
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 11 December 2024
Sec. Pediatric Infectious Diseases
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1480514
Introduction and importance: Paroxysmal sympathetic hyperactivity (PSH) syndrome often occurs with severe traumatic brain injury. However, it can also occur during infections, such as severe bacterial meningoencephalitis in children. Streptococcus intermedius is an aggressive, virulent, opportunistic pathogen. This species can cause meningoencephalitis in children, as reported in a few cases.
Case information: A five-year-old boy with no relevant past medical history was admitted to a hospital because of a fever and progressive disturbance of consciousness. His head computed tomography scan and magnetic resonance imaging revealed extensive brain damage and an intraventricular abscess. A next-generation sequencing technology test performed on his cerebrospinal fluid revealed that the child's meningoencephalitis was caused by S. intermedius. During treatment, the child had clinical manifestations such as fever, tachycardia, tachypnea, diaphoresis, and hypertension. Changes in muscle tone and abnormal posture, which were misdiagnosed as epilepsy at the early treatment stage, were also observed; however, anti-epileptic treatment was ineffective. The child was diagnosed with PSH and received the appropriate treatment, and his symptoms eventually improved.
Conclusions: To our knowledge, this is the first case report on PSH induced by S. intermedius meningoencephalitis. Early identification, diagnosis, and treatment of PSH are crucial.
Paroxysmal sympathetic hyperactivity (PSH) is a clinical syndrome characterized by hyperactive sympathetic nerve activity accompanied by posture or dystonia, and it mainly occurs in traumatic brain injury (TBI), anoxic brain injury, cerebrovascular disease, and other diseases. Having inadequate understanding, some patients do not receive timely and appropriate diagnosis and treatment (1). Streptococcus intermedius is a Gram-positive member of the β-hemolytic opportunistic bacterium family that causes infections ranging from mild (e.g., dental abscesses and sinusitis) to severe infections involving the head and neck, lungs, abdomen, and soft tissues. In children, S. intermedius often causes head and neck infections, and, in rare cases, meningitis (2). Herein, we present the case of a five-year-old child with PSH induced by S. intermedius meningoencephalitis misdiagnosed as epilepsy at the early stage.
A five-year-old boy with no relevant past medical history was admitted to a hospital because of a fever and headache for 1 week and unconsciousness for 2 h. During physical examination, his body temperature, blood pressure, respiratory rate, pulse, and Glasgow Coma Scale score were 38.5°C, 105/60 mmHg, 30 beats per min, 108 per min, and 6 points, respectively. The boy also had neck stiffness (+), bilateral Pasteurelle sign (+), Krill's sign (+), increased limb muscle tone, and muscle strength (not cooperating). A computed tomography (CT) scan of the boy's head revealed multiple low-density shadows in the right frontal lobe and bilateral basal ganglia (Figure 1A). Hemorrhage soon appeared in the right basal ganglia (Figure 1B). The laboratory findings were as follows: white blood cell (WBC) count of 27.8 × 109 /L, NE% = 88%, and C-reactive protein (CRP) level of 67.4 mg/L. Cerebrospinal fluid (CSF) examination revealed bacterial infection, and next-generation sequencing showed that the patient tested positive for S. intermedius, as shown in Table 1. The final diagnosis was S. intermedius meningoencephalitis.
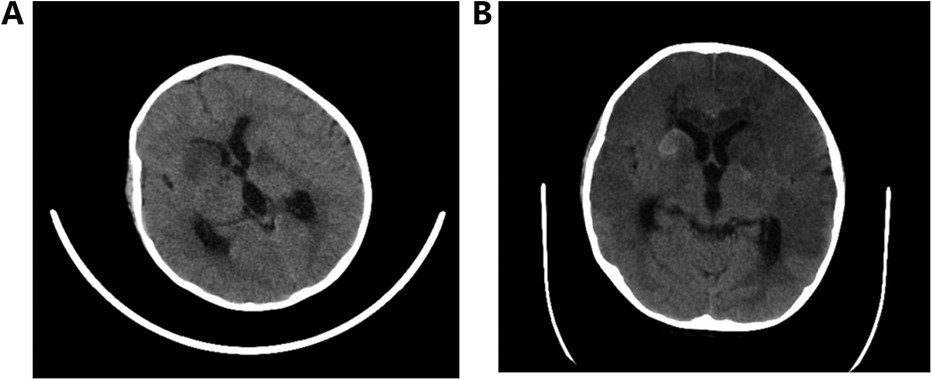
Figure 1. (A) Multiple low-density shadows in the right frontal lobe and bilateral basal ganglia and (B) hemorrhage in the right basal ganglia.
After the identification of S. intermedius as the cause of the intermediate streptococcal infection, the patient was treated with vancomycin for two weeks. Subsequently, the patient's condition showed gradual improvement, with a decrease in fever, WBC count (reduced to 14.7 × 109 /L), CRP (reduced to 26.1 mg/L), and CSF cell count (decreased to 183 × 106 /L). However, despite these improvements, the patient continued to experience recurring fever and exhibited signs/symptoms, such as staring to the left, increased lower limb muscle tone, opisthotonic posture, tremors in the upper limbs, noticeable laryngeal phlegm, accelerated heart rate (up to 180 bpm), and frequent episodes lasting for several minutes multiple times a day. Although epileptic seizure was suspected, the 24 h continuous Video electroencephalogram (EEG) monitoring did not show any epileptic discharge. Treatment with midazolam, valproic acid, oxcarbazepine, and levetiracetam failed to control the seizures. Subsequent magnetic resonance imaging (MRI) revealed diffuse cerebral hemisphere lesions, pus in the cisterna and ventricle, midbrain aqueduct stenosis, and supratentorial hydrocephalus (Figure 2).
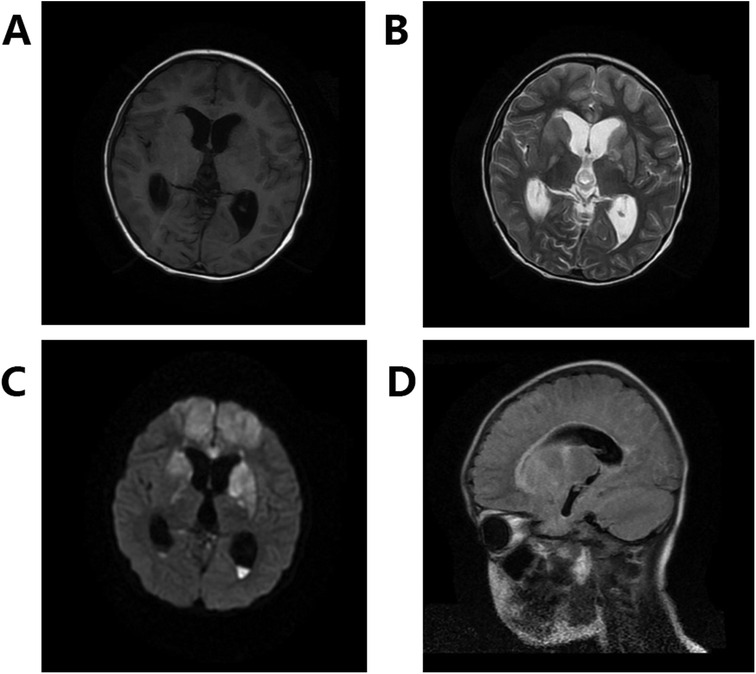
Figure 2. Diffuse cerebral hemispherical lesions, pus in the cisterna and ventricle, and midbrain aqueduct stenosis with supratentorial hydrocephalus. (A) Shows T1, (B) shows T2, (C) shows DWI, and (D) shows Flair.
The patient underwent bilateral lateral ventricular irrigation and drainage following a referral to neurosurgery. After surgery, the child experienced intensified attacks characterized by fever (above 39°C), profuse sweating, a rapid heart rate of 180–200 bpm, elevated blood pressure of 180/100 mmHg, rapid breathing of 50–60 times per min, phlegm in the larynx, muscle stiffness, and opisthotonus. These attacks lasted 30–60 min, occurred dozens of times a day, and were often triggered or worsened by actions, such as suctioning phlegm, changing positions, patting on the back, and nasogastric feeding. Treatment with dexmedetomidine and propofol proved ineffective. After a multidisciplinary consultation involving neurosurgery, neurology, and pediatric neurology and based on Baguley's diagnostic criteria (1), the patient was determined to have a Clinical Feature Scale (CFS) score of 17 points, a Diagnosis Likelihood Tool (DLT) Scale score of 10 points, and a PSH Assessment Measure (PSH-AM) Scale score of 27. The final diagnosis was PSH.
Treatment included micropump-delivered midazolam and nasally administered propranolol, gabapentin, bromointine, and other medications. After surgery, linezolid was utilized to combat infection, resulting in a gradual decrease in body temperature to normal levels within a week and a reduction in the frequency of attacks. After six weeks of anti-infection therapy, a lateral ventriculoperitoneal shunt procedure was performed to address hydrocephalus. Subsequently, occasional attacks were managed with nightly oral clonazepam intake, along with hyperbaric oxygen therapy and rehabilitation training. A two-year follow-up MRI revealed bilateral cerebral hemisphere atrophy, partial softening, and ventricle enlargement post-encephalitis (Figure 3). The patient experienced residual motor and cognitive impairments, with no PSH recurrence.
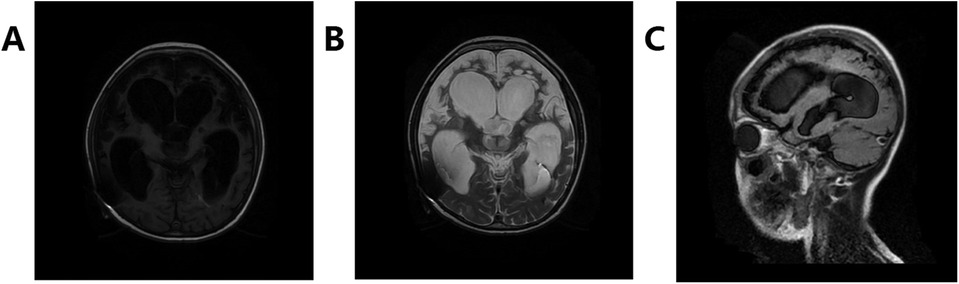
Figure 3. Changes in sequelae of encephalitis, bilateral cerebral hemisphere atrophy, partial softening, ventricle enlargement, and changes after drainage tube operation. (A) Shows T1, (B) shows T2, and (C) shows Flair.
As an opportunistic pathogen, S. intermedius poses a high risk in individuals undergoing oral surgical procedures or those with sinusitis. The majority of infections are associated with abscess formation, with certain genotypes increasing the likelihood of spreading to the brain and causing brain abscesses (3). Reported cases also include brain abscesses (4) in children and meningitis (5). Treatment typically involves ceftriaxone and metronidazole, either alone or in combination with vancomycin (3). However, in some instances, aggressive anti-infective therapy may effectively halt disease progression. For localized abscesses, abscess drainage and surgery are considered the primary interventions, and surgical irrigation and drainage are utilized to achieve optimal infection control.
PSH is a severe clinical syndrome with an unknown pathogenesis that is characterized by mydriasis, shortness of breath, tachycardia, high fever, profuse sweating, increased blood pressure, and increased limb muscle tone (1). In adults, PSH is often secondary to central nervous system diseases, and children typically develop the condition as a result of brain injury. PSH has an overall prevalence of 13%–14% in children with acquired brain injury (6). PSH presents neither specific imaging characteristics nor laboratory indications. Various evaluation standards exist; however, the main diagnostic method is PSH-AM (consisting of CFS and DLT), which was proposed by Baguley et al. in 2014. When the total score of all symptoms (CFS + DLT) is greater than 17, a likely diagnosis is given (1). In the case presented in this study, the final score was 27 points, and the diagnosis was confirmed after ruling out symptoms caused by other conditions.
Therapeutic drugs primarily function by inhibiting hypersensitivity reactions in sensory pathways, reducing central sympathetic nerve outflow, and blocking peripheral organ responses to sympathetic nerves. Commonly used drugs include opioids, gamma-aminobutyric acid agonists, dopamine receptor agonists, beta-receptor antagonists, and benzodiazepines (7). For instance, propranolol acts as a β-receptor antagonist, leading to decreased blood pressure and heart rate. Bromocriptine, an agonist of dopamine receptors in the hypothalamus and pituitary gland, targets D2R, D3R, and D4R to induce a cooling effect (8). It is primarily utilized to address fever and dystonia in patients with PSH syndrome. Clonazepam, which does not cause sedation in small doses, can relax skeletal muscles, reduce muscle tension, lower the heart rate, ease respiratory muscles, and alleviate shortness of breath. Hence, it is used as a maintenance drug for late stages. Recent studies have shown that hydrogen sulfide levels in the paraventricular nucleus of the hypothalamus play a role in regulating sympathetic nerve efferent activity, thus offering a potential new strategy and target for PSH prevention and treatment (9).
In clinical practice, PSH is frequently misunderstood, which leads to potential missed or incorrect diagnoses. In this particular case, the child was initially misdiagnosed with epileptic seizures and did not respond to various antiepileptic medications, ultimately leading to prolonged hospitalization and increased medical costs.
PSH has been sporadically observed in adults with TBI; however, it rarely occurs in children. Herein, we present the first reported case of PSH induced by S. intermedius meningoencephalitis. PSH can be mistakenly diagnosed as epileptic seizures, malignant syndrome, or central hyperthermia at the initial stages (10). Hence, prompt identification and specific treatment are crucial to minimize hospitalization durations and medical costs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Huzhou Central Hospital, Zhejiang, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
QH: Conceptualization, Data curation, Methodology, Writing – original draft. RZ: Writing – original draft, Investigation, Project administration. YZ: Writing – review & editing, Methodology, Project administration. JL: Writing – review & editing, Conceptualization. FY: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Baguley IJ, Perkes IE, Fernandez-Ortega J-F, Rabinstein AA, Dolce G, Hendricks HT. Paroxysmal sympathetic hyperactivity after acquired brain injury: consensus on conceptual definition, Nomenclature, and diagnostic criteria. J Neurotrauma. (2014) 31(17):1515–20. doi: 10.1089/neu.2013.3301
2. Furuichi M, Horikoshi Y. Sites of infection associated with Streptococcus anginosus group among children. J Infect Chemother. (2018) 24(2):99–102. doi: 10.1016/j.jiac.2017.09.011
3. Issa E, Salloum T, Tokajian S. From normal flora to brain abscesses: a review of Streptococcus intermedius. Front Microbiol. (2020) 11:826. doi: 10.3389/fmicb.2020.00826
4. Miloudi M, Arsalane L, Nakhli R, El Ghouat G, El Kamouni Y, Zouhair S. Brain abscess caused by Streptococcus intermedius: a case report. Ann Biol Clin. (2019) 77(2):184–6. doi: 10.1684/abc.2019.1421
5. Tkacz K, Piwowarczyk A, Podsiadły E, Kuchar E. Streptococcus intermedius acute meningitis in an immunocompetent child. Pediatr Infect Dis J. (2022) 41(10):e428–e9. doi: 10.1097/INF.0000000000003613
6. Alofisan TO, Algarni YA, Alharfi IM, Miller MR, Charyk Stewart T, Fraser DD, et al. Paroxysmal sympathetic hyperactivity after severe traumatic brain injury in children: prevalence, risk factors, and outcome*. Pediatr Crit Care Med. (2019) 20(3):252–8. doi: 10.1097/PCC.0000000000001811
7. Gilbert BW, Faber RK, Evans K, Patel I, Finnick M, Reeder J, et al. Pharmacological treatment for paroxysmal sympathetic hyperactivity. Crit Care Nurse. (2020) 40(3):e9–e16. doi: 10.4037/ccn2020348
8. O’Keefe LM, Mui G. Treating paroxysmal sympathetic hyperactivity with enteral baclofen in anoxic brain injury. Neurologist. (2020) 25(2):24–5. doi: 10.1097/NRL.0000000000000258
9. Mahmoud RSG. Involvement of hydrogen sulfide in the pathogenesis of ischemic stroke-induced paroxysmal sympathetic hyperactivity. Hypertens Res. (2024) 47(7):1987–8. doi: 10.1038/s41440-024-01691-x
Keywords: paroxysmal sympathetic hyperactivity, children, streptococcus intermedius meningoencephalitis, diagnosis, case report
Citation: Huang Q, Zhou R, Zhang Y, Li J and Yu F (2024) Paroxysmal sympathetic hyperactivity syndrome caused by Streptococcus intermedius meningoencephalitis in children: a case report and literature review. Front. Pediatr. 12:1480514. doi: 10.3389/fped.2024.1480514
Received: 14 August 2024; Accepted: 25 November 2024;
Published: 11 December 2024.
Edited by:
Josette Raymond, Hôpital de Bicêtre, FranceReviewed by:
Syed Ahmed Zaki, All India Institute of Medical Sciences, IndiaCopyright: © 2024 Huang, Zhou, Zhang, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, TEpBSTA1MTRAMTYzLmNvbQ==; Feng Yu, eXVmZW5nd2luZDA2MTdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.