
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 04 March 2025
Sec. Pediatric Immunology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1440366
This article is part of the Research TopicAntibody-Mediated Maternal and Fetal Conditions of PregnancyView all articles
Background: Currently, tics and Tourette's disorder are burdensome neurological disorders that manifest in vocal and motor tics with onset during childhood. Previous studies have demonstrated that maternal autoimmune diseases may cause several neurodevelopmental disorders in offspring via maternal immune activation. However, the association between them has never been thoroughly researched. Thus, in this study, we aimed to explore whether maternal autoimmune diseases are associated with the risk of tics and Tourette's disorder in offspring in a real-world nationwide population-based cohort study.
Methods: We analyzed offspring with or without autoimmune disease exposure between 2009 and 2016 from national population databases in Taiwan. Multivariate analysis, multiple Cox regression analyses, and stratified analyses were conducted in the study.
Results: In total, 76,411 offspring with autoimmune disease exposure and 1,211,936 offspring without maternal autoimmune disease exposure were selected and analyzed in this study. The incidence of childhood tics and Tourette's disorder was 2.35 [95% confidence interval (CI) 2.23–4.86] and 1.89 (95% CI 1.86–1.92) per 10,000 person-months in children exposed to maternal autoimmune disease and non-exposed children, respectively. The children whose mothers had an autoimmune disease had a 1.26-fold risk of tics and Tourette's disorder compared to children whose mothers did not have an autoimmune disease [crude hazard ratio: 1.26; 95% CI, 1.20–1.34, adjusted hazard ratio (aHR): 1.22; 95% CI, 1.15–1.29]. Offspring of mothers with rheumatoid arthritis (aHR: 1.46, 95% CI, 1.07–1.97), system lupus erythematosus (aHR: 1.57, 95% CI, 1.18–2.09), Sjogren's syndrome (aHR: 1.28, 95% CI, 1.09–1.50), ankylosing spondylitis (aHR: 1.49, 95% CI, 1.07–2.09), Graves’ disease (aHR: 1.26, 95% CI, 1.15–1.37), Hashimoto's thyroiditis (aHR: 1.59, 95% CI, 1.29–1.98), and type I diabetes (aHR: 1.68, 95% CI, 1.13–2.50) had a significantly higher risk of developing tics and Tourette's disorder. Aside from maternal autoimmune diseases, mothers with urinary tract infections, diabetes mellitus, hyperlipidemia, anemia, a sleep disorder, endometriosis, and depression were also associated with childhood tics and Tourette's disorder.
Conclusion: Maternal autoimmune diseases appeared to be associated with tics and Tourette's disorder in offspring, especially in mothers with the abovementioned diseases. Further research is warranted to investigate the possible pathogenetic mechanisms of these associations.
Tics and Tourette's disorder are chronic neurodevelopmental conditions characterized by sudden non-rhythmic motor and vocal tics typically emerging in childhood and persisting for over a year (1). According to the Centers for Disease Control and Prevention (CDC) in the United States, Tourette's disorder affects approximately 0.6% of children, with a three-fold higher prevalence in boys than in girls (2–4). In Taiwan, from 2007 to 2015, the overall incidence of TD increased from 5.34 to 6.87 per 100,000 person-years. Among children and adolescents, the age- and sex-standardized incidence rose from 19.58 to 31.79 per 100,000 person-years, while the prevalence surged from 37.51 per 100,000 people in 2007 to 84.18 per 100,000 in 2015. These trends highlight a growing public health concern (5). Tourette's disorder typically begins between 4 and 10 years of age, significantly affecting children's behavior, academic performance, and social interactions (6, 7). Although hyperkinetic symptoms are mostly ameliorated in adulthood (8), approximately 85% of affected children experience comorbidities such as depression, anxiety, attention deficit hyperactivity disorder (ADHD), autism spectrum disorder, obsessive-compulsive disorder (OCD), and even rage attacks and self-injurious behavior (9). The etiology of Tourette's disorder is multifactorial, including genetic and epigenetic changes, prenatal insults, environmental exposure, emotional stress, and immune-mediated risk factors (10). For example, Tourette's disorder among family members, prenatal emetic drug exposure, assisted reproduction, prenatal or early infections, diet, gut microbiota, sleeping time, exercise, pollution exposure, allergy, and socioeconomic status have been noted as possible risk factors for neurodevelopmental disorders in previous studies (11–16).
Growing evidence suggests that maternal immune activation (MIA) during pregnancy—triggered by infection, autoimmune disease, or dysregulation—may contribute to neurodevelopmental disorders in offspring (17–19). Studies have linked MIA to autism spectrum disorder, ADHD, OCD, schizophrenia, and other behavioral conditions. In addition, maternal autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and antiphospholipid syndrome have been associated with higher rates of psychiatric disorders in offspring (20–25). One exception to this rule is represented by maternal multiple sclerosis which is not associated with a higher risk of neurodevelopmental disorders in offspring, perhaps due to the organ-specific characteristic of this central nerve system (CNS) immune-mediated disease (26). Taken together, most of these studies indicate that maternal autoimmune diseases seem to play an important role in the activation of the immune system, through the placenta or circulation, causing neuroinflammatory processes in the fetus and causing neurodevelopmental or mental problems in childhood.
To manage tics and Tourette's disorder, physicians apply behavioral therapies, medication, acupuncture, and neurosurgery such as deep brain stimulation, but the treatment strategy is complex and the response varies (27–31). Recent research has focused on identifying the risk factors and pathophysiological pathways to develop a treatment strategy. Although inflammation is speculated to play a role, evidence also suggests metabolic dysfunction and dysbiosis during early neurodevelopment may contribute (11, 32–35). However, the exact maternal-fetal pathway remains unclear. A 2016 review established a positive bidirectional association between autoimmune diseases and OCD and tic disorders (36). However, few epidemiological research studies have explored maternal autoimmune diseases as a risk factor (23, 37–39). We hypothesized that maternal autoimmune diseases, through immune-inflammatory activation processes, increase the risk of tics and Tourette's disorder in offspring and conducted a population-based, nationwide cohort study using Taiwan's National Health Insurance Research Database (NHIRD).
We retrieved the family health history for multiple generations and the relationship between children and their birth parents by linking the 2009–2019 NHIRD, Birth Registration Database, Maternal and Child Health Database, and the National Death Index Database (40), which are regulated by the Health and Welfare Data Science Center (HWDC) in Taiwan. The NHIRD contains all outpatient and inpatient medical claims, including drug medications, medical operations, procedures, and fees. The Birth Registration Database contains birth weight, gestational weeks, delivery type, live birth, stillbirth, multiple births, and nationality of the mother. The Maternal and Child Health Database contains the parent's and child's de-identified numbers. By linking these databases, we could trace each mother's comorbidities and medications during pregnancy. Our study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (approval no. CS2-21006).
This study was a retrospective cohort study design. We retrieved individuals from the birth certificate application database from 2009 to 2016. The following were excluded: mother's identification missing, nationality missing, foreign nationality, multiple births, and stillbirth. The exposure group was offspring with autoimmune disease exposure. The definition was a diagnosis of autoimmune disease (including rheumatoid arthritis, system lupus erythematosus, Sjogren's syndrome, ankylosing spondylitis, psoriasis, Graves’ disease, Hashimoto's thyroiditis, polyarteritis nodosa, uveitis, inflammatory bowel diseases, and type I diabetes, which were coded as follows: International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 714, 710, 710.2, 720, 696, 696.1, 242, 245.2, 446, 360.12, 363.0x, 363.1x, 363.20, 363.21, 363.22, 364.0x, 364.1x, 364.2x, 364.3, 555, 556, and 250.01; ICD-10-CM code M05, M06, M07, M09, M32, M35, M45, L40, E05, E06.3, M30, H44.11, H30, H20, K50, K51, and E10) in the mother during pregnancy or 1 year before pregnancy. The comparison group was the offspring of mothers never diagnosed with an autoimmune disease during pregnancy or 1 year before pregnancy. The index date was set as the birth date.
The outcome variable was defined as a diagnosis of tics and Tourette's disorder (ICD-9-CM code: 307.2, 37.20, 37.21, 37.22, 37.23; ICD-10-CM code: F95.0, F95.1, F95.2, F95.8, F95.9) during the observational period in NHIRD. Patients seeking medical care exhibiting symptoms or such abnormal behaviors, who had three outpatient department visits or one admission record with any type of tic or Tourette's disorder, were enrolled as the outcome in this study. Both groups, offspring of mothers with autoimmune diseases or without, were followed up until the onset of tics and Tourette's disorder, death, or 31 December 2019, whichever occurred first.
The baseline characteristics were birth year, child's sex, birth weight (<2,500, 2,500–3,499, ≥3,500 g), gestational weeks (<36, 36–40, ≥41 weeks), delivery [normal spontaneous delivery, NSD, cesarean section (C/S)], parity, parents’ ages, congenital defects, urbanization, insurance unit, maternal and paternal comorbidities, children or parents died within 1 year after birth, medication exposure during pregnancy, and maternal comorbidities, including asthma (ICD-CM = 493, J44, J45), hypertension (ICD-CM = 401–405, I10–I15), diabetes mellitus (ICD-CM = 250, E10, E11, E12, E13, E14), hyperlipidemia (ICD-CM = 272, E78), gestational diabetes (ICD-CM = 648.8, O99.81, O24.41, O24.42, O24.43), preeclampsia or eclampsia (ICD-CM = 6,424.4, 642.5, 642.6, 642.7, O11, O14,O15), cancer (ICD-CM = 140–199, 200–208, C00–C97), urinary tract infection (ICD-CM = 599, N39), anemia (ICD-CM = 281–285, D60–D64), endometriosis (ICD-CM = 617.0–617.9, 621.3, N80), sleep disorder (ICD-CM = 327, 780.5, G47), depression (ICD-CM = 293.83, 296.2, 296.3,300.4, 311, F06.3, F32.0, F32.1, F32.2, F32.3, F32.4, F32.5, F32.9, F33.0, F33.1, F33.2, F33.3, F33.4, F33.9, F34.1), postpartum depression (ICD-CM = 648.44, F53), and seizure disorder (ICD-CM = 345, G40–41). In addition, paternal comorbidities, including asthma, hypertension, diabetes mellitus, hyperlipidemia, cancers, urinary tract infection, anemia, sleep disorder, depression, and seizure disorder, were also considered for further multivariate analysis. These parental comorbidities were defined within 2 years before the child’s birth.
Comparisons between the maternal autoimmune disease cohort and the non-maternal autoimmune disease cohort were performed using absolute standardized difference (ASD). The characteristics of the groups were considered balanced when the ASD value was less than 0.1 (41). The incidence density was calculated as the number of newly diagnosed cases per 10,000 person-months. The confidence intervals of incidence density were calculated using the Rothman–Greenland method (42). Kaplan–Meier analysis was used to calculate the 10-year cumulative incidence of tics and Tourette's disorder between the two cohorts. The log-rank test was used to test the significance. To determine the independent risk of the maternal autoimmune disease group, a multivariable Cox proportional hazard model was used to estimate the adjusted hazard ratios (HRs). The propensity score-matched (PSM) cohorts were selected to further balance potential confounders between the two study cohorts (41). The propensity score (probability) of maternal autoimmune disease exposure was calculated using logistic regression, incorporating covariates such as baseline perinatal characteristics, including the child’s birth year, child’s sex, delivery method, parity, parental age, urbanization, insurance coverage, comorbidities, and aspirin exposure during pregnancy. Subsequently, each child with maternal autoimmune disease exposure was matched with two non-exposed children with a similar propensity score, achieved through the nearest neighbor greedy algorithm with a caliper of 0.01. However, PSM still fails to address unmeasured confounding effects, and its application when selecting the study population is associated with numerous limitations and potential biases (43). In studies involving a large sample size, the traditional covariate adjustment method performs similarly to the propensity score methods, which include propensity score stratification, propensity score matching, and propensity score inverse probability weighting (44). Therefore, we based our primary results on the traditional covariate adjustment method. In addition, we performed stratified analyses to observe the HRs within different subgroups. The statistical software used for the analyses was SAS version 9.4 (SAS Institute Inc., NC, USA).
In total, 1,459,093 individuals were retrieved from the birth certificate applications database from 2009 to 2016 and 1,288,347 offspring passed the exclusion criteria. We selected 76,411 offspring with maternal autoimmune disease exposure and 1,211,936 individuals without maternal autoimmune disease exposure for analysis (Figure 1).
Among the demographic and perinatal characteristics of both cohorts, the maternal autoimmune disease exposure cohort had higher prevalence rates of maternal comorbidities, such as diabetes mellitus (4.71% vs. 2.56%, with ASD = 0.115), hyperlipidemia (1.63% vs. 0.45%, with ASD = 0.116), anemia (17.82% vs. 8.4%, with ASD = 0.282), sleep disorder (7.15% vs. 4.45%, with ASD = 0.116), and depression (4.62% vs. 2.68%, with ASD = 0.103), and higher proportions of aspirin, hydroxychloroquine, azathioprine, or sulfasalazine users when compared with those in the without maternal autoimmune disease exposure group. The other comorbidities and risk factors, including preterm or low birth weight, birth year, child's sex, birth weight, delivery mode, parity, Apgar score, congenital defects, urbanization, and insurance unit showed no difference between the maternal autoimmune disease exposure and without maternal autoimmune exposure groups (all ASD < 0.1). We used propensity score matching to balance the baseline perinatal characteristics, including the child’s birth year, child’s sex, delivery method, parity, parental age, urbanization, insurance coverage, comorbidities, and aspirin exposure during pregnancy between the two study cohorts (Table 1).
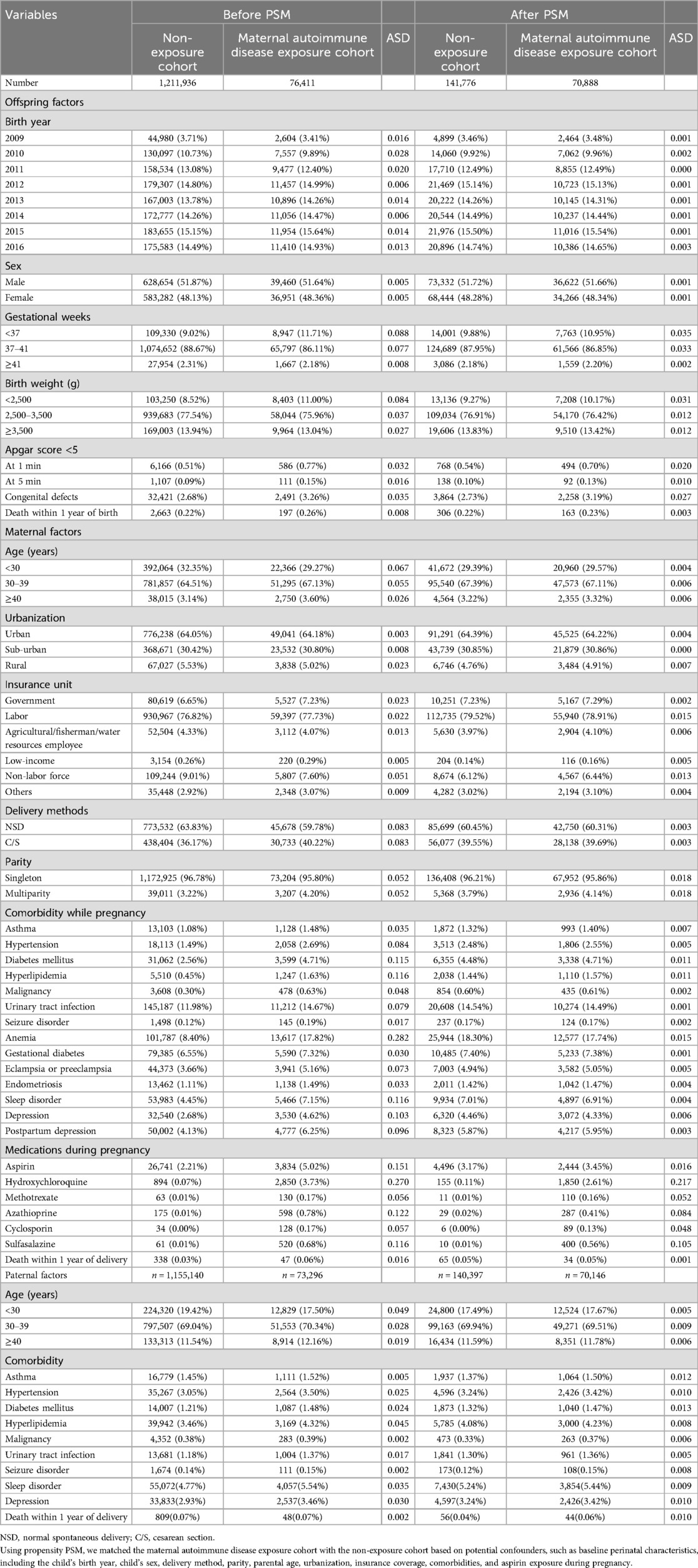
Table 1. Demographic and perinatal characteristics of the non-exposure cohort and maternal autoimmune disease exposure cohort.
Table 2 displays the incidence rates per 10,000 person-months of childhood tics and Tourette's disorder before propensity score matching. The maternal autoimmune disease exposure cohort exhibited a significantly higher incidence rate (2.35, 95% CI = 2.23–2.48) compared to the non-exposure cohort (1.89, 95% CI = 1.86–1.92).
In Figure 2A, the 10-year cumulative probability of tics and Tourette's disorder was higher in the maternal autoimmune disease exposure cohort (4.00%) than in the non-exposure cohort (3.13%), with the log-rank test yielding a p-value of <0.001. The unadjusted hazard ratio for tics and Tourette's disorder was 1.26 (95% CI = 1.20–1.34) in children born to mothers with autoimmune disease, in comparison to those born to mothers without autoimmune disease. After adjusting for perinatal characteristics, the adjusted hazard ratio (aHR1, the model recommended by experts) was calculated as 1.22 (95% CI = 1.15–1.29), and aHR2 (the full model) was determined to be 1.21 (95% CI = 1.15–1.29) (Table 2). Following propensity score matching, the hazard ratio was found to be 1.25 (95% CI = 1.17–1.35), and the 10-year cumulative probability of tics and Tourette's disorder within the propensity score-matched cohorts is illustrated in Figure 2B.
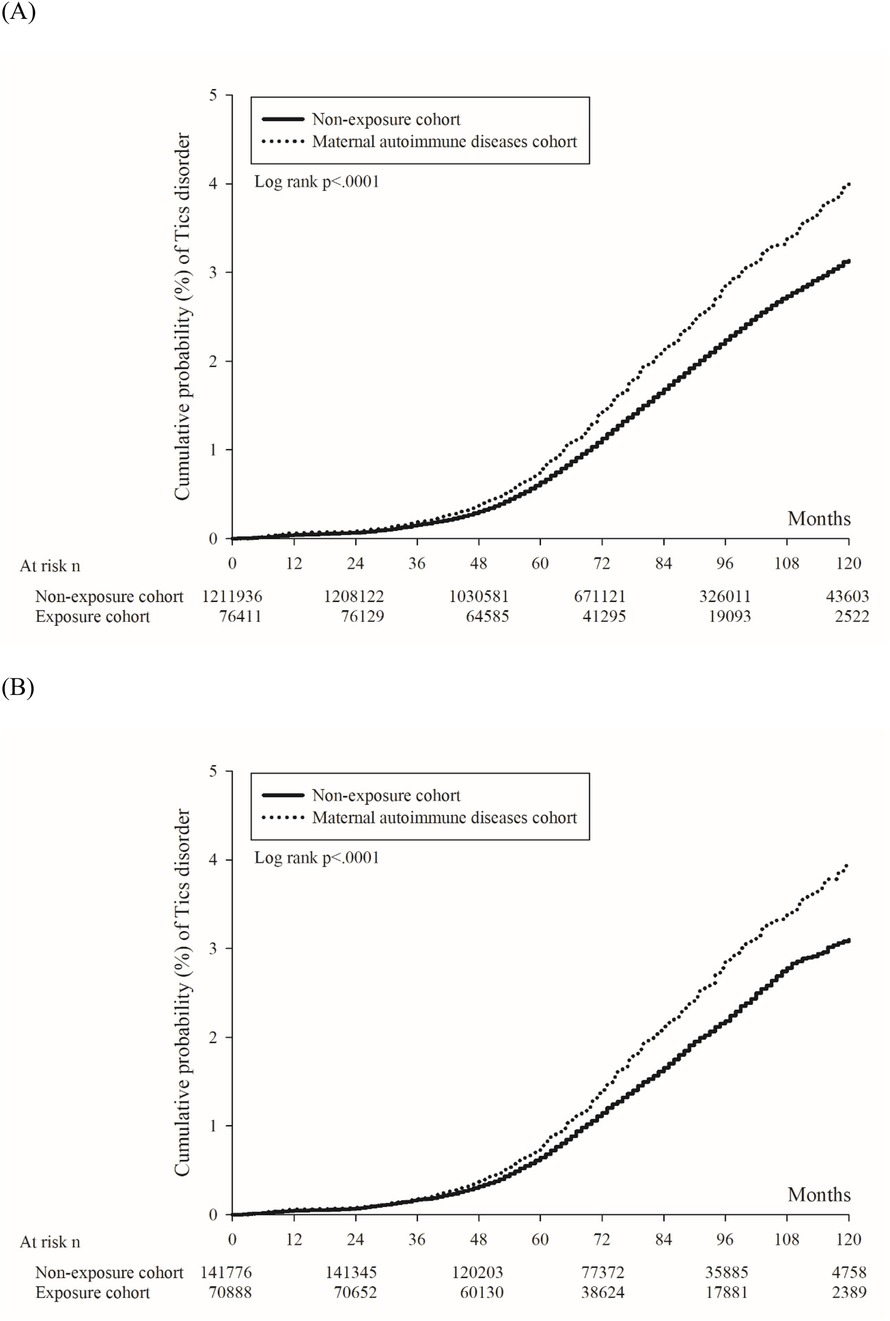
Figure 2. Kaplan–Meier curve of the cumulative probability of tics and Tourette's disorder in offspring. (A) Before propensity score matching. (B) After propensity score matching.
Regardless of the child's sex, gestational age, or maternal age strata, children in the maternal autoimmune disease exposure cohort showed an elevated likelihood of developing tics and Tourette's disorder (Figure 3).
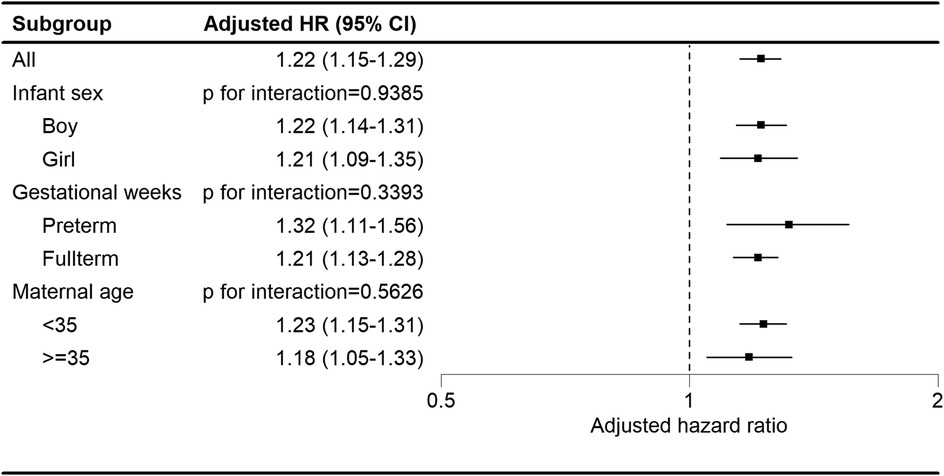
Figure 3. Subgroup analysis of the association between maternal autoimmune disease and development of tics and Tourette's disorder in offspring.
As shown in Figure 4, in the analysis stratified by the type of maternal autoimmune disease exposure, offspring exposed to maternal rheumatoid arthritis (aHR: 1.46, 95% CI, 1.07–1.97), systemic lupus erythematosus (aHR: 1.57, 95% CI, 1.07–1.97), Sjogren's syndrome (aHR: 1.28, 95% CI, 1.09–1.50), ankylosing spondylitis (aHR: 1.49, 95% CI, 1.07–2.09), Graves’ disease (aHR: 1.26, 95% CI, 1.15–1.37), Hashimoto's thyroiditis (aHR: 1.59, 95% CI, 1.29–1.98), or type I diabetes (aHR: 1.68, 95% CI, 1.13–2.50) had a significantly elevated risk of developing tics and Tourette's disorder.
To the best of our knowledge, this study is the first to analyze a large, nationwide cohort using the Maternal and Child Health Database to explore the link between maternal autoimmune disorders and childhood tics and Tourette's disorders in an Asian population. We found a significantly higher incidence and cumulative risk of these neurobehavior disorders in children born to mothers with autoimmune diseases, especially rheumatoid arthritis, systemic lupus erythematosus, Sjogren's syndrome, ankylosing spondylitis, Graves’ disease, Hashimoto's thyroiditis, and type I diabetes. These findings highlight the importance of early monitoring for neurodevelopmental issues in children of affected mothers.
The etiology of tics and Tourette's disorder is multifactorial, involving multiple genetic (45, 46), environmental, or immunological factors. Studies have suggested that autoimmune diseases may lead to excessive immune response, abnormal cytokine levels, dysregulation of neurotransmitters, and microglial dysfunction, potentially disrupting fetal brain development. While these mechanisms are well-studied in animal models and neuroimage and postmortem analyses, direct evidence in humans remains limited (47–53). Children with tics and Tourette's disorder sometimes have a sensory premonitory urge prior to the onset of the motor component (6), implying that neurotransmitter dysregulation, including gamma-aminobutyric acid (GABA), glutamate, and dopamine pathways, is involved in neuroinflammation. For example, the generation of unplanned movement or sounds is associated with disruption of GABA-related disinhibition or poor-regulated glutamate excitation in the basal ganglia and dopaminergic system dysregulation in the substantia nigra (54, 55).
Maternal autoimmune diseases may cause excessive immune-cellular responses, dysregulated serum cytokines, and poor long-lasting imprinting of the immune system, especially during pregnancy (56–58). The MIA, via a dysfunctional and inflammatory intrauterine phenomenon, may lead to neuroinflammation in fetal brain development or early childhood, leading to neurodevelopmental disease (16, 39, 59, 60). For example, microglial cells were believed to be involved in OCD, Tourette’s disorder, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), or psychiatric disease (19, 61–65). Abnormal cytokines, such as monocyte chemotactic factor-1 (MCP-1), interleukin-2 (IL-2), and protein tyrosine phosphatase receptor-N (PTPR-N) were noted in patients with Tourette’s disorder (66). In a recent animal model study, alpha-enolase-specific antibody, which is often detected in autoimmune disease, was found to be highly expressed in the maternal circulation and in the brain tissues of offspring, causing impaired learning and memory abilities through complement-dependent cytotoxicity (67). These pathophysiological mechanisms have been well-studied in animal models, adults, and postmortem studies, indicating that abnormal immunity promotes the development of neurogenic disease, but there is still limited evidence of transfer from the mother across the placenta to the fetal brain in human beings, manifesting as a neurogenic disorder in early childhood, the developmental stage in which the onset of tics and Tourette's disorder occurs. It is worth mentioning that a recent study presents a new concept of neurodevelopmental disorders in the case of autism. Sotgiu et al. (65) indicated that, aside from altered maternal immune factors, intrinsic fetal susceptibility factors also play important roles in disturbed neuroimmune crosstalk through the placenta. This can lead to chronic fetal microglial activation, which may ultimately result in altered neurogenesis, synapse formation, and pruning. In our study, a higher prevalence of childhood tics and Tourette's disorder resulted from overall maternal autoimmune disease, especially in mothers with rheumatoid arthritis, systemic lupus erythematosus, Sjogren's syndrome, ankylosing spondylitis, Graves’ disease, Hashimoto's thyroiditis, and type I diabetes. The results indicate that the maternal autoimmune diseases triggered immune-inflammatory processes involved in the development of tics and Tourette's disorder in the offspring.
Aside from these autoimmune diseases, our study showed that children born to mothers with urinary tract infections, diabetes mellitus, hyperlipidemia, anemia, sleep disorder, endometriosis, and depression had a higher risk of tics and Tourette's disorder (Supplement Table S1), which implies that infections, metabolic conditions, dysbiosis-induced gut-brain axis, and emotional or environmental stress could explain the neuroinflammatory condition facilitating tics and Tourette's disorder, as noted in previous studies (11, 33, 68). A higher prevalence was noted in boys and children with congenital defects in our study, suggesting crucial roles for hereditary, genetic, or epigenetic factors in the development of tics and Tourette's disorder. Interestingly, depressed fathers appear to somehow play a role in the neurobehavioral disorders of their offspring, while other paternal factors showed no association, which serves as a reminder that postnatal education, environment, emotional stress, and poor communication may also contribute to this neurodevelopmental disorder.
The study's strengths include its large sample size and long follow-up period. The availability of comprehensive historical medical service records for all cases has enabled us to mitigate potential selection or recall biases, ensuring a robust evaluation of our hypothesis. However, there were still some potential limitations in our study. First, the NHIRD lacks data on various covariates, including personal lifestyle, family history, education status, social adversity, laboratory data, genetic sequencing, and environmental factors. Despite our efforts to adjust for several comorbidities and match propensity scores, the presence of these unmeasured confounding factors may have potentially impacted our results. Second, the diagnoses of maternal autoimmune diseases, childhood tics, Tourette's disorder, and comorbidities depend on the ICD codes in the dataset. A comprehensive review of all medical records was not conducted to confirm the accuracy of the diagnoses, potentially leading to some instances of misclassification. Because of this, we did not categorize the outcome into different kinds of tics or Tourette's disorder. That is, patients seeking medical care exhibiting similar symptoms or such abnormal behaviors, who had three outpatient department visits or one admission record with any type of tic or Tourette's disorder, were enrolled as the outcome in this study. Second, we do not know whether the underlying maternal autoimmune diseases were active during pregnancy, which may also influence or strengthen the results. Third, neurological autoimmune disorders such as multiple sclerosis were not included in the study. As a study suggested that maternal multiple sclerosis is not associated with a higher risk of neurodevelopmental disorders in offspring, perhaps due to the organ-specific characteristic of this CNS immune-mediated disease, there may be minimal influence on our study results (26). Moreover, it 's important to note that the vast majority of these patients were residents of Taiwan, predominantly of Asian ethnicity. As a result, the applicability of these findings to other countries or ethnic groups may require careful consideration. Finally, it is worth highlighting that the precise mechanisms through which maternal autoimmune diseases might impact the risk of Tourette's and tic disorders were not explored in this analysis, indicating a need for further research. Exploring the association between Tourette's disorder, neurodevelopmental disorders, and maternal autoimmune disorder would also have additional synergistic value to this research topic.
In conclusion, maternal autoimmune diseases can be considered a risk factor for developing tics and Tourette's disorder in offspring, especially in mothers with rheumatoid arthritis, system lupus erythematosus, Sjogren's syndrome, ankylosing spondylitis, Graves’ disease, Hashimoto's thyroiditis, or type I diabetes. We hope that the epidemiological observational results presented in this study can raise clinicians’ awareness of the relationship between tics and Tourette's disorder in offspring and maternal autoimmune diseases, as early diagnosis and treatment can improve outcomes for individuals with tics and Tourette's disorder. Further research is warranted to investigate the possible pathogenetic mechanisms of this association.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: the LHID is a subset of the NHIRD, a database of all medical claims in Taiwan's NHI system. The usage of NHIRD is limited to research purposes only. Only Taiwanese citizens who fulfill the requirements for conducting research projects are eligible to apply for access to the National Health Insurance Research Database (NHIRD). Applicants must follow the Personal Data Protection Act (https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=I0050021) and related regulations of the National Health Insurance Administration and NHRI (National Health Research Institutes), and an agreement must be signed by the applicant and his/her supervisor upon application submission. The datasets generated and analyzed during the current study are available from the authors on reasonable request.
The studies involving humans were approved by the Institutional Review Board of Chung Shan Medical University Hospital (approval no. CS2-21006). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Y-FL: Writing – original draft, Writing – review & editing. M-CW: Writing – review & editing. Y-CH: Conceptualization, Writing – review & editing. J-YH: Data curation, Formal analysis, Methodology, Writing – review & editing. JC-CW: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1440366/full#supplementary-material
1. Johnson KA, Worbe Y, Foote KD, Butson CR, Gunduz A, Okun MS. Tourette syndrome: clinical features, pathophysiology, and treatment. Lancet Neurol. (2023) 22(2):147–58. doi: 10.1016/S1474-4422(22)00303-9
2. Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. (2012) 47(2):77–90. doi: 10.1016/j.pediatrneurol.2012.05.002
3. Charania SN, Danielson ML, Claussen AH, Lebrun-Harris LA, Kaminski JW, Bitsko RH. Bullying victimization and perpetration among US children with and without Tourette syndrome. J Dev Behav Pediatr. (2022) 43(1):23–31. doi: 10.1097/DBP.0000000000000975
4. Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. Mental health surveillance among children—United States, 2013–2019. MMWR Supplements. (2022) 71(2):1. doi: 10.15585/mmwr.su7102a1
5. Chou IJ, Hung P-C, Lin J-J, Hsieh M-Y, Wang Y-S, Kuo C-Y, et al. Incidence and prevalence of Tourette syndrome and chronic tic disorders in Taiwan: a nationwide population-based study. Soc Psychiatry Psychiatr Epidemiol. (2022) 57(8):1711–21. doi: 10.1007/s00127-022-02253-7
6. Dale RC. Tics and Tourette: a clinical, pathophysiological and etiological review. Curr Opin Pediatr. (2017) 29(6):665–73. doi: 10.1097/MOP.0000000000000546
7. Claussen AH, Bitsko RH, Holbrook JR, Bloomfield J, Giordano K. Impact of Tourette syndrome on school measures in a nationally representative sample. J Dev Behav Pediatr. (2018) 39(4):335–42. doi: 10.1097/DBP.0000000000000550
8. Mittal SO. Tics and Tourette’s syndrome. Drugs Context. (2020) 9:2019-12-2. doi: 10.7573/dic.2019-12-2
9. Mol Debes NM. Co-morbid disorders in Tourette syndrome. Behav Neurol. (2013) 27(1):7–14. doi: 10.1155/2013/237513
10. Hoekstra PJ, Dietrich A, Edwards MJ, Elamin I, Martino D. Environmental factors in Tourette syndrome. Neurosci Biobehav Rev. (2013) 37(6):1040–9. doi: 10.1016/j.neubiorev.2012.10.010
11. Geng J, Liu C, Xu J, Wang X, Li X. Potential relationship between Tourette syndrome and gut microbiome. J Pediatr (Rio J). (2023) 99(1):11–6. doi: 10.1016/j.jped.2022.06.002
12. Blaty JL, DelRosso LM. Tourette disorder and sleep. Biomed J. (2022) 45(2):240–9. doi: 10.1016/j.bj.2022.01.002
13. Chen H-L, Zhang R, Tsai SC-S, Chou R-H, Hsu Y-C, Fan H-C, et al. Ambient air pollution exposure and risk of developmental delay in children and teenagers in Taiwan. Atmosphere (Basel). (2021) 12(8):1039. doi: 10.3390/atmos12081039
14. Tsai LH, Lin JW, Lue KH. Study protocol to investigate the correlation between Tourette syndrome and allergy in children and adolescents. J Int Med Res. (2020) 48(12):300060520973921. doi: 10.1177/0300060520973921
15. Choi W, Hong SB, Kim JI, Lee J, Jang S, Ahn YD, et al. Association of pre- and perinatal risk factors with Tourette syndrome or chronic tic disorders in a Korean school-age population. Soa Chongsonyon Chongsin Uihak. (2023) 34(1):37–44. doi: 10.5765/jkacap.220024
16. Zhang T, Brander G, Isung J, Isomura K, Sidorchuk A, Larsson H, et al. Prenatal and early childhood infections and subsequent risk of obsessive-compulsive disorder and tic disorders: a nationwide, sibling-controlled study. Biol Psychiatry. (2022) 93(11):1023–30. doi: 10.1016/j.biopsych.2022.07.004
17. Usui N, Matsumoto-Miyai K, Koyama Y, Kobayashi Y, Nakamura Y, Kobayashi H, et al. Social communication of maternal immune activation-affected offspring is improved by Si-based hydrogen-producing agent. Front Psychiatry. (2022) 13:872302. doi: 10.3389/fpsyt.2022.872302
18. Zhu J, Naughton S, Bowman N, LeRoith T, Luo X, Leeth C. Maternal antibody repertoire restriction modulates the development of lupus-like disease in BXSB offspring. Int Immunol. (2023) 35(2):95–104. doi: 10.1093/intimm/dxac049
19. Lampiasi N, Bonaventura R, Deidda I, Zito F, Russo R. Inflammation and the potential implication of macrophage-microglia polarization in human ASD: an overview. Int J Mol Sci. (2023) 24(3):2703. doi: 10.3390/ijms24032703
20. Nielsen TC, Nassar N, Shand AW, Jones HF, Han VX, Patel S, et al. Association of maternal autoimmune disease and early childhood infections with offspring autism spectrum disorder: a population-based cohort study. Autism Res. (2022) 15(12):2371–80. doi: 10.1002/aur.2824
21. Marder W, Romero VC, Ganser MA, Hyzy MA, Gordon C, McCune WJ, et al. Increased usage of special educational services by children born to mothers with systemic lupus erythematosus and antiphospholipid antibodies. Lupus Sci Med. (2014) 1(1):e000034. doi: 10.1136/lupus-2014-000034
22. Nielsen TC, Nassar N, Shand AW, Jones H, Guastella AJ, Dale RC, et al. Association of maternal autoimmune disease with attention-deficit/hyperactivity disorder in children. JAMA Pediatr. (2021) 175(3):e205487. doi: 10.1001/jamapediatrics.2020.5487
23. Murphy TK, Storch EA, Turner A, Reid JM, Tan J, Lewin AB. Maternal history of autoimmune disease in children presenting with tics and/or obsessive-compulsive disorder. J Neuroimmunol. (2010) 229(1-2):243–7. doi: 10.1016/j.jneuroim.2010.08.017
24. Isung J, Isomura K, Williams K, Zhang T, Lichtenstein P, de la Cruz L F, et al. Association of primary immunodeficiencies in parents with psychiatric disorders and suicidal behavior in their offspring. JAMA Psychiatry. (2023) 80(4):323–30. doi: 10.1001/jamapsychiatry.2022.4786
25. He H, Yu Y, Liew Z, Gissler M, László KD, Valdimarsdóttir UA, et al. Association of maternal autoimmune diseases with risk of mental disorders in offspring in Denmark. JAMA Netw Open. (2022) 5(4):e227503-e. doi: 10.1001/jamanetworkopen.2022.7503
26. Carta A, Zarbo IR, Scoppola C, Pisuttu G, Conti M, Melis MC, et al. Maternal multiple sclerosis is not a risk factor for neurodevelopmental disorders in offspring. Mult Scler J Exp Transl Clin. (2021) 7(2):20552173211017301. doi: 10.1177/20552173211017301
27. Zimmerman-Brenner S, Pilowsky-Peleg T, Rachamim L, Ben-Zvi A, Gur N, Murphy T, et al. Group behavioral interventions for tics and comorbid symptoms in children with chronic tic disorders. Eur Child Adolesc Psychiatry. (2022) 31(4):637–48. doi: 10.1007/s00787-020-01702-5
28. Zhu PY, Zuo X, Jiang B, Sun ST. [SUN Shen-tian’s clinical experience in treating Tourette’s syndrome with acupuncture]. Zhongguo Zhen Jiu. (2023) 43(3):261–4. doi: 10.13703/j.0255-2930.20221017-k0010
29. Cavanna AE. Current and emerging pharmacotherapeutic strategies for Tourette syndrome. Expert Opin Pharmacother. (2022) 23(13):1523–33. doi: 10.1080/14656566.2022.2107902
30. Tuleasca C, Régis J, Martinez-Alvarez R, Levivier M, Hariz M. Neurosurgical lesioning for Tourette syndrome. Lancet Neurol. (2023) 22(4):292. doi: 10.1016/S1474-4422(23)00078-9
31. Wang T, Wang Z, Qi W, Jiang G, Wang G. The role, targets and mechanisms of traditional Chinese medicine in regulating the balance of T helper type 17/regulatory T cells in rheumatoid arthritis. Int J Rheum Dis. (2023) 26(4):613–24. doi: 10.1111/1756-185X.14560
32. Alshammery S, Patel S, Jones HF, Han VX, Gloss BS, Gold WA, et al. Common targetable inflammatory pathways in brain transcriptome of autism spectrum disorders and Tourette syndrome. Front Neurosci. (2022) 16:999346. doi: 10.3389/fnins.2022.999346
33. Widomska J, De Witte W, Buitelaar JK, Glennon JC, Poelmans G. Molecular landscape of Tourette’s disorder. Int J Mol Sci. (2023) 24(2):1428. doi: 10.3390/ijms24021428
34. Shprecher DR, Gannon K, Agarwal N, Shi X, Anderson JS. Elucidating the nature and mechanism of tic improvement in Tourette syndrome: a pilot study. Tremor Other Hyperkinet Mov (N Y). (2014) 4:217. doi: 10.5334/tohm.190
35. Lee Y-F, Wu M-C, Ma KS-K, Huang J-Y, Wei JC-C. Association of early childhood constipation with the risk of autism spectrum disorder in Taiwan: real-world evidence from a nationwide population-based cohort study. Front Psychiatry. (2023) 14:1116239. doi: 10.3389/fpsyt.2023.1116239
36. Pérez-Vigil A, Fernández de la Cruz L, Brander G, Isomura K, Gromark C, Mataix-Cols D. The link between autoimmune diseases and obsessive-compulsive and tic disorders: a systematic review. Neurosci Biobehav Rev. (2016) 71:542–62. doi: 10.1016/j.neubiorev.2016.09.025
37. Dalsgaard S, Waltoft BL, Leckman JF, Mortensen PB. Maternal history of autoimmune disease and later development of Tourette syndrome in offspring. J Am Acad Child Adolesc Psychiatry. (2015) 54(6):495–501.e1. doi: 10.1016/j.jaac.2015.03.008
38. Mataix-Cols D, Frans E, Pérez-Vigil A, Kuja-Halkola R, Gromark C, Isomura K, et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol Psychiatry. (2018) 23(7):1652–8. doi: 10.1038/mp.2017.215
39. Jones HF, Han VX, Patel S, Gloss BS, Soler N, Ho A, et al. Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: transcriptomic data show common enriched innate immune pathways. Brain Behav Immun. (2021) 94:308–17. doi: 10.1016/j.bbi.2020.12.035
40. Hamm NC, Hamad AF, Wall-Wieler E, Roos LL, Plana-Ripoll O, Lix LM. Multigenerational health research using population-based linked databases: an international review. Int J Popul Data Sci. (2021) 6(1):1686. doi: 10.23889/ijpds.v6i1.1686
41. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28(25):3083–107. doi: 10.1002/sim.3697
42. Andersen PK. Modern Epidemiology. 2nd ed. Kenneth J. Rothman and Sander Greenland, Lippincott-Raven, U.S.A., 1998. No. of pages: 737. Price: $74.50. ISBN 0-316-75780-2. Statistics in Medicine. 19:881–2. (2000).
43. King G, Nielsen R. Why propensity scores should not be used for matching. Polit Anal. (2019) 27(4):435–54. doi: 10.1017/pan.2019.11
44. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. (2017) 69(3):345–57. doi: 10.1016/j.jacc.2016.10.060
45. Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. (2013) 18(6):721–8. doi: 10.1038/mp.2012.69
46. Baldan LC, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes Tourette syndrome: parallel findings in humans and mice. Neuron. (2014) 81(1):77–90. doi: 10.1016/j.neuron.2013.10.052
47. Zhongling K, Yanhui C, Guofeng C, Yanyan L. Neuroinflammation in a rat model of Tourette syndrome. Front Behav Neurosci. (2022) 16:710116. doi: 10.3389/fnbeh.2022.710116
48. Rapanelli M, Frick L, Bito H, Pittenger C. Histamine modulation of the basal ganglia circuitry in the development of pathological grooming. Proc Natl Acad Sci U S A. (2017) 114(25):6599–604. doi: 10.1073/pnas.1704547114
49. Yael D, Israelashvili M, Bar-Gad I. Animal models of Tourette syndrome—from proliferation to standardization. Front Neurosci. (2016) 10. doi: 10.3389/fnins.2016.00132
50. Muellner J, Delmaire C, Valabrégue R, Schüpbach M, Mangin JF, Vidailhet M, et al. Altered structure of cortical sulci in Gilles de la Tourette syndrome: further support for abnormal brain development. Mov Disord. (2015) 30(5):655–61. doi: 10.1002/mds.26207
51. Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. (2003) 60(4):415–24. doi: 10.1001/archpsyc.60.4.415
52. Jackson SR, Loayza J, Crighton M, Sigurdsson HP, Dyke K, Jackson GM. The role of the insula in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. Cortex. (2020) 126:119–33. doi: 10.1016/j.cortex.2019.12.021
53. He JL, Mikkelsen M, Huddleston DA, Crocetti D, Cecil KM, Singer HS, et al. Frequency and intensity of premonitory urges-to-tic in Tourette syndrome is associated with supplementary motor area GABA+ levels. Mov Disord. (2022) 37(3):563–73. doi: 10.1002/mds.28868
54. Ramteke A, Lamture Y. Tics and Tourette syndrome: a literature review of etiological, clinical, and pathophysiological aspects. Cureus. (2022) 14(8):e28575.36185878
55. Ganos C. Tics and Tourette’s: update on pathophysiology and tic control. Curr Opin Neurol. (2016) 29(4):513–8. doi: 10.1097/WCO.0000000000000356
56. Sukhikh GT, Safronova VG, Vanko LV, Matveeva NK, Belyaeva AS, Fedorova EV, et al. Phagocyte activity in the peripheral blood of pregnant women with systemic lupus erythematosus and in the cord blood of their newborns. Int J Rheum Dis. (2017) 20(5):597–608. doi: 10.1111/1756-185X.13085
57. Gönenli MG, Kaymaz Tahra S, Kara M, Keser G, Yazıcı A, Erden A, et al. Pregnancy in Takayasu’s arteritis has a high risk of hypertension-related fetomaternal complications: a retrospective study of a Turkish cohort. Int J Rheum Dis. (2022) 25(2):140–6. doi: 10.1111/1756-185X.14247
58. Lee Y-T, Shih H-L, Chang R, Yong S-B. Maternal autoimmune disease associated with a higher risk of offspring with type 1 diabetes: a nationwide mother–child cohort study in Taiwan. Int J Rheum Dis. (2024) 27(1):e14922. doi: 10.1111/1756-185X.14922
59. Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. (2021) 17(9):564–79. doi: 10.1038/s41582-021-00530-8
60. Gomathy S, Panigrahi B, Tirlangi PK, Wig N, Brijwal M, Sharma MC, et al. Progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus and autoimmune hepatitis. Int J Rheum Dis. (2022) 25(6):705–13. doi: 10.1111/1756-185X.14331
61. Gagliano A, Carta A, Tanca MG, Sotgiu S. Pediatric acute-onset neuropsychiatric syndrome: current perspectives. Neuropsychiatr Dis Treat. (2023) 19:1221–50. doi: 10.2147/NDT.S362202
62. Frick L, Pittenger C. Microglial dysregulation in OCD, Tourette syndrome, and PANDAS. J Immunol Res. (2016) 2016:8606057. doi: 10.1155/2016/8606057
63. Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. (2013) 2013:608654. doi: 10.1155/2013/608654
64. Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human Striatum in Tourette syndrome. Biol Psychiatry. (2016) 79(5):372–82. doi: 10.1016/j.biopsych.2014.07.018
65. Sotgiu S, Manca S, Gagliano A, Minutolo A, Melis MC, Pisuttu G, et al. Immune regulation of neurodevelopment at the mother-foetus interface: the case of autism. Clin Transl Immunology. (2020) 9(11):e1211. doi: 10.1002/cti2.1211
66. Morer A, Chae W, Henegariu O, Bothwell AL, Leckman JF, Kawikova I. Elevated expression of MCP-1, IL-2 and PTPR-N in basal ganglia of Tourette syndrome cases. Brain Behav Immun. (2010) 24(7):1069–73. doi: 10.1016/j.bbi.2010.02.007
67. Sun W, Feng Y, Li H, He X, Lu Y, Shan Z, et al. The effects of maternal anti-alpha-enolase antibody expression on the brain development in offspring. Clin Exp Immunol. (2022) 210(2):187–98. doi: 10.1093/cei/uxac086
Keywords: maternal autoimmune diseases, maternal immune activation, tics and Tourette's disorder, maternal and child health database, Taiwan NHIRD
Citation: Lee Y-F, Wu M-C, Huang Y-C, Huang J-Y and Wei JC-C (2025) Maternal autoimmune diseases and the risk of tics and Tourette's disorder in offspring: insights from Taiwan's real-world data. Front. Pediatr. 13:1440366. doi: 10.3389/fped.2025.1440366
Received: 29 May 2024; Accepted: 11 February 2025;
Published: 4 March 2025.
Edited by:
Anne M. Stevens, Century Therapeutics, United StatesReviewed by:
Stefano Sotgiu, University of Sassari, ItalyCopyright: © 2025 Lee, Wu, Huang, Huang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-Yang Huang, d2NoaW55YW5nQGdtYWlsLmNvbQ==; James Cheng-Chung Wei, amNjd2VpQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.