
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 21 February 2025
Sec. Pediatric Surgery
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1465278
This article is part of the Research TopicRecent Advances in Our Understanding of NEC Pathogenesis, Diagnosis, and Treatment - Volume IIView all 9 articles
Background: Early identification of infants with necrotizing enterocolitis (NEC) at risk of surgery is essential for an effective treatment. This study aims to clarify the risk factors of surgical NEC and establish a prediction model by machine learning algorithm.
Methods: Infants with NEC were split into two groups based on whether they had surgery or not. Clinical data was collected and compared between the groups. Variables were analyzed with one-way logistic regression and predictive models were built using logistic regression and CatBoost algorithm. The models were evaluated and compared using Receiver Operating Characteristic (ROC) curves and feature importance. Feature importance was ranked using the SHapley Additive exPlanation method and model optimization was performed using feature culling. Final model was selected and a user-friendly GUI software was created for clinical use.
Results: The Catboost model performed better than the logistic regression model in terms of discriminative power. An interpretable final model with 14 features was built after the features were reduced according to the feature importance level. The final model accurately identified Surgicel NEC in the internal validation (AUC = 0.905) and was translated into a convenient tool to facilitate its use in clinical settings.
Conclusions: Catboost machine learning model related to infants with surgical NEC was successfully developed. A GUI interface was developed to assist clinicians in accurately identifying children who would benefit from surgery.
Necrotizing enterocolitis (NEC) is an acute critical bowel disease commonly occurring in preterm infants. The incidence of NEC in preterm and very low birth weight neonates ranges from 2% to 13%, with an average mortality rate of 20%–30% (1, 2). The survival rate of preterm infants has significantly increased with the improvement of perinatal medicine and neonatal critical care. The incidence of NEC has also increased, and has become one of the diseases that seriously threaten the life and health of preterm newborn. However, there is a lack of specific treatment for this disease. The conservative medical treatment is the mainstay, and some severe cases require surgical treatment, but the mortality rate of children treated with surgery is as high as 50% (3).
Notably, infants requiring surgical intervention often present with more advanced and severe disease, which significantly contributes to their elevated mortality rates, poorer long-term prognosis, and diminished quality of life compared to those managed with conservative medical care (4, 5). This represents a substantial clinical and emotional burden for both the patients and their families (6). The etiological hypothesis of NEC is still incomplete, and its pathogenesis is highly complex and multifactorial. It is currently associated with an excessive inflammatory response and necrosis of the intestinal tissue due to multiple perinatal risk factors that act on the immature intestinal tract of preterm infants, including perinatal asphyxia, antibiotic use, no breastfeeding, infections, and abnormal fixation of the intestinal flora (3, 7). Therefore, the early identification of this disease and the need for surgery are essential, as they enable timely intervention in patients with severe disease, reduce surgical risk in patients with NEC, and improve the overall prognosis.
Currently, most of the studies focus on risk prediction models for the occurrence and severity of NEC, and only few studies are available on the models for the early prediction of the need of surgery in NEC patients. Moreover, the existing prediction models are heterogeneous, the indicators included are not comprehensive enough, and the clinical operability and accuracy are not good. The rapid development of precision medicine has allowed the use of machine learning methods featuring deep analysis in the construction of clinical prediction models, but few reports are available on the construction of models to predict whether NEC patients need surgery. Furthermore, the existing models show limitations, mainly represented by low predictive efficacy, poor operability, and insufficiently comprehensive incorporation of metrics (8–10).
Therefore, in this work, more comprehensive perinatal clinical data, laboratory tests and imaging examinations were included according to the pathophysiological mechanism of NEC, which were analyzed using machine algorithms. A scientific risk prediction model was then constructed by selecting the risk variables with the greatest predictive efficacy according to the importance of the characteristics and clinical operability. Thus, a theoretical foundation was provided to further improve the clinical identification of high-risk NEC, ensure timely treatment, and prevent further deterioration of the disease for further improving the long-term quality of life of children.
Clinical data of all children with NEC admitted to the neonatal unit of Children's Hospital of Soochow University from 1 January 2012 to 31 December 2021 were retrospectively analyzed, and the study was pre-approved by the institutional review board. The inclusion criteria for this study were the modified Bell staging ≥ stage II. The exclusion criteria were the following: children with immunodeficiencies, inherited metabolic disorders, congenital malformations of the intestine, and patients with pneumoperitoneum detected during preoperative examination, regardless of the presence of NEC. Additionally, cases of infants transferred from other hospitals who underwent a change in treatment from conservative to surgical without adequate clinical data were excluded. Enrollment in all cases was independently reviewed by two experts, with a third expert consulted in case of disagreements. All clinical and demographic data utilized in this study were retrospectively extracted from medical records. These data encompass patient characteristics and clinical parameters recorded at the time of NEC diagnosis, as well as subsequent clinical deterioration or preoperative status prior to surgical intervention. The study was approved by the Ethics Committee of the Children's Hospital of Soochow University (2023CS189; Suzhou, China), informed consent was waived by the ethics committee. The study was adhered to the tenets of the Declaration of Helsinki.
Variables including general data, maternal prenatal general data, underlying disease and comorbidities, treatment before diagnosis of NEC, laboratory testing and imaging, nutritional management before NEC diagnosis were collected and analyzed. Cases with more than 50% missing data for any variable were excluded from the analysis. Additionally, variables with more than 50% missing values were also excluded. For the remaining missing values, mean imputation was applied for continuous variables, and mode imputation was used for categorical variables.
Certain indicators, such as WBC, PLT, lymphocyte count, CRP, and procalcitonin, were categorized due to their clear normal ranges in neonates on the first day of life, facilitating the detection of abnormalities. In contrast, variables like hemoglobin and albumin were kept as continuous data to better capture subtle changes and their correlation with disease progression. This approach also allows the CatBoost algorithm to model nonlinear relationships more effectively, thereby improving the model's predictive accuracy.
Binary labels were constructed based on the dataset after dividing the population into two groups: conservative treatment NEC and surgical NEC using the revised Bell staging to define the different treatment modalities in the model. The conservative group involved conservative management, which included cessation of feeding, parenteral nutrition, and empirical antibiotic therapy. Surgical indications were defined by radiographic evidence of significant bowel perforation (pneumoperitoneum) or, in the absence of imaging findings, clinical deterioration despite prolonged conservative management. Infants who died in the conservative group were classified into the surgical group to mitigate potential bias, as these infants were likely candidates for surgery but were unable to receive it in a timely manner due to the progression of their condition. Surgical intervention was defined as including abdominal drainage and exploratory laparotomy. If the disease outcome was in the surgical group, the label was 1, otherwise, the label was 0. This binary labelling approach was used for both the training set and the validation set.
The goal of this study was to accurately predict whether a child would progress to NEC requiring surgery based on the clinical data (clinical indicators, test and examination results). The outcome was a binary categorical problem in which the target variable was the binary short-term progression of NEC. The explanatory variables were the parameters included in the measurement. Prediction was performed using two machine learning algorithms, including LR and CatBoost. The LR and Catboost models were implemented using Python packages.
The dataset was randomly divided into two subsets: 70% of the data was allocated for training the models, while the remaining 30% was reserved for the validation cohort. This division allowed for internal validation and ensured that model evaluation was conducted on data not seen during training, thus minimizing the risk of overfitting.
Model performance was assessed exclusively on the validation cohort to evaluate the models' generalizability to unseen data. Key performance metrics included the area under the ROC curve and its corresponding 95% confidence intervals. The AUC score was used to quantify the models' ability to distinguish between infants requiring surgical vs. conservative treatment for NEC. Additionally, other performance measures, such as sensitivity, specificity, and accuracy, were calculated to provide a comprehensive evaluation of the models' predictive capabilities.
A modern tool for interpretable AI called SHapley Additive exPlanations (SHAP) was used to determine the most important factors and their contribution to the prediction. This so-called SHAP value enabled the interpretation of the results of a machine learning model using game theoretic concepts: the Shapley value. The SHAP value quantified the marginal contribution of each feature to the final prediction. This study used the CatBoost machine learning algorithm for the final model, which was interpreted using the SHAP value.
The Graphics User Interface (GUI) was developed using the Python-based Tkinter module.
Statistical analysis was performed using Python version 3.6.5 and SPSS 26.0 software. Count data were expressed as cases and percentages (%), and the comparison between the two groups was made using the χ2 test or Fisher's exact probability method. Measurement information was expressed as median (interquartile spacing) [M (P25, P75)], and comparison between groups was performed using the Mann–Whitney U test. Covariance was assessed on the results of univariate logistic regression analysis (all tolerances ≥ 0.1 and VIF values ≤ 10). Variables with statistically significant difference in univariate logistic regression analysis were included in logistic regression analysis and Catboost regression analysis. Predictive efficacy was evaluated using the AUC, and the optimal threshold was established by maximizing the Youden index (sensitivity + specificity − 1). A two-tailed value of P < 0.05 was considered statistically significant.
This study involved 449 NEC cases in the cohort, all Bell stage II or higher, for predictive modelling. Thirty-five children with pneumoperitoneum on preoperative examination, thirty-one children with more than 50% missing case records, three children with congenital intestinal malformations (congenital megacolonopathy, intestinal atresia, and intestinal malrotation), and one child with spontaneous intestinal perforation were excluded during the study period. A total of 379 children were finally enrolled in this study, with 142 receiving surgical treatment (including 39 infants who died in the conservative treatment group) and 237 receiving conservative treatment. Details of the study design are shown in Figure 1. These children were randomly assigned to either the training or validation set (7:3). The clinical characteristics of the two groups of patients are listed in Table 1.
Among the general data, the gestational age and birth weight of NEC infants treated with surgery were significantly lower than those of NEC infants treated with conservative treatment. Specifically, the gestational age in the conservative treatment group ranged from 25.14 to 41.14 weeks (median: 31.57 weeks), while in the surgery group, it ranged from 27.00 to 41.71 weeks (median: 30.86 weeks). Regarding birth weight, the conservative treatment group showed a range from 750 g to 5,000 g (median: 2,000 g), while the surgery group ranged from 600 g to 4,500 g (median: 1,500 g). The proportion of preterm infants in the surgical group was higher than that in the conservative group. As regards the maternal prenatal general data, the proportion of cesarean delivery, antenatal corticosteroids treatment, and premature rupture of membranes ≥18 h in the surgery group were higher than those in the conservative group. As regards the underlying diseases or comorbidities before the diagnosis of NEC and treatment measures, the surgical treatment group had significantly higher proportion including frequent sleep apnea, type II respiratory failure, hyponatremia, anemia, use of drugs to close PDA, NRDS, sepsis, pulmonary alveolar surfactant, aminophylline, caffeine, and vasoactive drugs compared with the conservative group. As regards the laboratory results of blood test, the surgical treatment group had significantly higher parameters including WBC < 5 × 109/L, PLT < 100 × 109/L, lymphocyte count < 2.0 × 109/L, CRP ≥ 20 mg/L, and PH < 7.25 compared with the conservative group. And the surgical treatment group had significantly lower parameters including hemoglobin, albumin, and base excess compared with the conservative group. The results of abdominal x-ray examination showed that patients with intestinal wall thickening and portal vein gas in the surgery group were more than those in the conservative group. In terms of nutritional management before NEC diagnosis, The surgical group had significantly fewer cases of initiating breast feeding before the onset of NEC and fewer cases of feeding speed > 25 ml/(kg·day) compared to the medical treatment group.
A significant difference in the following variables between the two groups was found by univariate analysis: birth weight, gestational age, premature infants, cesarean delivery, antenatal corticosteroids treatment, premature rupture of membranes >18 h, frequent sleep apnea, type II respiratory failure, hyponatremia, anemia, use of drugs to close PDA, NRDS, sepsis, alveolar surfactant, aminophylline, caffeine, vasoactive drugs, WBC < 5 × 109/L, hemoglobin, PLT < 100 × 109/L, lymphocyte count < 2 × 109/L, albumin, CRP ≥ 20, PH < 7.25, base excess, intestinal wall thickening, portal vein gas, breastfeeding initiation, and feeding speed > 25 ml/(kg·day). The Collinearity test was performed on the results of univariate analysis, and the results showed that VIF values were less than 10, suggesting no significant collinearity between the variables.
The Catboost model (AUC = 0.910) predicted NEC surgery better than Logistic regression (AUC = 0.848). The discriminative performance of these 2 models is shown in Figure 2, where the best cut-off value with the largest Youden index was taken to calculate the sensitivity, specificity, precision and accuracy. The sensitivity of Catboost and LR were 61.5% and 64.1%, the specificity was 82.6% and 82.5%, the precision was 0.857 and 0.735, and the accuracy was 0.833 and 0.798, respectively. The above results demonstrated that the Catboost model performed better in the prediction of surgical intervention in NEC between the above 2 models.
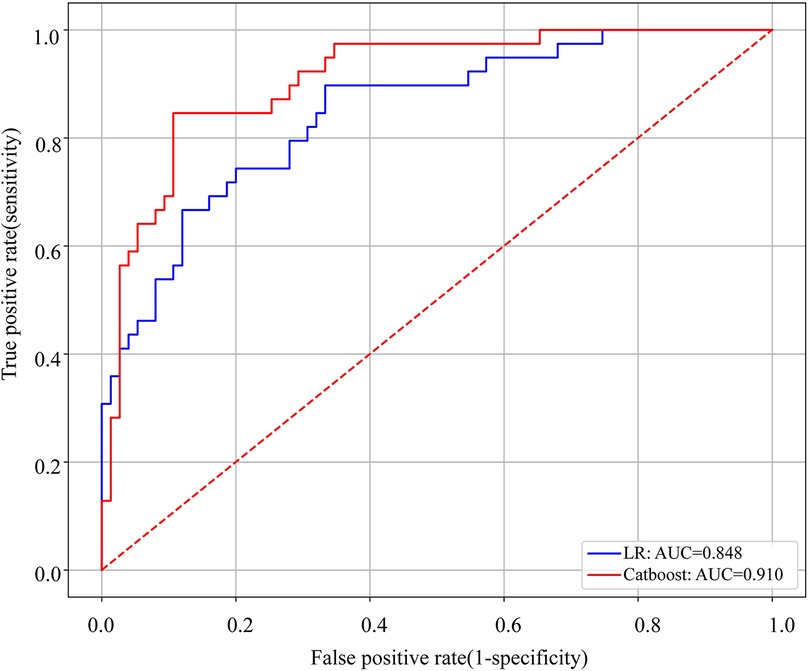
Figure 2. ROC curves of the Catboost and LR models to predict surgical NEC. AUC, area under the curve.
The final model was identified during the feature dimension reduction of the Catboost model. The 29 feature models were significantly better than the 10 feature models (AUC = 0.872) and 15 feature models (AUC = 0.902) in predicting NEC surgical intervention, but not significantly better than the 14 feature models (AUC = 0.905) in Figure 3. The 14 feature models have better net benefits and higher threshold probabilities compared with the 29 feature models. Therefore, our attention focused on the characteristics of the 14 Catboost model. These included base excess, WBC < 5 × 109/L, vasoactive drugs before NEC, gestational age, serum albumin, sepsis, birth weight, PH < 7.25, hemoglobin, portal vein gas, feeding speed > 25 ml/(kg day), type-2 respiratory failure, use of drugs to close PDA before NEC, and frequent sleep apnea, which were used as the final model for further analysis. The AUC, specificity, sensitivity, precision, and F1 score of the Catboost model for predicting NEC were 0.905, 0.833, 0.641, 0.858 and 0.734, respectively.
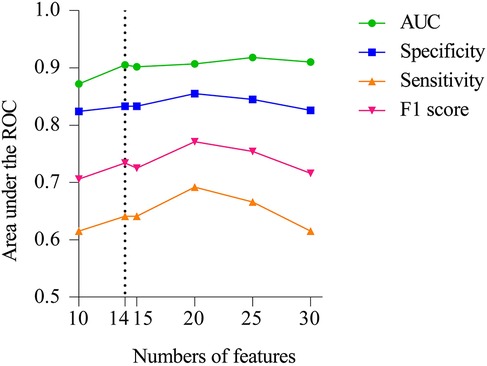
Figure 3. Performance of Catboost model to predict surgical NEC. AUC, sensitivity, specificity, and F1 score of the model with varied numbers of features are showed.
The output results of the final model were interpreted with the help of the SHAP method, and the average SHAP value was used to reflect the contribution of the features to the model. The influence of individual features on the model output and their importance ranking are shown in descending order in Figures 4A,B. Dot is made for SHAP value in the model for each infant, the colors of the dots demonstrate the actual values of the features for each one, as red means a higher feature value and blue means a lower feature value. The interpretation of the predictions using SHAP values are shown in Figure 4C. Red lines indicates features that drove a higher prediction probability of NEC surgery, and blue lines indicates features that drove a lower prediction probability. In addition, the length of the lever was proportional to the corresponding factors to predict the degree of contribution.
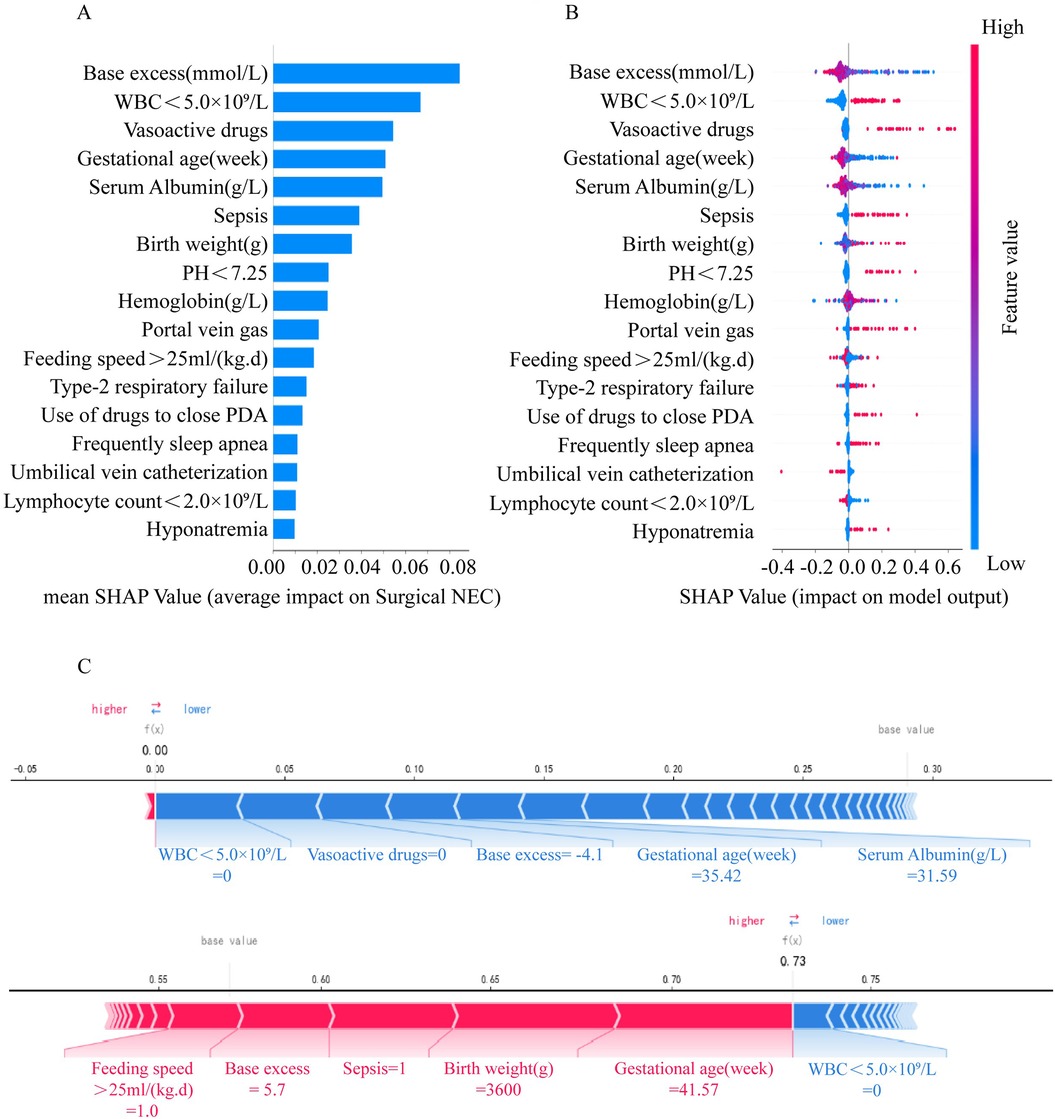
Figure 4. Interpretation of the effect of each feature on surgical NEC through SHAP values in the Catboost model. (A) SHAP summary bar plot. (B) SHAP summary dot plot. (C) SHAP force plot.
A Graphics User Interface (GUI) software was developed to facilitate clinicians to use the Catboost model. The GUI software screenshots are shown in Figure 5. The GUI software can be downloaded from https://nec-models.oss-cn-shanghai.aliyuncs.com/surgicalNECpredicted.exe and https://nec-models.oss-cn-shanghai.aliyuncs.com/model.pkl (please ensure that both files are placed in the same folder for the software to function properly). Clinicians or other users only need to input 14 feature values to directly obtain the prediction results to understand whether a child with NEC needs surgical intervention. This is beneficial to the choice of surgical timing and early intervention to improve the prognosis.
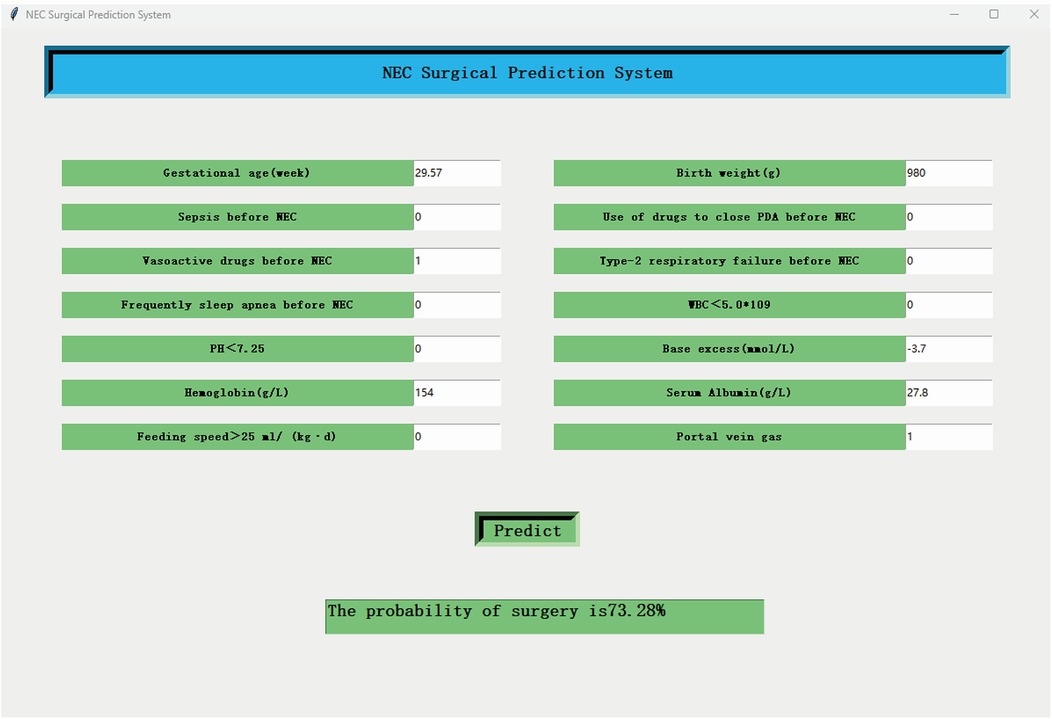
Figure 5. The screenshot of the GUI software. The final Catboost model with 14 features can be applied to surgical NEC prediction.
There is a lack of specific treatment for NEC, and 20%–40% of the children with NEC require surgery. The mortality rate of children who underwent surgery is 50%, which is much higher than that of patients who underwent conservative treatment with internal medicine. In this study, 27.62% of children with NEC underwent surgery, and the postoperative mortality rate was 23.15%, which was significantly higher than that of the conservative group. Patients with NEC who underwent surgery, were more likely to have growth retardation and neurodevelopmental disorders (11). Early identification of NEC patients who may progress to surgery and timely intervention could reduce the percentage of surgical patients and minimize the risk of the disease to preterm infants. Machine algorithmic models minimized the covariate multicollinearity limitation and have greater advantages in clinical decision-making and early prediction compared with traditional logistic regression (12, 13). Several studies have explored machine learning models for predicting necrotizing enterocolitis (NEC); however, models specifically designed for predicting the need for surgery in NEC are relatively scarce. A study on deep learning using abdominal x-rays in NEC patients demonstrated that a ResNet18-based model could achieve an accuracy of 0.919 in diagnosing surgical NEC, highlighting its potential in optimizing surgical decision-making (14). This study undoubtedly reinforces the significance of abdominal x-ray in the assessment of NEC and surgical NEC. However, it is well recognized that the diagnosis of NEC and the decision regarding surgical intervention require a comprehensive clinical judgment from physicians, taking into account not only radiographic findings but also the overall clinical condition of the infant, physical examination, and laboratory results.
This study, identified 29 risk factors including perinatal period as risk factors for surgery in children with NEC through multifactorial analysis, but the covariance between variables was poor. The identification of the final model was carried out in the feature dimensionality reduction process of the Catboost model, and 14 risk factors were finally screened to construct the Catboost final model, including birth weight, gestational age, frequent sleep apnea, type II respiratory failure, use of drugs to close PDA, NRDS, sepsis, vasoactive drugs, WBC < 5 × 109/L, hemoglobin, serum albumin, PH < 7.25, base excess, intestinal wall thickening, and portal vein gas. The Catboost model had better predictive efficacy for surgery in NEC patients than traditional multifactorial logistic regression analysis.
Previous studies showed that gestational age and birth weight are important risk factors for the occurrence of NEC and whether or not NEC patients are subjected to surgery (15, 16). It is well known that preterm infants with low birth weight have immature intestinal development and NEC is an ischemic and hypoxic injury of the intestinal mucosa due to multiple causes acting on the immature intestinal barrier (17). In addition, preterm birth and low birth weight may lead to reduced diversity of gut flora, and abnormal flora fixation (18). In this study, the NEC surgery group had a smaller gestational age and birth weight, which again confirmed that they were the key to understand whether or not a patient with NEC needed surgery.
Infection induces an excessive inflammatory response in the immature gut of preterm infants and plays an important role in the development of NEC. In this study, perinatal infection factors and related indicators (sepsis and WBC < 5 × 109/L) were significantly higher in the NEC surgery group than in the conservative group. Bacterial circulatory translocation and associated signal transduction resulting from infection leads to increased intestinal mucosal damage and decreased perfusion, further leading to intestinal tissue necrosis (19, 20).
Hypoxia is likewise another important risk factor in the occurrence and severity of NEC. In neonates, especially preterm infants, with lower intestinal vascular resistance and poor ability to respond to and regulate systemic circulatory perturbations, redistribution of blood flow is more pronounced in a hypoxic-anoxic environment, further increasing the susceptibility of the intestinal mucosa to hypoxic-ischemic injury (21, 22). In this study, the surgery group had a higher rate of type-2 respiratory failure, frequent sleep apnea, PH < 7.25, use of vasoactive drugs, use of drugs to close PDA and lower hemoglobin and base excess levels.
Nutritional information and related indicators of patients were also included in this study, and nutritional factors were found to be involved in the progression of NEC, with serum albumin included in the Catboost optimal model. Albumin reflects the nutritional and immune status of the body to a certain extent, and low albumin levels often indicate poor nutritional and immune status (23, 24). Sharif et al. found that serum albumin ≤ 20 g/L on the day after the diagnosis of NEC was a good predictor of the need of surgery (25). Serum albumin after the onset of NEC was not measured in this study but the results showed that lower serum albumin levels in the early postnatal period could be associated with surgery in patients with NEC, suggesting that surgically treated patients have poorer nutritional and immune status in the early stages of life. In this study, the feeding speed >25 ml/(kg·day) was less common in the surgical group compared to the medical group before the onset of NEC. This finding likely reflects the underlying vulnerability of surgical group patients, such as a higher proportion of preterm infants, poorer baseline nutritional status, or higher risk factors for NEC. The lower feeding speed might have been a clinical precaution to minimize gastrointestinal stress in these high-risk infants. Although this observation seems to diverge from standard feeding practices, it underscores the importance of individualized feeding strategies in the management of high-risk infants.
The present model incorporated more comprehensive perinatal factors, including prenatal and postnatal clinical data, laboratory tests, and imaging data than other ML-based surgical prediction models for NEC. The risk factors included in the optimal model included baseline characteristics of preterm infants, infection, hypoxia, nutrition, and imaging, and the ROC value of the model was 0.905, which was significantly higher than that of the existing ML-based NEC surgical prediction models (8–10). In addition, a simpler GUI software was developed to improve the clinical tractability of the predictive model, enabling the early clinical prediction of performing or not the surgery in patients with NEC based on identified characteristic factors.
Despite the encouraging results, this study has some limitations. Firstly, this was a single-center study with a limited sample size and a long-time frame, which may have biased the results. In addition, although our model showed a good predictive efficacy, it has not been externally validated by recruiting patients from other clinical centers, and a prospective multicenter cohort study should be performed to optimize the model and validate it.
In conclusion, our work based on a decade-long retrospective study of NEC in Suzhou, China, revealed that 37.47% of children with NEC in our clinical center were subjected to surgery. In addition, 14 key characteristics associated with surgery of NEC patients were considered and a CatBoost model and simple GUI software were developed to predict whether a patient with NEC requires surgical treatment better than other existing prediction methods.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Medical Ethics Committee of the Children's Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was waived by the ethics committee.
XJ: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft. WS: Conceptualization, Methodology, Writing – original draft. YL: Data curation, Formal Analysis, Writing – original draft. YS: Data curation, Writing – original draft. LX: Formal Analysis, Funding acquisition, Investigation, Writing – original draft. XZ: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the General Program of the National Natural Science Foundation of China (82271741); Suzhou Gusu Health Talent Plan Project (GSWS2022055); Key Medical Research Project of Jiangsu Provincial Health Commission (ZD2021013); Clinical Transformation of Scientific and Technological High-end Platform and Base Construction Project (ML13101523); Suiyuan Clinical Research Project of Children's Hospital of Soochow University (SY003) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_3341).
We thank all the children and their guardians who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13(10):590–600. doi: 10.1038/nrgastro.2016.119
2. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20(1):344. doi: 10.1186/s12887-020-02231-5
3. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364(3):255–64. doi: 10.1056/NEJMra1005408
4. Kum VTL, Chung PHY, Wong KKY. Quality of life in long-term survivors of surgical necrotizing enterocolitis. J Pediatr Surg. (2024) 59(4):553–6. doi: 10.1016/j.jpedsurg.2023.11.023
5. Garg PM, Lett K, Ansari MA, Pittman I, Riddick R, Varshney N, et al. Postoperative outcomes, and growth and brain injury outcomes in spontaneous intestinal perforation vs surgical necrotizing enterocolitis in preterm infants. Indian Pediatr. (2023) 60(11):922–6. doi: 10.1007/s13312-023-3037-4
6. Canvasser J, Patel RM, Pryor E, Green L, Hintz SR, Fagan M, et al. Long-term outcomes and life-impacts of necrotizing enterocolitis: a survey of survivors and parents. Semin Perinatol. (2023) 47(1):151696. doi: 10.1016/j.semperi.2022.151696
7. Kim W, Seo JM. Necrotizing enterocolitis. N Engl J Med. (2020) 383(25):2461–2461. doi: 10.1056/NEJMicm2020782
8. Kim SH, Oh YJ, Son J, Jung D, Kim D, Ryu SR, et al. Machine learning-based analysis for prediction of surgical necrotizing enterocolitis in very low birth weight infants using perinatal factors: a nationwide cohort study. Eur J Pediatr. (2024) 183(6):2743–51. doi: 10.1007/s00431-024-05505-7
9. Qi G, Huang S, Lai D, Li J, Zhao Y, Shen C, et al. An improved joint non-negative matrix factorization for identifying surgical treatment timing of neonatal necrotizing enterocolitis. Bosn J Basic Med Sci. (2022) 22(6):972–81. doi: 10.17305/bjbms.2022.7046
10. Song J, Li Z, Yao G, Wei S, Li L, Wu H. Framework for feature selection of predicting the diagnosis and prognosis of necrotizing enterocolitis. Kakulapati V, ed. PLoS One. (2022) 17(8):e0273383. doi: 10.1371/journal.pone.0273383
11. Okten EI, Frankl M, Wu S, Gamaty H, Thompson H, Yardley IE. Factors affecting neurodevelopmental outcome following surgical necrotising enterocolitis: a systematic review. Pediatr Surg Int. (2024) 40(1):71. doi: 10.1007/s00383-024-05651-x
12. Deng YH, Luo XQ, Yan P, Zhang NY, Liu Y, Duan SB. Outcome prediction for acute kidney injury among hospitalized children via eXtreme gradient boosting algorithm. Sci Rep. (2022) 12(1):8956. doi: 10.1038/s41598-022-13152-x
13. Ren Y, Loftus TJ, Datta S, Ruppert MM, Guan Z, Miao S, et al. Performance of a machine learning algorithm using electronic health record data to predict postoperative complications and report on a mobile platform. JAMA Netw Open. (2022) 5(5):e2211973. doi: 10.1001/jamanetworkopen.2022.11973
14. Wu Z, Zhuo R, Liu X, Wu B, Wang J. Enhancing surgical decision-making in NEC with ResNet18: a deep learning approach to predict the need for surgery through x-ray image analysis. Front Pediatr. (2024) 12:1405780. doi: 10.3389/fped.2024.1405780
15. Samuels N, Van De Graaf RA, De Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. (2017) 17(1):105. doi: 10.1186/s12887-017-0847-3
16. Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. (2003) 23(4):278–85. doi: 10.1038/sj.jp.7210892
17. Eaton S. Necrotizing enterocolitis symposium: epidemiology and early diagnosis. J Pediatr Surg. (2017) 52(2):223–5. doi: 10.1016/j.jpedsurg.2016.11.013
18. Korpela K, Blakstad EW, Moltu SJ, Strømmen K, Nakstad B, Rønnestad AE, et al. Intestinal microbiota development and gestational age in preterm neonates. Sci Rep. (2018) 8(1):2453. doi: 10.1038/s41598-018-20827-x
19. Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol. (2013) 190(7):3541–51. doi: 10.4049/jimmunol.1202264
20. Hackam DJ, Sodhi CP. Toll-like receptor–mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol. (2018) 6(2):229–238.e1. doi: 10.1016/j.jcmgh.2018.04.001
21. Wagner C, Torow N, Hornef MW, Lelouard H. Spatial and temporal key steps in early-life intestinal immune system development and education. FEBS J. (2022) 289(16):4731–57. doi: 10.1111/febs.16047
22. Ree IM, Smits-Wintjens VE, Rijntjes-Jacobs EG, Pelsma IC, Steggerda SJ, Walther FJ, et al. Necrotizing enterocolitis in small-for-gestational-age neonates: a matched case-control study. Neonatology. (2014) 105(1):74–8. doi: 10.1159/000356033
23. Aubin É, Roberge C, Lemieux R, Bazin R. Immunomodulatory effects of therapeutic preparations of human albumin. Vox Sang. (2011) 101(2):131–7. doi: 10.1111/j.1423-0410.2011.01475.x
24. Lustig JV, Rieger CHL, Kraft SC, Hunter R, Rothberg RM. Humoral and cellular responses to native antigen following oral and parenteral immunization with lipid-conjugated bovine serum albumin. Cell Immunol. (1976) 24(1):164–72. doi: 10.1016/0008-8749(76)90141-6
Keywords: necrotizing enterocolitis, surgical NEC, risk factors, CatBoost machine learning model, GUI interface
Citation: Jin X, Sun W, Li Y, Su Y, Xu L and Zhu X (2025) Use of CatBoost algorithm to identify the need for surgery in infants with necrotizing enterocolitis. Front. Pediatr. 13:1465278. doi: 10.3389/fped.2025.1465278
Received: 16 July 2024; Accepted: 7 February 2025;
Published: 21 February 2025.
Edited by:
Francesco Morini, Sapienza University of Rome, ItalyReviewed by:
George Bethell, University of Southampton, United KingdomCopyright: © 2025 Jin, Sun, Li, Su, Xu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Zhu, emh1eHVlcGluZzQ2MzdAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.