- 1Department of Pediatrics, College of Medicine, Qassim University, Buraydah, Saudi Arabia
- 2Department of Pediatrics, King Saud Hospital, Unaizah, Saudi Arabia
- 3Diabetic Center, King Saud Hospital, Unaizah, Saudi Arabia
- 4Department of Pathology, College of Medicine, Qassim University, Buraydah, Saudi Arabia
- 5Department of Obstetrics and Gynecology, College of Medicine, Qassim University, Buraydah, Saudi Arabia
- 6Department of Family and Community Medicine, College of Medicine, Qassim University, Buraydah, Saudi Arabia
Background: The association between 25-hydroxy-vitamin D [25(OH)D] levels and glycemic control in pediatric patients with type 1 diabetes mellitus (T1DM) is unclear. In this study, we aimed to investigate the association between 25(OH)D levels and glycemic control in Saudi pediatric patients' with T1DM in a region that is sunny year-round.
Materials and methods: A retrospective study was conducted in the Pediatric Department of King Saud Hospital in Unaizah, Saudi Arabia. A total of 218 children with T1DM were enrolled in the study and grouped according to their glycated hemoglobin (HbA1C) levels into the controlled T1DM (HbA1C ≤ 7.5%) and the uncontrolled T1DM (HbA1C > 7.5%). Their 25(OH)D levels and thyroid function were measured using standard methods.
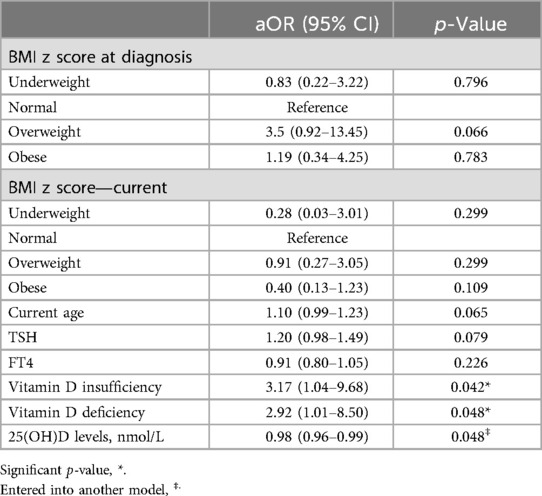
Results: Of the 218 children in this study, 182 (83.5%) had uncontrolled T1DM, while only 36 (16.5%) had controlled T1DM. The median (interquartile range) of 25(OH)D levels was significantly lower in the uncontrolled T1DM group compared with the controlled group [45.4 (31.2–59.7) nmol/L vs. 56.1 (37.5–77.6) nmol/L; p = 0.007], respectively. Vitamin D deficiency (<50.0 nmol/L) and insufficiency (50–74 nmol/L) were detected in 55.0% and 31.1% of all the enrolled children, respectively. Vitamin D deficiency was detected in 86.6% of the uncontrolled T1DM patients and in 16.5% of the controlled T1DM patients (p = 0.012). The multivariable analysis showed that both vitamin D deficiency [adjusted odds ratio (aOR) = 2.92, p = 0.048] and insufficiency [aOR = 3.17, p = 0.042] were risk factors for uncontrolled diabetes.
Conclusion: Vitamin D deficiency was highly prevalent in the studied group. Both vitamin D deficiency and insufficiency are associated with uncontrolled T1DM. Further study is needed.
Introduction
Type 1 diabetes mellitus (T1DM) in children is a chronic endocrinological disease that is characterized by hyperglycemia due to either relative or complete insulin deficiency (1). It is a worldwide concern, with its global incidence increasing annually by up to 4%. Saudia Arabia is no exception to this international pattern; the World Health Organization (WHO) ranks Saudi Arabia as the 7th leading country in T1DM prevalence worldwide (2).
T1DM can be described etiologically as an autoimmune disease in which self-immune cells target pancreatic cells. Hypothyroidism, which often presents as autoimmune hypothyroidism, has been identified as a contributing factor in the development of T1DM. It may proceed the development of diabetes or come later (3).
Vitamin D is known primarily for its crucial role in calcium metabolism and bone health. Its deficiency leads to rickets in children and osteomalacia in adults (4). It acts as a pro-hormone, exerting its action through a specific receptor expressed in various target tissues. The rapidly emerging and continuous research on vitamin D related functions has revealed that the biological and metabolic role of vitamin D is not limited to the skeletal tissues only but extends to other body tissues, including the pancreas and immune cells (5).
A growing body of evidence has pointed to the association between 25-hydroxy-vitamin D [25(OH)D] levels and T1DM (6, 7). Experimental evidence has shown that in the pancreatic tissues, insulin secretion is affected by 25(OH)D levels (8). Moreover, many studies have reported that the 25(OH)D levels is inversely correlated with the level of glycosylated hemoglobin (HbA1C), which indicates that 25(OH)D affects the level of glycemic control among children with T1DM (9, 10). Furthermore, interventional studies have shown that vitamin D supplementation improves the β-cell function (11) and the glycemic controls (12). Conversely, some studies have found no association between 25(OH)D levels and glycemic controls (13, 14). It has been reported that, the glycemic control of T1DM can be affected by the levels of thyroid hormones (15). Hyperthyroidism accelerates the catabolism of the insulin hormone, precipitating hyperglycemia and consequently deteriorates the levels of glycemic control (15). Moreover, an inverse correlation was observed between the thyroid stimulating hormone (TSH) and HbA1C (16). Additionally, a recent study reported that children with T1DM who have hypothyroidism have higher levels of HbA1c compare with patients without hypothyroidism (17). This study aimed to investigate the factors that may affect the HbA1c level in pediatric patients with T1DM. These factors include 25(OH)D levels, TSH and Free T4 (FT4) levels, as well as body mass index. To the best of our knowledge, this is the first study that investigated these factors in association with T1DM with children in our region of Qassim in Saudi Arabia. The findings of this study is expected to help clinicians and caregivers to enhance the glycemic control of their pediatric patients.
Materials and methods
This study was a retrospective cross-sectional study conducted between March 2020 and February 2023 in the Pediatrics Department of King Saud Hospital in Unaizah, Qassim region in Saudi Arabia. It included Saudi children aged 2–17 years who had been diagnosed with T1DM, whose 25(OH)D level was recently measured, and who had regular follow-ups for six months. T1DM was diagnosed based on the guidelines of the American Diabetic Association (18). Patients with HbA1C ≤ 7.5% were regarded as having controlled diabetes, and those with HbA1C > 7.5%, as having uncontrolled diabetes (19).
Sociodemographic and anthropometric data were collected from the patients using a structured questionnaire. The data included the patients' age, weight, and height, as well as clinical and biochemical data, such as vitamin D, HbA1C, and thyroid hormone function levels. The body mass index (BMI) was calculated as the weight in kilograms divided by the squared height in meters. Then, the calculated BMI was converted into the BMI z-score using the WHO international reference chart for children (20). The BMI z-score was interpreted as follows: severe thinness (SD <−3, thinness-underweight (SD − 2 to − 3), normal (SD − 2 to + 1), overweight (SD + 1 to + 2), and obesity (SD > + 2).
Laboratory measurements
The 25(OH)D level was measured using enzyme-linked immunosorbent assay (ELISA) kits (Euroimmun, Lubeck, Germany) according to the manufacturer's instructions. Six calibrators were used with a concentration range between 0 and 120 ng/ml, for which the manufacturer-recommended quality measures were strictly followed. A plasma 25(OH)D level of >75 nmol/L was categorized as adequate; 50–74 nmol/L, insufficient; and <50.0 nmol/L, deficient (21). The blood levels of serum thyroid profile (FT4 and TSH) levels were measured using the immunoassay analyser AIA 360 (Tosoh Bioscience, San Francisco, CA, USA), guided by the manufacturer's instructions.
Sample size calculation
The sample size was calculated using the OpenEpi calculator (22), guided by the reported mean 25(OH)D concentration of 35.15 nmol/L among Saudi children with diabetes (14). We assumed that the mean 25(OH)D concentration of the children with uncontrolled T1DM would be 28.0 nmol/L and that would differ from the mean 25(OH)D concentration of the children with controlled T1DM by 7 nmol/L. We found that to achieve 80% power and 5% precision, a sample size of 110 in each group was needed.
Statistical analysis
The data were entered into a computer and cleaned using the Microsoft Excel software. Statistical analysis was performed using the STATA software (version 16). The patients were divided into the following two groups based on their glycemic control level: into the controlled diabetes group for the patients with an HbA1c ≤ 7.5% and the uncontrolled diabetes group for the patients with HbA1c > 7.5%. The Kolmogorov-Smirnov test was used to determine the distribution of the data. Normally distributed continuous data were expressed as means [i.e., standard deviations (SDs)], and non-normally distributed data were expressed as medians [i.e., interquartile ranges (IQRs)]. Categorical data were expressed as numbers (%).
A multivariable analysis model was constructed to determine the factors associated with uncontrolled T1DM in children. Variables with a p value < 0.25 in the bivariable analysis were considered in the model. Two models were constructed in multivariable analysis, the first one includes 25(OH)D level as a categorical variable and the second one as a continuous variable to avoid multicollinearity. The crude odds ratios (cORs) and adjusted odds ratios (aORs), along with the 95% confidence intervals (95% CIs), were reported, and p values < 0.05 were considered statistically significant.
Results
A total of 218 children diagnosed with T1DM were included in this study, of whom 117 (53.7%) were boys. More than half (56.4%) of the children were underweight at the time of their T1DM diagnosis, but this figure has declined to 47.3% currently. In contrast, 21.6% and 13.3% of the children were overweight and obese, respectively, at the time of their T1DM diagnosis, but these figures have risen to 22.9% and 27.9%, respectively, at present. Fifty-five percent of all the children were deficient in vitamin D, and 31.1% had insufficient vitamin D. The overall median IQR age at diagnosis was 9 (5–11) years, and the current age (IQR) was 12 (9–17) years. The median (IQR) of the TSH, FT4, and 25(OH)D levels in all the studied samples are shown in Table 1.
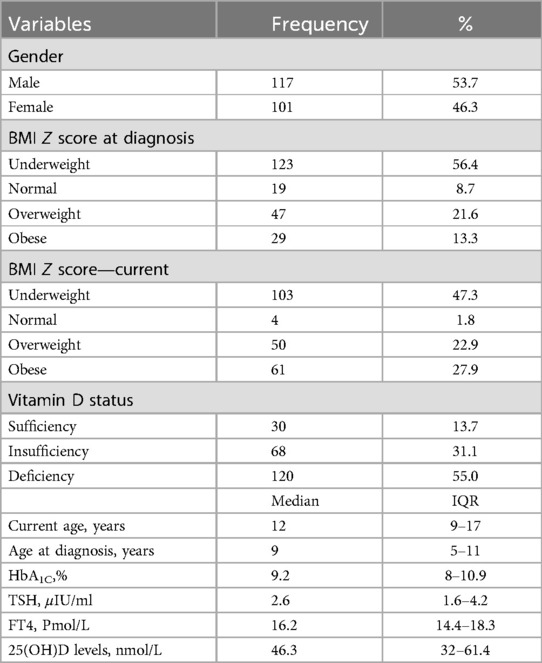
Table 1. Sociodemographic and clinical characteristics of the pediatrics participant with diabetes mellitus type 1.
Bivariate and multivariable analyses of factors associated with uncontrolled diabetes mellitus
Of the 218 children included in this study, 182 (83.5%) had uncontrolled T1DM (HbA1C ≥ 7.5%), and only 36 (16.5%) had controlled T1DM (HbA1C ≤ 7.5%). The bivariable analysis showed that only the current age (cOR = 1.09; p value = 0.025), FT4 level (cOR = 0.88; p value = 0.038), median 25(OH)D level (cOR = 0.98; p value = 0.007), vitamin D deficiency (cOR = 3.25; p value = 0.012), and vitamin D insufficiency (cOR = 2.90; p value = 0.039) were significantly associated with uncontrolled T1DM (Table 2). In the multivariable analysis, the model results revealed that the aORs of vitamin D deficiency (2.92; p = 0.048) and insufficiency (3.17; p = 0.042) were significant risk factors for uncontrolled T1DM. The other factors in the model, such as the BMI, child age, and TSH and FT4 levels, were not statistically significant (Table 3). To further confirm the association of vitamin D with the glycemic control, another model was constructed by adding the 25(OH)D levels as a median and 25(OH)D level showed a protective effect against uncontrolled diabetes (aOR = 0.98; p = 0.048), other factors remained not statistically significant, Table 3.
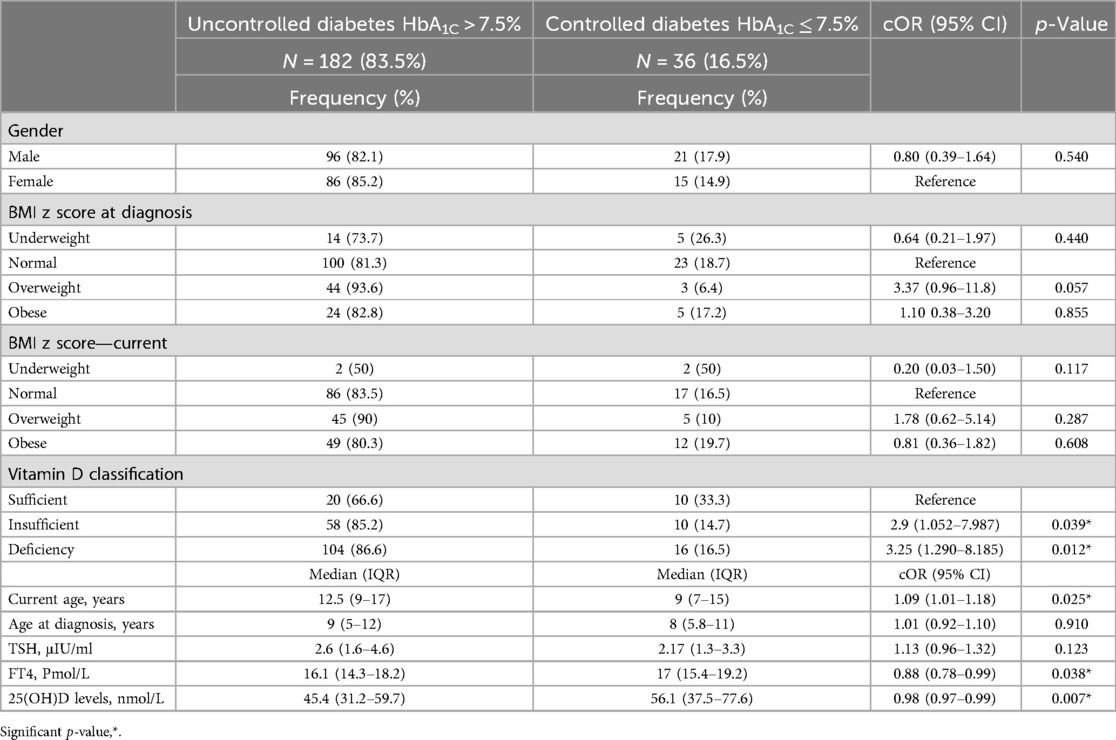
Table 2. Bivariate analysis and crude odds ratio (cOR) of the factors possibly associated with uncontrolled diabetes.
Discussion
The main finding of the current study is that vitamin D deficiency and insufficiency are considered risk factors for uncontrolled T1DM in children. This finding aligns with the findings from ALkharashi study on Saudi children with T1DM, as well as with those of studies from Kuwait and Spain (23–25). This finding is partially explained by the molecular function of in body cells, including those in the pancreas and immune systems. T1DM is an autoimmune disease characterized by immune cell infiltration into the pancreatic β-cells (1). The major immune cells involved in this infiltration are cytotoxic and regulatory T cells (26). It is worth mentioning that the vitamin D receptor is expressed in T lymphocytes, allowing it to modulate their function. Specifically, vitamin D reduces the production of Th1 cells over Th2 cells, thereby decreasing the production of pro-inflammatory cytokines, namely IL-1β, TNF-α and others (27). Noteworthy, immunomodulation therapy aimed at reducing proinflammatory cytokines such as IL-1β and TNF-α has shown a promising results in both the treatment and prevention of T1DM (27). Additionally, vitamin D regulates the production of antibodies from β-cells by inhibiting antigen presentation to antigen-presenting cells (28). In the case of vitamin D deficiency, the modulatory effect on T helper cells is significantly diminished, leading to increased cellular immunity and autoantibody production. In islet cells of the pancreas, vitamin D plays a protective role by downregulating gene expression related to apoptosis (29). Animal models have shown that vitamin D deficiency directly reduces the pancreas' insulin secretion in response to hyperglycemia. The insulin secretion process is well known as dependent on the calcium level, which is regulated by the availability of vitamin D (30). Correcting the 25(OH)D level appears to improve glycemic control in children with T1DM (31). Considering these premises, it is plausible that vitamin D deficiency degrades the glycemic control in children with T1DM.
The second finding of this study is that the prevalence of both vitamin D deficiency & insufficiency among all the participants was 86.1%. This figure closely matches the 81% reported by Al-Daghri in a study among mixed Saudi participant who included children (32). Despite the year-round sunny weather in this study's setting, the high prevalence of vitamin D deficiency among the study participants can be attributed to the hot environmental conditions in the Gulf region (33), which reduce outdoor activities and direct sun exposure (34). Another possible factor is the high percentage of overweight children among our study participants at their T1DM diagnosis (20%), as it has been reported that overweight children are more prone to vitamin D deficiency (35). Researchers have hypothesized that since vitamin D is fat-soluble, its bioavailability decreases due to its distribution and sequestration in adipose tissue compartment (36). However, in the multivariable model, being overweight showed borderline statistical significance, possibly due to our small sample size. Of interest, both vitamin D deficiency and obesity have been reported to exert a synergistic negative effect on glycemic control in pediatric patients (31, 37, 38). Additionally, adopting weight reduction strategies has been positively infleunce the outdoor activity, glycemic control and vitamin D levels (39).
From another perspective, recent studies have pointed out to the association between genetic variants, such as of the variants in DHCR7 gene, and vitamin D deficiency (40, 41). This variant has been reported among Saudi families with vitamin D deficiency (42). Moreover, these variants have also been associated with vitamin D deficiency in conjunction with autoimmune T1DM (43). However, in this study, we didn't conduct a genetic analysis; otherwise, our findings would be more informative. Regardless of the genetic components of our patients, the presence of diabetes mellitus per se is considered an independent risk factor for vitamin D deficiency. Diabetes decreases the hepatic synthesis of 25(OH)D, which is a pre-requisite step in the vitamin D synthesis pathway, and instead, hepatocytes start to degrade the active vitamin D metabolites (44). Moreover, diabetes has been proven to inhibit the synthesis of the vitamin D binding protein, which is necessary for transporting vitamin D between body tissues (45)These factors, taken together, may explain the high prevalence of vitamin D deficiency in the current study.
In this study, we observed a statistical borderline effect of TSH on uncontrolled diabetes. Vitamin D deficiency has been well linked to hypothyroidism and autoimmune hypothyroidism (46). Moreover, among children with autoimmune thyroiditis, the 25(OH)D levels are inversely well correlated with the TSH levels (47). Furthermore, clinical trials have shown that vitamin D supplementation can normalize TSH levels in patients with hypothyroidism (48, 49). However, the exact mechanism by which vitamin D improves thyroid function is not yet fully understood.
In conclusion, high prevalence of vitamin D deficiency and insufficiency was observed in this study. Both vitamin D deficiency and insufficiency were associated with poor glycemic control among children with T1DM. The current findings are important for healthcare providers, emphasizing the needs to screen for vitamin D deficiency in children with T1DM and correcting the deficiencies when identified. Up to date, this screening is not widely implemented, therefore mor studies are needed at community levels to evaluate and consolidate this association in a large scale. However, this study had limitations that should be considered when interpreting our findings. Firstly, we did not investigate antithyroid hormone antibodies to determine the exact prevalence of autoimmune hypothyroidism. Second, since this study was a retrospective study, the participants' 25(OH)D levels at their T1DM diagnosis were not measured, so whether they already had vitamin D deficiency at their T1DM diagnosis or developed it afterward is unknown. This also limited our ability to track glycemic control in correlation with 25(OH)D level during the disease course. Additionally, due to the retrospective design of this study, causality cannot be inferred. Therefore, further study is needed with a prospective design and a larger sample size, and that will also consider autoimmune thyroid disease and investigate the genetic makeup of the patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Regional Research Ethics Committee, Qassim Province, Saudi Arabia, Reference no.607456181. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because the data was collected retrospectively.
Author contributions
SA: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing. AAls: Data curation, Investigation, Writing – review & editing. MA: Data curation, Investigation, Writing – review & editing. AS: Data curation, Investigation, Writing – review & editing. HZH: Formal Analysis, Validation, Writing – original draft. AA-N: Methodology, Validation, Writing – review & editing. AsA: Methodology, Validation, Writing – review & editing. IA: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing. OA-W: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aOR, Adjusted odds ratio; cOR, Crude odds ratio; ELISA, Enzyme-linked immunosorbent assay; FT4, Free T4; HbA1C, Hemoglobin A1C; IQR, Inter-quartile range; T1DM, Type 1 diabetes mellitus; TSH, Thyroid Stimulating Hormone; WHO, World Health Organization.
References
1. Siddiqui K, Nawaz SS, Alfadda AA, Mujammami M. Islet autoantibodies to pancreatic insulin-producing Beta cells in adolescent and adults with type 1 diabetes Mellitus: a cross-sectional study. Diagnostics (Basel). (2023) 13(10):1736. doi: 10.3390/diagnostics13101736
2. Alaqeel AA. Pediatric diabetes in Saudi Arabia: challenges and potential solutions. A review article. Int J Pediatr Adolesc Med. (2019) 6:125. doi: 10.1016/j.ijpam.2019.05.008
3. Rogowicz-Frontczak A, Falkowski B, Grzelka-Wozniak A, Uruska A, Araszkiewicz A, Zozulinska-Ziolkiewicz D. Does autoimmune hypothyroidism increase the risk of neurovascular complications in type 1 diabetes? J Endocrinol Invest. (2020) 43:833–9. doi: 10.1007/s40618-019-01171-x
4. Joshi M, Uday S. Vitamin D deficiency in chronic childhood disorders: importance of screening and prevention. Nutrients. (2023) 15:2805. doi: 10.3390/nu15122805
5. Zittermann A, Gummert JF. Nonclassical vitamin D action. Nutrients. (2010) 2:408. doi: 10.3390/nu2040408
6. Infante M, Ricordi C, Sanchez J, Clare-Salzler MJ, Padilla N, Fuenmayor V, et al. Influence of vitamin D on islet autoimmunity and Beta-cell function in type 1 diabetes. Nutrients. (2019) 11:2185. doi: 10.3390/nu11092185
7. Altieri B, Grant WB, Della Casa S, Orio F, Pontecorvi A, Colao A, et al. Vitamin D and pancreas: the role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. (2017) 57:3472–88. doi: 10.1080/10408398.2015.1136922
8. Norman AW, Frankel BJ, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. (1980) 209:823–5. doi: 10.1126/science.6250216
9. Wulandari D, Cahyono H A, Widjajanto E, Puryatni A. Low levels of vitamin D correlate with hemoglobin A1c and interleukin-10 levels in pediatric type 1 diabetes Mellitus patients. J Trop Life Sci. (2014) 4:182–6. doi: 10.11594/jtls.04.03.04
10. Bayan AS, El-Aziz Nosair NA, Salamah AM. Assessment of Serum vitamin D level in children with type 1 diabetes Mellitus: a cross-sectional study. J Pak Med Assoc. (2023) 73(Suppl 4):S317–21. doi: 10.47391/JPMA.EGY-S4-61
11. Dahlquist G. Vitamin D supplement in early childhood and risk for type I (insulin-dependent) diabetes mellitus. The EURODIAB substudy 2 study group. Diabetologia. (1999) 42:51–4. doi: 10.1007/s001250051112
12. Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann Saudi Med. (2010) 30:454. doi: 10.4103/0256-4947.72265
13. Carakushansky M, Patel P, Ben Khallouq BA, Gurnurkar S. Prevalence of vitamin D deficiency in children with type 1 diabetes Mellitus. Cureus. (2020) 12(4):e7836. doi: 10.7759/cureus.7836
14. Al Zahrani AM, Al Shaikh A. Glycemic control in children and youth with type 1 diabetes Mellitus in Saudi Arabia. Clin Med Insights Endocrinol Diabetes. (2019) 12. doi: 10.1177/1179551418825159
15. Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res. (2011) 2011:439463. doi: 10.4061/2011/439463
16. Yuan C, Sun X, Liu Y, Wu J. The thyroid hormone levels and glucose and lipid metabolism in children with type 1 diabetes: a correlation analysis. Transl Pediatr. (2021) 10:276–82. doi: 10.21037/tp-20-204
17. Metwalley K, El-Saied A-R-H. Thyroid abnormalities in Egyptian children and adolescents with type 1 diabetes mellitus: a single center study from upper Egypt. Indian J Endocrinol Metab. (2014) 18:637. doi: 10.4103/2230-8210.139218
18. Doyle-Delgado K, Chamberlain JJ, Shubrook JH, Skolnik N, Trujillo J. Pharmacologic approaches to glycemic treatment of type 2 diabetes: synopsis of the 2020 American diabetes association’s standards of medical care in diabetes clinical guideline. Ann Intern Med. (2020) 173:813–21. doi: 10.7326/M20-2470
19. Redondo MJ, Libman I, Maahs DM, Lyons SK, Saraco M, Reusch J, et al. The evolution of hemoglobin A1c targets for youth with type 1 diabetes: rationale and supporting evidence. Diabetes Care. (2021) 44:301–12. doi: 10.2337/dc20-1978
20. Croft TN, Marshall AM, Allen CK, Arnold F, Assaf S, Balian S. Guide to DHS Statistics. Rockville, MA: Rockville (2018).
21. Saggese G, Vierucci F, Prodam F, Cardinale F, Cetin I, Chiappini E, et al. Vitamin D in pediatric age: consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital J Pediatr. (2018) 44(1):51. doi: 10.1186/s13052-018-0488-7
22. Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health, version. Available online at: www.OpenEpi.com (Accessed March 18, 2024).
23. Elsayed A, Mohamed G. Vitamin D deficiency and its correlation to hemoglobin A1C in adolescent and young adult type 1 diabetes mellitus patients. Al-Azhar Assiut Medical Journal. (2016) 14:76. doi: 10.4103/1687-1693.192643
24. Segovia-Ortí R, Bennassar AB, De Sotto-Esteban D, Cortés PS. Vitamin D status is related to severity at onset of diabetes and worse glycemic control. J Pediatr Endocrinol Metab. (2020) 33:1265–71. doi: 10.1515/jpem-2020-0149
25. ALkharashi NA. Estimation of vitamin D deficiency prevalence among Saudi children in armed forces hospital and Riyadh care hospital in Riyadh, kingdom of Saudi Arabia and its relation to type 1 diabetes mellitus. Saudi Med J. (2019) 40:1290–3. doi: 10.15537/smj.2019.12.24643
26. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. (2019) 15:635–50. doi: 10.1038/s41574-019-0254-y
27. Rak K, Bronkowska M. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of type 1 diabetes Mellitus-A narrative review. Molecules. (2018) 24(1):53. doi: 10.3390/molecules24010053
28. Geldmeyer-Hilt K, Heine G, Hartmann B, Baumgrass R, Radbruch A, Worm M. 1,25-dihydroxyvitamin D3 impairs NF-κB activation in human naïve B cells. Biochem Biophys Res Commun. (2011) 407:699–702. doi: 10.1016/j.bbrc.2011.03.078
29. Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, et al. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the fas receptor. Apoptosis. (2006) 11:151–9. doi: 10.1007/s10495-006-3558-z
30. Milner RDG, Hales CN. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. (1967) 3:47–9. doi: 10.1007/BF01269910
31. Savastio S, Cadario F, Genoni G, Bellomo G, Bagnati M, Secco G, et al. Vitamin D deficiency and glycemic status in children and adolescents with type 1 diabetes Mellitus. PLoS One. (2016) 11:e0162554. doi: 10.1371/journal.pone.0162554
32. Al-Daghri NM. Vitamin D in Saudi Arabia: prevalence,distribution and disease associations. J Steroid Biochem Mol Biol. (2018) 175:102–7. doi: 10.1016/j.jsbmb.2016.12.017
33. Golbahar J, Al-Saffar N, Altayab Diab D, Al-Othman S, Darwish A, Al-Kafaji G. Predictors of vitamin D deficiency and insufficiency in adult Bahrainis: a cross-sectional study. Public Health Nutr. (2014) 17:732–8. doi: 10.1017/S136898001300030X
34. Singh P, Kumar M, Al Khodor S. Vitamin D deficiency in the gulf cooperation council: exploring the triad of genetic predisposition, the gut microbiome and the immune system. Front Immunol. (2019) 10:1042. doi: 10.3389/fimmu.2019.01042
35. Corsello A, Macchi M, D’Oria V, Pigazzi C, Alberti I, Treglia G, et al. Effects of vitamin D supplementation in obese and overweight children and adolescents: a systematic review and meta-analysis. Pharmacol Res. (2023) 192:106793. doi: 10.1016/j.phrs.2023.106793
36. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. (2000) 72:690–3. doi: 10.1093/ajcn/72.3.690
37. Özkaya V, Özkaya ŞÖ, Adal SE. Relationship between visceral adiposity index and glycemic and metabolic control in children and adolescents with type 1 diabetes mellitus. Ir J Med Sci. (2024) 193:181–9. doi: 10.1007/s11845-023-03375-w
38. Tee PP, Wong JSL, Selveindran NM, Hong JYH. Effect of obesity and excessive body fat on glycaemic control in paediatric type 1 diabetes. J Pediatr Endocrinol Metab. (2022) 35:1474–80. doi: 10.1515/jpem-2022-0151
39. Mondkar S, Oza C, Dange N, Soren P, Kajale N, Kardile M, et al. Assessment of vitamin D Status, its determinants and relationship with bone health in Indian children and young adults with type-1 diabetes. Indian J Endocrinol Metab. (2024) 28:405–12. doi: 10.4103/ijem.ijem_141_23
40. Almesri N, Das NS, Ali ME, Gumaa K, Giha HA. Independent associations of polymorphisms in vitamin D binding protein (GC) and vitamin D receptor (VDR) genes with obesity and plasma 25OHD3 levels demonstrate sex dimorphism. Appl Physiol Nutr Metab. (2016) 41:345–53. doi: 10.1139/apnm-2015-0284
41. Thacher TD, Levine MA. CYP2R1 Mutations causing vitamin D-deficiency rickets. J Steroid Biochem Mol Biol. (2017) 173:333–6. doi: 10.1016/j.jsbmb.2016.07.014
42. Alharazy S, Naseer MI, Alissa E, Robertson MD, Lanham-New S, Chaudhary AG. Whole-Exome sequencing for identification of genetic variants involved in vitamin D metabolic pathways in families with vitamin D deficiency in Saudi Arabia. Front Genet. (2021) 12:677780. doi: 10.3389/fgene.2021.677780
43. Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. (2011) 60:1624–31. doi: 10.2337/db10-1656
44. Bouillon R, Bikle D. Vitamin D metabolism revised: fall of dogmas. J Bone Miner Res. (2019) 34:1985–92. doi: 10.1002/jbmr.3884
45. Nyomba BL, Bouillon R, Bidingija M, Kandjingu K, De Moor P. Vitamin D metabolites and their binding protein in adult diabetic patients. Diabetes. (1986) 35:911–5. doi: 10.2337/diab.35.8.911
46. Nettore IC, Albano L, Ungaro P, Colao A, Macchia PE. Sunshine vitamin and thyroid. Rev Endocr Metab Disord. (2017) 18:347–54. doi: 10.1007/s11154-017-9406-3
47. Metwalley KA, Farghaly HS, Sherief T, Hussein A. Vitamin D status in children and adolescents with autoimmune thyroiditis. J Endocrinol Invest. (2016) 39:793–7. doi: 10.1007/s40618-016-0432-x
48. Chahardoli R, Saboor-Yaraghi AA, Amouzegar A, Khalili D, Vakili AZ, Azizi F. Can supplementation with vitamin D modify thyroid autoantibodies (anti-TPO ab, Anti-Tg Ab) and Thyroid profile (T3, T4, TSH) in hashimoto’s thyroiditis? A double blind, randomized clinical trial. Horm Metab Res. 2019;51:296–301.31071734
Keywords: prevalence, diabetes Mellitus, child, vitamin D, HbA1c, glycemic control, thyroid function
Citation: Almansour S, Alsalamah A, Almutlaq M, Sheikh A, Hamdan HZ, Al-Nafeesah A, AlEed A, Adam I and Al-Wutayd O (2025) Association of vitamin D deficiency and insufficiency with uncontrolled type 1 diabetes Mellitus among Saudi pediatric patients; a hospital-based retrospective study. Front. Pediatr. 12:1479815. doi: 10.3389/fped.2024.1479815
Received: 12 August 2024; Accepted: 18 December 2024;
Published: 7 January 2025.
Edited by:
Seyed-Amir Tabatabaeizadeh, Islamic Azad University of Mashhad, IranReviewed by:
Tushar Bharat Jagzape, All India Institute of Medical Sciences Raipur, IndiaPradeep Kumar Dabla, G B Pant Institute of Postgraduate Medical Education and Research (GIPMER), India
Copyright: © 2025 Almansour, Alsalamah, Almutlaq, Sheikh, Hamdan, Al-Nafeesah, AlEed, Adam and Al-Wutayd. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osama Al-Wutayd, by5hbHd1dGF5ZEBxdS5lZHUuc2E=
 Salman Almansour
Salman Almansour Abdullrahman Alsalamah2
Abdullrahman Alsalamah2 Hamdan Z. Hamdan
Hamdan Z. Hamdan Abdullah Al-Nafeesah
Abdullah Al-Nafeesah Ashwaq AlEed
Ashwaq AlEed Ishag Adam
Ishag Adam Osama Al-Wutayd
Osama Al-Wutayd