- 1Department of Immunology of College of Medicine, Wuhan Wuchang Hospital, Wuhan University of Science and Technology, Wuhan, China
- 2Department of Pharmacy, Beijing Hospital, Beijing, China
- 3Department of Pharmacy, Beijing Children’s Hospital, Capital Medical University, Beijing, China
- 4Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen, China
- 5Pediatric Department, Wuhan Asian General Hospital, Wuhan, China
- 6Pediatric Department, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 7Pediatric Department, Jinan Maternity and Child Care Hospital, Jinan, China
- 8Department of Otolaryngology Head and Neck Surgery, Shandong Maternity and Child Care Hospital, Jinan, China
- 9Department of Obstetrics and Gynecology, First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 10Department of Immunology, Tongji Medical College Huazhong University of Science and Technology, Wuhan, China
1 Introduction
The upper respiratory tract is a crucial site for host defense, as it is home to bacterial communities that both modulate host immune defense and serve as a reservoir of potential pathogens. During the first few years of life, much like the gastrointestinal tract microbiome, nasopharyngeal microbiota in young children changes from an immature state to a more diverse state as it matures to resemble the adult microbiota, resulting in a higher risk of respiratory illness in young children (1). According to a systematic analysis of respiratory infections in 33 provinces in China from 1990 to 2019, a consistently increasing trend in the number of RTi cases was observed. RTi cases in 2019 were 3%, 5%, and 11% higher than those in 2010, 2000, and 1990, respectively. The incidence rate of URTis was the largest in younger children indicating that the future URTi and LRTi prevention strategies should focus on maternal and child health, especially in young children (2). URTIs, although they are generally mild and resolve spontaneously, can significantly impact the quality of life, school attendance of children, and work absence for caregivers. Pediatricians and caregivers require prevention strategies to reduce the recurrence incidence in children prone to frequent RTIs to reduce medical visits. Risk factors that are significantly associated through odds ratio (OR) with recurrent respiratory tract infection include asthma [OR = 8.31 (P < 0.001)], allergy [OR = 2.31 (P < 0.001)], initial use of antibiotics [OR = 1.72 (P < 0.001)], breastfeeding duration <6 months [OR = 1.24 (P < 0.002)], and maternal body mass index 1.19 (P < 0.001) (3). In general, distinct microbial maturation patterns involved with early asymptomatic respiratory viral presence and dynamics in gene expression profiles (4) translate into either a beneficial microbiota or susceptibility to RTI development and/or asthma and/or allergic rhinitis. A “three-hit” model for chronic RTI development begins with an establishment of non-beneficial respiratory microbiota as the first hit, suffering RTi with varying degrees of severity as the second hit, and progression to a long-term inflammatory airway status as the third hit (5). The strategies for managing pediatric recurrent respiratory tract infections (RRTi) can be built on several key approaches. One approach focuses on modulating non-specific immune responses to strengthen the body's natural defenses against infectious agents. Another involves enhancing specific immune responses to directly combat respiratory pathogens. Additionally, managing inflammation through anti-inflammatory responses can help reduce infection- or pathogen-induced airway inflammation. Modulating respiratory microflora through the administration of probiotics that colonize the airway is also promising. This approach aims to improve the interaction between host commensal bacteria and the immune system, ultimately reducing susceptibility to RRTi.
To date, unnecessary antibiotics are widely prescribed for pediatric RTIs and most pediatric patients with RTIs do not receive guideline-recommended antibiotic classes in Chinese primary healthcare facilities (6). Meanwhile, parents often resort to self-medication with antibiotics for their children in China (7). Unfortunately, microbiome recovery lost diversity after short courses of antibiotics and can be delayed by azithromycin; the long-term effect of altered diversity, resistance, and composition is considered “antibiotic scarring” (8).
Well-documented evidence suggests that early antibiotic exposure affects the development of infant gut microbiota and the disturbances in the host increase the susceptibility to a variety of diseases later in life including respiratory infections (9). In addition, antibiotic use in early life preferentially impairs the development of lung mucosal-associated invariant T (MAIT) cells which plays an important role in recognizing a broad array of respiratory pathogens (10).
Antibiotic-induced microbial disruption in early life can be exacerbated by the vertical transmission of resistance genes from mother to offspring during pregnancy and lactation (11) and is associated with higher risks of childhood metabolic disorders (12); neurobehavioral conditions including autism spectrum disorder, intellectual disorder, language disorder, and epilepsy (13); and juvenile idiopathic arthritis (JIA) (14).
Although most recent reviews reveal variable outcomes, knowledge gaps, and insufficient evidence to recommend probiotics for the prevention or management of RTi conditions (15–18), a specific formula with consistent positive clinical results should be recommended and be considered a possible supportive approach in selected patients to improve their quality of life and reduce the burden of RTIs.
Oropharyngeal probiotic Bactoblis has clinical and real-world evidence for indications of selected respiratory disorders in children, adolescents, and adults. These clinical studies were technically reviewed to provide insight into the potential of oropharyngeal probiotics as a clinical modality targeted at recurrent and non-recurrent respiratory infectious diseases. As published data demonstrate that only a single formulation of oral probiotic species complies with the required stability requirements established by Chinese medical practitioners, other evidence was excluded from the examined information used as a basis for this consensus. Indeed, investigators of a New Zealand study of a Streptococcus salivarius K12 strain for prevention of the development of symptomatic Group A Streptococcus pharyngitis faced confounding factors arising from their choice of the final product used in the study, including contamination of the placebo formulation with active strain administered to the test group (19). These observations underscore the importance of selecting clinically validated probiotic formulas that meet established standards, to ensure efficacy and reliability in managing respiratory health.
Therefore, it is extremely important for healthcare professionals to be properly educated and updated on the knowledge of oropharyngeal probiotics even though their awareness toward gut probiotic has been well-established (20), with the purpose of assisting pediatricians or practitioners who are willing to offer their patients an alternative option to safely and effectively manage their respiratory conditions and appropriately recommend the use of oropharyngeal probiotics. The following professional recommendations are meant to be broadly applicable and should be viewed as the preferred alternative and adjunctive approach. However, they are not meant to replace standard approaches and clinical management strategies depend on individual clinical scenarios. This project aims to support decision-making in respiratory health based on scientific evidence and the daily life of both the pediatric patients with respiratory infections and their caregivers.
A meeting of our consensus panel consisting of clinical and scientific experts (pediatrician, obstetrician and gynecologist, oncologist, pharmacist, immunology and microbiology scientists) was convened to review, examine, select, and synthesize 38 evidence-based publications on oropharyngeal probiotics, which were organized into two main topics, which are the existing evidence-based science and the recommendation for future researches. Participants in the meeting jointly considered key questions and generated and approved the outcomes hereby summarized. We hope that this consensus statement will provide consensus views on the appropriate use of oropharyngeal probiotics and recommendations for future research associated with oropharyngeal probiotics and human respiratory health.
2 Expert consensus statement based on existing evidence-based science
2.1 Recurrent respiratory tract infections
Very few convincing dietary measures for reducing the frequency and clinical relevance of recurrent respiratory episodes in RTi-prone children have been developed. Until July 2024, professional societies and groups of experts technically reviewed 10 clinical trials conducted with oropharyngeal probiotics with documented efficacy for the management of RRTi published since 2012 with a total of 832 children participating. Oropharyngeal probiotic Bactoblis was given to subjects in the form of slowly dissolving oral lozenges, to be administered before bedtime after brushing their teeth every evening, and they were required to suck the lozenge until fully dissolved (approximately 4–5 min) and not to chew or directly swallow it. They were suggested not to drink or swallow any substance for at least 1 h after the administration of oropharyngeal probiotic lozenges. If the subjects were prescribed with antibiotics by study practitioners during RTIs, they are requested to continuously take the oropharyngeal probiotics during the days taking antibiotics, but making sure that oropharyngeal probiotics and antibiotics had been taken 2 h apart. Oropharyngeal probiotics with recommendations for use in clinical practices were discussed based on the evaluation of 10 trials in terms of population, burden of RRTi, current treatment strategies, probiotic dose, and administration schedule. Overall, oropharyngeal probiotic, as a general group, reduced the risk of developing new episodes of respiratory tract infections and antibiotic exposure in children with RRTi. A conditional recommendation based on level 2 evidence criteria graded by study type of randomized trials with consistent effect according to the latest World Gastroenterology Organisation Global Guidelines: Probiotics and Prebiotics 2024 (21), on the adjunctive use of oropharyngeal probiotics supportive of standard treatment in children with recurrent respiratory tract infections was made, in the context of the corresponding 10 clinical trials. Most of the 10 trials are homogeneous in regards to study subjects, dosage, schedule of administration, and formulation. Oropharyngeal probiotic Bactoblis has been found to have a good safety profile. These findings revealed that compared to controls, people with a history of recurrent RTIs receiving oropharyngeal probiotic Bactoblis had a significantly lower risk of new episodes of RTi during the study period reduced by around 90%. The protective benefit was observed during both the intervention period and maintained several months after the end of the intervention. The risk of antibiotic exposure was significantly higher among controls than among subjects administrating oropharyngeal probiotic Bactoblis. The limitations of the 10 studies were the use of non-blinded method and that the pathogen type and severity of RTIs were not defined by molecular tests and RTi-related quality of life. The type of antibiotic used by the subjects were not described in the article, and the size of the studies was generally small. However, the baseline clinical characteristics of the enrolled subjects were precisely defined in all of 10 trials. Among the 10 trials, 6 were published in English, of which 5 were carried out in Europe and 1 was carried out in China, and 4 were carried out in Ukraine and published in Ukrainian with the abstract in English and in most cases were published in Ukrainian journals. Clinical studies evidencing that oropharyngeal probiotic Bactoblis can provide clinical benefit on recurrent RTi management are listed in Table 1.
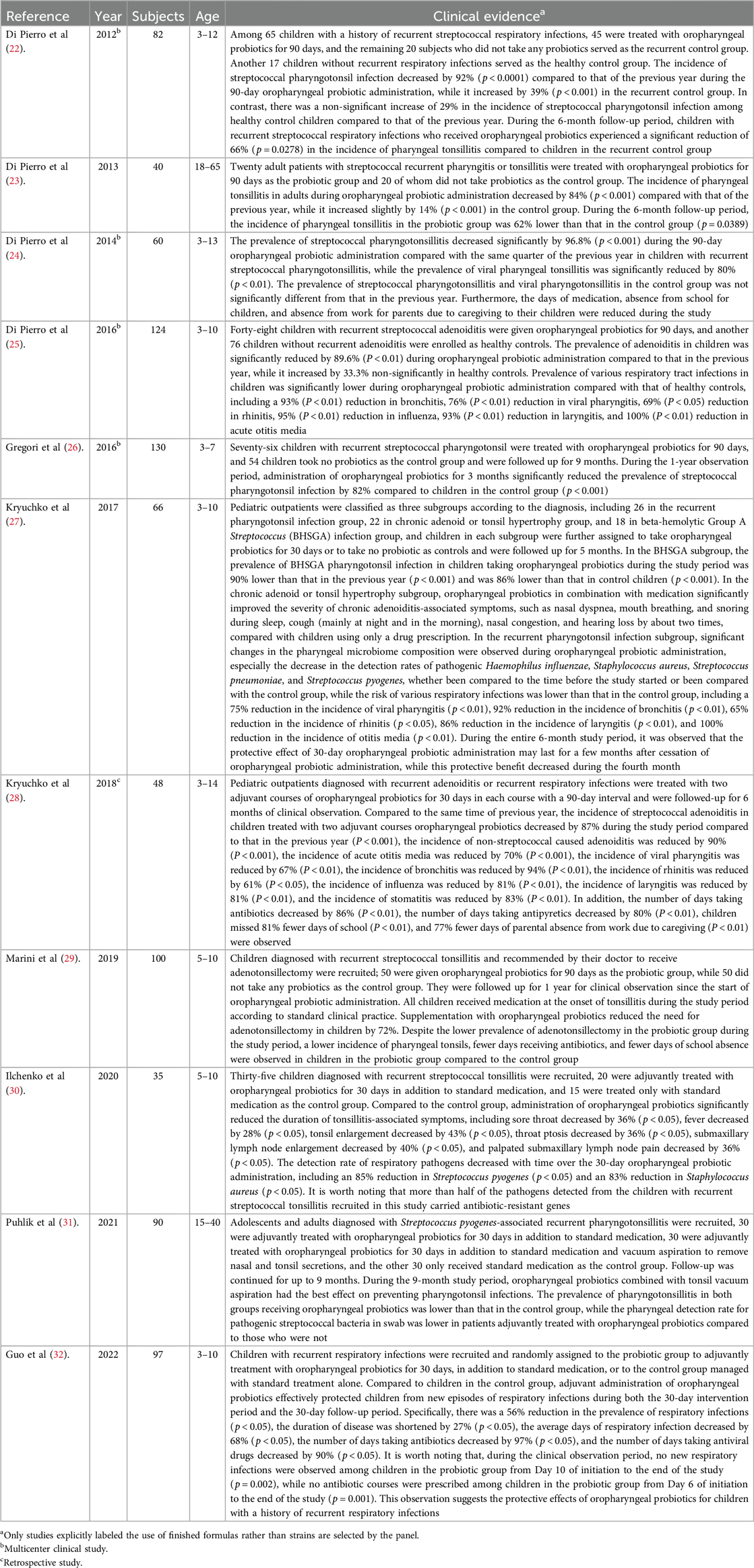
Table 1. Studies providing evidence that the oropharyngeal probiotic formula, Bactoblis, can provide clinical benefit on recurrent RTi management.
2.2 Otitis Media
Otitis media represents a significant burden on children, their families, and the healthcare system and is the major cause of hearing loss and serious life-long sequelae, such as behavior, attention (33), anxiety, learning, and speech-language problems in early and late childhood (34), if left untreated. Chronic and recurrent otitis media are recalcitrant to current therapies due to the formation of biofilms and intracellular biofilm pods by otopathogens on the middle ear mucosa and within the middle ear fluid (35). Antibiotics are generally used empirically for treating chronic suppurative otitis media, which may lead to the emergence of resistant bacterial strains (36). In addition, official recommendations differ regarding tympanostomy-tube placement that could favor the time to a first episode of acute otitis media and various episode-related clinical findings in young children with recurrent acute otitis media, but the rate of acute otitis media episodes, the percentage of episodes considered to be severe, and antimicrobial resistance among respiratory isolates were not significantly lower than with medical management (37). Relative abundances of potential pathogens, such as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis in the upper respiratory tract, might lead to further investigation into new preventive measurements for acute, secretory, and recurrent otitis media (38). The seven studies with evidence for the oropharyngeal probiotic formula, Bactoblis, providing a clinical benefit for otitis media management are listed in Table 2.
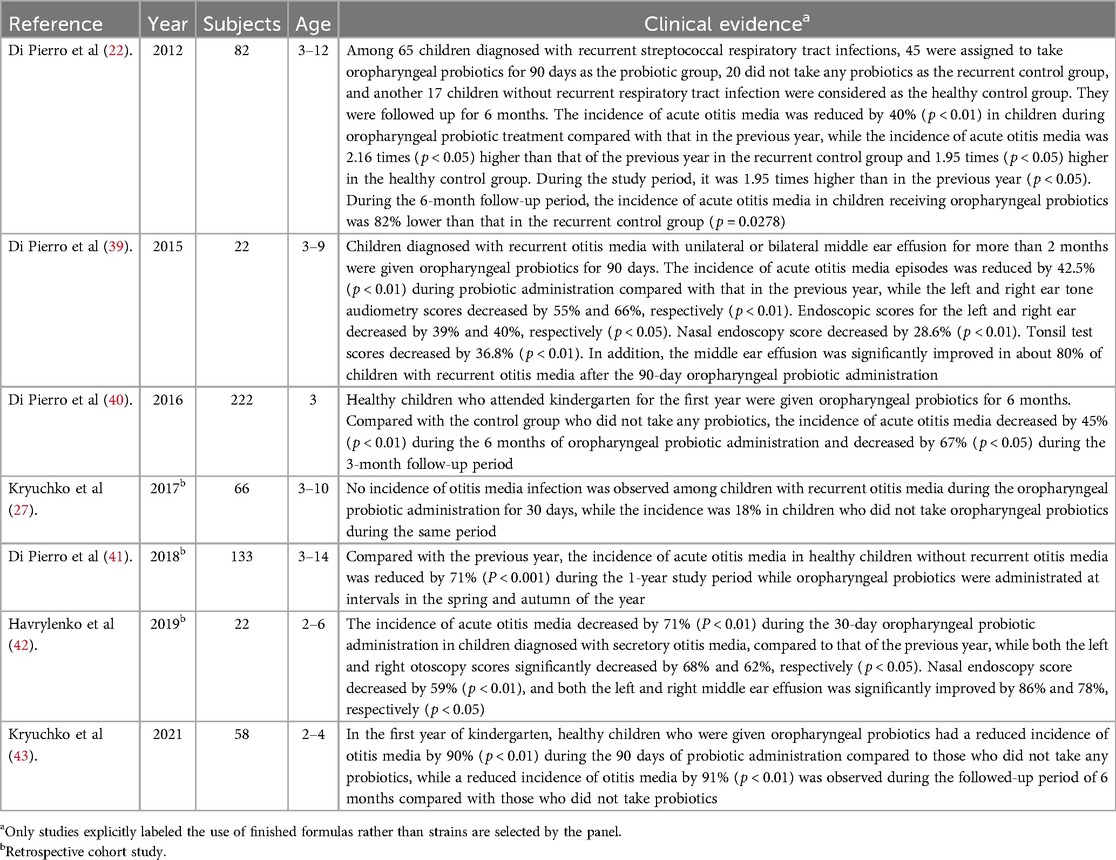
Table 2. Studies providing evidence that oropharyngeal probiotic Bactoblis can provide clinical benefit on otitis media management.
2.3 Acute respiratory tract infections
Respiratory viral infections are the most common type of acute respiratory infection, predisposing patients to secondary bacterial infections that often have a more severe clinical course. Antiviral immune responses induced by acute RTi are associated with dysbiosis in the respiratory tract, which in turn alters subsequent immune function against secondary bacterial infection or the dynamics of inter-microbial interactions, thereby enhancing the proliferation of potentially pathogenic bacterial species (44). Increased microbial diversity and growth rates of specific pathogens in the upper respiratory tract were observed. Oropharyngeal microbiota is a type specifically disrupted by the infection of influenza A virus (FluA), influenza B virus (FluB), respiratory syncytial virus (RSV), and human rhinovirus (HRV) (45), as well as omicron or other variant of SARS-CoV-2 viruses (46). Non-pathogenic commensal organisms colonized at nasopharynx and oropharynx possess the ability to interfere with the growth of potential pathogens such as S. pneumoniae, H. influenzae, and M. catarrhalis, the carriage of which increases during nasopharyngitis or pharyngitis, as well as symptomatic and asymptomatic viral URTi (47). Even though respiratory microbiota is shaped during the critical window of early life, season, and RTIs, evidence indicates a reduced niche differentiation preceding confirmed RTIs. This loss of ecological topography is further augmented by the start of daycare and linked to the consecutive development of symptomatic RTIs (48). In summary, restoring the loss of topography is linked to the prevention of subsequent development of RTi episodes. The four studies suggesting the clinical benefit of preventing acute RTi via administration of oropharyngeal probiotic Bactoblis are listed in Table 3.
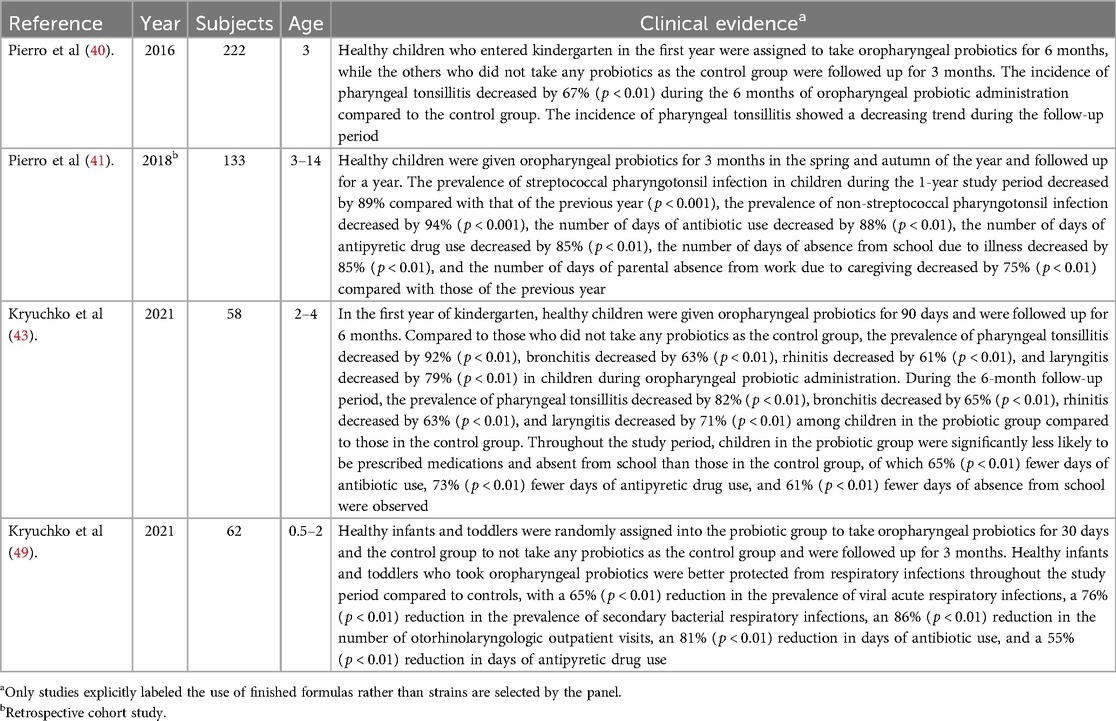
Table 3. Studies providing evidence of the clinical benefit of preventing acute RTi via administration of oropharyngeal probiotic Bactoblis.
2.4 Chronic adenoiditis and chronic tonsillitis
Repeated use of antibiotics is common during the treatment of tonsillitis, and prior antibiotic use is a major contributor to subsequent antibiotic prescribing (50), except for recurrent antibiotic prescriptions. Tonsillectomy remains a common pediatric surgery for recurrent and chronic tonsillitis or recurrent otitis media; however, severe postoperative pain is common, and some patients will have postoperative complications of bleeding (51). Importantly, a cohort study of 1.2 million patients followed up for 30 years showed that childhood adenoidectomy or tonsillectomy was associated with a significantly increased relative risk of respiratory infections and allergies later in life. Increases in long-term absolute disease risks were considerably larger than changes in risk for the disorders these surgeries aim to treat, suggesting that it is important to consider long-term risks when making decisions to perform tonsillectomy or adenoidectomy (52). Children who had adenoidectomy for adenoid-related diseases are mostly due to obstructive sleep-disordered breathing, otitis media with effusion, and chronic sinusitis, while respiratory pathogens H. influenzae, S. aureus, S. pneumoniae, and M. catarrhalis are commonly found in adenoids (53). Surgical removal of adenoids and tonsils to treat obstructed breathing or recurrent middle ear infections remains a common pediatric procedure; however, a meta-analysis of current literature that included more than a thousand subjects demonstrated that pediatric sleep apnea is often not cured by tonsillectomy and adenoidectomy (54).
Chronic adenoiditis occurs frequently in children and is complicated by the subsequent development of recurrent or chronic middle ear diseases, such as recurrent acute otitis media, persistent otitis media with effusion, and chronic otitis media that fail to respond to traditional antibiotic therapy, which may predispose a child to long-term functional sequalae and auditory impairment (55).
In the treatment of children with chronic adenoiditis, it is necessary to take into account the features of the normal microbiota of the nasopharynx, by acting on opportunistic and pathogenic microorganisms. Favorable conditions for stimulating the growth and development of representatives of the indigenic microbiota can be created, which in turn will contribute to the patient's speedy recovery from chronic adenoiditis and absence of relapses (56). Further investigation of individual microbiomes in a longitudinal design with implantation of protective oropharyngeal probiotics may have the potential to lead to new strategies as an alternative to adenoidectomy. The four studies demonstrating that the oropharyngeal probiotic Bactoblis can provide clinical benefit on chronic adenoiditis and tonsillitis management are listed in Table 4.
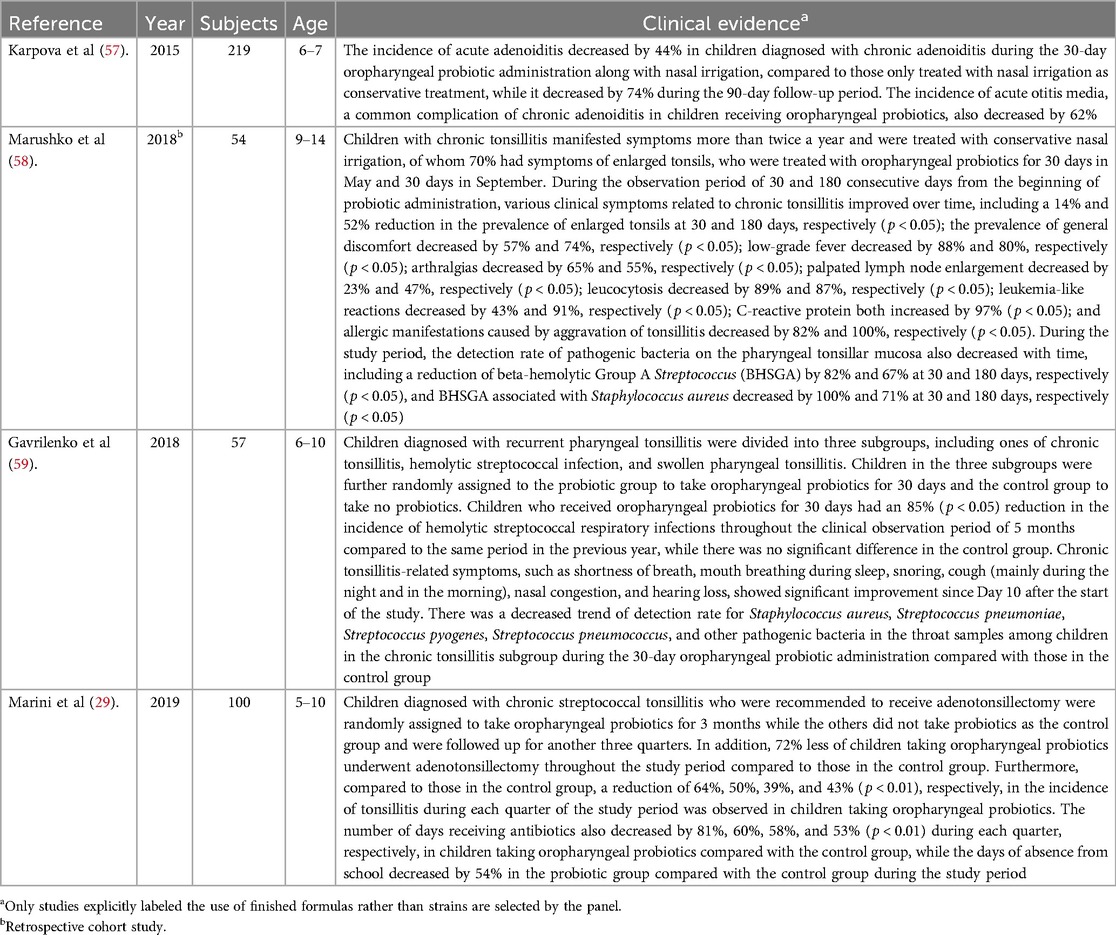
Table 4. Studies providing evidence that oropharyngeal probiotic Bactoblis can provide clinical benefit on chronic adenoiditis and tonsillitis management.
2.5 PFAPA (periodic fever, aphthous stomatitis, pharyngitis, and adenitis) syndrome
The microbiota of the tonsils removed from PFAPA patients differed significantly from those of the non-PFAPA patients, indicating that tonsillar microbiota may play a role in triggering the inflammatory processes that lead to symptoms of PFAPA (60). Furthermore, tonsil dysbiosis may be associated with altered antimicrobial peptide expression on tonsil surface epithelium as in other autoinflammatory diseases which was not evident in recurrent tonsillitis (61). The two studies evidencing that oropharyngeal probiotic Bactoblis can provide clinical benefit on PFAPA management are listed in Table 5.
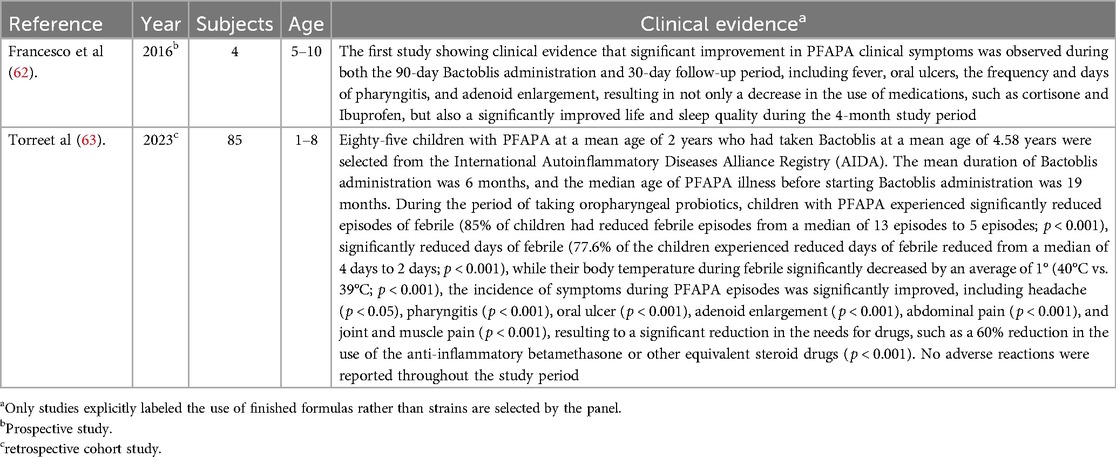
Table 5. Studies evidencing that oropharyngeal probiotic Bactoblis can provide clinical benefit on PFAPA management.
3 Recommendations for future research on oropharyngeal probiotics
3.1 Allergic rhinitis
Repeated cycles of infection-associated lower airway inflammation drive the pathogenesis of persistent wheezing disease in children. The occurrence of viral acute is accompanied by a shift in the nasopharyngeal microflora toward dominance by a small range of pathogenic bacterial genera. However, this change frequently precedes the detection of viral pathogens and acute symptoms. Colonization of illness-associated bacteria coupled with early allergic sensitization is associated with persistent wheeze in school-aged children, which is the hallmark of the asthma phenotype (64). Furthermore, pediatric chronic rhinosinusitis is a condition commonly encountered in otolaryngology practice, a proportion of which progresses from acute bacterial sinusitis induced by upper respiratory tract infections (65). Intranasal corticosteroids remain the first-line treatment for chronic rhinosinusitis. The study results of the effects of intranasal corticosteroids on the composition of the respiratory microbiome were highly heterogeneous (66), due to their immunosuppressive properties. It is worthwhile considering the possibility of the long-term use of inhaled corticosteroids associated with an increased risk of bacterial infections (67). Children admitted with asthma exacerbations harbor a microbiome characterized by overgrowth of Staphylococcus and oral microbes and an underrepresentation of beneficial niche-appropriate commensals (68, 69), which also present seasonally (70). Restoring the beneficial nasopharyngeal microbiota could be an alternative approach for self-management in pediatric patients with allergic respiratory conditions. Evidence from observational studies of children attending daycare revealed that nasopharyngeal probiotic Bactoblis administration in children was associated with an increased abundance of commensal S. salivarius in saliva and a lower abundance of otopathogens, Moraxella, in the nasopharynx which is strongly associated with the exacerbation of asthma (71). More research on oropharyngeal microflora intervention might help improve the quality of life for people with allergic rhinitis and reduce the risk of developing respiratory infections during high allergy seasons.
3.2 Systemic autoimmune diseases
Despite decades of research, systemic autoimmune diseases (SADs) continue to be a major global health concern, and the etiology of these diseases remain unclear. Oral microbial dysbiosis has been identified in SADs including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren's syndrome (SS), although the dysbiosis features were different among studies (72). Although several investigations failed to establish causal relationships, with the exception of Group A Streptococcus in rheumatic arthritis, microbial contributions to SAD initiation and propagation are plausible and likely, especially via the connective tissue and primary vasculitides (73). Indeed, accumulating evidence shows that antimicrobial peptides are induced or upregulated by dysbiosis in SADs while the host immune system attacks self-tissues with complex pathogenesis (74). Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease in children. Infectious agents are suspected to be environmental triggers involved with molecular mimicry between bacterial molecules and self-antigens (75). In addition, DNA from oral and gut microbiota can be identified in RA synovium, possibly due to the translocation through circulation from the oral cavity (76, 77).
3.3 Occupational respiratory health for health care workers
The World Health Organization (WHO) and the International Labour Organization (ILO) have published a new guide on developing and implementing stronger occupational health and safety programs for health workers in 2022. Even before the COVID-19 pandemic, the health sector was among the most hazardous sectors to work in due to the suffering from infections and allergies from the working environment (78). Since individuals spend most of their lives at work, occupational exposures may have an impact on the microbiota. Chronic respiratory diseases are ranked as the most common occupational disease (79), and the concept of WORKbiota has been recently emphasized by European scientists in 2022 (80). A randomized controlled study conducted during the early COVID-19 pandemic showed that oropharyngeal probiotic Bactoblis protects frontline healthcare workers from respiratory tract infections during the month of taking care of critically ill COVID-19 patients (81). Establishing a homeostasis status of respiratory microflora has been recognized as a safe and positive approach to achieving occupational-related respiratory health by international experts (82). Occupational allergens are one of the risk factors for allergic rhinitis (83), and healthcare workers are exposed to a range of allergens including cleaners and disinfectants, natural rubber latex, and various medications. Studies have shown that exposed healthcare workers are at risk for work-related rhinitis and asthma. For example, high prevalence rates of occupational asthma are found in nurses (10.7%) in Japan according to the Japanese Guidelines for Occupational Allergic Diseases published in 2020 (84). A review of cross-sectional studies indicated that occupational rhinitis affects 10%∼60% of healthcare workers, and occupational anaphylaxis was most frequently triggered by natural rubber latex, chemicals, disinfectants, and medications (85). The inflammatory response of allergic rhinitis continues to interact with the imbalance of nasal flora. Exposure to allergens will induce changes in the bacterial flora of the nasal mucosa, leading to acute sinusitis, nasal eosinophilia, and more serious nasal symptoms, which will reduce the quality of life (70). In addition to occupation-related allergens, the adhesion or colonization of specific opportunistic pathogens in the nasal mucosa is also an important risk factor for inducing chronic airway inflammation leading to allergic respiratory diseases (86). It has been reported that the oropharyngeal colonization of Streptococcus pneumoniae and Haemophilus influenzae in healthcare workers working in hospitals is as high as two times compared to non-healthcare workers (87, 88). It has also been documented that healthcare workers (89), medical laboratory staff due to direct and dense contact with the pathogens, and those living with hospital staff have a higher prevalence of Methicillin-resistant S. aureus (MRSA) nasal colonization. The isolates also appeared more virulent while all isolates were β-lactamases positive (90). Another study indicated that the carriage rates of S. aureus and MRSA among surgical HCWs (32.4%) and nurses (30.8%) were relatively higher, while the highest MRSA rate was detected in nurses (91). The presence of Staphylococcus spp. was more prevalently in the hands of HCWs working in the internal medicine ward and the surgical ward, which is about six times compared to personnel in the neonatal unit, while those with multidrug-resistant or extensively drug-resistant strains were isolated (92). It was also demonstrated that Staphylococcus spp. were most frequently (40%) isolated from the cellphones of hospital staff, while Gram-positive isolates were all susceptible to the antibiotic used and Gram-negative isolates were all resistant to ceftazidime (93). The establishment of a balanced and healthier respiratory microflora through the intervention of oropharyngeal probiotics among healthcare workers and their families is expected to help protect their long-term respiratory health and reduce the risk of transmission of resistance genes to their family members.
3.4 Mode of action of oropharyngeal probiotics on protecting hosts from respiratory infections
Antiviral microbiome mechanisms include the following: (1) enhancement of mucosal barrier function which provides a physical barrier between invasive pathogens and host epithelial cells where the tight junctions and mucosal permeability are maintained by commensal microbiome; (2) antimicrobial compounds, such as bacteriocins, produced by commensal microbiome; (3) inhibition of viral attachment to host epithelial cells by various cross-immune reactions; and (4) modulation of the immune system, such as downregulating inflammatory immune pathways and/or enhancing innate and/or adopted immune pathways and cytokine signaling (94). In children with regurgitation and microaspiration syndromes, the use of oropharyngeal probiotic Bactoblis had a pronounced positive effect on the respiratory microbial composition, of which the pathogenic Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and Klebsiella pneumoniae were significantly reduced (95). A study conducted on young athletes performing a high-intensity training program showed that short-term administration of oropharyngeal probiotic Bactoblis significantly increased salivary immunoglobulin (sIgA) secretion in young athletes, an indication of a potential immune enhancement (96). Since the oropharynx is a primary source of the lung microbiota community which contributes to the susceptibility to viral infections, a preliminary study showed that a 14-day administration of oropharyngeal probiotic Bactoblis reduced the mortality rate of hospitalized COVID-19 patients, improving the clinical parameters associated with pulmonary inflammation caused by viral infections and immune responses (97). These clinical evidence-based findings reflected these mechanisms and suggested that improving the respiratory microbiota may enhance immune function and anti-inflammatory effects. Therefore, more research on respiratory microecological intervention and immune regulation mechanisms will provide a more solid theoretical foundation for clinical application and evidence-based medicine.
3.5 Reducing the carriage of antibiotic resistance genes in human
The rise of antibiotic resistance and a dwindling antimicrobial pipeline have been recognized as emerging threats to public health (98). Enormous therapeutic challenges may present in specific groups of children who have a higher risk of acquiring antibiotic-resistant genes. For example, a major proportion of pneumococci isolated from the nasopharyngeal aspirates of inpatient children with respiratory infections were resistant to more than three types of antibiotics in China (99). Resistant bacteria are present in significant amounts in the adenoids of children with middle ear disease and rhinosinusitis symptoms compared with healthy children (100). A large-scale multicenter study showed that the resistance rate of ampicillin and azithromycin in H. influenzae isolated from the respiratory tract in Chinese children showed an increasing trend through the years, and the major multidrug resistance pattern was resistant to β-lactams, macrolides, and sulfonamides (101). Group A Streptococcus (GAS) is an important cause of acute pharyngitis, and its positive rate in throat culture was about 20% in younger children (102). A retrospective study conducted for 20 years in Taiwan indicated that there was a significant increase by three times to reach about 60% (p < 0.0001) in macrolide-resistant Group A Streptococcus in children with URTi, especially for those diagnosed with scarlet fever (103). The outcome was supported by a similar result from a study conducted in Beijing that prevalent strain of Group A Streptococcus collected from pediatric outpatients who were diagnosed with scarlet fever has a high resistance rate to macrolides at over 90% (104). In a recent study, a strong seasonal epidemiological association between respiratory syncytial virus (RSV) and Streptococcus pneumoniae (pneumococcus) was confirmed by a parallel decrease and a subsequent resurgence during and after the COVID-19 pandemic, respectively (105). Considering the increased concurrent pediatric RSV infections and GAS pharyngitis and the high prevalence of resistant genes in children, the intervention of oropharyngeal probiotics has great potential to support the management of an important public health condition. From 1990 to 2021, deaths due to antimicrobial resistance increased by over 80% among individuals aged 70 years and older globally (106). This indicates the growing challenge of antibiotic resistance, which is now recognized as a global public health crisis. It is essential that everyone, from individuals to countries, actively respond to and take action to address this emergency. Clinicians should play a central role in addressing antibiotic resistance, and medical training is a key factor in this effort. However, there remain gaps between the knowledge and practices among healthcare workers regarding antibiotic use. There is a clear need for multifaceted interventions targeting the public to improve behaviors of rational use of antibiotics.
4 Conclusion
The panel conducted a technical review of evidence-based clinical studies and completed the first expert consensus on the adjuvant use of oropharyngeal probiotics for the management of pediatric respiratory tract infections and otitis media. This consensus was based on the clinical studies that explicitly labeled the use of evidence-based finished formulas rather than formulas with only strains described of which the clinical benefit is undefined.
The consensus process aims to help doctors better understand how to use the evidence-based oropharyngeal probiotic Bactoblis as a dietary supplement and an assistive tool, adjunctively with standard treatment, to manage pediatric respiratory health. This is important for managing refractory pediatric recurrent and chronic respiratory tract infections, such as recurrent and suppurative otitis media, recurrent tonsillitis, chronic adenoiditis, etc. When adjuvantly or prophylactically supplemented, oropharyngeal probiotics can safely reduce the incidence of respiratory tract infections and shorten the course of infectious episodes. Meanwhile, children and parents or caregivers can benefit from reduced absence from school due to illness, absence due to caregiving, and reduced need for prescriptions of antibiotics and antiviral drugs. This expert consensus can be considered as a widely applicable strategy for self-health management that is accepted according to the patient's active will. The recommendation of oropharyngeal probiotics is not intended to replace any standard treatment.
5 Annotation
The oropharyngeal probiotic Bactoblis, which is available in China, contains Streptococcus salivarius subsp. thermophilus Biohalo23. The recommended daily effective dosage is no less than 1 billion CFUs for individuals of all ages, including toddlers, children, adolescents, and adults.
Author contributions
QW: Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation. YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – review & editing. XC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – review & editing. ZG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – review & editing. YL: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Software, Visualization, Writing – review & editing. L-hX: Conceptualization, Investigation, Resources, Software, Visualization, Writing – review & editing. ZL: Conceptualization, Formal Analysis, Investigation, Project administration, Software, Writing – review & editing. JZ: Data curation, Funding acquisition, Methodology, Resources, Visualization, Writing – review & editing. ZZ: Conceptualization, Investigation, Resources, Software, Writing – review & editing. KS: Conceptualization, Formal Analysis, Investigation, Software, Writing – review & editing. GS: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The expert consensus provided by this panel serves only as a reference to best practice, and the diagnosis and treatment of the disease is determined by the physician on a case-by-case basis.
References
1. Stearns JC, Davidson CJ, McKeon S, Whelan FJ, Fontes ME, Schryvers AB, et al. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. Isme J. (2015) 9(5):1246–59. doi: 10.1038/ismej.2014.250
2. Ruan Z, Qi J, Qian ZM, Zhou M, Yang Y, Zhang S, et al. Disease burden and attributable risk factors of respiratory infections in China from 1990 to 2019. Lancet Reg Health West Pac. (2021) 11:100153. doi: 10.1016/j.lanwpc.2021.100153
3. Zhou B, Niu W, Liu F, Yuan Y, Wang K, Zhang J, et al. Risk factors for recurrent respiratory tract infection in preschool-aged children. Pediatr Res. (2021) 90(1):223–31. doi: 10.1038/s41390-020-01233-4
4. de Steenhuijsen Piters WAA, Watson RL, de Koff EM, Hasrat R, Arp K, Chu M, et al. Early-life viral infections are associated with disadvantageous immune and microbiota profiles and recurrent respiratory infections. Nat Microbiol. (2022) 7(2):224–37. doi: 10.1038/s41564-021-01043-2
5. de Steenhuijsen Piters WAA, Binkowska J, Bogaert D. Early life microbiota and respiratory tract infections. Cell Host Microbe. (2020) 28(2):223–32. doi: 10.1016/j.chom.2020.07.004
6. Fu M, Gong Z, Li C, Ling K, Zhu Y, Li H, et al. Appropriate use of antibiotics for acute respiratory infections at primary healthcare facilities in China: a nationwide cross-sectional study from 2017 to 2019. Lancet Reg Health West Pac. (2023) 40:100880. doi: 10.1016/j.lanwpc.2023.100880
7. Qu W, Wang X, Liu Y, Mao J, Liu M, Zhong Y, et al. Self-medication with antibiotics among children in China: a cross-sectional study of Parents’ knowledge, attitudes, and practices. Infect Drug Resist. (2023) 16:7683–94. doi: 10.2147/IDR.S431034
8. Anthony WE, Wang B, Sukhum KV, D’Souza AW, Hink T, Cass C, et al. Acute and persistent effects of commonly used antibiotics on the gut microbiome and resistome in healthy adults. Cell Rep. (2022) 39(2):110649. doi: 10.1016/j.celrep.2022.110649
9. Huang H, Jiang J, Wang X, Jiang K, Cao H. Exposure to prescribed medication in early life and impacts on gut microbiota and disease development. EClinicalMedicine. (2024) 68:102428. doi: 10.1016/j.eclinm.2024.102428
10. Sobel AL, Melamed J, Haas D, LeBlanc G, Cirone A, Constantinides MG. Antibiotic use in early life subsequently impairs MAIT cell-mediated immunity. bioRxiv. (2024) 2024.05.10.593643. doi: 10.1101/2024.05.10.593643
11. Patangia DV, Ryan CA, Dempsey E, Stanton C, Ross RP. Vertical transfer of antibiotics and antibiotic resistant strains across the mother/baby axis. Trends Microbiol. (2022) 30(1):47–56. doi: 10.1016/j.tim.2021.05.006
12. Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol. (2015) 11(3):182–90. doi: 10.1038/nrendo.2014.210
13. Choi A, Lee H, Jeong HE, Lee SY, Kwon JS, Han JY, et al. Association between exposure to antibiotics during pregnancy or early infancy and risk of autism spectrum disorder, intellectual disorder, language disorder, and epilepsy in children: population based cohort study. Br Med J. (2024) 385:e076885. doi: 10.1136/bmj-2023-076885
14. Kindgren E, Ludvigsson J. Infections and antibiotics during fetal life and childhood and their relationship to juvenile idiopathic arthritis: a prospective cohort study. Pediatr Rheumatol Online J. (2021) 19(1):145. doi: 10.1186/s12969-021-00611-4
15. Emre IE, Eroğlu Y, Kara A, Dinleyici EC, Özen M. The effect of probiotics on prevention of upper respiratory tract infections in the paediatric community - a systematic review. Benef Microbes. (2020) 11(3):201–11. doi: 10.3920/BM2019.0119
16. Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. (2022) 8(8):Cd006895. doi: 10.1002/14651858.CD006895.pub4
17. Lehtoranta L, Latvala S, Lehtinen MJ. Role of probiotics in stimulating the immune system in viral respiratory tract infections: a narrative review. Nutrients. (2020) 12(10). doi: 10.3390/nu12103163
18. Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, et al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). (2016) 95(31):e4509. doi: 10.1097/MD.0000000000004509
19. Doyle H, Pierse N, Tiatia R, Williamson D, Baker M, Crane J. Effect of oral probiotic Streptococcus salivarius K12 on group A Streptococcus pharyngitis: a pragmatic trial in schools. Pediatr Infect Dis J. (2018) 37(7):619–23. doi: 10.1097/INF.0000000000001847
20. Pettoello-Mantovani M, Çullu Çokuğraş F, Vural M, Mestrovic J, Nigri L, Piazzolla R, et al. Pilot study for the understanding and use of probiotics by different paediatric healthcare professionals working in different European countries. Ital J Pediatr. (2019) 45(1):57. doi: 10.1186/s13052-019-0648-4
21. Guarner F, Sanders ME, Szajewska H, Cohen H, Eliakim R, Herrera-deGuise C, et al. World gastroenterology organisation global guidelines: probiotics and prebiotics. J Clin Gastroenterol. (2024) 58(6):533–53. doi: 10.1097/MCG.0000000000002002
22. Di Pierro F, Donato G, Fomia F, Adami T, Careddu D, Cassandro C, et al. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med. (2012) 5:991–7. doi: 10.2147/IJGM.S38859
23. Di Pierro F, Adami T, Rapacioli G, Giardini N, Streitberger C. Clinical evaluation of the oral probiotic Streptococcus salivarius K12 in the prevention of recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes in adults. Expert Opin Biol Ther. (2013) 13(3):339–43. doi: 10.1517/14712598.2013.758711
24. Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf. (2014) 6:15–20. doi: 10.2147/DHPS.S59665
25. Di Pierro F, Colombo M, Zanvit A, Rottoli AS. Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: a pilot investigation. Drug Healthc Patient Saf. (2016) 8:77–81. doi: 10.2147/DHPS.S117214
26. Gregori G, Righi O, Risso P, Boiardi G, Demuru G, Ferzetti A, et al. Reduction of group A beta-hemolytic streptococcus pharyngo-tonsillar infections associated with use of the oral probiotic Streptococcus salivarius K12: a retrospective observational study. Ther Clin Risk Manag. (2016) 12:87–92. doi: 10.2147/TCRM.S96134
27. Kryuchko TO, Tkachenko YO. Possibilities of using lantibiotics to prevent recurrent upper respiratory tract infections in children. Clin Pediatr. (2017) 12(8):27–32.
28. Kryuchko T, Tkachenko O. Clinical experience of Streptococcus salivarius K12 use for the prevention of pharyngotonsillitis and respiratory infections in children. Child’s Health. (2018) 13(7):629–34. doi: 10.22141/2224-0551.13.7.2018.148915
29. Marini G, Sitzia E, Panatta ML, De Vincentiis GC. Pilot study to explore the prophylactic efficacy of oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngo-tonsillar episodes in pediatric patients. Int J Gen Med. (2019) 12:213–7. doi: 10.2147/IJGM.S168209
30. Ilchenko S, Fialkovska A, Ivanus S. The effectiveness of using respiratory probiotic Streptococcus salivarius K12 in children with recurrent tonsillitis. Actual Infectology. (2020) 8(2):25–9. doi: 10.22141/2312-413x.8.2.2020.199732
31. Puhlik SM, Аndreev АV, Gushcha SG, Tagunova IK, Volyanska VS, Balashova IV, et al. Experience with the use of oral probiotic Streptococcus salivarius K12 for the prevention of recurrence of pharyngotonsillar. Pharmacologyonline. (2021) 1:120–4. https://repo.odmu.edu.ua:443/xmlui/handle/123456789/9472
32. Guo H, Xiang X, Lin X, Wang Q, Qin S, Lu X, et al. Oropharyngeal probiotic ENT-K12 as an effective dietary intervention for children with recurrent respiratory tract infections during cold season. Front Nutr. (2022) 9:900448. doi: 10.3389/fnut.2022.900448
33. Altamimi AAH, Robinson M, McKinnon EJ, Alenezi EMA, Veselinović T, Choi RSM, et al. The association between otitis media in early childhood with later behaviour and attention problems: a longitudinal pregnancy cohort. Int J Pediatr Otorhinolaryngol. (2023) 168:111545. doi: 10.1016/j.ijporl.2023.111545
34. Altamimi AA, Robinson M, Alenezi EM, Veselinović T, Choi RS, Brennan-Jones CG. Recurrent otitis media and behaviour problems in middle childhood: a longitudinal cohort study. J Paediatr Child Health. (2024) 60(1):12–7. doi: 10.1111/jpc.16518
35. Thornton R, Seppanen E, Clark S. Biofilms and intracellular infection in otitis media. Microbiol Aust. (2023) 44(2):88–91. doi: 10.1071/MA23025
36. Xu J, Du Q, Shu Y, Ji J, Dai C. Bacteriological profile of chronic suppurative otitis media and antibiotic susceptibility in a tertiary care hospital in Shanghai, China. Ear Nose Throat J. (2021) 100(9):Np391–6. doi: 10.1177/0145561320923823
37. Dedhia K, Tasian GE, Forrest CB. Tympanostomy tubes or medical management for recurrent acute otitis media. N Engl J Med. (2021) 385(9):860–1. doi: 10.1056/NEJMc2109725
38. Fagö-Olsen H, Dines LM, Sørensen CH, Jensen A. The adenoids but not the palatine tonsils serve as a reservoir for Bacteria associated with secretory otitis media in small children. mSystems. (2019) 4(1). doi: 10.1128/msystems.00169-18
39. Pierro FD, Pasquale DD, Cicco MD. Oral use of Streptococcus salivarius K12 in children with secretory otitis media: preliminary results of a pilot, uncontrolled study. Int J Gen Med. (2015) 8:303–8. doi: 10.2147/IJGM.S92488
40. Di Pierro F, Colombo M, Giuliani MG, Danza ML, Basile I, Bollani T, et al. Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children. Eur Rev Med Pharmacol Sci. (2016) 20(21):4601–6. Available online at: https://www.europeanreview.org/article/1169627874935
41. Di Pierro F, Risso P, Poggi E, Timitilli A, Bolloli S, Bruno M, et al. Use of Streptococcus salivarius K12 to reduce the incidence of pharyngo-tonsillitis and acute otitis media in children: a retrospective analysis in not-recurrent pediatric subjects. Minerva Pediatr. (2018) 70(3):240–5. doi: 10.23736/S0026-4946.18.05182-4
42. Havrylenko YV. Experience with the clinical use of the respiratory probiotic Bactobolis in children with secretory otitis media. Child’s Health. (2019) 14(5):90–3.
43. Kryuchko TO, Tkachenko OY. An open-label study to evaluate the effects of Streptococcus salivarius K12 given as a powder formula to prevent respiratory infections in young children. Nutrafoods. (2021) 1:246–53. doi: 10.17470/NF-021-0032
44. Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. (2018) 9:2640. doi: 10.3389/fimmu.2018.02640
45. Li H, Wu X, Zeng H, Chang B, Cui Y, Zhang J, et al. Unique microbial landscape in the human oropharynx during different types of acute respiratory tract infections. Microbiome. (2023) 11(1):157. doi: 10.1186/s40168-023-01597-9
46. Cui G, Sun Y, Zou Y, Sun R, Gao Y, Liu X, et al. Dynamic changes of bacterial microbiomes in oropharynx during infection and recovery of COVID-19 omicron variant. PLoS Pathog. (2024) 20(4):e1012075. doi: 10.1371/journal.ppat.1012075
47. DeMuri GP, Gern JE, Eickhoff JC, Lynch SV, Wald ER. Dynamics of bacterial colonization with Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis during symptomatic and asymptomatic viral upper respiratory tract infection. Clin Infect Dis. (2018) 66(7):1045–53. doi: 10.1093/cid/cix941
48. Man WH, Clerc M, de Steenhuijsen Piters WAA, van Houten MA, Chu M, Kool J, et al. Loss of microbial topography between oral and nasopharyngeal microbiota and development of respiratory infections early in life. Am J Respir Crit Care Med. (2019) 200(6):760–70. doi: 10.1164/rccm.201810-1993OC
49. Kryuchko TO, Tkachenko OY. Efficacy of administering Streptococcus salivarius K12 as a powder formula in preventing respiratory infections in infants. Nutrafoods. (2021) 2:254–61. doi: 10.17470/NF-021-0033
50. Lalmohamed A, Venekamp RP, Bolhuis A, Souverein PC, van de Wijgert J, Gulliford MC, et al. Within-episode repeat antibiotic prescriptions in patients with respiratory tract infections: a population-based cohort study. J Infect. (2024) 88(4):106135. doi: 10.1016/j.jinf.2024.106135
51. Guntinas-Lichius O, Geißler K, Mäkitie AA, Ronen O, Bradley PJ, Rinaldo A, et al. Treatment of recurrent acute tonsillitis-a systematic review and clinical practice recommendations. Front Surg. (2023) 10:1221932. doi: 10.3389/fsurg.2023.1221932
52. Byars SG, Stearns SC, Boomsma JJ. Association of long-term risk of respiratory, allergic, and infectious diseases with removal of adenoids and tonsils in childhood. JAMA Otolaryngol Head Neck Surg. (2018) 144(7):594–603. doi: 10.1001/jamaoto.2018.0614
53. Ungkanont K, Jootakarn S, Leelaporn A, Kijsinthopchai U, Tanphaichitr A, Vathanophas V, et al. Association between adenoid bacteriology and clinical characteristics of adenoid-related diseases in children. SAGE Open Med. (2021) 9:20503121211006005. doi: 10.1177/20503121211006005
54. Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. (2009) 140(6):800–8. doi: 10.1016/j.otohns.2009.01.043
55. Torretta S, Drago L, Marchisio P, Ibba T, Pignataro L. Role of biofilms in children with chronic adenoiditis and middle ear disease. J Clin Med. (2019) 8(5). doi: 10.3390/jcm8050671
56. Karpova EP, Gurov AV, Burlakova KY. Analysis of the mucous microbiome of the nasopharynx in children with chronic adenoiditis and otitis media of effusion. Pediatrics. Consilium Medicum. (2021) 1:39–45.
57. Karpova EP, Karpycheva IE, Tulupov DA. Prophylaxis of chronic adenoiditis in the children. Vestn Otorinolaringol. (2015) 80(6):43–5. doi: 10.17116/otorino201580643-45
58. Marushko TV, Hliadielova NP, Onufriiv OE. Clinico-immunological efficacy of Streptococcus salivarius K12 in the prevention of exacerbations and treatment of chronic tonsillitis in children. Child’s Health. (2018) 13(8):82–8.
59. Gavrilenko YV. Current benefits of lantibiotics use in prevention of recurrent pharyngeal infections in children. Child’s Health. (2018) 13(6):89–94.
60. Tejesvi MV, Uhari M, Tapiainen T, Pirttilä AM, Suokas M, Lantto U, et al. Tonsillar microbiota in children with PFAPA (periodic fever, aphthous stomatitis, pharyngitis, and adenitis) syndrome. Eur J Clin Microbiol Infect Dis. (2016) 35(6):963–70. doi: 10.1007/s10096-016-2623-y
61. Gazi U, Agada ME, Ozkayalar H, Dalkan C, Sanlidag B, Safak MA, et al. Tonsillar antimicrobial peptide (AMP) expression profiles of periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) patients. Int J Pediatr Otorhinolaryngol. (2018) 110:100–4. doi: 10.1016/j.ijporl.2018.05.005
62. Francesco DP, Andrea C, Maria Laura P. The use of Streptococcus salivarius K12 in attenuating PFAPA syndrome, a pilot study. Altern Integr Med. (2016) 5(4):222. doi: 10.4172/2327-5162.1000222
63. La Torre F, Sota J, Insalaco A, Conti G, Del Giudice E, Lubrano R, et al. Preliminary data revealing efficacy of Streptococcus salivarius K12 (SSK12) in periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: a multicenter study from the AIDA network PFAPA syndrome registry. Front Med (Lausanne). (2023) 10:1105605. doi: 10.3389/fmed.2023.1105605
64. Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, et al. Airway Microbiota dynamics uncover a critical window for interplay of pathogenic Bacteria and allergy in childhood respiratory disease. Cell Host Microbe. (2018) 24(3):341–352.e5. doi: 10.1016/j.chom.2018.08.005
65. Belcher R, Virgin F. The role of the adenoids in pediatric chronic rhinosinusitis. Med Sci. (2019) 7(2):35. doi: 10.3390/medsci7020035
66. Hartmann JE, Albrich WC, Dmitrijeva M, Kahlert CR. The effects of corticosteroids on the respiratory microbiome: a systematic review. Front Med (Lausanne). (2021) 8:588584. doi: 10.3389/fmed.2021.588584
67. Martínez-García M, Oscullo G, García-Ortega A, Matera MG, Rogliani P, Cazzola M. Inhaled corticosteroids in adults with non-cystic fibrosis bronchiectasis: from bench to bedside. A narrative review. Drugs. (2022) 82(14):1453–68. doi: 10.1007/s40265-022-01785-1
68. van Beveren GJ, de Steenhuijsen Piters WAA, Boeschoten SA, Louman S, Chu ML, Arp K, et al. Nasopharyngeal microbiota in children is associated with severe asthma exacerbations. J Allergy Clin Immunol. (2024) 153(6):1574–1585.e14. doi: 10.1016/j.jaci.2024.02.020
69. Versi A, Ivan FX, Abdel-Aziz MI, Bates S, Riley J, Baribaud F, et al. Haemophilus influenzae and Moraxella catarrhalis in sputum of severe asthma with inflammasome and neutrophil activation. Allergy. (2023) 78(11):2906–20. doi: 10.1111/all.15776
70. Choi CH, Poroyko V, Watanabe S, Jiang D, Lane J, de Tineo M, et al. Seasonal allergic rhinitis affects sinonasal microbiota. Am J Rhinol Allergy. (2014) 28(4):281–6. doi: 10.2500/ajra.2014.28.4050
71. Sarlin S, Tejesvi MV, Turunen J, Vänni P, Pokka T, Renko M, et al. Impact of Streptococcus salivarius K12 on nasopharyngeal and Saliva microbiome: a randomized controlled trial. Pediatr Infect Dis J. (2021) 40(5):394–402. doi: 10.1097/INF.0000000000003016
72. Gao L, Cheng Z, Zhu F, Bi C, Shi Q, Chen X. The oral microbiome and its role in systemic autoimmune diseases: a systematic review of big data analysis. Front Big Data. (2022) 5:927520. doi: 10.3389/fdata.2022.927520
73. MF K. The microbiome in autoimmune rheumatic disease. Best Pract Res Clin Rheumatol. (2020) 34(1):101473. doi: 10.1016/j.berh.2019.101473
74. Zhang CY, Yang M. The role and potential application of antimicrobial peptides in autoimmune diseases. Front Immunol. (2020) 11:859. doi: 10.3389/fimmu.2020.00859
75. Frid P, Baraniya D, Halbig J, Rypdal V, Songstad NT, Rosèn A, et al. Salivary oral microbiome of children with juvenile idiopathic arthritis: a Norwegian cross-sectional study. Front Cell Infect Microbiol. (2020) 10:602239. doi: 10.3389/fcimb.2020.602239
76. Berthelot JM, Bandiaky ON, Le Goff B, Amador G, Chaux AG, Soueidan A, et al. Another Look at the contribution of oral microbiota to the pathogenesis of rheumatoid arthritis: a narrative review. Microorganisms. (2021) 10(1). doi: 10.3390/microorganisms10010059
77. Ilchenko SI, Fialkovska AA, Ivanus SG. The effectiveness application of the respiratory probiotic Streptococcus salivarius K12 for the correction of dysbiotic oral cavity disorders in children with juvenile rheumatoid arthritis. Clin Immunol. (2019) 3(116):30–4.
78. World Health Organization. New WHO/ILO guide urges greater safeguards to protect health workers EB/OL.(2022-02-21). Available online at: https://www.who.int/japan/news/detail-global/21-02-2022-new-who-ilo-guide-urgesgreater-safeguards-to-protect-health-workers
79. Bepko J, Mansalis K. Common occupational disorders: asthma, COPD, dermatitis, and musculoskeletal disorders. Am Fam Physician. (2016) 93(12):1000–6. https://www.aafp.org/pubs/afp/issues/2016/0615/p1000.htm27304769
80. Mucci N, Tommasi E, Chiarelli A, Lulli LG, Traversini V, Galea RP, et al. WORKbiota: a systematic review about the effects of occupational exposure on Microbiota and Workers’ health. Int J Environ Res Public Health. (2022) 19(3). doi: 10.3390/ijerph19031043
81. Wang Q, Lin X, Xiang X, Liu W, Fang Y, Chen H, et al. Oropharyngeal probiotic ENT-K12 prevents respiratory tract infections among frontline medical staff fighting against COVID-19: a pilot study. Front Bioeng Biotechnol. (2021) 9:646184. doi: 10.3389/fbioe.2021.646184
82. Picó-Monllor JA, Ruzafa-Costas B, Núñez-Delegido E, Sánchez-Pellicer P, Peris-Berraco J, Navarro-Lopez V. Selection of probiotics in the prevention of respiratory tract infections and their impact on occupational health: scoping review. Nutrients. (2021) 13(12). doi: 10.3390/nu13124419
83. Siddiqui ZA, Walker A, Pirwani MM, Tahiri M, Syed I. Allergic rhinitis: diagnosis and management. Br J Hosp Med (Lond). (2022) 83(2):1–9. doi: 10.12968/hmed.2021.0570
84. Dobashi K, Usami A, Yokozeki H, Tsurikisawa N, Nakamura Y, Sato K, et al. Japanese guidelines for occupational allergic diseases 2020. Allergol Int. (2020) 69(3):387–404. doi: 10.1016/j.alit.2020.03.010
85. Mazurek JM, Weissman DN. Occupational respiratory allergic diseases in healthcare workers. Curr Allergy Asthma Rep. (2016) 16(11):77. doi: 10.1007/s11882-016-0657-y
86. Kang HM, Kang JH. Effects of nasopharyngeal microbiota in respiratory infections and allergies. Clin Exp Pediatr. (2021) 64(11):543–51. doi: 10.3345/cep.2020.01452
87. Amritha GN, Meenakshi N, Selvabai RAP, Shanmugam P, Jayaraman P. A comparative profile of oropharyngeal colonization of Streptococcus pneumoniae and Haemophilus influenzae among healthcare workers (HCW) in a tertiary care hospital and non-healthcare individuals. J Prev Med Hyg. (2020) 61(3):E379–85. doi: 10.15167/2421-4248/jpmh2020.61.3.1479
88. Hosuru Subramanya S, Thapa S, Dwedi SK, Gokhale S, Sathian B, Nayak N, et al. Streptococcus pneumoniae and Haemophilus species colonization in health care workers: the launch of invasive infections? BMC Res Notes. (2016) 9:66. doi: 10.1186/s13104-016-1877-x
89. Ohadian Moghadam S, Modoodi Yaghooti M, Pourramezan N, Pourmand MR. Molecular characterization and antimicrobial susceptibility of the CA-MRSA isolated from healthcare workers, Tehran, Iran. Microb Pathog. (2017) 107:409–12. doi: 10.1016/j.micpath.2017.04.027
90. Xie X, Dai X, Ni L, Chen B, Luo Z, Yao Y, et al. Molecular epidemiology and virulence characteristics of Staphylococcus aureus nasal colonization in medical laboratory staff: comparison between microbiological and non-microbiological laboratories. BMC Infect Dis. (2018) 18(1):122. doi: 10.1186/s12879-018-3024-x
91. Kong Y, Ye J, Zhou W, Jiang Y, Lin H, Zhang X, et al. Prevalence of methicillin-resistant Staphylococcus aureus colonisation among healthcare workers at a tertiary care hospital in southeastern China. J Glob Antimicrob Resist. (2018) 15:256–61. doi: 10.1016/j.jgar.2018.08.013
92. Tselebonis A, Nena E, Nikolaidis C, Konstantinidis T, Kontogiorgis C, Panopoulou M, et al. Monitoring of frequency and antimicrobial susceptibility of pathogens on the hands of healthcare workers in a tertiary hospital. Folia Med (Plovdiv). (2016) 58(3):200–5. doi: 10.1515/folmed-2016-0028
93. Bayraktar M, Kaya E, Ozturk A, İbahim BMS. Antimicrobial susceptibility of bacterial pathogens isolated from healthcare workers’ cellphones. Infect Dis Now. (2021) 51(7):596–602. doi: 10.1016/j.idnow.2021.05.007
94. Harper A, Vijayakumar V, Ouwehand AC, Ter Haar J, Obis D, Espadaler J, et al. Viral infections, the microbiome, and probiotics. Front Cell Infect Microbiol. (2021) 10:596166. doi: 10.3389/fcimb.2020.596166
95. Ilchenko SI, Fialkovska AA, Mozheiko TV. Modern possibilities of correcting dysbiotic disorders of the mucous membranes of the upper respiratory tract in infants. Clin Pediatr. (2019) 7(4):559–65.
96. Bertuccioli A, Gervasi M, Annibalini G, Binato B, Perroni F, Rocchi MBL, et al. Use of Streptococcus salivarius K12 in supporting the mucosal immune function of active young subjects: a randomised double-blind study. Front Immunol. (2023) 14:1129060. doi: 10.3389/fimmu.2023.1129060
97. Di Pierro F, Iqtadar S, Mumtaz SU, Bertuccioli A, Recchia M, Zerbinati N, et al. Clinical effects of Streptococcus salivarius K12 in hospitalized COVID-19 patients: results of a preliminary study. Microorganisms. (2022) 10(10). doi: 10.3390/microorganisms10101926
98. Miller WR, Arias CA. ESKAPE pathogens: antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat Rev Microbiol. (2024) 22(10):598–616. doi: 10.1038/s41579-024-01054-w
99. Geng Q, Zhang T, Ding Y, Tao Y, Lin Y, Wang Y, et al. Molecular characterization and antimicrobial susceptibility of Streptococcus pneumoniae isolated from children hospitalized with respiratory infections in Suzhou, China. PLoS One. (2014) 9(4):e93752. doi: 10.1371/journal.pone.0093752
100. McClay JE. Resistant bacteria in the adenoids: a preliminary report. Arch Otolaryngol Head Neck Surg. (2000) 126(5):625–9. doi: 10.1001/archotol.126.5.625
101. Zhou M, Fu P, Fang C, Shang S, Hua C, Jing C, et al. Antimicrobial resistance of Haemophilus influenzae isolates from pediatric hospitals in mainland China: report from the ISPED program, 2017–2019. Indian J Med Microbiol. (2021) 39(4):434–8. doi: 10.1016/j.ijmmb.2021.09.001
102. Wi D, Choi SH. Positive rate of tests for group a Streptococcus and viral features in children with acute pharyngitis. Children (Basel). (2021) 8(7). doi: 10.3390/children8070599
103. Tsai WC, Shen CF, Lin YL, Shen FC, Tsai PJ, Wang SY, et al. Emergence of macrolide-resistant Streptococcus pyogenes emm12 in southern Taiwan from 2000 to 2019. J Microbiol Immunol Infect. (2021) 54(6):1086–93. doi: 10.1016/j.jmii.2020.08.019
104. Li H, Zhou L, Zhao Y, Ma L, Liu X, Hu J. Molecular epidemiology and antimicrobial resistance of group a streptococcus recovered from patients in Beijing, China. BMC Infect Dis. (2020) 20(1):507. doi: 10.1186/s12879-020-05241-x
105. Besteman SB, Bogaert D, Bont L, Mejias A, Ramilo O, Weinberger DM, et al. Interactions between respiratory syncytial virus and Streptococcus pneumoniae in the pathogenesis of childhood respiratory infections: a systematic review. Lancet Respir Med. (2024) 12(11):915–32. doi: 10.1016/S2213-2600(24)00148-6
Keywords: recurrent respiratory tract infections, rational use of antibiotics, otitis media, streptococcal pharyngitis/tonsilitis, oropharyngeal probiotic Bactoblis
Citation: Wang Q, Zhang Y, Cheng X, Guo Z, Liu Y, Xia L-h, Liu Z, Zheng J, Zhang Z, Sun K and Shen G (2025) Expert consensus on the use of oropharyngeal probiotic Bactoblis in respiratory tract infection and otitis media: available clinical evidence and recommendations for future research. Front. Pediatr. 12:1509902. doi: 10.3389/fped.2024.1509902
Received: 11 October 2024; Accepted: 25 November 2024;
Published: 28 January 2025.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Aleksander Zwierz, Nicolaus Copernicus University in Toruń, PolandCopyright: © 2025 Wang, Zhang, Cheng, Guo, Liu, Xia, Liu, Zheng, Zhang, Sun and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wang, d2FuZ3FpYW5nQHd1c3QuZWR1LmNu
 Qiang Wang
Qiang Wang Yatong Zhang
Yatong Zhang Xiaoling Cheng3
Xiaoling Cheng3 Zhi Guo
Zhi Guo Guanxin Shen
Guanxin Shen