- Key Laboratory of Birth Defects and Related Diseases of Women and Children of Ministry of Education (MOE), Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
This case is the first reported patient with a MEIS2 gene mutation who primarily exhibits pronounced inattention as the main manifestation and is diagnosed with ADHD, requiring methylphenidate treatment. It is characterized by unique clinical features that set it apart from previously reported cases with mutations in the MEIS2 gene. Here, we report a female child with a diagnosis of ADHD and comorbidities. She received treatment with methylphenidate, starting at a dose of 18 milligrams per day, which was gradually increased to 45 milligrams per day based on her attention performance, while also undergoing physical and language rehabilitation training. In addition, the parents involved the child in reading and retelling stories at home every day. After 2 years of treatment, the scale results indicated that the child still had a moderate degree of attention deficit. Therefore, she underwent whole exome sequencing (WES) showing that her MEIS2 gene carries a de novo frameshift mutation (c.934_937del, p. Leu312Argfs*11). After comparing the patient's features with those of other patients who also had the MEIS2 mutation, we discovered that the patient's cleft palate, heart abnormalities, and minor facial dysmorphism were all extremely comparable. A broad forehead, elongated and arched eyebrows, and a tent-shaped upper lip were examples of mild facial dysmorphic traits. Subtypes with phenotypes such as cleft palate, cardiac anomalies, or facial malformations were presented in all previously reported cases of MEIS2 mutations. Furthermore, less common characteristics include ADHD, learning difficulties, hearing loss, recurring respiratory infections, asthma, rhinitis, enuresis, and dental cavities. This case further supports the critical role of genetic testing in patients with ADHD who exhibit a suboptimal response to methylphenidate and present with multiple comorbidities. Furthermore, this case report expands the clinical symptom spectrum associated with MEIS2 gene mutations, providing a broader understanding of the condition.
1 Introduction
ADHD is one of the most prevalent neurodevelopmental disorders, affecting approximately 5%–10% of children and adolescents worldwide (1). The disorder is characterized by persistent inattention, hyperactivity, and impulsivity, leading to significant challenges in all aspects of life, including academic performance, social interactions, and overall daily functioning (2). In addition, approximately 43% of childhood ADHD persists into adulthood (3). With regard to the cause of the disease, research suggests that it is due to the interaction of genetic and environmental factors, but that genetics play a considerable role. Based on the results of family and twin studies, the estimated heritability of ADHD approximates 80% (4, 5). The fact that ADHD frequently co-occurs with other diseases such as learning disabilities, anxiety disorders, and oppositional defiant disorder complicates both the diagnosis and treatment of ADHD. However, the advancement of genetic testing technologies has made it possible to determine the genesis of neurodevelopmental disorders exhibiting intricate clinical manifestations.
The MEIS2 gene encodes a transcription factor belonging to the three-amino-acid-loop extension (TALE) protein superclass (6) and is an important transcription factor controlling embryonic development and cell differentiation. MEIS2 is involved in the development of the heart and craniofacial region based on phenotypes linked to the overexpression or deletion of the gene in animal models (7–9), limb growth and patterning (10, 11), and axial skeletal patterning (12). The function of MEIS2 in mouse palatal development has been thoroughly studied. Molecular and genomic analyses revealed that MEIS2 directly regulates important osteogenic genes, and specific inactivation of the MEIS2 gene in cranial neural crest cells resulted in complete cleft palate or submucous cleft and complete loss of palatal bone (9). In addition, Desiderio et al. (13) looked into the possibility that when crossing mouse strains to successfully deactivate MEIS2 in the neural crest, the resulting cleft palate in the newborn pups would be consistent with earlier findings that MEIS2 is essential for the development of the mouse's cranial and cardiac neural crest cells (7).
Additionally, MEIS2 has a role in nearly every facet of the development of the central nervous system, such as neural tube patterning, proliferation of neural progenitor cells, acquisition of cell destiny, maturation of neurons, neurite outgrowth, and synaptogenesis (14–17), and is required for the survival and function of different neuronal populations (18, 19). Furthermore, MEIS2 regulates the development of striatal neurons (20) and the maintenance of retinal progenitor cell pools (16). In the meantime, MEIS2 is required for inner ear formation and proper morphogenesis of the cochlea (6).
At least 17 distinct mutations in the MEIS2 gene have been linked to neurodevelopmental abnormalities in humans (21–23), highlighting the crucial role this gene plays in neuronal differentiation. Furthermore, MEIS2 has been reported to be a susceptibility gene for obsessive-compulsive behaviors in humans. Somatic mutations that produce de novo MEIS2 binding motifs are discovered in putative enhancer regions in the brains of people with autism spectrum disorders (24, 25). The characteristic features of a heterozygous missense mutation in the MEIS2 gene locus or a 15q14 microdeletion are a triad of cleft palate, congenital heart defects, and intellectual disability, which is referred to as MEIS2 syndrome (26).
This is the first report of an ADHD patient with a mutation in the MEIS2 gene (c.934_937del, p. Leu312Argfs*11). Her primary clinical manifestations are attention deficit and developmental delay, and she also presents with issues involving multiple organ systems, including the respiratory, urinary, cardiac, oral, ear, and nasal systems. Additionally, her attention deficit did not improve significantly after treatment with methylphenidate. This case further extends the clinical phenotype of mutations in the MEIS2 gene and confirms the importance of genetic testing in finding the etiology of ADHD in patients who do not respond well to methylphenidate treatment and have comorbidities.
2 Materials and methods
The study was approved by the Ethics Committee of West China Second Hospital of Sichuan University. In addition, written informed consent was obtained from the patient's parents before whole-exome sequencing was performed.
Peripheral blood samples (2–4 ml) from the pre-certified patient and her parents were collected into ethylenediaminetetraacetic acid (EDTA) anticoagulated blood sample tubes. Genomic DNA from this patient and parents was extracted from the blood according to the manufacturer's instructions (QIAamp DNA Blood Minikit). And WES was performed using the Illumina NovaSeq6000 platform. Sequencing reads were then aligned to the human reference genome GRCh38/HG38 and variants annotated using Burrows-Wheeler Aligner (BWA) software. Reads were aligned and locally recalibrated using GATK. A series of principles were used to screen for pathogenic mutations based on variant annotation, as follows: (1) Filtering for variants that are not seen to be carried by normal humans or have a carrier rate of less than 5% in databases such as gnomAD, 1,000 Genomes Project, and others. (2) Disease-causing mutation sites were evaluated using databases such as the Online Mendelian Inheritance in Man (OMIM) database, the National Institutes of Health (NIH), the Human Genetic Mutation Database (HGMD), and ClinVar. (3) Protein function prediction using software such as SIFT, PolyPhen2, and CADD. According to the American College of Medical Genetics and Genomics (ACMG) classification guidelines, the obtained single nucleotide variants (SNVs) were categorized into five categories, including pathogenic, possibly pathogenic, of uncertain significance, possibly benign, and benign. Finally, validation was performed using first-generation Sanger sequencing technology and samples from family members.
3 Case description
We report a 10-year-old girl who presented to the Department of Pediatric Rehabilitation, West China Second Hospital, Sichuan University, with what her parents described as inattention and developmental delay. The infant was born at 37 weeks of gestation, undersized for gestational age, weighing 2,100 g (below the 10th percentile), and measuring 47 cm (10th–25th percentile) in length. At birth, her parents noticed that she had a cleft palate, which was surgically corrected when she was one year old. A ventricular septal defect (VtSD), a patent foramen ovale (PFO), and mild tricuspid regurgitation were all detected by cardiac ultrasonography. Following that, the VtSD resolved on its own, and the foramen ovale has not yet closed. In addition, she developed full-mouth caries, asthma, rhinitis, enuresis, recurrent respiratory infections, and otitis media-related hearing loss. At the time of our initial evaluation, the child was 5 years and 2 months old, with a low bone age (equal to 4.9 years), a height of 105 cm (−2 SD), and a weight of 14.7 kg (between −2 and −3 SD). The patient also showed delayed motor development, beginning to walk at 18 months. She can no longer perform continuous movements, descends stairs slowly, and her coordination is weak. She also lags behind youngsters her age in fine motor skills, such as button fastening.
She also exhibits delayed language development. At 2 years and 10 months of age, she was only able to speak a few simple words, and her pronunciation was not accurate. Now at ten years old, her vocabulary in language communication is less than that of her peers, and she frequently experiences dysfluency in speech, which affects normal communication. During literacy reading, she skips words, and her logical thinking is poor. At school, multiple subject teachers have reported that she exhibits significant inattention during class, being able to concentrate for only about 20 min per lesson. Concurrently, she has learning difficulties and exhibits procrastination when doing homework, requiring parental supervision to complete tasks. Additionally, the child has poor social skills. Physical examination reveals mild facial dysmorphia, including a large forehead, extended and arched eyebrows, and a tent-shaped upper lip. These features are individually difficult to recognize.
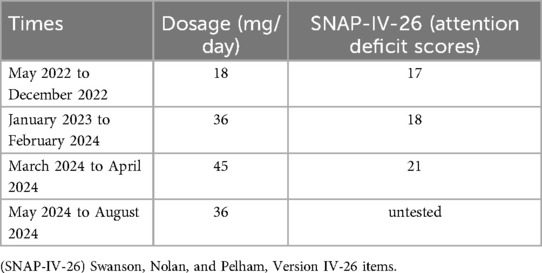
The Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) is used to assess children's level of intelligence, and the patient's total IQ score on the test was 92, which is at the normal level of intelligence for her age group. Based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the child was diagnosed with ADHD. The Swanson, Nolan, and Pelham, Version IV-26 items (SNAP-IV-26) consist of three parts: inattention, hyperactivity/impulsivity, and oppositional defiant. The scale uses a four-point scoring system ranging from 0 to 3, where 0 indicates the complete absence of such symptoms, and 3 represents the very frequent occurrence of these symptoms. It is used to assess the severity of ADHD symptoms, evaluate the effectiveness of treatment, and the degree of symptom improvement. The child showed moderately abnormal attention deficits using the SNAP-IV-26 test, while hyperactivity/impulsivity and oppositional defiance scores were at normal levels.
The Weiss Functional Impairment Rating Scales-Parent Report (WFIRS-P) is designed to assess the degree of impairment in the daily functioning of children and adolescents, encompassing areas such as family, school, life skills, self-management, social activities, and risky behaviors. The WFIRS-P assessment of the ADHD child revealed functional impairments across domains including family, school, life skills, and engagement in risky activities. Furthermore, the Child Behavior Check List (CBCL) categorizes behavioral problems into six primary behavioral symptom factors: social withdrawal, depression, sleep disturbances, somatic complaints, aggressive behavior, and destructive behavior. It aims to assess children's behavioral and emotional issues within the family, school, or community setting. The child's CBCL showed that she scored higher than normal in both social withdrawal and depression, implying that the child's performance in these specific domains tended to show symptoms of social withdrawal and depression more than most children of the same age. This may indicate that the child is having difficulty with social interactions, may feel isolated or unwilling to socialize with others, and may exhibit traits associated with depression in her emotional state, requiring close attention to the child's mental health.
The child's brain magnetic resonance imaging (MRI) at 9 months of age revealed delayed myelination maturation in comparison to peers. The most recent MRI of the brain revealed no anomalies. The child's blood routine, liver and kidney function, blood electrolytes, electrocardiogram, and electroencephalogram all show no abnormalities. Because the child had a history of recurrent cough, respiratory infections and wheezing, combined with allergic rhinitis, she needed to be alerted to the possibility of bronchial asthma. As a result, she underwent pulmonary ventilation testing that showed small airway airflow obstruction and impaired pulmonary ventilation.
4 Diagnostic inventory
The child was diagnosed with ADHD, cleft palate, VtSD, PFO, mild tricuspid regurgitation, delayed motor and language development, hearing loss, recurrent respiratory infections, asthma, rhinitis, enuresis, and dental caries based on her medical history, clinical symptoms, auxiliary examinations, and scale assessment results.
5 Patient's progress report
Given the clear diagnosis of ADHD in the child, she was treated with methylphenidate, starting at a dose of 18 mg/day, which was gradually increased to 36 mg/day based on her attentional performance, while also undergoing physical and language rehabilitation training. Additionally, the pediatrician advised the parents to engage the child in reading and retelling stories at home daily. The parents were fully involved in the treatment process. During the first year of treatment, the parents reported a positive impact, and the child's teacher noted an improvement in her attention span and the speed of completing homework. However, in the second year of treatment, both parents and the teacher reported that the child had difficulty concentrating again and exhibited learning difficulties, prompting an increase in the methylphenidate dose to 45 mg/day. This led to difficulties in falling asleep at night and poor sleep quality, so the dose was reduced back to 36 mg/day (Table 1). After two years of regular treatment, the child still met the diagnostic criteria for ADHD. Therefore, this suggests that methylphenidate was not effective in treating the child, and given her comorbid conditions, the possibility of inherited metabolic diseases should be considered. To further investigate the etiology and guide treatment, after obtaining informed consent from the parents, we conducted whole exome sequencing.
6 Results
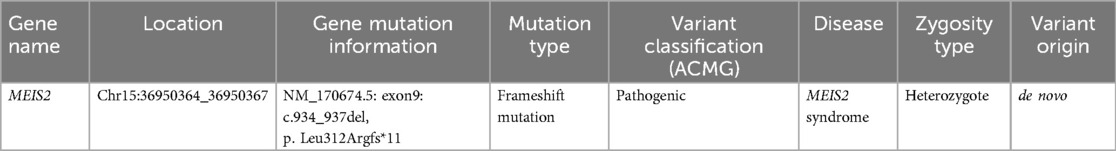
WES was performed to further elucidate the causes of multiple diseases in our patient. A heterozygous frameshift mutation in the MEIS2 gene (NM_170674.5: exon9: c.934_937del, p. Leu312Argfs*11) was identified through further genetic testing (Table 2). The MEIS2 gene is a de novo mutation, which means it is not present in the genetic sequence of the parents but emerges as a new mutation in the child. This variant is the only de novo protein coding variant found in this case. It has been confirmed by Sanger sequencing. This variant has been reported in the study by Verheije et al. (26) and has (27) been recorded in the ClinVar database. Furthermore, according to ACMG criteria (28), the c.934_937del variant in this patient is classified as pathogenic.
7 Discussion
We report a case of de novo heterozygous frameshift mutation in the MEIS2 gene that resulted in developmental delays and a phenotypic overlap of cardiac, craniofacial, and other abnormalities. The clinical presentation of this case included cleft palate, VtSD and PFO, ADHD, delayed motor and language development, mild facial dysmorphic features, learning difficulties, hearing loss, recurrent respiratory infections, asthma, rhinitis, enuresis, and dental caries. The clinical presentation of this patient shared some common clinical features with other previously described patients (21, 22, 26, 29–34), but also included some less common characteristics. Table 3 summarized the clinical phenotypes and genetic findings of this case and previously reported cases with MEIS2 gene variants.
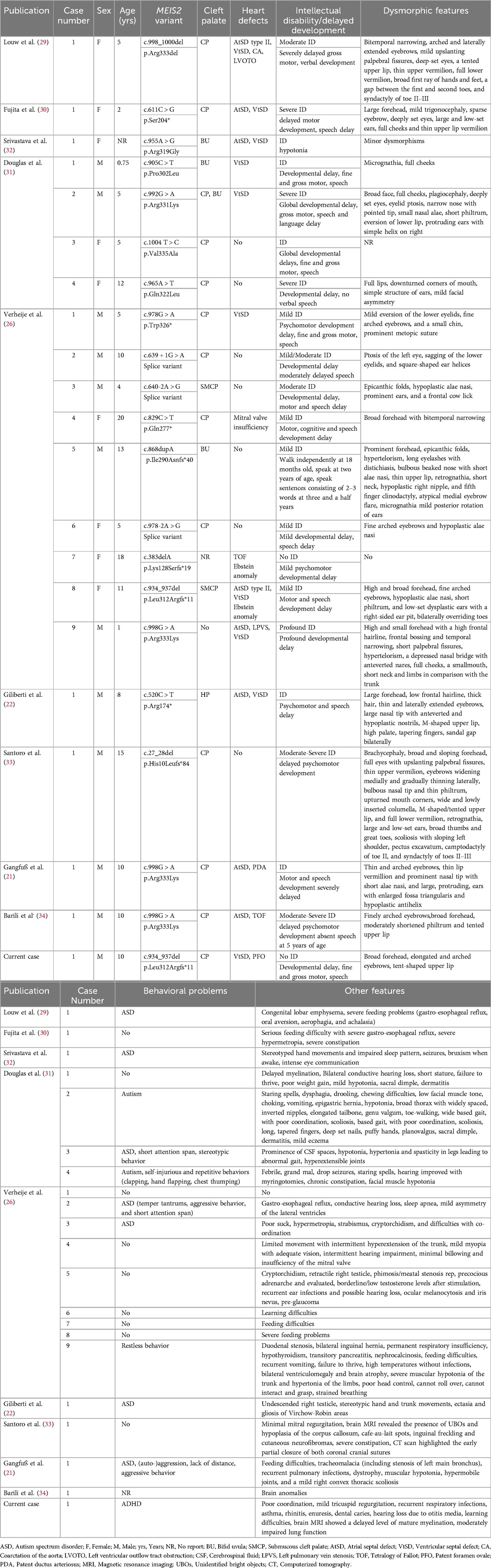
Table 3. The clinical phenotypes and genetic findings of this case and previously reported cases with MEIS2 gene variants.
The diagnosis and treatment of ADHD are further complicated by the fact that ADHD often co-exists with other disorders such as learning difficulties, anxiety disorders, and oppositional defiant disorder. Currently, the exact etiology of ADHD remains undetermined, but researchers generally agree that ADHD is the result of a combination of genetic and environmental factors (35, 36). With the advent of innovative genomics technologies, genetic evaluation of patients with complex clinical presentations has become possible. We report the patient diagnosed with ADHD, who also has a variety of other diseases, presenting with complex clinical manifestations. In addition, her treatment with methylphenidate showed no significant improvement in attention deficit. In search of the etiology, she underwent WES, which revealed a mutation in the MEIS2 gene. This further illustrates that genetic testing is an important clinical tool for identifying the causes in ADHD patients with multiple comorbidities and poor response to methylphenidate treatment.
To date, a total of 21 patients carrying MEIS2 gene mutations have been reported, with 20 patients detected through whole-exome sequencing, and only one patient in the study by Verheije et al. (26) identified using targeted sequencing. This suggests that the phenotype associated with MEIS2 gene mutations is not easily recognizable. Among the reported patients, most of them had intellectual disability, oral-facial clefts (18/21), similar facial malformation characteristics (19/21), and developmental delay (20/21), suggesting that intellectual disability, oral-facial clefts, facial malformations, and developmental delay are common characteristics. However, the patient we reported did not have an intellectual disability. Approximately half of the patients have cardiac defects (14/21), with ventricular septal defect being the most common feature. However, in three cases, more severe cardiac defects were reported, including CA, TOF, and Ebstein's anomaly. This is because these three severe cases carried a frameshift mutation or an intronic deletion of a single highly conserved amino acid (Arg333) (Patient by Louw et al. and Patients 7 and 8 by Verheije et al.) (26, 29). Our patient shared the same MEIS2 gene frameshift mutation with the patient 8 reported by Verheije et al. (26), yet the cardiac defects manifested only as a mild VtSD and PFO, with the ventricular septal defect resolving spontaneously over time.
ASD has been identified in some of the patients with MEIS2 gene mutations that have been documented thus far (9/21). But attention deficits are uncommon in patients with the MEIS2 gene mutation. The patient in this case report had attention deficits that met the diagnostic criteria for ADHD, a diagnosis that had never before been made in a case with a mutation in the MEIS2 gene. However, two additional patients with MEIS2 gene mutations have shown comparable results. Douglas et al. (31) and Verheije et al. (26) reported a 5-year-old girl and a 10-year-old boy, respectively, both diagnosed with ASD and showing short attention spans. Overall, more patients carrying MEIS2 gene mutations are needed to better define the genotype-phenotype correlations.
Recently, Verheije et al. (26) described a patient with the same frameshift mutation (c.934_937del, p. Leu312Argfs*11). This patient had right ventricular hypoplasia, VtSD, PFO, and tricuspid valve abnormalities, which resulted in severe cyanosis at birth. She also had severe eating issues, delayed motor and language development, a moderate intellectual handicap, a cleft soft palate, and a slight indentation of the hard palate, as well as facial malformations. This patient and the case we reported had comparable clinical symptoms, including delayed motor and verbal development, cleft palate, VtSD, PFO, and facial malformations. In addition, our patient had ADHD, recurrent respiratory infections, learning difficulties, hearing loss, asthma, rhinitis, enuresis, and dental caries. However, she did not exhibit intellectual disability, which further expands the spectrum of clinical phenotypes caused by the same MEIS2 gene mutation.
Research has demonstrated that the human, mouse, and chicken MEIS2 gene regulates specific areas of the developing brain, indicating its significance in neurocognitive development (27, 37, 38). In chicken and mouse heart tissue, DeLaughter et al. (39) showed how the MEIS2 gene contributes to the conversion of endothelial cells to endocardial mesenchyme. While research indicates that MEIS2 gene knockout mice are embryonically deadly, conditional knockout mice display aberrant development of the heart, cranial nerves, and craniofacial bones (7), providing evidence to further support that a phenotype of general developmental abnormalities was caused by a disruption of normal human MEIS2 gene function. Furthermore, research has shown a connection between the MEIS2 gene and neurodegenerative illnesses and intellectual disability (40, 41).
Additionally, research has demonstrated that the MEIS2 gene has a role in the etiology of human malignancies (42, 43). Abnormal expression of MEIS2 significantly impacts neuroblastoma cell proliferation and tumorigenicity (44). Wan et al. (45) first demonstrated that MEIS2 acts as a metastasis promoter in colorectal cancer. Xiao et al. (46) found that MEIS2 functions as a tumor suppressor in breast cancer development. A recent study revealed that targeting MEIS2 expression inhibits proliferation and invasion of prostate cancer cells (47). High levels of MEIS2 are correlated with poor survival rates in patients with liver cancer (48). MEIS2 expression is highly downregulated in thyroid cancer patients, as demonstrated by Wen et al. (49) suggesting that MEIS2 may be a target for early diagnosis and targeted therapy in these individuals. MEIS2 inhibits the expression of genes specific to the ciliary marginal zone and optic disc and increases the expression of genes specific to retinal progenitor cells (16). MEIS2 plays a role in acute myeloid leukemia (AML)-ETO-positive leukemia (50). Conversely, high expression of MEIS2 is associated with improved prognosis in ovarian cancer patients (51). Therefore, MEIS2 can be considered as one of the genes involved in neurodevelopmental disorders and cancers, such as those related to the RAS pathway genes or BAF complex genes (37, 52).
Our report demonstrates the importance of genetic testing to find the etiology of ADHD in patients with multiple comorbidities who are poorly treated with methylphenidate. In addition, this case further extends the clinical phenotype of mutations in the MEIS gene.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by West China Second Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
FS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. JL: Conceptualization, Data curation, Formal Analysis, Writing – original draft. DL: Conceptualization, Formal Analysis, Writing – original draft. HZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the family for their consent to the publication of the case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADHD, attention deficit hyperactivity disorder; WES, whole-exome sequencing; TALE, three-amino-acid-loop extension; EDTA, ethylenediaminetetraacetic acid; BWA, Burrows-Wheeler Aligner; NIH, National Institutes of Health; HGMD, Human Genetic Mutation Database; ACMG, American College of Medical Genetics and Genomics; SNVs, single nucleotide variants; VtSD, ventricular septal defect; PFO, patent foramen ovale; WISC-IV, Wechsler Intelligence Scale for Children-Fourth Edition; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; SNAP-IV-26, Swanson, Nolan, and Pelham, Version IV–26 items; WFIRS-P, Weiss Functional Impairment Rating Scales-Parent Report; CBCL, Child Behavior Check List.
References
1. Salari N, Ghasemi H, Abdoli N, Rahmani A, Shiri MH, Hashemian AH, et al. The global prevalence of ADHD in children and adolescents: a systematic review and meta-analysis. Ital J Pediatr. (2023) 49(1):48. doi: 10.1186/s13052-023-01456-1
2. Baker KG. Evaluation of DSM-5 and IWG-2 criteria for the diagnosis of Alzheimer’s disease and dementia with Lewy bodies. Diagnosis. (2016) 3(1):9–12. doi: 10.1515/dx-2015-0031
3. Di Lorenzo R, Balducci J, Poppi C, Arcolin E, Cutino A, Ferri P, et al. Children and adolescents with ADHD followed up to adulthood: a systematic review of long-term outcomes. Acta Neuropsychiatr. (2021) 33(6):283–98. doi: 10.1017/neu.2021.23
4. Kranz TM, Grimm O. Update on genetics of attention deficit/hyperactivity disorder: current status 2023. Curr Opin Psychiatry. (2023) 36(3):257–62. doi: 10.1097/yco.0000000000000852
5. Balogh L, Pulay AJ, Réthelyi JM. Genetics in the ADHD clinic: how can genetic testing support the current clinical practice? Front Psychol. (2022) 13:751041. doi: 10.3389/fpsyg.2022.751041
6. Durán Alonso MB, Vendrell V, López-Hernández I, Alonso MT, Martin DM, Giráldez F, et al. MEIS2 is required for inner ear formation and proper morphogenesis of the cochlea. Front Cell Dev Biol. (2021) 9:679325. doi: 10.3389/fcell.2021.679325
7. Machon O, Masek J, Machonova O, Krauss S, Kozmik Z. MEIS2 is essential for cranial and cardiac neural crest development. BMC Dev Biol. (2015) 15:40. doi: 10.1186/s12861-015-0093-6
8. Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. (2012) 151(1):221–32. doi: 10.1016/j.cell.2012.08.027
9. Wang L, Tang Q, Xu J, Li H, Yang T, Li L, et al. The transcriptional regulator MEIS2 sets up the ground state for palatal osteogenesis in mice. J Biol Chem. (2020) 295(16):5449–60. doi: 10.1074/jbc.RA120.012684
10. Delgado I, López-Delgado AC, Roselló-Díez A, Giovinazzo G, Cadenas V, Fernández-de-Manuel L, et al. Proximo-distal positional information encoded by an Fgf-regulated gradient of homeodomain transcription factors in the vertebrate limb. Sci Adv. (2020) 6(23):eaaz0742. doi: 10.1126/sciadv.aaz0742
11. Delgado I, Giovinazzo G, Temiño S, Gauthier Y, Balsalobre A, Drouin J, et al. Control of mouse limb initiation and antero-posterior patterning by meis transcription factors. Nat Commun. (2021) 12(1):3086. doi: 10.1038/s41467-021-23373-9
12. López-Delgado AC, Delgado I, Cadenas V, Sánchez-Cabo F, Torres M. Axial skeleton anterior-posterior patterning is regulated through feedback regulation between MEIS transcription factors and retinoic acid. Development. (2021) 148(1). doi: 10.1242/dev.193813
13. Desiderio S, Schwaller F, Tartour K, Padmanabhan K, Lewin GR, Carroll P, et al. Touch receptor end-organ innervation and function require sensory neuron expression of the transcription factor MEIS2. eLife. (2024) 12. doi: 10.7554/eLife.89287
14. Agoston Z, Schulte D. MEIS2 competes with the Groucho co-repressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrain-hindbrain boundary organizer. Development. (2009) 136(19):3311–22. doi: 10.1242/dev.037770
15. Conte I, Carrella S, Avellino R, Karali M, Marco-Ferreres R, Bovolenta P, et al. miR-204 is required for lens and retinal development via Meis2 targeting. Proc Natl Acad Sci U S A. (2010) 107(35):15491–6. doi: 10.1073/pnas.0914785107
16. Dupacova N, Antosova B, Paces J, Kozmik Z. Meis homeobox genes control progenitor competence in the retina. Proc Natl Acad Sci U S A. (2021) 118(12). doi: 10.1073/pnas.2013136118
17. Heine P, Dohle E, Bumsted-O'Brien K, Engelkamp D, Schulte D. Evidence for an evolutionary conserved role of homothorax/Meis1/2 during vertebrate retina development. Development. (2008) 135(5):805–11. doi: 10.1242/dev.012088
18. Frazer S, Prados J, Niquille M, Cadilhac C, Markopoulos F, Gomez L, et al. Transcriptomic and anatomic parcellation of 5-HT(3A)R expressing cortical interneuron subtypes revealed by single-cell RNA sequencing. Nat Commun. (2017) 8:14219. doi: 10.1038/ncomms14219
19. Jakovcevski M, Ruan H, Shen EY, Dincer A, Javidfar B, Ma Q, et al. Neuronal Kmt2a/Mll1 histone methyltransferase is essential for prefrontal synaptic plasticity and working memory. J Neurosci. (2015) 35(13):5097–108. doi: 10.1523/jneurosci.3004-14.2015
20. Su Z, Wang Z, Lindtner S, Yang L, Shang Z, Tian Y, et al. Dlx1/2-dependent expression of MEIS2 promotes neuronal fate determination in the mammalian striatum. Development. (2022) 149(4). doi: 10.1242/dev.200035
21. Gangfuß A, Yigit G, Altmüller J, Nürnberg P, Czeschik JC, Wollnik B, et al. Intellectual disability associated with craniofacial dysmorphism, cleft palate, and congenital heart defect due to a de novo MEIS2 mutation: a clinical longitudinal study. Am J Med Genet A. (2021) 185(4):1216–21. doi: 10.1002/ajmg.a.62070
22. Giliberti A, Currò A, Papa FT, Frullanti E, Ariani F, Coriolani G, et al. MEIS2 Gene is responsible for intellectual disability, cardiac defects and a distinct facial phenotype. Eur J Med Genet. (2020) 63(1):103627. doi: 10.1016/j.ejmg.2019.01.017
23. Shimojima K, Ondo Y, Okamoto N, Yamamoto T. A 15q14 microdeletion involving MEIS2 identified in a patient with autism spectrum disorder. Hum Genome Var. (2017) 4:17029. doi: 10.1038/hgv.2017.29
24. Nestadt G, Wang Y, Grados MA, Riddle MA, Greenberg BD, Knowles JA, et al. Homeobox genes in obsessive-compulsive disorder. Am J Med Genet B. (2012) 159b(1):53–60. doi: 10.1002/ajmg.b.32001
25. Roussel J, Larcher R, Sicard P, Bideaux P, Richard S, Marmigère F, et al. The autism-associated MEIS2 gene is necessary for cardiac baroreflex regulation in mice. Sci Rep. (2022) 12(1):20150. doi: 10.1038/s41598-022-24616-5
26. Verheije R, Kupchik GS, Isidor B, Kroes HY, Lynch SA, Hawkes L, et al. Heterozygous loss-of-function variants of MEIS2 cause a triad of palatal defects, congenital heart defects, and intellectual disability. Eur J Hum Genet. (2019) 27(2):278–90. doi: 10.1038/s41431-018-0281-5
27. Kondo T, Isono K, Kondo K, Endo TA, Itohara S, Vidal M, et al. Polycomb potentiates MEIS2 activation in midbrain by mediating interaction of the promoter with a tissue-specific enhancer. Dev Cell. (2014) 28(1):94–101. doi: 10.1016/j.devcel.2013.11.021
28. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
29. Louw JJ, Corveleyn A, Jia Y, Hens G, Gewillig M, Devriendt K. MEIS2 Involvement in cardiac development, cleft palate, and intellectual disability. Am J Med Genet A. (2015) 167a(5):1142–6. doi: 10.1002/ajmg.a.36989
30. Fujita A, Isidor B, Piloquet H, Corre P, Okamoto N, Nakashima M, et al. De novo MEIS2 mutation causes syndromic developmental delay with persistent gastro-esophageal reflux. J Hum Genet. (2016) 61(9):835–8. doi: 10.1038/jhg.2016.54
31. Douglas G, Cho MT, Telegrafi A, Winter S, Carmichael J, Zackai EH, et al. De novo missense variants in MEIS2 recapitulate the microdeletion phenotype of cardiac and palate abnormalities, developmental delay, intellectual disability and dysmorphic features. Am J Med Genet A. (2018) 176(9):1845–51. doi: 10.1002/ajmg.a.40368
32. Srivastava S, Desai S, Cohen J, Smith-Hicks C, Barañano K, Fatemi A, et al. Monogenic disorders that mimic the phenotype of Rett syndrome. Neurogenetics. (2018) 19(1):41–7. doi: 10.1007/s10048-017-0535-3
33. Santoro C, Riccio S, Palladino F, Aliberti F, Carotenuto M, Zanobio M, et al. A novel MEIS2 mutation explains the complex phenotype in a boy with a typical NF1 microdeletion syndrome. Eur J Med Genet. (2021) 64(5):104190. doi: 10.1016/j.ejmg.2021.104190
34. Barili V, Ambrosini E, Uliana V, Bellini M, Vitetta G, Martorana D, et al. Success and pitfalls of genetic testing in undiagnosed diseases: whole exome sequencing and beyond. Genes. (2023) 14(6). doi: 10.3390/genes14061241
35. Cortese S. The neurobiology and genetics of attention-deficit/hyperactivity disorder (ADHD): what every clinician should know. Eur J Paediatr Neurol. (2012) 16(5):422–33. doi: 10.1016/j.ejpn.2012.01.009
36. Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. (2021) 128:789–818. doi: 10.1016/j.neubiorev.2021.01.022
37. Agoston Z, Li N, Haslinger A, Wizenmann A, Schulte D. Genetic and physical interaction of MEIS2, PAX3 and PAX7 during dorsal midbrain development. BMC Dev Biol. (2012) 12:10. doi: 10.1186/1471-213x-12-10
38. Larsen KB, Lutterodt MC, Laursen H, Graem N, Pakkenberg B, Møllgård K, et al. Spatiotemporal distribution of PAX6 and MEIS2 expression and total cell numbers in the ganglionic eminence in the early developing human forebrain. Dev Neurosci. (2010) 32(2):149–62. doi: 10.1159/000297602
39. DeLaughter DM, Christodoulou DC, Robinson JY, Seidman CE, Baldwin HS, Seidman JG, et al. Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro. J Mol Cell Cardiol. (2013) 59:196–204. doi: 10.1016/j.yjmcc.2013.03.016
40. Huang M, Deng C, Yu Y, Lian T, Yang W, Feng Q. Spatial correlations exploitation based on nonlocal voxel-wise GWAS for biomarker detection of AD. NeuroImage Clin. (2019) 21:101642. doi: 10.1016/j.nicl.2018.101642
41. Liu H, Barnes J, Pedrosa E, Herman NS, Salas F, Wang P, et al. Transcriptome analysis of neural progenitor cells derived from Lowe syndrome induced pluripotent stem cells: identification of candidate genes for the neurodevelopmental and eye manifestations. J Neurodev Disord. (2020) 12(1):14. doi: 10.1186/s11689-020-09317-2
42. Bhanvadia RR, VanOpstall C, Brechka H, Barashi NS, Gillard M, McAuley EM, et al. MEIS1 and MEIS2 expression and prostate cancer progression: a role for HOXB13 binding partners in metastatic disease. Clin Cancer Res. (2018) 24(15):3668–80. doi: 10.1158/1078-0432.Ccr-17-3673
43. Jeong JH, Park SJ, Dickinson SI, Luo JL. A constitutive intrinsic inflammatory signaling circuit composed of miR-196b, MEIS2, PPP3CC, and p65 drives prostate cancer castration resistance. Mol Cell. (2017) 65(1):154–67. doi: 10.1016/j.molcel.2016.11.034
44. Zha Y, Xia Y, Ding J, Choi JH, Yang L, Dong Z, et al. MEIS2 Is essential for neuroblastoma cell survival and proliferation by transcriptional control of M-phase progression. Cell Death Dis. (2014) 5(9):e1417. doi: 10.1038/cddis.2014.370
45. Wan Z, Chai R, Yuan H, Chen B, Dong Q, Zheng B, et al. MEIS2 promotes cell migration and invasion in colorectal cancer. Oncol Rep. (2019) 42(1):213–23. doi: 10.3892/or.2019.7161
46. Xiao Y, Liu Y, Sun Y, Huang C, Zhong S. MEIS2 Suppresses breast cancer development by downregulating IL10. Cancer Rep. (2024) 7(5):e2064. doi: 10.1002/cnr2.2064
47. Dai X, Chen X, Chen W, Ou Y, Chen Y, Wu S, et al. CircDHRS3 inhibits prostate cancer cell proliferation and metastasis through the circDHRS3/miR-421/MEIS2 axis. Epigenetics. (2023) 18(1):2178802. doi: 10.1080/15592294.2023.2178802
48. Yan G, Chang Z, Wang C, Gong Z, Xin H, Liu Z. LncRNA ILF3-AS1 promotes cell migration, invasion and EMT process in hepatocellular carcinoma via the miR-628-5p/MEIS2 axis to activate the Notch pathway. Dig Liver Dis. (2022) 54(1):125–35. doi: 10.1016/j.dld.2021.04.036
49. Wen X, Liu M, Du J, Wang X. Meis homeobox 2 (MEIS2) inhibits the proliferation and promotes apoptosis of thyroid cancer cell and through the NF-κB signaling pathway. Bioengineered. (2021) 12(1):1766–72. doi: 10.1080/21655979.2021.1923354
50. Vegi NM, Klappacher J, Oswald F, Mulaw MA, Mandoli A, Thiel VN, et al. MEIS2 is an oncogenic partner in AML1-ETO-positive AML. Cell Rep. (2016) 16(2):498–507. doi: 10.1016/j.celrep.2016.05.094
51. Crijns AP, de Graeff P, Geerts D, Ten Hoor KA, Hollema H, van der Sluis T, et al. MEIS And PBX homeobox proteins in ovarian cancer. Eur J Cancer. (2007) 43(17):2495–505. doi: 10.1016/j.ejca.2007.08.025
Keywords: ADHD, MEIS2, cleft palate, congenital heart defect, developmental delay, case report
Citation: Shen F, Li J, Li D and Zhou H (2024) Cleft palate, congenital heart disease, and developmental delay involving MEIS2 heterozygous mutations found in the patient with attention deficit hyperactivity disorder: a case report. Front. Pediatr. 12:1500152. doi: 10.3389/fped.2024.1500152
Received: 22 September 2024; Accepted: 9 December 2024;
Published: 24 December 2024.
Edited by:
Xinxiu Xu, Vanderbilt University Medical Center, United StatesReviewed by:
Qianqian Liang, Geisinger Health System, United StatesVibha Acharya, University of Pittsburgh, United States
Copyright: © 2024 Shen, Li, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhou, MTEwMzU3MDQ4OUAgcXEuIGNvbQ==
 Fang Shen
Fang Shen Junyan Li
Junyan Li