to infection (5). Early detection of sepsis can be challenging, as blood cultures alone cannot confirm the presence of sepsis, and the diagnostic process relies on clinical criteria and biomarkers. Commonly used nonspecific markers, such as white blood cell count, offer suboptimal sensitivity and specificity. Additionally, emerging inflammatory markers like Interleukin 6 (IL-6), serum amyloid A, and CD64 face clinical limitations due to high costs and restricted detection conditions (6). Thus, the search for rapid, reliable, and specific biomarkers for NS diagnosis remains essential for improving clinical outcomes.
Recently, the NLR and PLR have been widely reported as reliable markers for various infectious diseases such as pneumonia and appendicitis. They have demonstrated value in the diagnosis, severity assessment, and prognosis of these diseases (7, 8). There have been reports indicating that NLR and PLR have good predictive roles in the diagnosis and assessment of adult sepsis (9). This suggests that NLR and PLR may also serve as predictive indicators for NS, providing a reference for early clinical diagnosis. In this study, we conducted a retrospective analysis of clinical data from 242 neonates, comparing the levels of NLR and PLR between those with LOS and those with systemic infections or non-infectious diseases. Moreover, these ratios were compared with the commonly used clinical marker CRP. The aim was to explore the potential application value of NLR and PLR in the early diagnosis of LOS.
Retrospective data were collected from January 1, 2017, to December 31, 2020, at the Neonatal Intensive Care Unit of Lianyungang Hospital affiliated with Xuzhou Medical University. A total of 142 full-term neonates with LOS admitted during this period were selected as the LOS group. Additionally, 50 newborns with systemic infections admitted during the same period (presenting with signs of infection upon admission, with sepsis diagnosis excluded during hospitalization, including 38 cases of neonatal pneumonia and 12 cases of neonatal omphalitis) were chosen as the systemic infection group. Furthermore, 50 newborns undergoing outpatient examinations (either in neonatal outpatient clinics or pediatric health check-up clinics) were included as the non-systemic infection group.
(1) LOS Group: Neonates aged between 7 and 28 days, diagnosed according to the diagnostic criteria for neonatal LOS established by the Neonatology Group of the Chinese Pediatric Society in 2019. The diagnosis requires clinical manifestations and positive blood culture results. If the blood culture identifies pathogenic bacteria, it must meet the criteria of two consecutive cultures yielding the same pathogenic strain or a single positive culture with elevated inflammatory markers, along with antibiotic treatment for 5 days or more. The pathogenic bacteria include, but are not limited to, Coagulase-negative staphylococci (CoNS), Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and other commonly recognized neonatal pathogens.
(2) Systemic infection Group: Neonates aged between 7 and 28 days with signs of infection upon admission, with sepsis diagnosis excluded during hospitalization.
(3) Non-systemic infection Group: Neonates aged between 7 and 28 days undergoing outpatient examinations at our hospital’s neonatal outpatient clinics or pediatric health check-up clinics.
(4) Blood samples from all patients were collected on the day of admission, before the initiation of antimicrobial treatment, under aseptic conditions. Clinical data were complete.
(1) Gestational age less than 37 weeks, age less than 7 days, or greater than 28 days.
(2) Newborns with genetic metabolic disorders, chromosomal diseases, or congenital developmental abnormalities.
(3) Newborns with concomitant immune system disorders, hematologic disorders, or impaired liver or kidney function.
(4) Newborns who received antimicrobial or antiplatelet drug therapy before blood sampling.
(5) Newborns with a history of maternal transfusion during delivery or postnatal transfusion.
(6) Positive blood culture without clinical evidence of sepsis, considered as specimen contamination in newborns.
We collected general clinical data for all newborns during hospitalization, including age, gestational age, gender, birth weight, delivery method, and Apgar scores at 1 and 5 min. Laboratory test results, including neutrophil count, lymphocyte count, platelet count, CRP, and PCT, were obtained for all three groups of newborns. The NLR, PLR, and PNR were calculated. All blood samples for testing were collected within 30 min of admission, prior to the initiation of antimicrobial treatment. Additionally, blood samples for blood culture, complete blood count, CRP, and PCT tests were collected simultaneously and sent for analysis.
Statistical analysis was performed using SPSS 24.0 software. Normally distributed quantitative data are expressed as mean ± standard deviation (±s), while non-normally distributed data are presented as median (interquartile range) [M(P25, P75)]. For normally distributed data with homogeneity of variance among the three groups, one-way ANOVA with LSD post-hoc tests was used for pairwise comparisons. For data without homogeneity of variance, Welch's ANOVA was applied, followed by Games-Howell post-hoc comparisons. Non-normally distributed data were analyzed using the Kruskal–Wallis test for overall comparison, with pairwise comparisons conducted via the Bonferroni method. Categorical data are presented as [number (%)] and compared using appropriate statistical tests. Multiple logistic regression was employed to identify independent risk factors for neonatal late-onset sepsis (LOS). The diagnostic value of each marker for neonatal LOS was assessed using receiver operating characteristic (ROC) curves. A significance level of P < 0.05 was considered statistically significant.
In the comparative analysis of newborns, various factors including age, gestational age, birth weight, gender, delivery method, 1 min Apgar score, and 5 min Apgar score were scrutinized across three groups: the LOS group, the systemic infection group, and the non-systemic infection group. The examination yielded no statistically significant differences in these parameters (P > 0.05), as meticulously outlined in Table 1.
Examining the data presented in Table 2, the comparison among Neonatal LOS group, systemic infection group, and non-systemic infection group newborns revealed noteworthy trends. Specifically, NLR, PLR, PNR, CRP, and PCT exhibited higher values in the LOS group in comparison to both the systemic infection and non-systemic infection groups. Furthermore, these parameters were elevated in the systemic infection group compared to the non-systemic infection group, with these differences proving statistically significant (P < 0.05).
Pairwise comparisons further showed that PNR values were lower in the LOS group than in both the systemic infection and non-systemic infection groups, with these differences also statistically significant (P < 0.05). However, no significant difference was found between the systemic infection and non-systemic infection groups (P > 0.05), as shown in Table 3.
Further analysis of PCT highlighted statistically significant variances between the LOS group and the non-systemic infection group, as well as between the systemic infection group and the non-systemic infection group (P < 0.05). Interestingly, no statistically significant difference was identified between the LOS group and the systemic infection group (P > 0.05), as depicted in Figure 1.
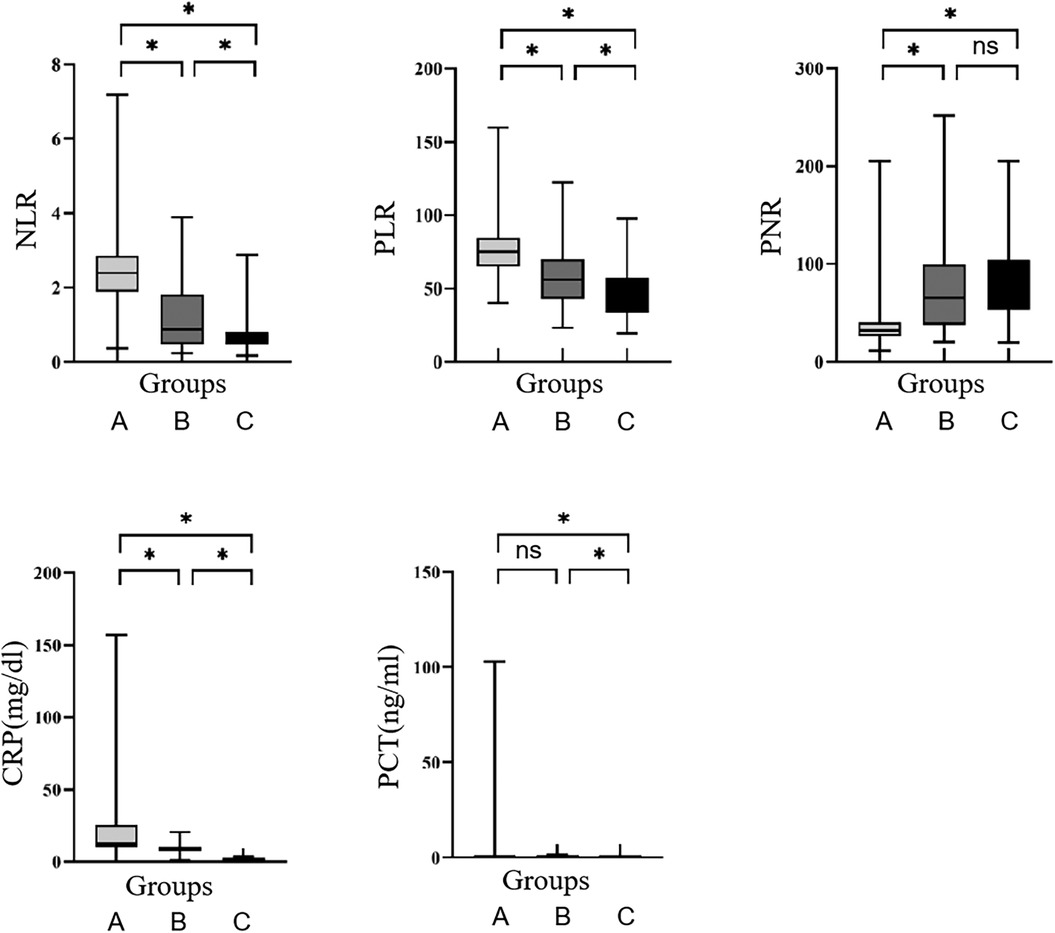
Figure 1. Comparison of NLR, PLR, PNR, CRP, and PCT among the three groups. (A) LOS group; (B) systemic infection group; (C) non-systemic infection group. *P < 0.05, ns, no significance.
To ascertain independent factors influencing neonatal LOS, a multiple logistic regression analysis was conducted. The results revealed that NLR, PLR, and CRP were independent risk factors for the occurrence of neonatal LOS, while PNR and PCT were not independent influencing factors for neonatal LOS (Table 4). These results contribute valuable insights into the specific markers that independently contribute to the risk profile of neonatal LOS, enhancing our understanding of the underlying factors associated with this condition.
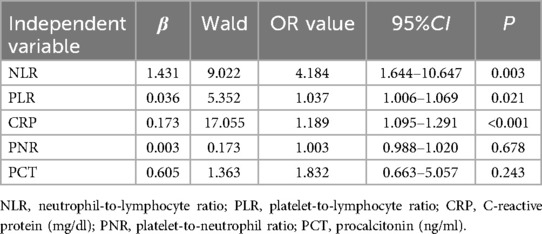
Table 4. Multiple logistic regression analysis of the independent factors influencing the occurrence of neonatal LOS.
Based on the results of the multiple regression analysis, Receiver Operating Characteristic (ROC) curves were constructed (Figure 2) to assess the predictive value of markers for neonatal late-onset sepsis (LOS). The Area Under the Curve (AUC) for NLR was 0.903, with an optimal cutoff of 1.30, achieving a sensitivity of 89.4% and specificity of 81.0%. For PLR, the AUC was 0.833, with an optimal cutoff of 57.86, yielding a sensitivity of 92.3% and specificity of 68.0%. CRP demonstrated an AUC of 0.876, with an optimal cutoff of 10.21 mg/L, resulting in a sensitivity of 76.8% and specificity of 87.0%. When combining NLR, PLR, and CRP, the AUC increased to 0.942, with sensitivity of 90.8% and specificity of 86.0%, as shown in Table 5.
Neonatal sepsis, particularly late-onset sepsis, continues to be a leading cause of morbidity and mortality in neonates, despite advances in neonatal care. Early diagnosis remains a critical challenge, and biomarkers such as the NLR and PLR have emerged as potential diagnostic tools. In this study, we focused on the diagnostic value of NLR and PLR in neonatal LOS, demonstrating their role as independent risk factors and providing new insights into their utility when combined with traditional inflammatory markers like CRP and PCT.
Elevated NLR has been identified as a significant marker in various forms of neonatal sepsis, with several studies pointing to its potential as an early diagnostic tool. Our study further supports this conclusion, finding that NLR was significantly higher in the LOS group compared to both systemic infection and non-systemic infection groups (P < 0.05). This reflects heightened neutrophil activity and lymphocyte depletion, both indicative of a robust inflammatory response in the context of neonatal sepsis. Our findings align with prior studies, such as those by C.D. Russell et al. in adult sepsis and E. Tamelytė et al. in pediatric sepsis, where elevated NLR was shown to correlate with disease severity and prognosis (10, 11).
A key contribution of our study is the specific focus on neonatal LOS, an area that has received less attention in the literature compared to early-onset sepsis (EOS). By examining the role of NLR in the diagnosis of LOS, we provide new evidence to support its diagnostic potential in this distinct population. Our results demonstrate that NLR is not only an early diagnostic marker but also an independent risk factor for neonatal LOS, as confirmed by multiple regression analysis. The ROC analysis, showing an AUC of 0.903 with sensitivity of 89.4% and specificity of 81.0%, further highlights NLR's strong diagnostic performance, comparable to findings in other studies on sepsis.
Interestingly, changes in NLR have also been reported in other co-infectious conditions, such as viral hepatitis. Xiao-Mao Li et al. have demonstrated that in patients with viral hepatitis, both NLR and PLR can also reflect the degree of inflammation and immune response (12, 13). In viral hepatitis, elevated NLR is often associated with more severe disease and worse prognosis, particularly in the setting of acute liver failure or chronic hepatitis. These findings underscore the broader relevance of NLR and PLR in various inflammatory and infectious diseases, further supporting their potential as universal markers of inflammation and immune dysfunction. However, the diagnostic thresholds and their specific roles in viral infections may differ from those in bacterial sepsis, as viral infections typically involve more complex immune responses, including altered lymphocyte subsets and cytokine profiles.
In addition to NLR, we explored the diagnostic utility of PLR in neonatal sepsis. Our study found that PLR was significantly elevated in the LOS group compared to the systemic infection and non-systemic infection groups (P < 0.05), suggesting its potential role as an inflammatory marker. PLR has been previously linked to disease severity in adult sepsis and has shown promise in predicting outcomes (14–17). In our study, logistic regression analysis identified PLR as an independent risk factor for neonatal LOS, with an AUC of 0.833, sensitivity of 92.3%, and specificity of 68.0%.However, while PLR showed promising diagnostic potential, our study also found that PNR did not serve as an independent risk factor for neonatal LOS, indicating its limited utility in this context. This suggests that, although platelet activation is central to inflammation and coagulation, PNR may not offer significant added value in the diagnosis of neonatal LOS, at least compared to NLR.
One of the major strengths of our study is the integrated approach of combining traditional inflammatory markers such as CRP and PCT with NLR and PLR. While CRP and PCT are widely used in clinical practice, they have limitations in early diagnostic accuracy, particularly for neonatal sepsis. Our study found that CRP was significantly elevated in the LOS group compared to the systemic infection and non-systemic infection groups (P < 0.05), confirming its diagnostic value as an independent risk factor for neonatal LOS (AUC = 0.876). PCT also showed elevated levels in the LOS and systemic infection groups compared to non-infected neonates (P < 0.05), though no significant difference was observed between the LOS and systemic infection groups.
Logistic regression further indicated that PCT was not an independent factor for neonatal LOS, highlighting the limitations of PCT as a sole diagnostic tool. However, when CRP was combined with NLR and PLR, the diagnostic efficacy was significantly enhanced, with an AUC of 0.942. This combined approach offers a novel contribution to the field, as it improves upon the diagnostic performance of individual markers. The integration of multiple biomarkers could provide a more robust tool for early and accurate detection of neonatal LOS, which is essential for improving clinical outcomes.
An important aspect of our study is the comparison of diagnostic criteria for neonatal sepsis between China and international standards. Our study followed the criteria established by the Neonatology Group of the Chinese Pediatric Society, which primarily relies on clinical manifestations and common inflammatory markers like CRP and PCT. However, international guidelines, such as the Phoenix Consensus, adopt a more comprehensive approach, incorporating multi-dimensional assessments including clinical scoring systems (e.g., pSOFA), organ dysfunction, and additional biomarkers like IL-6, IL-8, and PCT (5).
While our study demonstrated the significant diagnostic potential of NLR and PLR under the Chinese criteria, the broader, more dynamic diagnostic framework of the Phoenix Consensus may result in different performance for these markers. The international guidelines place greater emphasis on early organ dysfunction and inflammation beyond just neutrophil and platelet counts. This could potentially affect the relative weight and diagnostic thresholds of NLR and PLR in different clinical settings.
Despite the promising results of NLR and PLR, recent advancements in sepsis diagnostics have introduced a variety of novel biomarkers that could complement or even enhance these traditional indicators. New screening tools such as Presepsin (sCD14-ST), PTX3, nCD64, and Monocyte Distribution Width (MDW) have shown potential in improving sepsis detection (18–21). Among these, the LIP score, which incorporates biomarkers like lymphocyte count, INR, and PCT, is particularly noteworthy. Although the LIP score has shown promise in adult populations, its application to neonatal sepsis remains to be validated (22). The inclusion of these novel biomarkers alongside traditional ones could provide a more comprehensive diagnostic approach, offering both sensitivity and specificity in neonatal sepsis diagnosis (23). Future studies comparing the diagnostic performance of the LIP score and other emerging tools with the markers explored in our study would be beneficial in refining neonatal sepsis diagnostic protocols and improving clinical decision-making.
In conclusion, our study provides novel insights into the diagnostic value of NLR and PLR in neonatal LOS. These markers, when combined with traditional inflammatory markers like CRP and PCT, offer a promising approach to early and accurate diagnosis, which is critical for improving clinical outcomes. Our findings specifically address neonatal LOS, an area with less extensive research compared to early-onset sepsis. We believe that the integrated use of NLR, PLR, and traditional markers enhances diagnostic efficacy and may ultimately lead to more targeted interventions for neonates with suspected sepsis. As the field of sepsis diagnostics continues to evolve, future research will be essential to explore the role of emerging biomarkers and refine diagnostic protocols for neonatal sepsis.
Despite the promising results, this study has several limitations. Firstly, it was a single-center, retrospective study, which may limit the generalizability of the findings to broader populations. Future multi-center, prospective studies with larger sample sizes are needed to validate these results and assess their applicability across different neonatal care settings. Secondly, while we focused on a range of biomarkers, there may be other inflammatory markers or biomarkers that could further enhance diagnostic accuracy but were not included in this study. Additionally, the lack of follow-up data on long-term outcomes in neonates with LOS means that we could not evaluate the prognostic value of these markers in predicting clinical outcomes such as survival rates or neurological development. Lastly, although NLR and PLR were identified as independent risk factors for LOS, the underlying biological mechanisms contributing to these changes were not fully explored, and further research into the pathophysiological role of these markers is warranted.
This study highlights the significant diagnostic value of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in the early detection of neonatal late-onset sepsis (LOS). The ROC analysis revealed that NLR had an AUC of 0.903, with a sensitivity of 89.4% and specificity of 81.0%, while PLR demonstrated an AUC of 0.833, with a sensitivity of 92.3% and specificity of 68.0%. These results underscore the potential of NLR and PLR as reliable biomarkers for LOS diagnosis. Additionally, the combination of NLR, PLR, and CRP further improved diagnostic performance, yielding an AUC of 0.942. This combination may enhance clinical decision-making in identifying neonatal LOS at an early stage.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by Lianyungang Clinical Medical College of Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
QY: Conceptualization, Data curation, Investigation, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LS: Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. QZ: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. WX: Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1483522/full#supplementary-material
NS, NS; LOS, late-onset sepsis; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; CPR, C-reactive protein; PCT, procalcitonin; PNR, platelet-to-neutrophil ratio.
1. Flannery DD, Puopolo KM. Neonatal early-onset sepsis. Neoreviews. (2022) 23(11):756–70. doi: 10.1542/neo.23-10-e756
2. Fleiss N, Schwabenbauer K, Randis TM, Polin RA. What’s new in the management of neonatal early-onset sepsis? Arch Dis Child Fetal Neonatal Ed. (2023) 108(1):10–4. doi: 10.1136/archdischild-2021-323532
3. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and NS: a systematic review. Lancet Respir Med. (2018) 6(3):223–30. doi: 10.1016/S2213-2600(18)30063-8
4. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. (2016) 193(3):259–72. doi: 10.1164/rccm.201504-0781OC
5. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
6. Hauck W, Samlalsingh-Parker J, Glibetic M, Ricard G, Beaudoin MC, Noya FJ, et al. Deregulation of cyclooxygenase and nitric oxide synthase gene expression in the inflammatory cascade triggered by experimental group B streptococcal meningitis in the newborn brain and cerebral microvessels. Semin Perinatol. (1999) 23(3):250–60. doi: 10.1016/S0146-0005(99)80070-6
7. Ling Y, Ning J, Xu Y. Explore the predictive value of peripheral blood cell parameters in refractory Mycoplasma pneumoniae pneumonia in children over 6 years old. Front Pediatr. (2021) 9:659677. doi: 10.3389/fped.2021.659677
8. Kalayci T, Kartal M, Significance of NLR. PLR, serum albumin and prognostic nutritional index as predictors of morbidity in super-elderly patients operated on for acute appendicitis. Eur Rev Med Pharmacol Sci. (2022) 26(3):820–7. doi: 10.26355/eurrev_202202_27990 35179748
9. Kriplani A, Pandit S, Chawla A, de la Rosette J, Laguna P, Jayadeva Reddy S, et al. Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis. (2022) 50(3):341–8. doi: 10.1007/s00240-022-01319-0
10. Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect. (2019) 78(5):339–48. doi: 10.1016/j.jinf.2019.02.006
11. Tamelytė E, Vaičekauskienė G, Dagys A, Lapinskas T, Jankauskaitė L. Early blood biomarkers to improve sepsis/bacteremia diagnostics in pediatric emergency settings. Medicina (Kaunas). (2019) 55(4):99. doi: 10.3390/medicina55040099
12. Li XM, Ma L, Yang YB, Shi ZJ, Zhou SS. Prognostic factors of fulminant hepatitis in pregnancy. Chin Med J (Engl). (2005) 118(20):1754–7.16313765
13. Yang Y, Deng L, Li X, Shi Z, Jiang P, Chen D, et al. Analysis of prognosis-associated factors in fulminant viral hepatitis during pregnancy in China. Int J Gynaecol Obstet. (2011) 114(3):242–5. doi: 10.1016/j.ijgo.2011.03.017
14. Elbaset MA, Zahran MH, Hashem A, Ghobrial FK, Elrefaie E, Badawy M, et al. Could platelet to leucocytic count ratio (PLR) predict sepsis and clinical outcomes in patients with emphysematous pyelonephritis? J Infect Chemother. (2019) 25(10):791–6. doi: 10.1016/j.jiac.2019.04.008
15. Taşkın A, Can E, Hamilçıkan Ş. Suspected or proven early-onset sepsis and NLR, PLR, and MPV parameters in neonates with born through MSAF. Am J Perinatol. (2022) 39(6):609–15. doi: 10.1055/s-0040-1718369
16. Zhang S, Zhang W, Luan X, Jin Z. Diagnostic value of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in premature rupture of membranes complicated by sepsis. J Coll Physicians Surg Pak. (2022) 32(5):602–5. doi: 10.29271/jcpsp.2022.05.602
17. Tosson AMS, Koptan D, Abdel Aal R, Abd Elhady M. Evaluation of serum and salivary C-reactive protein for diagnosis of late-onset NS: a single center cross-sectional study. J Pediatr (Rio J). (2021) 97(6):623–8. doi: 10.1016/j.jped.2021.01.004
18. Lee S, Song J, Park DW, Seok H, Ahn S, Kim J, et al. Diagnostic and prognostic value of presepsin and procalcitonin in non-infectious organ failure, sepsis, and septic shock: a prospective observational study according to the sepsis-3 definitions. BMC Infect Dis. (2022) 22(1):8. doi: 10.1186/s12879-021-07012-8
19. Hamed S, Behnes M, Pauly D, Lepiorz D, Barre M, Becher T, et al. Diagnostic value of pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis. (2017) 17(1):554. doi: 10.1186/s12879-017-2606-3
20. Cong S, Ma T, Di X, Tian C, Zhao M, Wang K. Diagnostic value of neutrophil CD64, procalcitonin, and interleukin-6 in sepsis: a meta-analysis. BMC Infect Dis. (2021) 21(1):384. doi: 10.1186/s12879-021-06064-0
21. Hausfater P, Robert Boter N, Morales Indiano C, Cancella de Abreu M, Marin AM, Pernet J, et al. Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit Care. (2021) 25(1):227. doi: 10.1186/s13054-021-03622-5
22. Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. (2019) 20(21):5376. doi: 10.3390/ijms20215376
Keywords: neonatal, diagnostic, LOS, NLR, PLR
Citation: Yin Q, Yin J, Shen L, Zhou Q and Xu W (2025) The early diagnostic value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in neonatal late-onset sepsis. Front. Pediatr. 13:1483522. doi: 10.3389/fped.2025.1483522
Received: 20 August 2024; Accepted: 24 February 2025;
Published: 7 March 2025.
Edited by:
Zhongjie Shi, Wayne State University, United StatesReviewed by:
Rinawati Rohsiswatmo, RSUPN Dr. Cipto Mangunkusumo, IndonesiaCopyright: © 2025 Yin, Yin, Shen, Zhou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: WeiDong Xu, NDQ0ODAzMDQ1QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.