- 1Department of Pediatrics, The Second Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Xiaorong Luo’s National Renowned Expert Inheritance Studio, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
Introduction: Macrolide-unresponsive Mycoplasma pneumoniae pneumonia (MUMPP) cases have been rapidly increasing. The primary reason for this increased incidence is the pathogen's acquisition of resistance through mutations in 23S rRNA genes. Due to the unfeasibility of testing for macrolide susceptibility at the time of admission, this study aimed to assess the clinical features of pediatric MUMPP, using insights from laboratory tests and patterns of chest radiographic resolution.
Material and methods: We conducted a retrospective review of 161 patients with M. pneumoniae pneumonia (MPP) between January 2023 and December 2023. These patients were categorized into two groups based on their responsiveness to macrolides: 72 patients were in the MUMPP group, and 89 patients were in the macrolide-sensitive Mycoplasma pneumoniae pneumonia (MSMP) group.
Results: MUMPP patients experienced a longer duration of fever and hospital stay. A higher proportion of MUMPP patients had shortness of breath, transcutaneous blood oxygen saturation (SpO2) lower than 94%, bilateral lobar infiltrates, lobar pneumonia and pleural effusion. The serum level of serum ferritin (SF), interleukin-6(IL-6), D-dimer, lactate dehydrogenase to albumin rate (LAR), and neutrophil to lymphocyte rate (NLR) were higher in MUMPP group.
Conclusions: Our findings revealed that patients with MUMPP exhibit more severe initial radiographic indicators and clinical course compared to those with MSMP. Therefore, it is crucial to promptly administer alternative therapeutic agents besides macrolides for the management of MUMPP.
1 Introduction
Mycoplasma pneumoniae pneumonia (MPP) is a type of the community-acquired pneumonia (CAP), with M. pneumoniae being the primary cause of lower respiratory tract infection (LRTI) in children. MPP typically manifests as a benign, self-limiting condition associated with a positive prognosis. However, infrequent complications in other organs can pose significant health risks. Globally, M. pneumoniae epidemics occur every 3–7 years, with varying incidence rates (1–5). Recent report indicates a sharp rise in M. pneumoniae cases in several regions of China since June 2023 (6). Meanwhile, an increase of the incidence of infections with M. pneumoniae has also observed in 2023/2024 in Europe (7).These M. pneumoniae infection often leads to more severe clinical symptoms, which can not only impact the health of patients but also inflicts an economic burden on their families, often correlating with the severity of the infection and the effectiveness of antibiotic treatment.
After infection, M. pneumoniae adheres to the host's respiratory epithelium and produces numerous cytotoxic proteins, protecting itself from removal by mucociliary escalator mechanisms while damaging the host's pseudostratified epithelium (8). A recent study demonstrated that Club Cell Secretory Protein (CC16) plays an important role in the airway remodeling and pulmonary epithelium damage during respiratory infection with M. pneumoniae (9). Reports also indicate that the pathogenesis of M. pneumoniae infection is closely related to the stimulation of macrophages by this pathogen via toll-like receptors, which release immunomodulatory and inflammatory cytokines and chemokines (10). The severity of M. pneumoniae infection is related to the host immune system's response to the infection (11).
Conventionally, macrolides have been the preferred choice of antimicrobials for treating M. pneumoniae infections in children (11). However, recent observations indicate that despite treatment with macrolides for over 7 days, some cases still exhibit worsening clinical symptoms like persistent fever and aggravated lung imaging, leading to refractory MPP (RMPP) (12). Although macrolides are effective in treating RMPP, alternative treatments are required in cases of prolonged fever and hospitalization. The increasing prevalence of macrolide-resistant M. pneumoniae compromises treatment effectiveness. Additionally, excessive immune response, co-infections, and host conditions contribute to lung injury in MPP, further affecting the efficacy of macrolide therapy (13, 14). Timely and appropriate treatment is crucial to prevent complications like necrotizing pneumonia, bronchiolitis obliterans, thrombosis, and even death (15). Therefore, predicting macrolide responsiveness and susceptibility in the early stages of M. pneumoniae infection is essential.
Predicting treatment response at admission has recently become increasingly challenging. Although distinguishing between RMPP and MSMP has recently garnered attention, obtaining records of the gradually worsening syndrome or chest x-ray is difficult. Additionally, clinicians often switch treatments within 7 days of initial macrolide use for MPP patients. To confirm macrolide resistance, the drug resistance gene detection is required. However, drug resistance gene detection is not a routine test item and can be costly. Based on an actual treatment course, macrolide-unresponsive Mycoplasma pneumoniae pneumonia (MUMPP) is characterized by a persistent fever of 38.0℃ or higher, lasting for 72 h or more following macrolide therapy. Despite its frequency in clinical settings, few studies have characterized this condition. Patients with MUMPP face a higher risk of progressing to RMPP and/or severe clinical outcomes compared to MSMP patients, making it a critical issue in clinical practice (16). Although some reports indicate that switching to secondary treatments does not improve therapeutic efficacy compared to prolonged macrolide use in children with MUMPP (17), early administration of tetracycline antibiotics may effectively prevent the progression of MUMPP to RMPP and/or mitigate severe clinical outcomes (16).
In this study, patients were categorized into two distinct groups based on the duration of fever after macrolide administration: MUMPP (persistent fever of ≥38.0°C for ≥72 h after macrolide administration) and MSMP (fever subsided within 3 days). This study sought to compare and identify the differences in clinical and radiological characteristics between these two groups. Additionally, we evaluated potential markers such as IL-6, SF, D-dimer, and LDH for predicting MUMPP. This distinction may assist physicians in administering safe and effective treatment strategies, enhancing the efficacy of early-stage MPP management. Ultimately, this research aims to determine the distinguishing features of MUMPP from MSMP, thereby contributing to the advancement of MPP management strategies.
2 Materials and methods
2.1 Subjects and study design
General information: We conducted a retrospective analysis of the medical records of patients aged ≤18 years admitted to our department from January 1, 2023, to December 31, 2023, and diagnosed with MPP.
Inclusion criteria: (1) complete and comprehensive medical records; (2) hospitalized patients under 18 years of age; (3) Patients exhibiting signs and symptoms of CAP, including fever, acute respiratory symptoms (cough, tachypnoea, difficult breathing), or both, along with the presence of new infiltrates or consolidation on chest radiography; (4) positive result for M. pneumoniae. The diagnosis of M. pneumoniae infection was confirmed if any or all of the following conditions were met: (i) positive serologic tests, with the specific IgM titers against M. pneumoniae ≥1:160 or a four-fold or greater increase in IgM or IgG (or both) antibody titers between the acute and convalescent stages; (ii) Positive PCR or real-time PCR results for M. pneumoniae using nasopharyngeal aspiration or sputum samples.
Based on macrolide sensitivity, the MPP patients were divided into two groups: MUMPP and MSMP. MUMPP cases were clinically defined as those with persistent fever ≥38.0°C for at least ≥72 h after macrolide treatment. Conversely, MSMP cases were those whose fever subsided within 72 h after macrolide administration.
Exclusion criteria included: (1) patients with clinical symptoms and radiologic findings inconsistent with pneumonia, despite positive M. pneumoniae IgM; (2) patients who switched treatment within 72 h of the initial macrolide antibiotic administration; (3) patients with immunodeficiency disease, chronic pulmonary disease, kidney or liver disease, neoplasms, primary ciliary dyskinesia, cystic fibrosis, cardiovascular disease or malabsorption syndromes; (4) patients in the late stages of MPP.
2.2 Data collection
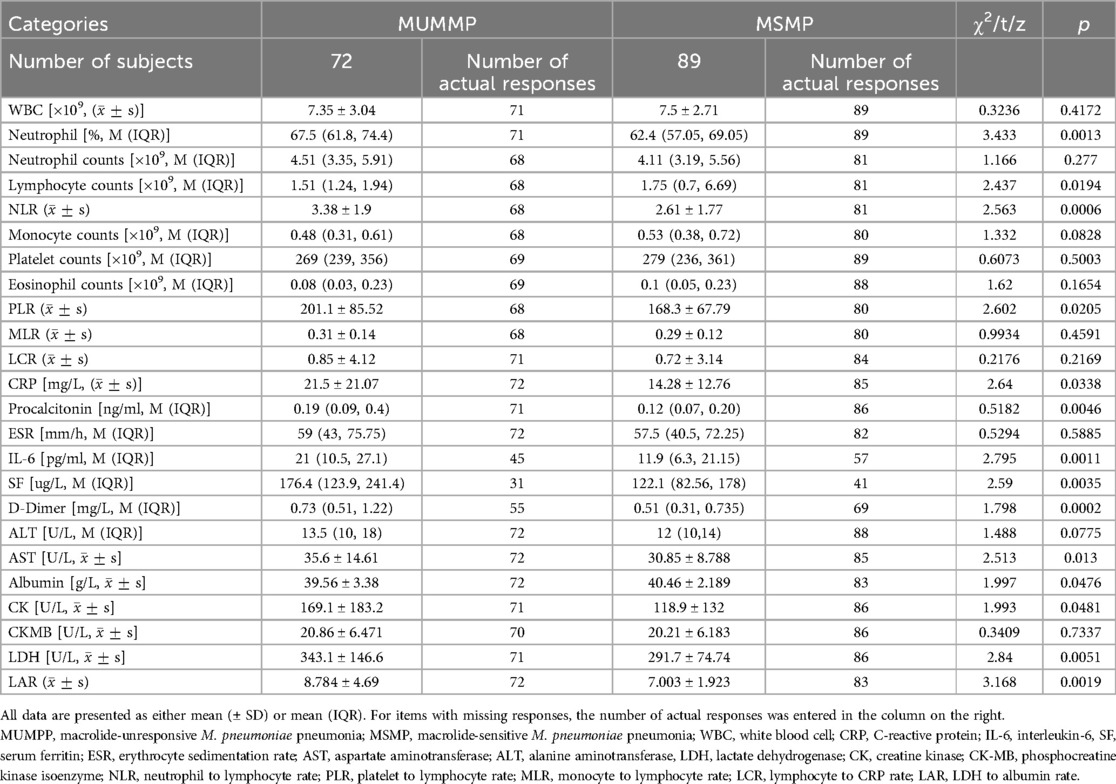
Demographic and clinical data were collected from medical histories, encompassing age, sex and BMI. Clinical characteristics included the duration and onset time of fever, catarrhal syndrome, hospital stay, transcutaneous blood oxygen saturation (SpO2), extrapulmonary complications and administered treatments. Upon admission, blood samples were assessed for total and differential cell counts, as well as serum levels of white blood cell (WBC), C-reactive protein (CRP); interleukin-6 (IL-6), serum ferritin (SF); erythrocyte sedimentation rate (ESR); aspartate aminotransferase (AST); alanine aminotransferase (ALT), lactate dehydrogenase (LDH); creatine kinase (CK); phosphocreatine kinase isoenzyme (CK-MB) and D-dimer. Additionally, the rates of certain serum indicators, including neutrophil to lymphocyte rate (NLR); platelet to lymphocyte rate (PLR); monocyte to lymphocyte rate (MLR); lymphocyte to CRP rate (LCR), and LDH to albumin rate (LAR), were evaluated.
Chest radiographs were conducted when clinically indicated, allowing for the detection of bronchopneumonia or lobar pneumonia, with or without pleural effusion and atelectasis. M. pneumoniae infection was diagnosed using DNA extracted from oropharyngeal swabs. Acute IgM serology and/or elevated IgG titers in serum were evaluated by using a commercial test kit. A comparative analysis was performed to assess the differences in the aforementioned indicators between the MUMPP and MSMP groups. The majority of clinical information and chest radiographs reported in this study were reviewed by pediatric pulmonary specialists.
2.3 Data analysis
Categorical variables were summarized in terms of frequency and expressed as percentages, while serial data were analyzed in two ways. When the assumption of normal distribution was met, serial data were analyzed using the mean and standard deviation. Conversely, when the data was not normally distributed, the median and interquartile range were used to represent the serial data.
Continuous variables were compared using the paired t-test (or Wilcoxon test, as applicable) for normally distributed data, whereas the Mann-Whitney test was used for non-normally distributed data. Categorical variables between the two groups were compared using the Chi-Square test, with Fisher's exact test being preferred in instances where at least one expected frequency was less than 5. The significant variables were further described using the receiver operating characteristic (ROC) curve, and optimal cut-off points were determined using the area under the curve (AUC). All statistical analyses were performed using SPSS software (version 22) and Prism GraphPad (version 10), with a significance level set at p < 0.05.
3 Results
3.1 Subject's demographics
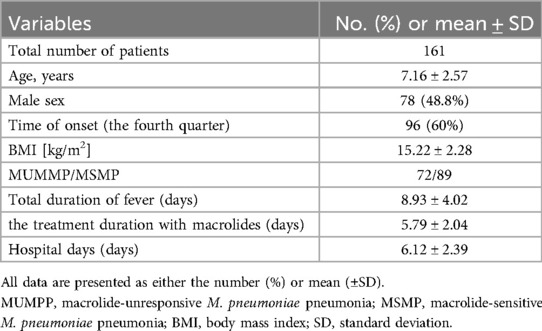
A total of 161 patients diagnosed with M. pneumonia and treated in our hospital's pediatrics department were included in the study. Table 1 outlines the patients' demographic details. Out of these, 48.8% (78) patients were male. The median age was 7.16 years (±2.57), with a BMI of 15.22 kg/m2 (±2.28), and a median duration of fever (from the initial onset) of 8.93 days (±4.02). The presence of M. pneumoniae was detected throughout 2023, with a significant peak observed in the fourth quarter. According to the inclusion criteria, the patients were further categorized into two groups: 72 patients belonged to the MUMPP group, whereas the remaining 89 patients were in the MSMP group. The average hospital stay was 6.12 days (±2.39) and the average duration of macrolide treatment was 5.79 days (±2.04).
3.2 Comparison of clinical characteristics between the two groups
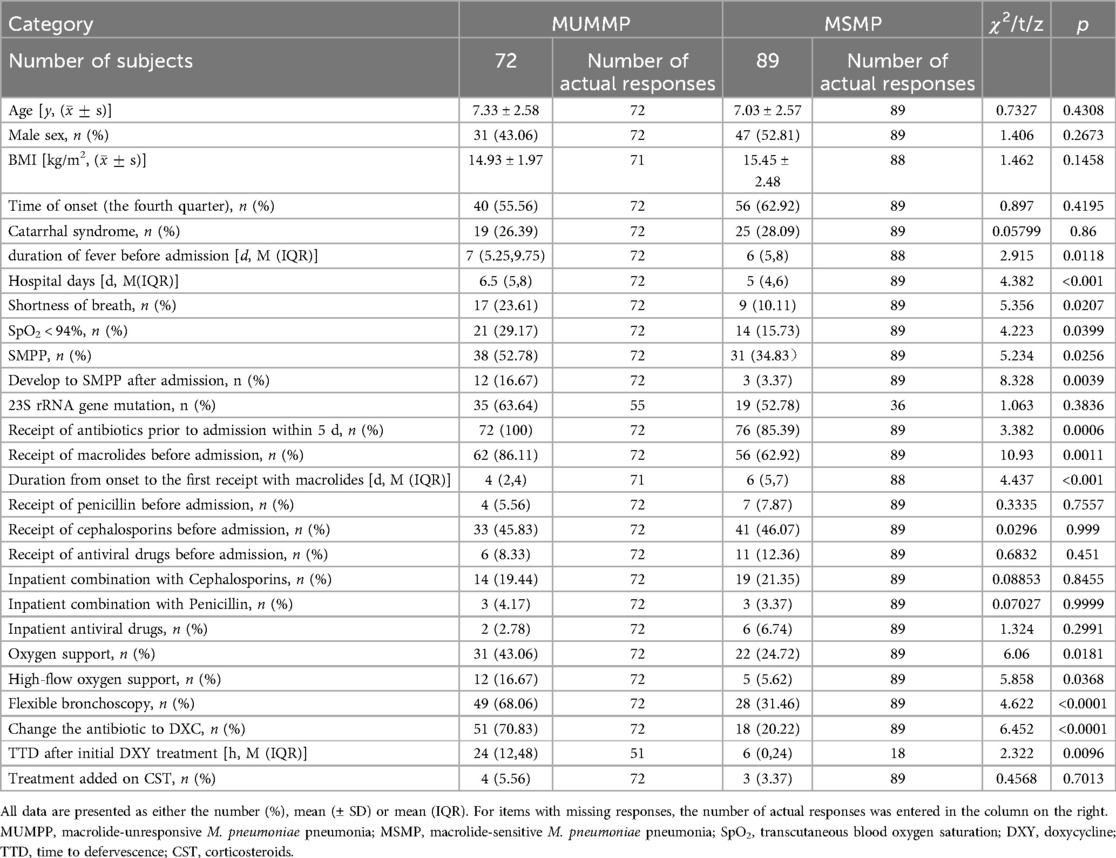
The clinical differences between MUMPP and MSMP children are outlined in Table 2. Both groups exhibited no significant difference in terms of age, gender, BMI, onset time, or catarrhal syndrome. However, the MUMPP group had a significantly longer median fever duration before admission, standing at 7 (5.25, 9.75) days, compared to the MSMP group's 6 (5, 8) days (p = 0.0118). Additionally, the median length of stay for MUMPP patients was significantly longer, standing at 6.5 (5, 8) days, in contrast to the 5 (4, 6) days for the MSMP group (p < 0.001).
Notably, 100% of MUMPP patients received antibiotics within 5 days prior to admission, compared to 85.39% in the MSMP group (p = 0.0006) (Table 2). While the difference in the proportion of patients treated with macrolides before admission was statistically significant between the two groups, no significant differences were observed in the proportions of patients receiving cephalosporins or penicillin. Furthermore, MUMPP patients experienced a shorter duration from onset to the first administration of macrolides compared to the MSMP group. Compared to antibiotics, antiviral drugs showed a lower administration rate before admission, with no significant difference between the two groups.
During hospitalization, the combination of antibiotics and antiviral drugs was not significantly different between the two groups. However, the MUMPP group exhibited a higher frequency of SpO2 < 94% and SMPP, which require more frequent use of flexible bronchoscopy and oxygen support, particularly heated and humidified high-flow oxygen. Among these SMPP patients, MUMPP group had a higher rate of transitioning to severe MPP(SMPP) after admission compared to MSMP patients (p < 0.01).
In this study, the primary secondary treatment measures included the addition of corticosteroids (CST) or switching to doxycycline as the antibiotic. Notably, the MUMPP group had a significantly higher rate of switching to doxycycline (p < 0.05). Following doxycycline therapy, a median time to defervescence (TTD) of 24 h (interquartile range 24–48) in all patients, with the MUMPP group experiencing a longer median duration of 24 h compared to 6 h in the MSMP group. The addition of CST was observed in only a small proportion of cases, with no significant difference between the two groups.
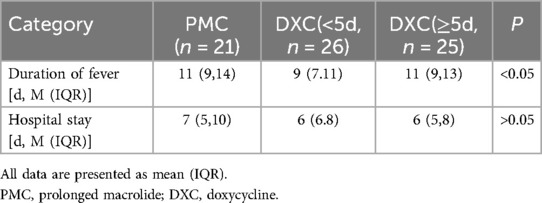
In the MUMPP group, with the exception of prolonged macrolide treatment (PMC), the primary therapeutic approach was switched to doxycycline (DXC). We categorized the MUMPP patients into two groups: the PMC group and the DXC group. Within the DXC group, patients were further divided based on the duration of macrolide treatment prior to DXC administration: those with less than 5 days and those with more than 5 days of macrolide treatment. Our findings indicate that early administration of DXC can improve both the duration of fever and hospital stay, with a statistically significant difference observed in the duration of fever. (p < 0.05) (Table 3).
3.3 Extrapulmonary manifestations
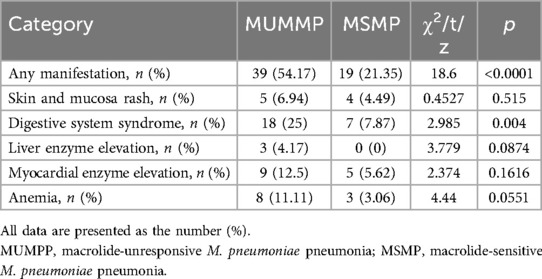
Different extrapulmonary manifestation in patients with M. pneumoniae and their relative distribution are shown in Table 4. Overall, 58 (36.6%) patients exhibited one or more extrapulmonary manifestation, with a notably higher prevalence in the MUMPP group, accounting for 39 (54.17%) cases, compared to the MSMP group with 19 (21.35%) cases. Notably, the MUMPP group exhibited a greater frequency of gastric syndrome than the MSMP group in this study. While there were no significant differences in skin and mucosal, hepatobiliary system, and cardiovascular involvement between the two groups, these manifestations were more common in the MUMPP group.
3.4 Comparison of serum markers at admission between the two groups
The serum levels of inflammatory cytokines were examined in the children with MUMPP group and MSMP. The findings revealed significantly elevated levels of NEUT% (67.5 vs. 62.4, p < 0.05), CRP (15.79 vs. 11.3 mg/L, p < 0.05), PCT (0.19 vs. 0.115 ng/ml, p < 0.05), IL-6 (21 vs. 11.9 pg/ml, p < 0.05), SF (176.4 vs. 122.1 ug/L, p < 0.05), and NLR (3.382 vs. 2.611, p < 0.05) in the MUMPP patients compared to the MSMP group.
Moreover, we observed significant difference in serval systemic indicators, as detailed in Table 5. Compared to the MSMP group, the MUMPP group showed significantly higher median values of serum total LDH (343.1 ± 146.6 vs. 291. 7 ± 74.74 IU/L, p = 0.0051), AST (35.6 vs. 30.85 IU/L, p < 0.05), albumin (39.56 vs. 40.46 g/L, p < 0.05), LAR (8.784 vs. 7.003, p < 0.05), CK (169.1 vs. 118.9, p < 0.05), and D-dimer (0.73 vs. 0.51 mg/L, p < 0.05). However, no significant differences were noted in WBC, neutrophil counts, platelet, EOS, monocytes, ESR, ALT, and CK-MB between the two groups.
ROC curves were generated to further evaluate the predictive value of these risk factors for MUMPP. The areas under the ROC curves for SF, IL-6, D-dimer, LAR, PCT, NLR, LDH and CRP were 0.7002, 0.6865, 0.6946, 0.6528, 0.631, 0.6616, 0.6218, and 0.5984 respectively (p < 0.01). Among these, SF exhibited the largest area under the ROC curve, indicating its highest predictive value for MUMPP in MPP patients. At the specified cut-off value, SF showed high specificity (90.32%) but relatively low sensitivity (39.02%). The cut-off values were: SF at 104.1 ug/L (39.02% sensitivity, 90.32% specificity), NLR was 3.212 (81.48% sensitivity, 48.53% specificity), LAR was 8.476 (86.59% sensitivity, 43.66% specificity), IL-6 was 16.81 pg/ml (66.67% sensitivity, 64.44% specificity), D-dimer was 0.435 mg/L (43.348% sensitivity, 87.27% specificity), PCT was 0.2151 ng/ml (76.74% sensitivity, 47.89% specificity), LDH was 291 U/L (60.47% sensitivity, 60.56% specificity) and CRP was 26.21 mg/L (89.41% sensitivity, 30.56% specificity). As demonstrated above, SF, IL-6, D-dimer, LAR, and NLR exhibited the highest areas under the ROC (Figure 1), meaning that these were the best indicators for predicting MUMPP.
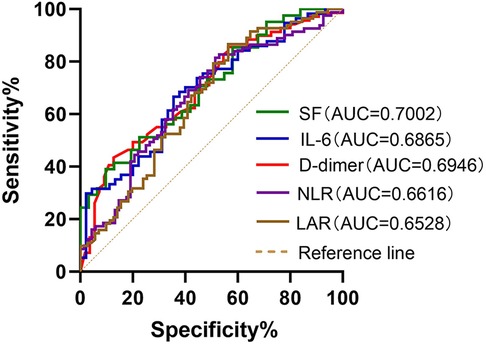
Figure 1. Receiver operating characteristic (ROC) curves of SF, IL-6, D-dimer, NLR and LAR for predicting MUMPP. SF had better predictive ability for differentiation of MUMPP. SF, serum ferritin; IL-6, interleukin-6; NLR, neutrophil to lymphocyte rate; LAR, lactate dehydrogenase to albumin rate; MUMPP, Macrolide-unresponsive Mycoplasma pneumoniae pneumonia; AUC, area under the curve.
3.5 Chest imaging
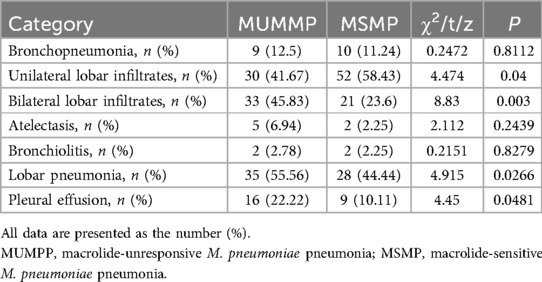
The features of chest radiographs obtained at admission and the distinguishing characteristics of each patient group are summarized in Table 6. The proportion of patients with bronchopneumonia was comparable in the MUMPP group (12.5%) and the MSMP group (11.24%). Compared with the MSMP group, the MUMPP group showed no significant difference in consolidation. However, the MUMPP group exhibited a significantly higher likelihood of unilateral lobar infiltrates and a lower tendency towards bilateral lobar infiltrates. Specifically, the percentage of patients with lobar pneumonia alone was significantly higher in the MUMPP group (55.56%) compared to the MSMP group (44.44%) (p < 0.01). Pleural effusion was observed in a total of 25 patients (15.23%), with a significantly greater proportion noted in the MUMPP group (22.22%) compared to the MSMP group (10.11%). Furthermore, bronchiolitis and atelectasis were detected in only a minor fraction of patients, showing no significant differences between the two groups (p > 0.05).
4 Discussion
The recent surge in MUMPP incidence, particularly in children, is concerning and linked to macrolides resistant Mycoplasma pneumoniae strains infection and potentially life-threatening conditions (14, 18). Beyond increased bacterial load, an excessive host immune response among patients with MUMPP may lead to lung injury (13, 14). Without proper treatment, these patients may experience poor outcomes and prolonged disease courses, with numerous complications during the acute stage and potential long-term sequelae such as post-infectious bronchiolitis obliterans, bronchial asthma, unilateral transparent lung, and bronchiectasis (3, 19). Therefore, analyzing predictive factors for MUMPP and establishing early detection and timely treatment is crucial to prevent complications and sequelae.
Consistent with other studies, the median age of MPP patients was 7 years, with no statistically significant gender difference. M. pneumoniae incidence peaks in the summer and fall seasons in America (20) and between August and January in China (21). Similarly, more than half of MPP patients in our study were detected in the fourth quarter. Longer median hospital stay (8.97 days) often burdens medical resources and clinical staff, especially during epidemics. The clinical symptoms associated with M. pneumoniae CAP in multivariable analysis resemble those of respiratory viral illnesses (22), making it challenging to distinguish M. pneumoniae from other pathogens. However, related syndromes such as catarrhal syndrome were less common in our study (27.3%). The median BMI was lower in the MUMPP group, suggesting that smaller individuals may be less sensitive to macrolides and prone to severe syndromes. However, BMI showed no significant difference between the groups, highlighting the need for a larger patient sample in future studies.
Compared to the MSMP group, the MUMPP group exhibited a greater proportion of severe clinical syndromes, characterized by significantly longer fever duration, prolonged hospital stays, and more pronounced symptoms, including a higher percentage of shortness of breath (23.61%) and SpO2 < 94% (29.17%). Furthermore, the ratio of SMPP ones was significantly higher in the MUMPP group than in the MSMP group (52.78% vs. 34.83%; p < 0.05), consistent with prior research (23). Although no patients required ICU admission with mechanical ventilation, a larger proportion of MUMPP patients required oxygen therapy, particularly heated and humidified high-flow oxygen support.
M. pneumoniae is known to induce a wide range of extrapulmonary manifestations affecting nearly every organ system, potentially leading to more severe medical complications than pneumonia (24). In our study, the MUMPP group exhibited a significantly higher proportion of extrapulmonary manifestations compared to the MSMP group. Gastrointestinal symptoms were the most prevalent extrapulmonary manifestation, particularly in the MUMPP group. However, MP-associated myocarditis, encephalitis, and other extrapulmonary complications were not observed, possibly due to the limited number of MPP patients included. In clinical practice, the possibility of MUMPP should be considered when similar intrapulmonary and extrapulmonary manifestations are observed in children.
Following M. pneumoniae infection, disruptions in innate and adaptive immunity lead to excessive inflammation in the lungs and throughout the body (25). In turn, the cytokines and chemokines released during these hyperinflammatory responses further amplify the inflammatory cascade (26). In our study, we assessed several serological markers to predict MUMPP. Our analysis revealed that the MUMPP group had significantly higher levels of N%, IL-6, SF, CRP, PCT, AST, D-dimer, and LDH compared to the MSMP group. Additionally, the neutrophil-to-lymphocyte ratio (NLR) and LDH-to-albumin ratio (LAR) were significantly higher in the MUMPP group. These findings suggest that excessive inflammatory and immune responses play crucial roles in the pathogenesis of MUMPP, consistent with previous studies (27, 28).
Ferritin is induced by activated macrophages, which produce tumor necrosis factor (TNF)-α (29). Serum ferritin (SF) often exhibits a non-specific increase in various infectious or inflammatory disorders, and its level can effectively reflect the body's immune defense ability (30). Previous studies have reported that the SF level rises progressively in children with MPP as their disease worsens (31). In our study, ROC curve analysis revealed that SF could be used as a predictor of MUMPP. SF had the largest AUC (0.7002) among all serum markers. Its predictive value for MUMPP was 104.1ug/L, with high specificity (90.32%) but low sensitivity (39.022%).
M. pneumoniae infection and abnormal immune response lead to systemic inflammation, vascular endothelial injury, subcutaneous collagen exposure, and vasoconstriction. This disrupts the balance of blood coagulation and anticoagulation, resulting in a hypercoagulable state and elevated D-dimer levels. Elevated D-dimer levels in children with MPP are linked to hypercoagulability and vascular endothelial dysfunction, correlating with disease severity (32, 33). Consistent with previous studies (34), our research shows that D-dimer is a predictive factor for MUMPP. Elevated D-dimer levels, especially >0.435 mg/L, contribute to the early diagnosis of MUMPP.
Lactic dehydrogenase (LDH), a non-specific inflammatory biomarker of tissue damage, is present in all tissue cytoplasm. Its release into the serum is linked to cell dissolution or membrane damage, serving as an important indicator of infection severity and inflammatory disease (35, 36). Previous studies suggest that LDH is a predictor of severity and a marker of glucocorticoid therapy efficacy in MPP patients (28, 37, 38). In the present study, we observed elevated LDH and decreased albumin levels, with the LDH to albumin ratio (LAR) having a higher AUC than LDH alone (0.6528 vs. 0.6218). The LAR is widely used as an indicator of tissue damage, nutritional status, and systemic inflammatory response. Previous studies have reported high LDH and low albumin levels in SMPP patients, with LAR assisting clinicians in evaluating the progression of severe M. pneumoniae infection (39). Our study identified an LAR cut-off of 8.476 for predicting MUMPP, with 86.59% sensitivity and 43.66% specificity.
Neutrophils, which participate in the first line of defense against infection, play a crucial role in the immune response to M. pneumoniae (40). Their numbers increase in peripheral blood (41), bronchoalveolar lavage fluid (25), and lung tissue (42) after M. pneumoniae infection. However, excessive inflammation may cause lymphocytes apoptosis, thereby reduce their numbers (43). This phenomenon has been observed in MPP patients (44). The neutrophil-to-lymphocyte ratio (NLR) in peripheral blood is a simple, rapid and widely available indicator that has been reported to be associated with poor prognosis in idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease (45), and COVID-2019 (46). Research indicates that NLR at admission can predict the prognosis of MPP (47). One retrospective study (48) concluded that an NLR >3.92 might be a valuable predictor value for RMPP in children over 6 years old. Our study also found NLR has predictive value for MUMPP, with an NLR >3.212 at admission indicating MUMPP with a sensitivity and specificity of 81.48% and 48.53%, respectively.
Despite observing some differences, certain indicators such as CRP, a commonly used marker for the early evaluation and identification of MPP with severe complications (33, 49), demonstrated low predictive ability for MUMPP due to their combined low specificity and sensitivity. This may be attributed to their nonspecific nature and reflection of whole-body inflammation levels.
These findings indicate that MUMPP exhibits more severe pulmonary inflammatory reaction or tissue damage. A robust cellular immune response leads to severe ciliary dysfunction, reduced airway immune function, and impaired ciliary mucus clearance. This results in large-scale infiltration, atelectasis, and mucus plug formation, hindering the recovery lung inflammation (49, 50). In a study of 393 hospitalized children with MPP, lobar or segmental consolidation was the most common radiological finding (37%) (51). Pneumonia involving two or more lobes is more common in macrolide-refractory MPP patients (52, 53). Our study found unilateral infiltrates more frequent in the MSMP group and bilateral infiltrates more frequent in the MUMPP group, with a higher incidence of lobar pneumonia in the MUMPP group, consistent with previous studies (23).
The early assessment of MPP severity through imaging strategies is crucial to prevent adverse outcomes in clinical practice. Kim et al. (2021) demonstrated that children with MPP and pleural effusion had more severe pneumonia lesions and poorer treatment outcomes, leading to prolonged resolution of lung abnormalities (54). Our study found a higher proportion of pleural effusion in the MUMPP group compared to the MSMP group. Radiologic findings of lobar pneumonia and pleural effusion in the MUMPP group suggest severe illness due to macrolide resistance, higher M. pneumoniae burden, severe host reactions, or other refractory response factors. Therefore, in M. pneumoniae patients, the possibility of an ensuing refractory response to macrolides should be considered if bilateral infiltrates, pleural effusion, or lobar pneumonia are detected on chest radiography during the initial hospitalization period.
Clinical practice guidelines recommend macrolide antibiotic treatment for patients with LRTIs compatible with atypical pathogens like M. pneumoniae (55). Physicians often prescribe macrolides without positive microbiology results (56). In our study, almost all patients received antibiotics prior to admission, with macrolides being the most frequent outpatient antibiotic. Despite receiving macrolides earlier than the MSMP group, MUMPP patients were more likely to progress to SMPP and require flexible bronchoscopy. Previous study also suggests that early macrolide treatment or resistance does not necessarily mitigate the clinical severity of MPP (57). Therefore, early macrolide treatment may not prevent severe MPP manifestations.
Secondary treatment agents like tetracyclines, fluoroquinolones, and corticosteroids have been reported to improve MUMPP prognosis. Doxycycline (DXY), a tetracycline, is effective against both macrolide-susceptible and -resistant strains (58). In our study, 70.83% of MUMPP patients switched to DXY, a significantly higher proportion than the MSMP group (p < 0.05). Patients who switched antibiotics tended to have severe symptoms, resulting in a longer TTD in the MUMPP group compared to the MSMP group (median 24 h vs. 6 h). However, the average TTD with DXY was significantly shorter than those with prolonged macrolide use (17). Notably, no patients received glucocorticoids when administered DXY, and previous studies have also reported lower glucocorticoid use in the DXY group compared to the azithromycin group (59).
Japanese guidelines recommend switching to a tetracycline antibiotic if defervescence is not achieved within 48 h of macrolide therapy (60). Early oral doxycycline treatment can quickly improve clinical symptoms and promote the resolution of pulmonary inflammation (58, 61). Furthermore, the early use of minocycline may offer better economic and clinical benefits, even without test results for drug resistance genes (16). Our study showed that early use of DXC could significantly improve the duration of fever. These findings underscore the importance of promptly initiating appropriate second-line agents in treating MUMPP.
Therefore, identifying the risk factors of MUMPP is of great significant, especially in settings without possibility of testing mutations in the 23S ribosomal RNA gene. Children with MUMPP were more likely to experience prolonged fever, intrapulmonary or extrapulmonary complications, severe lung imaging changes, and elevated inflammatory markers (SF, IL-6, D-dimer, LAR, NLR). If such manifestations are observed in children, MUMPP should be highly considered, and early use of secondary agents should be contemplated. The indicators included in our study are easily obtainable and conducive to clinical use.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Institutional Review Board of The Guangdong Provincial Hospital of Chinese Medicine (IRB number: YF2024-001-01).
Author contributions
YL: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal Analysis, Data curation. YWL: Writing – review & editing, Methodology, Formal Analysis, Data curation. XC: Writing – review & editing, Validation, Project administration, Methodology. XX: Writing – review & editing, Supervision, Project administration, Formal Analysis. YC: Writing – review & editing, Validation, Methodology. LW: Writing – review & editing, Investigation, Data curation. WJ: Writing – review & editing, Investigation, Data curation. JY: Writing – review & editing, Validation, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Department of Science and Technology of Guangdong provincial hospital of Chinese medicine, under the project “clinical research of Jianer Jiedu Formula for the treatment of refractory mycoplasma pneumoniae pneumonia in children” (NO. YN2023MS20) and the State Administration of Traditional Chinese Medicine, under the project “a project for Chinese Medicine on Ying Lv's Renowned Expert Inheritance Studio” (NO. E43729).
Acknowledgments
We are grateful to the dedicated participants, the researchers who shared their finding, the MPP, MUMPP and predictive indicators, all of which provided leads and possibilities for subsequent studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Uldum SA, Bangsborg JM, Gahrn-Hansen B, Ljung R, Mølvadgaard M, Føns Petersen R, et al. Epidemic of mycoplasma pneumoniae infection in Denmark, 2010 and 2011. Eurosurveillance. (2012) 17(5):20073. doi: 10.2807/ese.17.05.20073-en22321137
2. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. (2015) 373(5):415–27. doi: 10.1056/NEJMoa150024526172429
3. Gao LW, Yin J, Hu YH, Liu XY, Feng XL, He JX, et al. The epidemiology of paediatric mycoplasma pneumoniae pneumonia in north China: 2006 to 2016. Epidemiol Infect. (2019) 147:e192. doi: 10.1017/s095026881900083931364532
4. Beeton ML, Zhang XS, Uldum SA, Bébéar C, Dumke R, Gullsby K, et al. Mycoplasma pneumoniae infections, 11 countries in Europe and Israel, 2011 to 2016. Eurosurveillance. (2020) 25(2):1900112. doi: 10.2807/1560-7917.Es.2020.25.2.190011231964459
5. Otheo E, Rodríguez M, Moraleda C, Domínguez-Rodríguez S, Martín MD, Herreros ML, et al. Viruses and mycoplasma pneumoniae are the main etiological agents of community-acquired pneumonia in hospitalized pediatric patients in Spain. Pediatr Pulmonol. (2022) 57(1):253–63. doi: 10.1002/ppul.2572134633153
6. Yan C, Xue GH, Zhao HQ, Feng YL, Cui JH, Yuan J. Current status of mycoplasma pneumoniae infection in China. World J Pediatr. (2024) 20(1):1–4. doi: 10.1007/s12519-023-00783-x38185707
7. Meyer Sauteur PM, Beeton ML, ESGMAC the ESGMAC MAPS study group. Mycoplasma pneumoniae: gone forever?. Lancet Microbe. (2023) 4(10):e763. doi: 10.1016/S2666-5247(23)00182-937393927
8. He J, Liu M, Ye Z, Tan T, Liu X, You X, et al. [Corrigendum] insights into the pathogenesis of mycoplasma pneumoniae (review). Mol Med Rep. (2017) 17(3):4155. doi: 10.3892/mmr.2017.832429286101
9. Iannuzo N, Dy ABC, Guerra S, Langlais PR, Ledford JG. The impact of CC16 on pulmonary epithelial-driven host responses during mycoplasma pneumoniae infection in mouse tracheal epithelial cells. Cells. (2023) 12(15):1984. doi: 10.3390/cells1215198437566063
10. Inamura N, Miyashita N, Hasegawa S, Kato A, Fukuda Y, Saitoh A, et al. Management of refractory mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother. (2014) 20(4):270–3. doi: 10.1016/j.jiac.2014.01.00124486173
11. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. (2004) 17(4):697–728. doi: 10.1128/cmr.17.4.697-728.200415489344
12. Bi Y, Zhu Y, Ma X, Xu J, Guo Y, Huang T, et al. Development of a scale for early prediction of refractory mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep. (2021) 11(1):6595. doi: 10.1038/s41598-021-86086-5.33758243
13. Yang HJ, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. (2017) 60(6):167–74. doi: 10.3345/kjp.2017.60.6.16728690643
14. Ding Y, Chu C, Li Y, Li G, Lei X, Zhou W, et al. High expression of HMGB1 in children with refractory mycoplasma pneumoniae pneumonia. BMC Infect Dis. (2018) 18(1):439. doi: 10.1186/s12879-018-3346-830157804
15. Tong L, Huang S, Zheng C, Zhang Y, Chen Z. Refractory mycoplasma pneumoniae pneumonia in children: early recognition and management. J Clin Med. (2022) 11(10):2824. doi: 10.3390/jcm1110282435628949
16. Chen J, Qi X, Yin Y, Zhang L, Zhang J, Yuan S. Effects of minocycline on macrolide-unresponsive mycoplasma pneumoniae pneumonia in children: a singlecenter retrospective study. Transl Pediatr. (2021) 10(11):2997–3004. doi: 10.21037/tp-21-35634976765
17. Ha SG, Oh KJ, Ko KP, Sun YH, Ryoo E, Tchah H, et al. Therapeutic efficacy and safety of prolonged macrolide, corticosteroid, doxycycline, and levofloxacin against macrolide-unresponsive mycoplasma pneumoniae pneumonia in children. J Korean Med Sci. (2018) 33(43):e268. doi: 10.3346/jkms.2018.33.e26830344461
18. Lee E, Cho HJ, Hong SJ, Lee J, Sung H, Yu J. Prevalence and clinical manifestations of macrolide resistant mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr. (2017) 60(5):151–57. doi: 10.3345/kjp.2017.60.5.15128592978
19. Kumar S, Roy RD, Sethi GR, Saigal SR. Mycoplasma pneumoniae infection and asthma in children. Trop Doct. (2019) 49(2):117–19. doi: 10.1177/004947551881659130537911
20. Kutty PK, Jain S, Taylor TH, Bramley AM, Diaz MH, Ampofo K, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. (2019) 68(1):5–12. doi: 10.1093/cid/ciy41929788037
21. Wang X, Li M, Luo M, Luo Q, Kang L, Xie H, et al. Mycoplasma pneumoniae triggers pneumonia epidemic in autumn and winter in Beijing: a multicentre, population-based epidemiological study between 2015 and 2020. Emerg Microbes Infect. (2022) 11(1):1508–17. doi: 10.1080/22221751.2022.207822835582916
22. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. (2014) 371(17):1619–28. doi: 10.1056/NEJMra131288525337751
23. Choi YJ, Chung EH, Lee E, Kim CH, Lee YJ, Kim HB, et al. Clinical characteristics of macrolide-refractory mycoplasma pneumoniae pneumonia in Korean children: a multicenter retrospective study. J Clin Med. (2022) 11(2):306. doi: 10.3390/jcm1102030635054002
24. Narita M. Classification of extrapulmonary manifestations due to mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. (2016) 7:23. doi: 10.3389/fmicb.2016.0002326858701
25. Guo L, Liu F, Lu MP, Zheng Q, Chen ZM. Increased T cell activation in BALF from children with mycoplasma pneumoniae pneumonia. Pediatr Pulmonol. (2015) 50(8):814–9. doi: 10.1002/ppul.2309525157471
26. Lee YC, Chang CH, Lee WJ, Liu TY, Tsai CM, Tsai TA, et al. Altered chemokine profile in refractory mycoplasma pneumoniae pneumonia infected children. J Microbiol Immunol Infect. (2021) 54(4):673–79. doi: 10.1016/j.jmii.2020.03.03032299786
27. Kurata S, Osaki T, Yonezawa H, Arae K, Taguchi H, Kamiya S. Role of IL-17A and IL-10 in the antigen induced inflammation model by mycoplasma pneumoniae. BMC Microbiol. (2014) 14:156. doi: 10.1186/1471-2180-14-15624928272
28. Choi YJ, Jeon JH, Oh JW. Critical combination of initial markers for predicting refractory mycoplasma pneumoniae pneumonia in children: a case control study. Respir Res. (2019) 20(1):193. doi: 10.1186/s12931-019-1152-531443650
29. Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis. (2000) 59(Suppl 1):6i–16. doi: 10.1136/ard.59.suppl_1.i6
30. Montonen J, Boeing H, Steffen A, Lehmann R, Fritsche A, Joost HG, et al. Body iron stores and risk of type 2 diabetes: results from the European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetologia. (2012) 55(10):2613–21. doi: 10.1007/s00125-012-2633-y22752055
31. Kawamata R, Yokoyama K, Sato M, Goto M, Nozaki Y, Takagi T, et al. Utility of serum ferritin and lactate dehydrogenase as surrogate markers for steroid therapy for mycoplasma pneumoniae pneumonia. J Infect Chemother. (2015) 21(11):783–9. doi: 10.1016/j.jiac.2015.07.00926298038
32. Liu J, He R, Wu R, Wang B, Xu H, Zhang Y, et al. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing children’s hospital. BMC Infect Dis. (2020) 20(1):51. doi: 10.1186/s12879-020-4774-931948402
33. Wen J, Su Y, Sun H, Zhang H, Li H. The combination of initial markers to predict refractory mycoplasma pneumoniae pneumonia in Chinese children: a case control study. Respir Res. (2021) 22(1):89. doi: 10.1186/s12931-020-01577-933752670
34. Jin X, Zhu Y, Zhang Y, Chen J, Rong L, Zhao X. Assessment of levels of D-dimer and interferon-γ in pediatric patients with mycoplasma pneumoniae pneumonia and its clinical implication. Exp Ther Med. (2018) 16(6):5025–30. doi: 10.3892/etm.2018.687330546408
35. Esteves F, Calé SS, Badura R, de Boer MG, Maltez F, Calderón EJ, et al. Diagnosis of pneumocystis pneumonia: evaluation of four serologic biomarkers. Clin Microbiol Infect. (2015) 21(4):379.e1–10. doi: 10.1016/j.cmi.2014.11.02525630458
36. Vogel M, Weissgerber P, Goeppert B, Hetzel J, Vatlach M, Claussen C, et al. Accuracy of serum LDH elevation for the diagnosis of pneumocystis jiroveci pneumonia. Swiss Med Wkly. (2011) 141:w13184. doi: 10.4414/smw.2011.1318421528464
37. Liu TY, Lee WJ, Tsai CM, Kuo KC, Lee CH, Hsieh KS, et al. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol. (2018) 59(5):501–06. doi: 10.1016/j.pedneo.2017.12.00829337082
38. Lee E, Lee YY. Predictive factors of the responses to treatment of mycoplasma pneumoniae pneumonia. J Clin Med. (2021) 10(6):1154. doi: 10.3390/jcm1006115433801856
39. Luo XQ, Luo J, Wang CJ, Luo ZX, Tian DY, Xie XH. Clinical features of severe mycoplasma pneumoniae pneumonia with pulmonary complications in childhood: a retrospective study. Pediatr Pulmonol. (2023) 58(10):2815–22. doi: 10.1002/ppul.2659337431970
40. Zhang Z, Wan R, Yuan Q, Dou H, Tu P, Shi D, et al. Cell damage and neutrophils promote the infection of mycoplasma pneumoniae and inflammatory response. Microb Pathog. (2022) 169:105647. doi: 10.1016/j.micpath.2022.10564735724831
41. Zhu Y, Luo Y, Li L, Jiang X, Du Y, Wang J, et al. Immune response plays a role in mycoplasma pneumoniae pneumonia. Front Immunol. (2023) 14:1189647. doi: 10.3389/fimmu.2023.118964737304280
42. Tamiya S, Yoshikawa E, Ogura M, Kuroda E, Suzuki K, Yoshioka Y. Neutrophil-mediated lung injury both via TLR2-dependent production of IL-1α and IL-12 p40, and TLR2-independent CARDS toxin after mycoplasma pneumoniae infection in mice. Microbiol Spectr. (2021) 9(3):e0158821. doi: 10.1128/spectrum.01588-2134937175
43. Tezol O, Bozlu G, Sagcan F, Tuncel Daloglu F, Citak C. Value of neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio and red blood cell distribution width in distinguishing between reactive lymphadenopathy and lymphoma in children. Bratisl Lek Listy. (2020) 121(4):287–92. doi: 10.4149/bll_2020_04532356444
44. Rodríguez F, Ramírez AS, Castro P, Poveda JB. Pathological and immunohistochemical studies of experimental mycoplasma pneumoniae in Gerbils (Meriones unguiculatus). J Comp Pathol. (2021) 184:37–43. doi: 10.1016/j.jcpa.2021.01.011
45. Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, et al. Associations between the neutrophil-to-lymphocyte ratio and diabetic complications in adults with diabetes: a cross-sectional study. J Diabetes Res. (2020) 2020:6219545. doi: 10.1155/2020/621954532405503
46. Dymicka-Piekarska V, Dorf J, Milewska A, Łukaszyk M, Kosidło JW, Kamińska J, et al. Neutrophil/lymphocyte ratio (NLR) and lymphocyte/monocyte ratio (LMR)—risk of death inflammatory biomarkers in patients with COVID-19. J Inflamm Res. (2023) 16:2209–22. doi: 10.2147/jir.S40987137250103
47. Li D, Gu H, Chen L, Wu R, Jiang Y, Huang X, et al. Neutrophil-to-lymphocyte ratio as a predictor of poor outcomes of mycoplasma pneumoniae pneumonia. Front Immunol. (2023) 14:1302702. doi: 10.3389/fimmu.2023.130270238169689
48. Ling Y, Ning J, Xu Y. Explore the predictive value of peripheral blood cell parameters in refractory mycoplasma pneumoniae pneumonia in children over 6 years old. Front Pediatr. (2021) 9:659677. doi: 10.3389/fped.2021.65967734869089
49. Gong H, Sun B, Chen Y, Chen H. The risk factors of children acquiring refractory mycoplasma pneumoniae pneumonia: a meta-analysis. Medicine. (2021) 100(11):e24894. doi: 10.1097/md.000000000002489433725960
50. Zheng Y, Mao G, Dai H, Li G, Liu L, Chen X, et al. Early predictors of delayed radiographic resolution of lobar pneumonia caused by mycoplasma pneumoniae in children: a retrospective study in China. BMC Infect Dis. (2024) 24(1):414. doi: 10.1186/s12879-024-09289-x38641804
51. Cho YJ, Han MS, Kim WS, Choi EH, Choi YH, Yun KW, et al. Correlation between chest radiographic findings and clinical features in hospitalized children with mycoplasma pneumoniae pneumonia. PLoS One. (2019) 14(8):e0219463. doi: 10.1371/journal.pone.021946331461462
52. Zhou Y, Hu M, Ye B, Chen Z, Zhang Y. Early prediction of necrotizing pneumonia from mycoplasma pneumoniae pneumonia with large pulmonary lesions in children. Sci Rep. (2020) 10(1):19061. doi: 10.1038/s41598-020-76083-533149220
53. Zhang Y, Zhou Y, Li S, Yang D, Wu X, Chen Z. The clinical characteristics and predictors of refractory mycoplasma pneumoniae pneumonia in children. PLoS One. (2016) 11(5):e0156465. doi: 10.1371/journal.pone.015646527227519
54. Kim SH, Lee E, Song ES, Lee YY. Clinical significance of pleural effusion in mycoplasma pneumoniae pneumonia in children. Pathogens. (2021) 10(9):1075. doi: 10.3390/pathogens1009107534578108
55. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. (2011) 53(7):e25–76. doi: 10.1093/cid/cir53121880587
56. Bandell RAM, Dekkers T, Semmekrot BA, de Wildt SN, Fleuren H, Warlé-van Herwaarden MF, et al. Macrolide prescription in Dutch children: compliance with guidelines. Eur J Clin Microbiol Infect Dis. (2019) 38(4):675–81. doi: 10.1007/s10096-019-03473-730680574
57. Yang D, Chen L, Chen Z. The timing of azithromycin treatment is not associated with the clinical prognosis of childhood mycoplasma pneumoniae pneumonia in high macrolide-resistant prevalence settings. PLoS One. (2018) 13(1):e0191951. doi: 10.1371/journal.pone.019195129377957
58. Lee H, Choi YY, Sohn YJ, Kim YK, Han MS, Yun KW, et al. Clinical efficacy of doxycycline for treatment of macrolide-resistant mycoplasma pneumoniae pneumonia in children. Antibiotics. (2021) 10(2):192. doi: 10.3390/antibiotics1002019233671151
59. Chen Y, Zhang Y, Tang QN, Shi HB. Efficacy of doxycycline therapy for macrolide-resistant mycoplasma pneumoniae pneumonia in children at different periods. Ital J Pediatr. (2024) 50(1):38. doi: 10.1186/s13052-024-01615-y38439015
60. Uehara S, Sunakawa K, Eguchi H, Ouchi K, Okada K, Kurosaki T, et al. Japanese guidelines for the management of respiratory infectious diseases in children 2007 with focus on pneumonia. Pediatr Int. (2011) 53(2):264–76. doi: 10.1111/j.1442-200x.2010.03316.x21648118
Keywords: macrolide-unresponsive Mycoplasma pneumoniae pneumonia (MUMPP), macrolide-sensitive Mycoplasma pneumoniae pneumonia (MSMP), clinical characteristics, predictive indictors, early and appropriate treatment
Citation: Li Y, Liu Y, Chen X, Xiao X, Chen Y, Wang L, Jiang W and Yang J (2024) Clinical characteristics and predictive indictors of macrolide-unresponsive Mycoplasma pneumoniae pneumonia in children: a retrospective study. Front. Pediatr. 12:1489389. doi: 10.3389/fped.2024.1489389
Received: 1 September 2024; Accepted: 18 November 2024;
Published: 3 December 2024.
Edited by:
Jochen G. Mainz, Cystic Fibrosis Center, GermanyReviewed by:
Tobias Ankermann, Städtisches Krankenhaus Kiel, GermanyEitan Naaman Berezin, Santa Casa of Sao Paulo, Brazil
Copyright: © 2024 Li, Liu, Chen, Xiao, Chen, Wang, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinghua Yang, ZG91bWlhb21hbWFAMTI2LmNvbQ==
 Yun Li
Yun Li Yunwei Liu
Yunwei Liu Xinying Chen
Xinying Chen Xiaolan Xiao1
Xiaolan Xiao1 Yiting Chen
Yiting Chen Jinghua Yang
Jinghua Yang