- 1Research Centre for Medical Genetics, Moscow, Russia
- 2Oncology and Immunology, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Moscow, Russia
- 3Regional Children’s Clinical Hospital No. 1, Yekaterinburg, Russia
- 4Republican Perinatal Center, Grozny, Russia
- 5State Budgetary Institution “Maternity Hospital” of the Ministry of Healthcare of the Chechen Republic, Grozny, Russia
- 6Department of Maternity and Childhood, Ministry of Health Chechen Republic, Grozny, Russia
- 7S.V. Ochapovsky Regional Clinical Hospital №1, Krasnodar, Russia
- 8Orenburg Regional Clinical Hospital No. 2, Orenburg, Russia
- 9Ryazan Regional Clinical Perinatal Center, Ryazan, Russia
- 10North-Ossetian State Medical Academy, Vladikavkaz, Russia
- 11Republican Childrens Clinical Hospital of the Republic of North Ossetia-Alania, Vladikavkaz, Russia
- 12The State Budgetary Healthcare Institution of the Vladimir Region “Regional Clinical Hospital”, Vladimir, Russia
- 13Republican Center for Medical Genetics, Ufa, Russia
- 14Bashkir State Medical University, Ufa, Russia
- 15Clinical Diagnostic Center “Maternal and Child Health”, Yekaterinburg, Russia
Newborn screening (NBS) for severe combined immunodeficiency (SCID) has been widely implemented to enable early detection and intervention. Trisomy 21, commonly known as Down syndrome (DS), poses unique challenges in NBS due to its frequent association with T and/or B cell lymphopenia. The pilot NBS screening program recently conducted in Russia was aimed to identify both severe T and B cell deficiencies by measuring TREC and KREC. This study aims to evaluate the incidence of DS in newborns who participated in the pilot program, assess their TREC and KREC values, and determine the proportion of DS newborns potentially identifiable through T/B lymphopenia NBS. We conducted a retrospective analysis of the data obtained during the pilot NBS program, involving 202,908 newborns from eight regions of Russia. The study identified 157 patients with trisomy 21 among the screened cohort, resulting in a DS birth prevalence of 1:1,284. Median TREC and KREC values did not significantly differ between full-term and pre-term subgroups of DS patients. TREC values in DS newborns were decreased and comparable to those of the extremely preterm newborns. DS newborns also demonstrated significant differences in KREC values as compared to the general cohort regardless of gestational age. Our data suggests abnormalities of T- and B-cell lineages development and requires further investigation. This article highlights the need for increased awareness of the intrinsic immunological defects associated with DS. The findings underscore the importance of continued follow-up and comprehensive support by healthcare teams for individuals with DS.
1 Introduction
Primary immunodeficiencies (PIDs), also known as inborn errors of immunity (IEIs), encompass a spectrum of genetically determined disorders characterized by defects in various components of the immune system (1). One notable PID is severe combined immunodeficiency (SCID), typified by the absence or markedly reduced numbers of T-lymphocytes, and in certain instances, deficient or malfunctioning B- and NK-cells (2). Affected newborns with SCID typically exhibit no overt clinical symptoms at birth, but between 3 and 6 months of age, they manifest severe, recurrent infections of bacterial, viral, and fungal origins, which, in the absence of appropriate intervention, can culminate in mortality within the first one to two years of life (3). Newborn screening for SCID, involving the quantification of T-cell receptor excision circles (TRECs) and/or kappa-deleting recombination excision circles (KRECs) in dried blood spots collected on filter paper test cards, is effectively implemented in numerous countries worldwide (4). This screening facilitates the early detection of affected individuals, enhancing the prospects for successful therapeutic intervention during the presymptomatic phase of the disease (5). It is noteworthy that beyond SCID, syndromic forms of PID, such as 22q11.2 deletion syndrome, CHARGE syndrome, and trisomy 21, among others, can also be identified through newborn screening (6). The incorporation of KREC measurements into NBS for PIDs also facilitates the identification of various conditions characterized by B-cell lymphopenia, with agammaglobulinemia being the most severe form (7). This underscores the importance of comprehensive screening strategies to encompass a broad spectrum of immunodeficiency disorders, enabling early recognition and intervention to improve clinical outcomes in affected individuals.
Down syndrome (DS, OMIM #190685) is a prevalent chromosomal disorder resulting from partial or complete trisomy of chromosome 21. In the world population, DS prevalence is assessed as 1 per 700–1,000 people (8, 9). In Russia, the observed live birth prevalence is 1:1,209 which is primarily linked to the performed prenatal screening and family planning choices (10). DS is characterized by distinctive facial features, intellectual disability, and systemic involvement. Individuals with DS commonly experience neurological issues, and eye abnormalities leading to reduced visual acuity, hearing loss, congenital heart defects, pulmonary complications, renal anomalies, thyroid dysfunction, diabetes, and other symptoms. Furthermore, DS is associated with an increased risk of myeloid leukemia, growth delay, obesity, and other health conditions (11, 12). Immunological deficits are also described in DS patients (13, 14). Individuals with DS often exhibit hypoplastic thymus and a diminished number of mature thymocytes, leading to reduced T-cell populations in peripheral blood (15). Newborns with DS constitute a distinct subset of patients identified through newborn screening (NBS) for primary immunodeficiency disorders (PID) constituting up to 11% of syndromic cases identified (16). However, it remains unclear which subset of DS patients would be test-positive on NBS for T and B cell lymphopenia.
Recently, we conducted a pilot NBS program in Russia, involving the detection of TREC and KREC, encompassing a cohort of more than 202,908 newborns. Notably, the NBS program identified one patient with DS among the screened cohort (17). The objectives of the current study were to assess the incidence of DS, evaluate TREC/KREC levels in individuals with trisomy 21, and determine the proportion of newborns with DS that can be potentially identified through NBS by performing the retrospective analysis of DS patients within the cohort of infants who participated in the pilot NBS project.
2 Materials and methods
2.1 NBS pilot program
The NBS pilot program was carried out as described earlier (17). In summary, TREC/KREC-based screening was carried out from January 1, 2022, to February 19, 2023, across eight regions of the Russian Federation. These regions included the Krasnodar Territory, Vladimir, Orenburg, Ryazan, Sverdlovsk Regions, Republic of Bashkortostan, Republic of North Ossetia-Alania, and the Chechen Republic. The total cohort screened comprised 202,908 neonates. Written parental informed consent was obtained for all newborns enrolled in the pilot NBS program.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Research Centre for Medical Genetics (protocol №1/1 dated 02 March 2022).
We collected data on the newborns’ sex, gestational age, and birth weight from the eight regions participating in the study. Unfortunately, not all regions provided complete data, resulting in some gaps in the information. The male-to-female ratio was 106.8 males per 100 females. The proportion of premature infants, defined as those born on or before 37 weeks of gestation, was 5.86%, with a 95% confidence interval ranging from 5.74% to 5.99%. The median birth weight for the entire cohort was 3,360 g, with a minimum weight of 450 grams and a maximum weight of 5,500 grams. Notably, the percentages of preterm newborns and the male-to-female ratio observed in our study were consistent with the demographic data available for the entire Russian Federation during the same period.
The NBS tests were conducted utilizing the Eonis™ SCID-SMA kit (Wallac Oy, Turku, Finland) on the JANUS Extraction instrument (Perkin Elmer, Turku, Finland). Subsequently, real-time PCR analysis was performed using Applied Biosystems QuantStudio 5 Dx instruments (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's guidelines and recommendations. The cut-off values for TREC and KREC were set at 100 copies per 100,000 cells for this pilot study, in accordance with the manufacturer's recommendations and published data. Newborns with values below this threshold were flagged as positive in the screening, necessitating further analysis.
Retrospective data on newborns with chromosomal abnormalities, including DS, were collected throughout the duration of the NBS pilot project in the respective regions. The diagnosis of DS was based on clinical presentation and confirmed by karyotyping at the Genetic Counseling Centers in each participating region.
2.2 Statistical analysis
The data collected from the study was analyzed using GraphPad Prism 8.0.1 (GraphPad Software, San Diego, California, USA). Throughout the analysis, data were presented as median with interquartile range, unless specified otherwise. Additionally, Fisher's 95% confidence interval (95%CI) for proportional data was computed using WinPepi v. 11.65 software (18).
3 Results
During the pilot NBS study conducted in Russia, a cohort of 202,908 newborns from eight regions was analyzed. Upon retrospective examination, our study identified 157 patients presenting with various forms of trisomy 21, encompassing both translocational and nondisjunctional forms of the condition. This yielded a birth prevalence of DS during the analyzed period of 1:1,284 (95%CI: 1:1,105–1:1,521), with prevalence rates ranging from 1:789 to 1:1,561 across different regions. However, these variations in prevalence rates between regions did not reach statistical significance. The gender distribution analysis revealed a male-to-female ratio of 69 males to 62 females, which corresponds to the ratio observed in the screened cohort (p-value = 0.8156, z-test for two proportions). 22.62% (95% CI: 18.60–41.83) of the DS patients were born at less than 37 weeks of gestation, which significantly differs from the proportion observed in the general cohort (5.86%; 95%CI: 5.74%–5.99%) (p-value = 6.5925 × 10−11, z-test for two proportions).
We subsequently analyzed the distributions of TREC and KREC values in patients with DS compared to the general cohort, stratified by different gestational ages (Figure 1, Table 1). It is worth noting that the values of TREC and KREC did not exhibit significant differences between full-term and pre-term subgroups of DS patients (p-value = 0.1522 and 0.1565 for TREC and KREC, respectively). As such, we opted not to stratify DS patients based on their gestational age. Our analysis revealed significantly lower median TREC values in DS newborns as compared to both full-term and preterm infants in the large cohort (p < 0.0001). DS TREC values were as low as such of the extremely preterm newborns (<32 weeks of gestation) (p = 0.0723, Dunn's multiple comparisons test). DS newborns also demonstrated dramatic differences in KREC values that were significantly lower than in the general cohort regardless of gestational age.
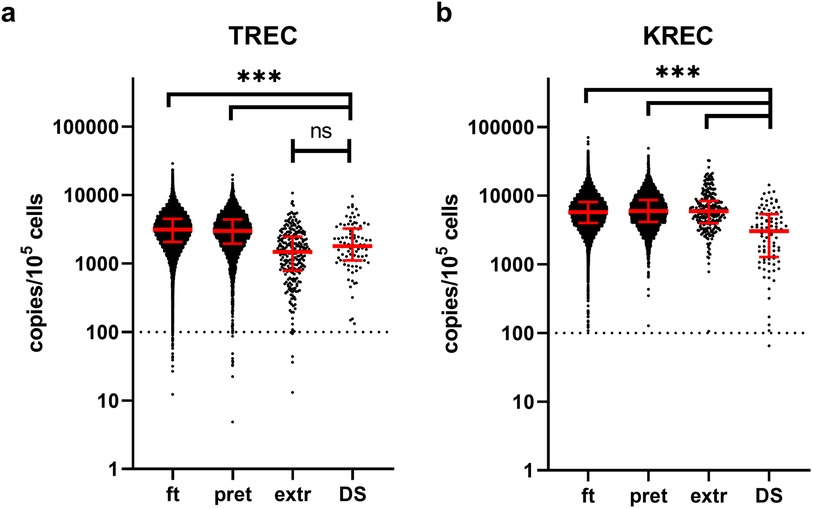
Figure 1. TREC (a) and KREC (b) concentrations (in copies per 105 cells) in newborns of different gestational age. Red lines represent the median and interquartile range. The dotted line represents a cut-off value of concentrations of analytes (100 copies/105 cells). Note the log10 scale on the Y axis. ft, full term (≥37 weeks of gestation), pret, preterm (32–37 weeks), extr, extremely preterm (<32 weeks), DS, down syndrome patients.
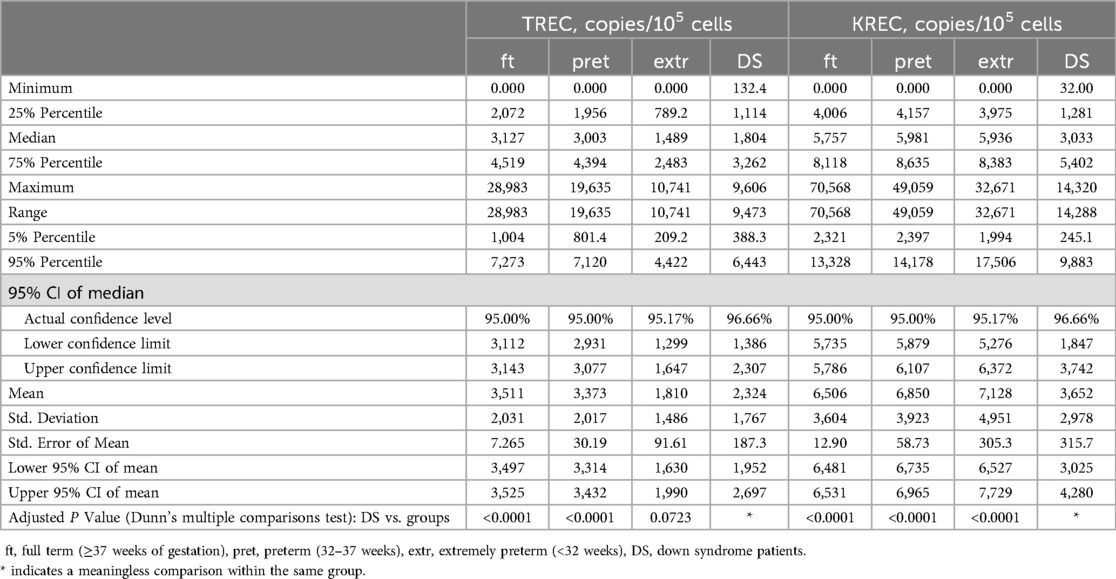
Table 1. Descriptive statistics of TREC and KREC levels (in copies per 105 cells) in newborns of different groups.
It is noteworthy that only one child within the DS newborns group was screen-positive at NBS, with 1st tier test TREC and KREC values of 253 and 32 copies/105 cells, respectively. Our findings estimate the ratio of TREC/KREC screen-positive DS newborns to be 1.12% (95%CI: 0.03%–6.10%). This ratio is significantly higher than the screen-positive group in full-term newborns (0.19%, 95%CI: 0.16%–0.22%; p-value = 0.0414, z-test for two proportions) and does not differ from the screen-positive group in preterm and extremely preterm group (0.38%, 95%CI: 0.22%–0.61%, p-value = 0.2690; 2.66%, 95%CI: 1.08%–5.41%, p-value = 0.4000, respectively).
The male patient with trisomy 21 was born at 36 weeks of gestation with a weight of 2,310 g. At birth, he was diagnosed with valvular pulmonary artery stenosis without circulatory insufficiency, muscle hypotonia, and bilateral cryptorchidism.
On day 34 of life, his TREC and KREC levels at retest were low with values of 156 and 32 copies/105 cells, respectively. By day 41, he exhibited low B cell counts (0.06 × 109/L), while maintaining normal T-cell counts. Immunoglobulin replacement therapy and antimicrobial prophylaxis were initiated. However, his family refused to continue the treatment and further genetic testing. By the age of 24 months, he had suffered severe infections, including enterocolitis complicated by sepsis and three episodes of pneumonia, and had been diagnosed with tuberculosis of the intrathoracic lymph nodes and started on anti-tuberculosis chemotherapy. At the immunological follow-up at the age of 25 months, his CD19+ count was still low (0.22 × 109/L) with decreased class-switched CD27 + IgM−IgD− memory B cells (5.3%). T cell lineage was normal [CD3+ 2.09 × 109/L, CD4+ 0.86 × 109/L (naïve 65%), CD8+ 1.17 × 109/L]. Serum immunoglobulin levels were normal (IgA 0.39 g/L, IgM 0.49 g/L, IgG 7.93 g/L,), without immunoglobulin substitution.
4 Discussion
Trisomy 21, commonly known as Down syndrome, manifests as a complex condition impacting multiple organs and systems, necessitating a comprehensive, multidisciplinary approach to follow-up and treatment (11). Trisomy 21 is associated with an elevated risk of preterm birth (<37 weeks; 22.62% vs. 5.86%), as evidenced by our study findings, which are consistent with previous research (19).
Our findings indicate that newborns with DS may show impairments in either TREC or KREC levels, regardless of gestational age, aligning with observations from other studies (13). Specifically, we found that TREC levels in DS patients are comparable to those in extremely preterm newborns. However, KREC levels in DS patients are consistently and significantly lower than those in non-DS newborns across all gestational ages. Despite this, our estimations suggest that only about 1% of DS patients could be identified through newborn screening, as they do not exhibit the profound decreases in TREC/KREC levels typically seen in conditions like SCID or agammaglobulinemia (20).
Although, in our pilot study, the sole newborn detected through NBS demonstrated a notable decrease in KREC levels and CD19+ cell counts, others have demonstrated T-cell lineage impairment in DS (21).
DS syndrome patients are known to develop many immunological problems such as increased risk of infections, poorer clinical outcomes, and chronic inflammation (14). According to the findings of the current study, newborns with DS exhibit general defects of T-cell and B-cell lineages. Given these observations, it is recommended that all newborns with DS undergo evaluation by an immunologist. Additionally, slightly more than 1% of DS newborns will present with significant lymphopenia, suggesting the necessity for more than just observation; these individuals may require prompt intervention and treatment. On a nationwide scale, this would translate to approximately 11–12 newborns with DS being detected through nationwide newborn screening (NBS) in 2023 (22). Although our data suggests that newborns with DS demonstrate signs of immunological defects, it is important to note that there are data indicating that TREC and KREC levels could potentially be restored later in life in some of them (23).
5 Conclusions
Newborns with DS exhibit a decrease in TREC/KREC values that suggests abnormalities of T-and B-cell lineages development and requires further investigation. Given these observations, it is recommended that all newborns with DS undergo evaluation by an immunologist. Slightly more than 1% of DS newborns will present with significant lymphopenia which can be detected with newborn screening measuring both TREC and KREC.
Data availability statement
The datasets presented in this article are not readily available because no new data was generated. Requests to access the datasets should be directed to the corresponding author,bWFyYWtob25vdkBnZW5lcmVzZWFyY2gucnU=.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of the Research Centre for Medical Genetics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AMa: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AMu: Conceptualization, Formal Analysis, Validation, Writing – original draft, Writing – review & editing. EV: Resources, Writing – review & editing. IE: Data curation, Formal Analysis, Writing – review & editing. NB: Formal Analysis, Writing – review & editing. YR: Formal Analysis, Writing – review & editing. DP: Formal Analysis, Writing – review & editing. ZM: Formal Analysis, Writing – review & editing. MM: Formal Analysis, Writing – review & editing. NS: Data curation, Formal Analysis, Validation, Writing – review & editing. DM: Resources, Writing – review & editing. DS: Resources, Writing – review & editing. TI: Resources, Writing – review & editing. SM: Resources, Writing – review & editing. EB: Resources, Writing – review & editing. GY: Resources, Writing – review & editing. IT: Resources, Writing – review & editing. YG: Resources, Writing – review & editing. MI: Resources, Writing – review & editing. NI: Resources, Writing – review & editing. LN: Resources, Writing – review & editing. ES: Resources, Writing – review & editing. TB: Resources, Writing – review & editing. OR: Resources, Writing – review & editing. SV: Project administration, Supervision, Writing – review & editing. RZ: Supervision, Writing – review & editing. AS: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. SK: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the state assignment of the Ministry of Science and Higher Education of the Russian Federation.
Acknowledgments
The authors extend their sincere thanks to Andrey Reznikov for invaluable assistance in processing NBS cards and compiling information on newborns. We also are grateful to Dr. Varvara Galkina for fruitful discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. (2022) 42:1473–507. doi: 10.1007/s10875-022-01289-3
2. Bousfiha A, Moundir A, Tangye SG, Picard C, Jeddane L, Al-Herz W, et al. The 2022 update of IUIS phenotypical classification for human inborn errors of immunity. J Clin Immunol. (2022) 42:1508–20. doi: 10.1007/s10875-022-01352-z
3. Van Der Burg M, Mahlaoui N, Gaspar HB, Pai SY. Universal newborn screening for severe combined immunodeficiency (SCID). Front Pediatr. (2019) 7:373. doi: 10.3389/fped.2019.00373
4. Lev A, Somech R, Somekh I. Newborn screening for severe combined immunodeficiency and inborn errors of immunity. Curr Opin Pediatr. (2023) 35:692–702. doi: 10.1097/MOP.0000000000001291
5. Dorsey MJ, Dvorak CC, Cowan MJ, Puck JM. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J Allergy Clin Immunol. (2017) 139:733–42. doi: 10.1016/j.jaci.2017.01.005
6. Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AA, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California, 2010–2017. Pediatrics. (2019) 143:e20182300. doi: 10.1542/peds.2018-2300
7. Cardenas-Morales M, Hernandez-Trujillo VP. Agammaglobulinemia: from X-linked to autosomal forms of disease. Clin Rev Allergy Immunol. (2022) 63:22–35. doi: 10.1007/s12016-021-08870-5
8. De Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with down syndrome in the United States. Am J Med Genet A. (2015) 167A:756–67. doi: 10.1002/ajmg.a.37001
9. Hughes-Mccormack LA, Mcgowan R, Pell JP, Mackay D, Henderson A, O'leary L, et al. Birth incidence, deaths and hospitalisations of children and young people with down syndrome, 1990–2015: birth cohort study. BMJ Open. (2020) 10:e033770. doi: 10.1136/bmjopen-2019-033770
10. Volodin NN, Demikova NS, Asanov AY, Podolnaya MA, Lapina AS. Trisomy 21 (down syndrome) incidence dynamics in the regions of the Russian federation in 2011–2017. Pediatria n.a. G.N. Speransky. (2019) 98:43–8. doi: 10.24110/0031-403X-2019-98-2-42-48 (In Russian).
11. Lagan N, Huggard D, Mc Grane F, Leahy TR, Franklin O, Roche E, et al. Multiorgan involvement and management in children with down syndrome. Acta Paediatr. (2020) 109:1096–111. doi: 10.1111/apa.15153
12. Vasilyeva TA, Sukhanova NV, Marakhonov AV, Kuzina NY, Shilova NV, Kadyshev VV, et al. Co-Occurrence of congenital aniridia due to nonsense PAX6 variant p.(Cys94*) and chromosome 21 trisomy in the same patient. Int J Mol Sci. (2023) 24:15527. doi: 10.3390/ijms242115527
13. Verstegen RH, Borte S, Bok LA, Van Zwieten PH, Von Dobeln U, Hammarstrom L, et al. Impact of down syndrome on the performance of neonatal screening assays for severe primary immunodeficiency diseases. J Allergy Clin Immunol. (2014) 133:1208–11. doi: 10.1016/j.jaci.2013.10.010
14. Huggard D, Doherty DG, Molloy EJ. Immune dysregulation in children with down syndrome. Front Pediatr. (2020) 8:73. doi: 10.3389/fped.2020.00073
15. Marcovecchio GE, Bortolomai I, Ferrua F, Fontana E, Imberti L, Conforti E, et al. Thymic epithelium abnormalities in DiGeorge and down syndrome patients contribute to dysregulation in T cell development. Front Immunol. (2019) 10:447. doi: 10.3389/fimmu.2019.00447
16. Puck JM. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia. Immunol Rev. (2019) 287:241–52. doi: 10.1111/imr.12729
17. Marakhonov AV, Efimova IY, Mukhina AA, Zinchenko RA, Balinova NV, Rodina Y, et al. Newborn screening for severe T and B cell lymphopenia using TREC/KREC detection: a large-scale pilot study of 202,908 newborns. J Clin Immunol. (2024) 44:93. doi: 10.1007/s10875-024-01691-z
18. Abramson JH. WINPEPI Updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. (2011) 8:1. doi: 10.1186/1742-5573-8-1
19. Brock JK, Walsh JD, Allen VM. The effect of fetal trisomy 21 on adverse perinatal obstetrical outcomes in Nova Scotia, 2000–2019. J Obstet Gynaecol Can. (2021) 43:583–8. doi: 10.1016/j.jogc.2020.09.019
20. Wakamatsu M, Kojima D, Muramatsu H, Okuno Y, Kataoka S, Nakamura F, et al. TREC/KREC newborn screening followed by next-generation sequencing for severe combined immunodeficiency in Japan. J Clin Immunol. (2022) 42:1696–707. doi: 10.1007/s10875-022-01335-0
21. Vogel BH, Bonagura V, Weinberg GA, Ballow M, Isabelle J, Diantonio L, et al. Newborn screening for SCID in New York state: experience from the first two years. J Clin Immunol. (2014) 34:289–303. doi: 10.1007/s10875-014-0006-7
22. Voronin SV, Zakharova EY, Baydakova GV, Marakhonov AV, Shchagina OA, Ryzhkova OP, et al. Advanced neonatal screening for hereditary diseases in Russia: first results and future prospects. Pediatria n.a. G.N. Speransky. (2024) 103:16–29. (In Russian). doi: 10.24110/0031-403X-2024-103-1-16-29
Keywords: newborn screening, TREC, KREC, lymphopenia, Down syndrome, trisomy 21
Citation: Marakhonov A, Mukhina A, Vlasova E, Efimova I, Balinova N, Rodina Y, Pershin D, Markova Z, Minzhenkova M, Shilova N, Mudaeva D, Saydaeva D, Irbaieva T, Matulevich S, Belyashova E, Yakubovskiy G, Tebieva I, Gabisova Y, Ikaev M, Irinina N, Nurgalieva L, Saifullina E, Belyaeva T, Romanova O, Voronin S, Zinchenko R, Shcherbina A and Kutsev S (2024) Decreased TREC and KREC levels in newborns with trisomy 21. Front. Pediatr. 12:1468635. doi: 10.3389/fped.2024.1468635
Received: 22 July 2024; Accepted: 20 September 2024;
Published: 17 October 2024.
Edited by:
Luis Ignacio Gonzalez-Granado, University Hospital October 12, SpainReviewed by:
Eduardo Lopez-Granados, University Hospital La Paz, SpainMaaike Kusters, Great Ormond Street Hospital for Children NHS Foundation Trust, United Kingdom
Copyright: © 2024 Marakhonov, Mukhina, Vlasova, Efimova, Balinova, Rodina, Pershin, Markova, Minzhenkova, Shilova, Mudaeva, Saydaeva, Irbaieva, Matulevich, Belyashova, Yakubovskiy, Tebieva, Gabisova, Ikaev, Irinina, Nurgalieva, Saifullina, Belyaeva, Romanova, Voronin, Zinchenko, Shcherbina and Kutsev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrey Marakhonov, bWFyYWtob25vdkBnZW5lcmVzZWFyY2gucnU=
 Andrey Marakhonov
Andrey Marakhonov Anna Mukhina
Anna Mukhina Elena Vlasova3
Elena Vlasova3 Natalya Balinova
Natalya Balinova Dmitry Pershin
Dmitry Pershin Grigoriy Yakubovskiy
Grigoriy Yakubovskiy Anna Shcherbina
Anna Shcherbina