- 1Department of Medical Nursing, School of Nursing, College of Medicine and Health Sciences University of Gondar, Gondar, Ethiopia
- 2Department of Reproductive Health, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Health Systems and Policy, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4National Centre for Epidemiology and Population Health, The Australian National University, Canberra, ACT, Australia
- 5School of Public Health, College of Health Science, Woldia University, Woldia, Ethiopia
- 6Department of Health Informatics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 7Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Science, Wollo University, Wollo, Ethiopia
- 8Department of Pediatrics and Child Health Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: One of the most common measures of society's health is mortality among neonates. Developing and developed countries still differ significantly in neonatal mortality rates. While there are about 18 neonatal deaths worldwide for every 1,000 live births during the first month of life, less is known about neonatal mortality in developing countries, especially in East Africa. Understanding the extent of mortality during the post-neonatal period and its determinants is crucial for developing appropriate policies and strategies that could help solve the issue. Thus, the aim of this study was to identify the prevalence of post-neonatal mortality in East African countries and the factors that are associated with it.
Methods: Secondary data analysis was conducted using data from the most recent Demographic and Health Surveys, which included 11 East African countries between 2014 and 2022. A weighted sample of 225,635 live births had been used in the study. STATA/SE 14 was used for data analysis. The multilevel mixed-effects logistic regression model was applied to determine the factors associated with post-neonatal mortality. In the multilevel logistic regression model, significant factors were deemed to be associated with post-neonatal mortality at p-values <0.05. The data were interpreted using the adjusted odds ratio (AOR) and confidence interval (CI). The best-fit model has been found to be the one with the lowest deviance and highest logliklihood ratio.
Results: In East Africa, post-neonatal mortality was found to be 15 per 1,000 live births. Pregnancy type (AOR = 3.09, 95% CI: 2.30, 4.13), birth weight (AOR = 1.58, 95% CI: 1.25, 2.01), maternal age (AOR = 1.58, 95% CI: 1.32, 1.90), maternal education (AOR = 1.82, 95% CI: 1.14, 2.92), tetanus shots prior to delivery (AOR = 1.23; 95% CI: 1.06–1.42), birth order (AOR = 5.68, 95% CI: 4.48, 7.24), those born in Uganda (AOR = 1.33, 95% CI: 1.03, 1.73), and Burundi (AOR = 1.48, 95% CI: 1.11, 1.98) had the highest odds of post-neonatal death.
Conclusion: According to this study, post-neonatal mortality is higher in developing countries, particularly in East Africa. It was discovered that factors at the individual and community levels associated with post-neonatal mortality. Consequently, focus should be paid to babies born to mothers in the lowest age group, those born of multiple pregnancies, without formal educations, who did not receive tetanus shots prior to birth, and who were born in the first birth order.
Introduction
The number of infant deaths between the ages of 28 days and 11 months is known as post-neonatal mortality, and it is determined by dividing the total number of post-neonatal deaths by the number of live births in a particular year (1). Infant mortality is a good and affordable way to measure population health. The health of the child and society at large are both indicated by neonatal mortality (2). The high postnatal mortality rate is a result of the predominance of unfavorable social, economic, and environmental situations during the first year of life (3). Because they have considerably weaker immune systems than adults, newborns are far more susceptible to challenges in the environment and in community. They also require assistance because they are unable to care for themselves. Infants typically suffer the most from poor living conditions as a result (4).
Worldwide, there is a huge disparity in the post-neonatal death rate. Diarrhea, acute respiratory illness, measles, tetanus, and malaria are among the diseases that can be readily treated or prevented and are the main reasons of post-neonatal mortality. In developing countries, a high and significantly variable proportion of infants still die each year from these and other reasons (5–7). A child's first month of existence carries the biggest risk of death; in 2021, the average global rate of deaths per 1,000 live births was 18 (down 51% from 37 in 1990). In contrast, it was calculated that there were 11 deaths for every 1,000 people who lived past the first month of life and before turning 1 year (8, 9). Only a small decline in postnatal deaths is seen globally each year. However, a number of developing countries, such as those in Eastern Africa, are still far left behind (10).
The risk of post-neonatal death is 55 per 1,000 live births in African countries, which is more than five times higher than the rate of 10 per 1,000 live births in European countries (11, 12). Post-neonatal mortality in Sub-Saharan Africa, particularly in East Africa, has increased gradually as a result of the region's ongoing high rates of pneumonia, diarrhea, malaria, and vaccine-preventable infections (13, 14).
Research conducted globally has shown a substantial association between post-neonatal mortality with place of delivery (15), means of delivery (16), number of antenatal visits (17), birth interval (18), educational status of the mother (19–21), type of place of residence (15), distance to health institution (22, 23), birth order number (24), pregnancy status (25), child weight at birth (26), type of pregnancy (27), and child sex (28).
To the best of our knowledge and literature search, no research has been done on the post-neonatal mortality rate in East Africa, despite the fact that those countries bear a significant portion of the world's infant mortality burden. Thus, the current study uses multilevel mixed effect analysis of the most recent Demographic and Health Survey data to investigate the prevalence and factors associated with post-neonatal mortality in East Africa.
Methods and materials
Study design and period
Through secondary analysis, a population-based cross-sectional study was carried out using data from the Demographic and Health Survey (DHS) of 11 East African countries between 2014 and 2022. To generate updated health and health-related indicators, a community-based cross-sectional Demographic and health survey is conducted every 5 years.
Data source, study population and sampling technique
Based on the most recent East African countries Demographic Health Surveys (DHS) datasets from 2014 to 2022, a secondary data analysis was carried out. The DHS surveys from eleven East African countries such as Burundi, Ethiopia, Comoros, Uganda, Rwanda, Tanzania, Mozambique, Zimbabwe, Kenya, Zambia, and Malawi were employed in our analysis. To determine the prevalence and factors associated with post-neonatal mortality in Eastern Africa, the data were appended together. Every country's survey contains a variety of datasets, including those related to men, women, children, births, and households. Using a stratified two-stage cluster technique, DHS selects enumeration areas (first stage) and then draws a sample of households from each selected enumeration area (second stage). The age at death (b7) variable was recoded from the kid's Record (KR) dataset in order to calculate the outcome variable (post-neonatal mortality).
The factors associated with post-neonatal mortality were determined using a binary logistic regression model. In the bivariable analysis, unadjusted odds ratios (ORs) with a 95% confidence interval were calculated to identify potential candidate variables for the multivariable analysis. Variables with p-values less than 0.25 in the bivariable analysis were considered suitable for inclusion in the multivariable analysis. In the multivariable analysis, adjusted odds ratios (AORs) with a 95% confidence interval were reported to account for potential confounders, and variables with p-values less than 0.05 were considered statistically significant. We used the weighting variable (v005) as a relative weight normalized to make the analysis survey-specific. For the pooled data, we denormalized the post-neonates’ individual standard weight variable by dividing it by the sampling fraction of each country. The post-neonates’ adjusted weight was calculated as follows: Post-neonates’ adjusted weight = V005 × (number of post-neonates aged 28 days to 11 months in the country at the time of the survey)/(total number of post-neonates aged 28 days to 11 months interviewed in the survey). In all, 225,635 live births were included in the weighted sample for this study (Table 1).
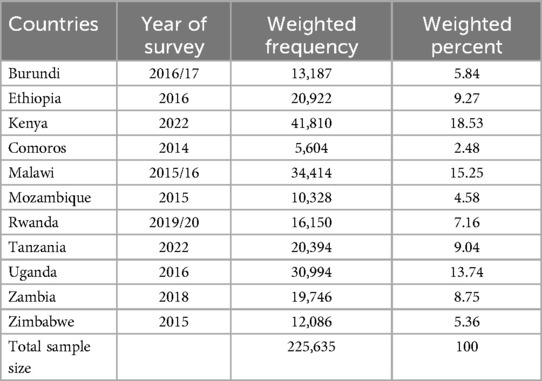
Table 1. Sample size for post-neonatal mortality and its determinants among post-neonates in East Africa, DHS 2014–2022.
Study variables
Dependent variables
Post-neonatal death in months was the study's outcome variable. The number of infant deaths between the ages of 28 days and 11 months is known as post-neonatal mortality, and it is measured as the number of neonatal deaths per 1,000 live births in a year. Neonatal deaths were dichotomized into “yes = 1” for those who passed away between the ages of 28 days and 11 months and “no = 0” for those who lived (29).
Independent variables
The independent factors taken into consideration for this study was obtained from two sources (individual-level and community-level variables), due to the hierarchical nature of DHS data. Maternal age (15–24, 25–34, 35–49), maternal education (no formal education, primary, secondary, or higher), and maternal occupation (has no occupation, has occupation), the mother's marital status (single, married, other), wealth index (poor, middle, rich), child's sex (male, female), sex of the head of the household (male, female), Birth weight (Normal, High, or Low), delivery location (home, health facility), delivery mode (CS) (Yes, No), pregnancy type (single, multiple), birth interval (≤24, >24) breast feeding duration (ever breastfed, not currently breastfeeding, never breastfed, still breastfeed, others), give a youngster something besides breast milk (Yes, No). The number of ANC visits (<4, ≥4), shots of tetanus prior to birth (Yes, No), Ever received a vaccination (Yes, No), birth order (First-order, 2–4, greater than 4), the number of children still alive (less than or equal to three, more than three), Presence of a toilet (Yes, No). The community-level factors were country of residence (Burundi, Ethiopia, Kenya, Comoros, Malawi, Mozambique, Rwanda, Tanzania, Uganda, Zambia, Zimbabwe), place of residence (Urban, Rural), community women's illiteracy (Low, High), and community poverty (Low, High) (Figure 1; Table 2).
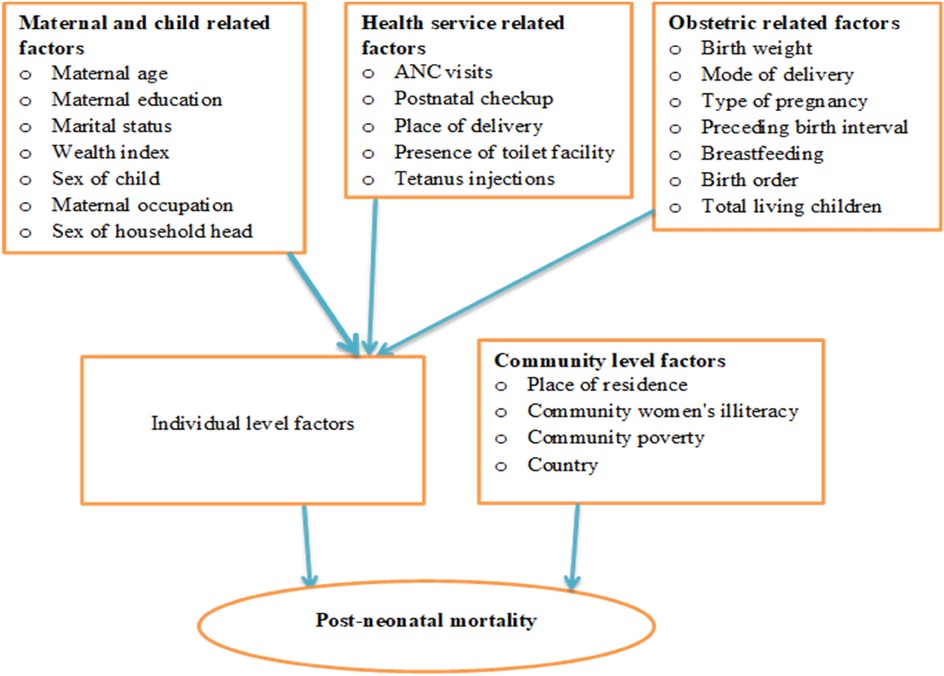
Figure 1. Conceptual framework for variables associated with post-neonatal mortality in East Africa.
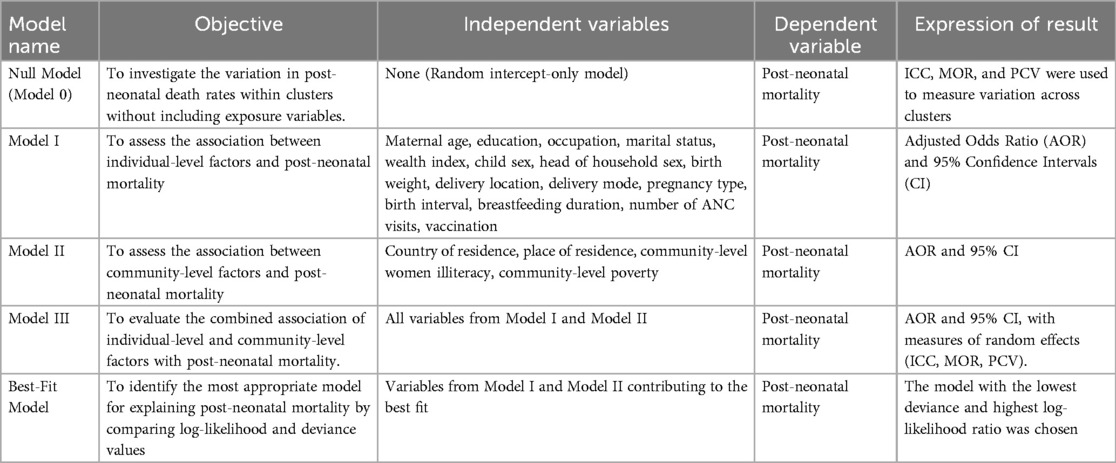
Table 2. Summary of model name, objective, independent variables, dependent variable, and expression of result.
Description of independent variables
Wealth index
The wealth index is a composite indicator of a household's total standard of living. The wealth index is created using conveniently collected data on the items that a household owns, like televisions and bicycles, the materials used to build a home, the types of water access, and the availability of sanitary facilities (30).
Community-level women illiteracy
Data on respondents’ educational attainment is used to calculate the percentage of women with at least a primary education. Following the calculation of cross-tabulating the individual level of women's education with the cluster number (v001), it was then classified using the national mean value: low community level women's illiteracy (communities with ≥50% of the national mean value of women's education) and high community-level women's illiteracy (communities with <50% of the national mean value of women's education).
Community-level poverty
It is produced by taking into account the percentage of women in the rich and middle-class categories. It was then categorized using the national mean value of the wealth index following the computation of the cross-tabulating individual-level combined wealth index with the cluster number (v001): low community-level poverty (communities with ≥50% of the national mean value of the community wealth index) and high community-level poverty (communities with <50% of the national mean value of the community wealth index).
Data management and statistical analysis
The data extracted from recent DHS data sets were cleaned, entered, and analyzed with the statistical software STATA/SE version 14. The DHS data contains nested clusters of variables, and the similarities between variables inside a cluster are greater than those between variables outside of it. To use a standard logistic regression model, the assumptions of independent data and equal variance across clusters were broken. This suggests that an advanced model must be used to account for between-cluster variations (31).
In light of this, post-neonatal mortality was determined by using multilevel mixed-effects logistic regression to identify the associated factors. Four models are used in multilevel mixed effect logistic regression: model I (which only includes individual level variables), model II (which only includes community level factors), and model III (which includes both individual and community level variables) (32). The null model, which does not include exposure factors, was employed to investigate the variation in post-neonatal death rates within the cluster.
The association between community-level and individual-level variables and the outcome variable (Model II) and Model I, respectively, was evaluated. The association between the individual and community-level factors and the outcome variable (post-neonatal mortality) was fitted simultaneously in the final model, or Model III. The models were compared using the deviance and log-likelihood tests; the model with the highest log-likelihood ratio and the lowest deviance was found to be the best-fitting one. Additionally, the variance inflation factor (VIF) was used to test for multicollinearity. In this analysis, missing data were addressed using the STATA command “drop if variable ==.” which ensures the exclusion of observations with missing values for the specified variable(s) from the analysis. The results show that there was no significant multicollinearity across the independent variables, with a mean VIF of 1.74 and a VIF of less than five for each independent variable.
Random effects
The random effects or measures of variation of the outcome variables were measured using the intra-class correlation coefficient (ICC), proportional change in variance (PCV), and median odds ratio (MOR). A proportional change in variance (PCV) and intra-class correlation coefficient (ICC) were calculated to determine the difference between the clusters. Using clusters as a random variable, the ICC indicates that the difference in post-neonatal death rates across clusters can be calculated as follows: . When two clusters are randomly selected, using clusters as a random variable, the MOR is the median value of the odds ratio between the area with the highest risk and the area of the lowest risk for post-neonatal mortality: MOR = e0.95√VC.
Additionally, the PCV shows how variables account for the variation in the prevalence of post-neonatal mortality, which is calculated as: ; where VC is the cluster level variance and Vnull is the variance of the null model (32–34). The likelihood of post-neonatal mortality was estimated using random effects and independent variables at the individual and community levels. With a p-value of less than 0.05, the adjusted odds ratio (AOR) and 95% confidence intervals were used to judge it and show its strength. Because the data set is nested, a deviance = −2 log likelihood ratio was used to compare the models; the model with the lowest deviance was chosen as the best-fit model. By calculating the variance inflation factors (VIF), the multi-collinearity of the variables employed in the models was confirmed, and the results were found to be within reasonable bounds of one to ten.
Results
Socio-demographic and economic characteristics of post-neonates in East Africa
The analysis comprised 225,641 live births, of which 113,708 were male and 111,933 were female. The majority of the babies (32.16%) were born to moms who were unemployed. 73,468 (45.41%) babies were born whose mothers attended less than four antenatal care visits during their pregnancy, and more than one-third (75.61%) of the participants were born to mothers who lived in rural areas of east Africa. Over half (54.91%) of the babies were delivered to mothers who didn't have a high degree of literacy in their community (Table 3).
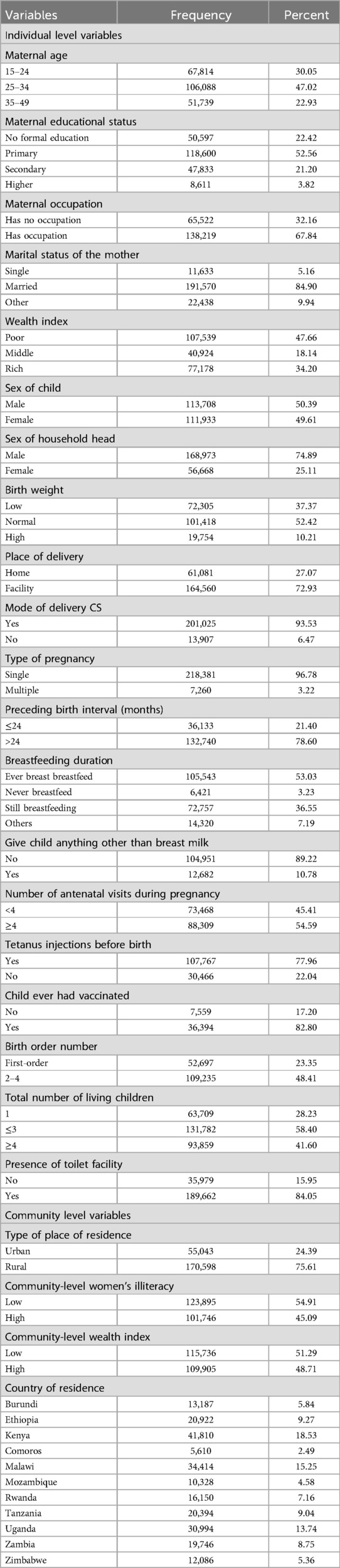
Table 3. Socio-demographic and economic characteristics of respondents in east Africa, DHS 2014–2022.
Prevalence of post-neonatal mortality among post-neonates in east African countries
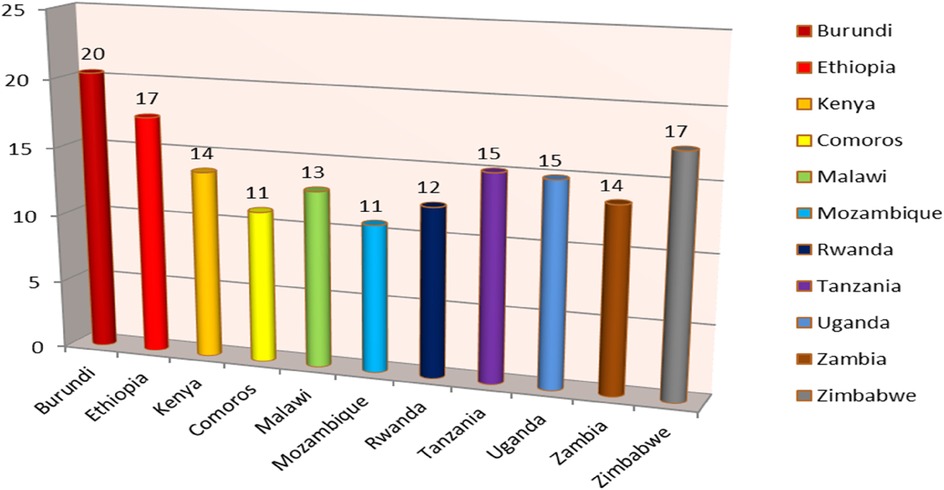
In East Africa, the prevalence of post-neonatal mortality was 15 per 1,000 live births. In east Africa, the prevalence of post-neonatal mortality has been found to be 13 and 15 deaths per 1,000 live births, respectively, in urban and rural areas (Figure 2). In East Africa, Burundi had the greatest rate of post-neonatal mortality (20 deaths per 1,000 live births) while Comoros had the lowest rate (11 deaths per 1,000 live births) (Figure 3).
Random effect and model fitness
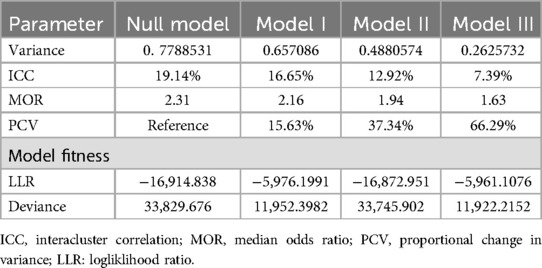
In order to test whether the information supported the choice to evaluate randomness at the community level, a null model was conducted. The null model's findings demonstrated that the post-neonatal death rate differed greatly between communities, with variance = 0.7788531 and a p value of <0.001. According to the null model's ICC value, variance across clusters accounted for 19.14% of the variation in post-neonatal mortality, whereas within-cluster variation accounted for 80.86% of the variation. The probabilities of post-neonatal mortality in the null model were 2.31 times different between higher and lower risk clusters. According to Model I's intraclass correlation value, 16.65% of the variations in post-neonatal mortality were accountable for the variations between communities. Then we built Model II using only community-level variables in the null model; based on the ICC value from Model II, cluster distinctions explained 12.92% of the variation in post-neonatal mortality. In the final model (model III), the risks of post-neonatal mortality varied 1.63 times across low and high risk clusters. This model attributed approximately 66.29% of the variation in odds of post-neonatal mortality to both individual and community-level determinants (PCV = 66.29%) (Table 4).
Factors associated with post-neonatal mortality in East Africa
Maternal age (15–24), maternal education (no formal education), low birth weight, type of pregnancy (multiple), not receiving tetanus shots prior to birth, birth order (>4), number of living children (>3), and countries (Burundi and Uganda) were significantly associated with post-neonatal mortality in the best-fit model of multivariable multilevel logistic regression at a p-value of <0.001.
Children born to mothers between the ages of 15 and 24 had a 1.58 higher risk of post-neonatal death than children born to mothers between the ages of 25 and 34 (AOR = 1.58, 95% CI: 1.32, 1.90). The odds of post-neonatal death were 1.82 times greater for babies born to mothers with no formal education (AOR = 1.82, 95% CI: 1.14, 2.92) than for babies born to mothers with higher levels of education. Low birth weight babies had a 1.59 times greater chance of dying between the ages of 1 and 11 months compared to children born at a normal birth weight (AOR = 1.58, 95% CI: 1.25, 2.01). The odds of post-neonatal death were 3.09 times higher for post-neonates born of multiple pregnancies than for those born of a single pregnancy (AOR = 3.09, 95%.
Compared to newborns delivered to women who had tetanus shots before to delivery, the odds of post-neonatal mortality were 1.23 times higher for babies born to mothers who did not receive tetanus injections (AOR = 1.23; 95% CI: 1.06–1.42). Compared to babies in the 2–4 birth order, those in the first birth order had a 5.68 higher chance of dying between the ages of 1 and 11 months (AOR = 5.68, 95% CI: 4.48, 7.24). Children born to mothers in Burundi (AOR = 1.48, 95% CI: 1.11, 1.98) and Uganda (AOR = 1.33, 95% CI: 1.03, 1.73) had 1.48 and 1.33 times a higher risk of post-neonatal mortality, respectively, than babies born to mothers in Ethiopia (Table 5).
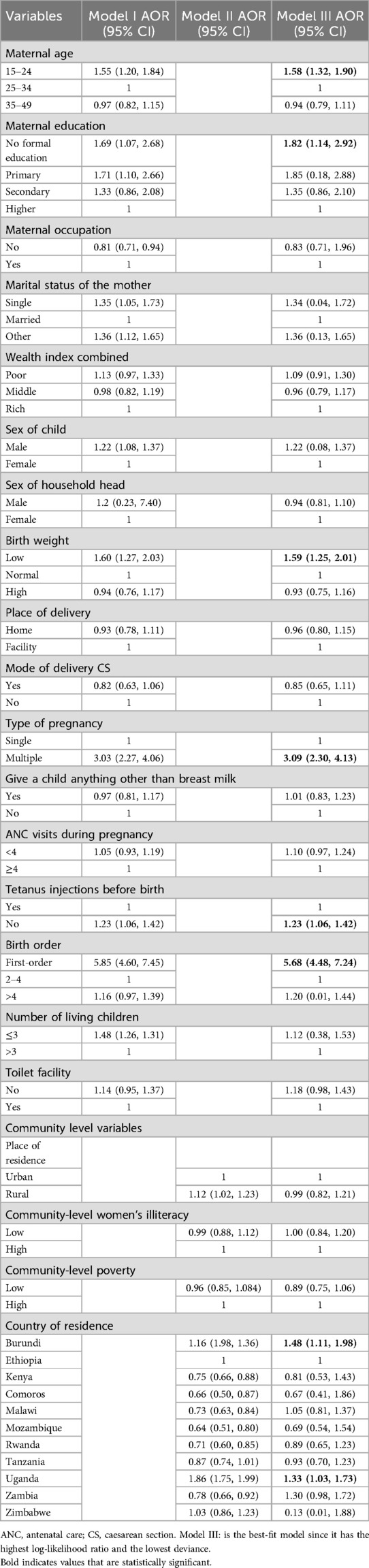
Table 5. Multivariable multilevel logistic regression analysis of individual-level and community level factors associated with post-neonatal mortality in east Africa, DHS 2014–2022.
Discussion
In developing countries like East Africa, post-neonatal deaths play a significant role in increasing childhood mortality. The purpose of this study was to determine the prevalence and determinants of post-neonatal mortality in East Africa. In East Africa, the prevalence of post-neonatal mortality was found to be 15 post-neonatal mortality per 1,000 live births. The finding is higher than the previous studies conducted in north African countries such as South Africa (35) and Gambia (36). The higher prevalence of post-neonatal mortality in this study than previous findings in South Africa and Gambia could be due to differences in socio-economic status and variability in health infrastructure and health policy.
The finding is lower than the previous studies conducted in African countries, such as Ethiopia (29), Kenya (37), and Tanzania (38). These variations might be due to differences in aggregate data and individual data. We used appended or aggregated data from individual countries that is averaged by geographic area and year. So individual data are disaggregated results which show the highest individual results compared to appended data.
In the multivariable logistic regression; Maternal age (15–24), maternal education (No formal education), low birth weight, type of pregnancy (multiple), not receiving tetanus shots prior to birth, Birth order (first birth order), Burundi, and Uganda were shown to be strongly associated with post-neonatal mortality in East Africa.
A significant predictor of post-neonatal death in this study was the mother's age. The odds of post-neonatal mortality were 1.58 times higher among babies born to mothers aged 15 and 24 years compared to babies born to mothers in age groups between 25 and 34. This finding is consistent with earlier findings (29, 39). The association between post-neonatal mortality and belonging to the 15–24 year old maternal age group could perhaps stem that the woman was not yet fully developed physically or physiologically for pregnancy. Along with physical or physiological immaturity, another factor can be related to a lack of prior childcare experience. Furthermore, post-neonatal deaths are more frequent in younger mothers because they are more likely to have preterm deliveries, low birth weight babies, and babies with congenital abnormalities (40, 41).
Compared to newborns born to mothers with greater levels of education, the risks of post-neonatal mortality were 1.82 times higher for babies born to mothers with no formal education. The results of earlier studies corroborate the conclusions of this finding (42, 43). This could be a feasible explanation for why raising maternal education levels is one of the most crucial steps to enhancing not just maternal and child health but also household production and the mother's and family's socio-emotional status (44). Achieving good maternal education will enhance women's socioeconomic status and health outcomes, which will have a favorable impact on child survival.
In relation to birth weight, Low-birth-weight babies had a 1.59 times greater chance of passing away between the ages of 1 and 11 months than babies born at a normal weight. This result is consistent with prior investigations (43, 45). Prematurity, intrauterine growth restriction, or both might result in low birth weight. Thus, one explanation for low birth weight children could be because they have immature organs and medical illnesses such as down syndrome, congenital heart disease, and diabetes mellitus (DM), which could raise their risk of death in the post-neonatal period (46).
The odds of post-neonatal mortality were 3.09 times higher among multiple pregnancies compared with singleton pregnancies. This finding is supported by the study findings (47, 48). This might be a logical explanation for why newborns from multiple pregnancies typically have restricted growth, poor Apgar scores, and extremely low birth weights. Furthermore, complications during pregnancy, labor, and postpartum are more likely in multiple pregnancies. In addition, due to increased food consumption, multiple pregnancies result in lower weight competition (49). The odds of post-neonatal mortality were 1.23 times higher among babies born to mothers who did not receive tetanus injections compared babies delivered to mothers who received tetanus shots before birth. The outcome of this study is in line with findings (50, 51). A possible explanation for this is that the tetanus vaccine creates antibodies that are protective against post-neonatal tetanus.
First-born babies had a 5.68 times higher risk of dying between the ages of 1 and 11 months as compared to children in birth orders two to four. This finding is consistent with (29, 52, 53). One explanation for this could be that babies born as firstborns are more vulnerable to pregnancy-related problems such Antepartum Hemorrhage (APH), preeclampsia, preterm, and fetal distress, which can raise their chance of dying between the ages of 1–11 months (54).
Furthermore, statistically, geographical regions (countries in East Africa) were associated with post-neonatal mortality. The odds of post-neonatal mortality were 1.48 times higher among babies born to mothers in Burundi and 1.33 times higher in Uganda compared to the reference country Ethiopia. The possible justification might be due to the difference in socioeconomic status, the health system, and health infrastructure variations.
The main strength of this study is the use of data from an adequately representative sub continental population. This is the first DHS-based study on post-neonatal mortality and factors in East Africa, as far as we are aware. The study's conclusions have a big impact on policy, especially when it comes to figuring out what measures are needed to consistently lower post-neonatal mortality. While our study highlights the influence of geographic disparities in outcomes, we acknowledge that the reliance on secondary DHS data precludes the inclusion of nuanced variables, such as maternal psychological health and cultural practices, which might significantly influence outcomes. Furthermore, we recognize that our analysis does not delve into the health system differences between countries, which could also play a critical role in shaping these disparities.
Conclusion
According to this study, post-neonatal mortality is higher in developing countries, particularly in East Africa. Post-neonatal mortality was influenced by factors such as earliest gestational ages, multiple pregnancies, low birth weight, lack of formal education, failure to receive tetanus shots before birth, first birth order, and birthplaces in Burundi and Uganda. Therefore, emphasis should be given on children born to mothers in the lowest age group, those born of multiple pregnancies, without formal educations, who did not receive tetanus shots prior to birth, and who were born in the first birth order.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.dhsprogram.com.
Author contributions
AZ: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. DA: Investigation, Methodology, Writing – original draft. WN: Software, Supervision, Writing – review & editing. TB: Formal Analysis, Investigation, Methodology, Writing – original draft. EF: Conceptualization, Methodology, Writing – original draft. AK: Data curation, Formal Analysis, Writing – original draft. TB: Software, Supervision, Writing – review & editing. SF: Investigation, Methodology, Writing – review & editing. BA: Resources, Visualization, Writing – original draft. SM: Software, Visualization, Writing – review & editing. AE: Funding acquisition, Investigation, Writing – original draft. TT: Investigation, Methodology, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to the DHS programmes for letting us use the relevant DHS data in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. (2019) 7(6):e710–20. doi: 10.1016/S2214-109X(19)30163-9
2. Reidpath DD, Allotey P. Infant mortality rate as an indicator of population health. J Epidemiol Community Health. (2003) 57(5):344–6. doi: 10.1136/jech.57.5.344
3. Lewis ME, Gowland R. Brief and precarious lives: infant mortality in contrasting sites from medieval and post-medieval England (AD 850–1859). Am J Phys Anthropol. (2007) 134(1):117–29. doi: 10.1002/ajpa.20643
4. Zewdie SA, Adjiwanou V. Multilevel analysis of infant mortality and its risk factors in South Africa. Int J Popul Stud. (2017) 3(2):43–56. doi: 10.18063/ijps.v3i2.330
5. Heft-Neal S, Burney J, Bendavid E, Burke M. Robust relationship between air quality and infant mortality in Africa. Nature. (2018) 559(7713):254–8. doi: 10.1038/s41586-018-0263-3
6. Frey RS, Field C. The determinants of infant mortality in the less developed countries: a cross-national test of five theories. Soc Indic Res. (2000) 52:215–34. doi: 10.1023/A:1007093631977
7. Kotsadam A, Østby G, Rustad SA, Tollefsen AF, Urdal H. Development aid and infant mortality. Micro-level evidence from Nigeria. World Dev. (2018) 105:59–69. doi: 10.1016/j.worlddev.2017.12.022
8. Sharrow D, Hug L, You D, Alkema L, Black R, Cousens S, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN inter-agency group for child mortality estimation. Lancet Glob Health. (2022) 10(2):e195–206. doi: 10.1016/S2214-109X(21)00515-5
9. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. (2014) 384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7
10. Demisse AG, Alemu F, Gizaw MA, Tigabu Z. Patterns of admission and factors associated with neonatal mortality among neonates admitted to the neonatal intensive care unit of university of Gondar hospital, northwest Ethiopia. Pediatric Health Med Ther (2017) 8:57–64. doi: 10.2147/PHMT.S130309
11. Dadi AF. A systematic review and meta-analysis of the effect of short birth interval on infant mortality in Ethiopia. PLoS One. (2015) 10(5):e0126759. doi: 10.1371/journal.pone.0126759
12. Helleringer S, Liu L, Chu Y, Rodrigues A, Fisker AB. Biases in survey estimates of neonatal mortality: results from a validation study in urban areas of Guinea-Bissau. Demography. (2020) 57(5):1705–26. doi: 10.1007/s13524-020-00911-6
13. Wang H, Bhutta ZA, Coates MM, Coggeshall M, Dandona L, Diallo K, et al. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388(10053):1725–74. doi: 10.1016/S0140-6736(16)31575-6
14. Bart KJ, Lin KF-YC. Vaccine-preventable disease and immunization in the developing world. Pediatr Clin N Am. (1990) 37(3):735–56. doi: 10.1016/S0031-3955(16)36913-9
15. Adetunji JA. Infant mortality in Nigeria: effects of place of birth, mother’s education and region of residence. J Biosoc Sci. (1994) 26(4):469–77. doi: 10.1017/S002193200002160X
16. Högberg U, Holmgren P. Infant mortality of very preterm infants by mode of delivery, institutional policies and maternal diagnosis. Acta Obstet Gynecol Scand. (2007) 86(6):693–700. doi: 10.1080/00016340701371306
17. Dube L, Taha M, Asefa H. Determinants of infant mortality in community of Gilgel Gibe field research center, southwest Ethiopia: a matched case control study. BMC Public Health. (2013) 13:401. doi: 10.1186/1471-2458-13-401
18. Rutstein SO. Effects of preceding birth intervals on neonatal, infant and under-five years mortality and nutritional status in developing countries: evidence from the demographic and health surveys. Int J Gynaecol Obstet. (2005) 89:S7–24. doi: 10.1016/j.ijgo.2004.11.012
19. Peña R, Wall S, Persson L-A. The effect of poverty, social inequity, and maternal education on infant mortality in Nicaragua, 1988-1993. Am J Public Health. (2000) 90(1):64. doi: 10.2105/AJPH.90.1.64
20. Hobcraft JN, McDonald JW, Rutstein SO. Socio-economic factors in infant and child mortality: a cross-national comparison. Popul Stud. (1984) 38(2):193–223. doi: 10.1080/00324728.1984.10410286
21. Desai S, Alva S. Maternal education and child health: is there a strong causal relationship? Demography. (1998) 35(1):71–81. doi: 10.2307/3004028
22. Gruber J, Hendren N, Townsend RM. The great equalizer: health care access and infant mortality in Thailand. Am Econ J Appl Econ. (2014) 6(1):91–107. doi: 10.1257/app.6.1.91
23. Frankenberg E. The effects of access to health care on infant mortality in Indonesia. Health Transit Rev. (1995) 5:143–63.10159677
24. Cabrera R. The influence of maternal age, birth order and socioeconomic status on infant mortality in Chile. Am J Public Health. (1980) 70(2):174–7. doi: 10.2105/AJPH.70.2.174
25. Singh A, Singh A, Mahapatra B. The consequences of unintended pregnancy for maternal and child health in rural India: evidence from prospective data. Matern Child Health J. (2013) 17:493–500. doi: 10.1007/s10995-012-1023-x
26. Scrimshaw SC. Infant mortality and behavior in the regulation of family size. Popul Dev Rev. (1978) 4:383–403. doi: 10.2307/1972856
27. Uthman OA, Uthman MB, Yahaya I. A population-based study of effect of multiple birth on infant mortality in Nigeria. BMC Pregnancy Childbirth. (2008) 8:41. doi: 10.1186/1471-2393-8-41
28. Tesema GA, Seifu BL, Tessema ZT, Worku MG, Teshale AB. Incidence of infant mortality and its predictors in East Africa using Gompertz gamma shared frailty model. Arch Public Health. (2022) 80(1):195. doi: 10.1186/s13690-022-00955-7
29. Fentaw KD, Fenta SM, Biresaw HB, Yalew MM. Factors associated with post-neonatal mortality in Ethiopia: using the 2019 Ethiopia mini demographic and health survey. PLoS One. (2022) 17(7):e0272016. doi: 10.1371/journal.pone.0272016
30. Demographic and Health Surveys Program. Available online at: https://www.dhsprogram.com/topics/wealth-index/ (Accessed September 16, 2023).
31. Asmamaw DB, Belachew TB, Negash WD. Multilevel analysis of early resumption of sexual intercourse among postpartum women in sub-Saharan Africa: evidence from demographic and health survey data. BMC Public Health. (2023) 23(1):733. doi: 10.1186/s12889-023-15687-8
32. Sommet N, Morselli D. Correction: keep calm and learn multilevel logistic modeling: a simplified three-step procedure using Stata, R, Mplus, and SPSS. Int Rev Soc Psychol. (2017) 30(1):1–3. doi: 10.5334/irsp.90
33. Belay DG, Aragaw FM, Teklu RE, Fetene SM, Negash WD, Asmamaw DB, et al. Determinants of inadequate minimum dietary diversity intake among children aged 6–23 months in sub-Saharan Africa: pooled prevalence and multilevel analysis of demographic and health survey in 33 sub-Saharan African countries. Front Nutr. (2022) 9:894552. doi: 10.3389/fnut.2022.894552
34. Tesema GA, Mekonnen TH, Teshale AB. Individual and community-level determinants, and spatial distribution of institutional delivery in Ethiopia, 2016: spatial and multilevel analysis. PLoS One. (2020) 15(11):e0242242. doi: 10.1371/journal.pone.0242242
35. Nannan N, Dorrington R, Laubscher R, Zinyakatira N, Prinsloo M, Darikwa T, et al. Under-5 Mortality Statistics in South Africa. Cape Town: South African Medical Research Council (2010).
36. Miyahara R, Jasseh M, Mackenzie GA, Bottomley C, Hossain MJ, Greenwood BM, et al. The large contribution of twins to neonatal and post-neonatal mortality in the Gambia, a 5-year prospective study. BMC Pediatr. (2016) 16:39. doi: 10.1186/s12887-016-0573-2
37. Kaguthi G, Nduba V, Borgdorff MW, Verver S. Predictors of post neonatal mortality in Western Kenya: a cohort study. Pan Afr Med J. (2018) 31(1):1. doi: 10.11604/pamj.2018.31.114.16725
38. Ogbo FA, Ezeh OK, Awosemo AO, Ifegwu IK, Tan L, Jessa E, et al. Determinants of trends in neonatal, post-neonatal, infant, child and under-five mortalities in Tanzania from 2004 to 2016. BMC Public Health. (2019) 19:1243. doi: 10.1186/s12889-018-6343-3
39. Tesema GA, Teshale AB, Tessema ZT. Incidence and predictors of under-five mortality in East Africa using multilevel weibull regression modeling. Arch Public Health. (2021) 79(1):196. doi: 10.1186/s13690-021-00727-9
40. Kang G, Lim JY, Kale AS. Adverse effects of young maternal age on neonatal outcomes. Singapore Med J. (2015) 56(3):157. doi: 10.11622/smedj.2014194
41. Sari RP, Astuti VW. Teenage pregnancy in Indonesia: determinants and outcomes. J Aisyah J Ilmu Kesehatan. (2022) 7(3):949–56. doi: 10.30604/jika.v7i3.1258
42. Armstrong Schellenberg JR, Nathan R, Abdulla S, Mukasa O, Marchant TJ, Tanner M, et al. Risk factors for child mortality in rural Tanzania. Trop Med Int Health. (2002) 7(6):506–11. doi: 10.1046/j.1365-3156.2002.00888.x
43. Ezeh OK, Agho KE, Dibley MJ, Hall JJ, Page AN. Risk factors for postneonatal, infant, child and under-5 mortality in Nigeria: a pooled cross-sectional analysis. BMJ Open. (2015) 5(3):e006779. doi: 10.1136/bmjopen-2014-006779
44. Ahmed S, Creanga AA, Gillespie DG, Tsui AO. Economic status, education and empowerment: implications for maternal health service utilization in developing countries. PLoS One. (2010) 5(6):e11190. doi: 10.1371/journal.pone.0011190
45. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10(1):1–14. doi: 10.1186/1742-4755-10-1
47. Gebremedhin S. Multiple births in sub-Saharan Africa: epidemiology, postnatal survival, and growth pattern. Twin Res Hum Genet. (2015) 18(1):100–7. doi: 10.1017/thg.2014.82
48. Nwankwo T, Aniebue U, Ezenkwele E, Nwafor M. Pregnancy outcome and factors affecting vaginal delivery of twins at university of Nigeria teaching hospital, Enugu. Niger J Clin Pract. (2013) 16(4):490–5. doi: 10.4103/1119-3077.116895
49. Fenta SM, Fenta HM. Risk factors of child mortality in Ethiopia: application of multilevel two-part model. PLoS One. (2020) 15(8):e0237640. doi: 10.1371/journal.pone.0237640
50. Mekonnen Y, Tensou B, Telake DS, Degefie T, Bekele A. Neonatal mortality in Ethiopia: trends and determinants. BMC Public Health. (2013) 13(1):483. doi: 10.1186/1471-2458-13-483
51. Kc A, Jha AK, Shrestha MP, Zhou H, Gurung A, Thapa J, et al. Trends for neonatal deaths in Nepal (2001–2016) to project progress towards the SDG target in 2030, and risk factor analyses to focus action. Matern Child Health J. (2020) 24:5–14. doi: 10.1007/s10995-019-02826-0
52. Kayode GA, Adekanmbi VT, Uthman OA. Risk factors and a predictive model for under-five mortality in Nigeria: evidence from Nigeria demographic and health survey. BMC Pregnancy Childbirth. (2012) 12(1):10. doi: 10.1186/1471-2393-12-10
53. Singh R, Tripathi V. Maternal factors contributing to under-five mortality at birth order 1 to 5 in India: a comprehensive multivariate study. Springerplus. (2013) 2:284. doi: 10.1186/2193-1801-2-1
Keywords: determinants, East Africa, mortality, post-neonatal, prevalence
Citation: Zegeye AF, Asmamaw DB, Negash WD, Belachew TB, Fentie EA, Kidie AA, Baykeda TA, Fetene SM, Addis B, Maru Wubante S, Endawkie A and Tamir TT (2025) Prevalence and determinants of post-neonatal mortality in East Africa: a multilevel analysis of the recent demographic and health survey. Front. Pediatr. 13:1380913. doi: 10.3389/fped.2025.1380913
Received: 13 February 2024; Accepted: 10 January 2025;
Published: 23 January 2025.
Edited by:
Zhangbin Yu, Shenzhen People's Hospital, The Second Clinical Medical College of Jinan University, First Affiliated Hospital of Southern University of Science and Technology, ChinaReviewed by:
Chienchung Lee, Linkou Chang Gung Memorial Hospital, TaiwanJ. Mark Ansermino, University of British Columbia, Canada
Copyright: © 2025 Zegeye, Asmamaw, Negash, Belachew, Fentie, Kidie, Baykeda, Fetene, Addis, Maru Wubante, Endawkie and Tamir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alebachew Ferede Zegeye, YWxleGZlcmVkZTI0QGdtYWlsLmNvbQ==
†Present Address Tsegaw Amare Baykeda, School of Public Health, The University of Queensland, Brisbane, QLD, Australia
 Alebachew Ferede Zegeye
Alebachew Ferede Zegeye Desale Bihonegn Asmamaw
Desale Bihonegn Asmamaw Wubshet D. Negash
Wubshet D. Negash Tadele Biresaw Belachew
Tadele Biresaw Belachew Elsa Awoke Fentie
Elsa Awoke Fentie Atitegeb Abera Kidie
Atitegeb Abera Kidie Tsegaw Amare Baykeda
Tsegaw Amare Baykeda Samrawit Mihret Fetene
Samrawit Mihret Fetene Banchlay Addis
Banchlay Addis Sisay Maru Wubante
Sisay Maru Wubante Abel Endawkie
Abel Endawkie Tadesse Tarik Tamir
Tadesse Tarik Tamir