- 1Faculty of Medicine, Al-Quds University, Jerusalem, Palestine
- 2Department of Pediatric Surgery, Al-Makassed Islamic Charitable Hospital, Jerusalem, Palestine
Teratomas are germ cell tumors that arise from the derivatives of the three germ cell layers. They are categorized into subtypes by the extent of maturation, with mature teratomas being the most common subtype. While they can arise in various extragonadal regions, including the retroperitoneum, their occurrence in the retroperitoneal space is extremely rare. It is even more exceptional for these tumors to be located within the adrenal gland. In this report, we describe an 18-day-old female infant who presented with a left suprarenal mass. The mass was detected during prenatal screening via ultrasound at 30 weeks of pregnancy. Evaluation after birth, including a chest and abdomen computed tomography (CT) scan, revealed a large, well-defined left suprarenal mass. The mass was surgically resected and found to measure 9 cm × 7 cm × 5 cm. Histopathological examination confirmed a cystic mature teratoma containing a variety of well-differentiated tissues. The patient has shown excellent progress over the 1-year follow-up, with no evidence of recurrence. Only a few cases of mature adrenal teratoma have been reported, highlighting the importance of this case report.
Introduction
Retroperitoneal teratomas (RPTs) are the third most prevalent retroperitoneal tumors in the pediatric population, following neuroblastoma and Wilms tumors (1). However, it is important to note that the majority of these lesions are benign in nature. Interestingly, teratomas are exceedingly rare and uncommon tumors, with an estimated incidence rate of only 0.9 per 100,000 in the general population (2, 3). Moreover, primary adrenal teratomas in infants represent an exceptionally rare subset of this already uncommon category. As a result, only a limited number of cases have been documented and published in the medical literature, underscoring the unique and elusive nature of these tumors.
In this report, we present a compelling case of an 18-day-old female infant diagnosed with a mature adrenal teratoma. This case report has been reported in accordance with the SCARE criteria (4).
Case presentation
An 18-day-old female infant, born to healthy and non-consanguineous parents, was delivered via normal vaginal delivery and had no previous medical history, presented with a left suprarenal mass that was detected during prenatal screening. The mass was initially identified during a routine antenatal ultrasound scan at 30 weeks of pregnancy, which indicated a sizable cystic solid mass in the left adrenal region, raising concerns for potential malignancy. The mass was monitored throughout the remainder of the pregnancy, with subsequent ultrasounds confirming its size and characteristics, showing no significant changes in appearance or maternal health during this period. After birth, ultrasound examination revealed a sizable cystic solid mass in the left adrenal region, measuring approximately 7.9 cm × 4.7 cm. The mass was observed to cause indentation and displacement of the left kidney downwards, while the rest of the examination was unremarkable, except for fullness in the left abdomen.
Routine biological assessments, including liver and kidney function tests as well as a complete blood count, were unremarkable. Additionally, laboratory tests, including tumor markers (α-fetoprotein and β-human chorionic gonadotropin), were all within normal ranges. Further evaluation was conducted through urine gas chromatography-mass spectrometry (GC-MS) analysis, which did not reveal any diagnostic abnormalities, including the absence of homovanillic acid (HVA) or vanillylmandelic acid (VMA) excretion. It is important to note that normal values do not exclude the presence of catecholamine-secreting tumors. Subsequently, a chest and abdominal computed tomography (CT) scan was performed, revealing a large, well-defined left suprarenal mass measuring 8.5 cm × 6.5 cm (Figure 1). The mass displayed a variety of tissue components, including calcifications, fat, solid areas, and cystic regions. The lesion exerted a significant mass effect on the surrounding structures, anteriorly displacing the spleen, splenic vessels, pancreas, and portions of the stomach body. Medially, it pushed against the superior mesenteric artery and inferiorly displaced the kidney to the left iliac fossa, resulting in a transverse kidney position. The renal vein was displaced inferiorly and medially. Both kidneys exhibited normal excretion, and no distant metastases were observed. The mass was determined to be resectable and did not invade any vessels.
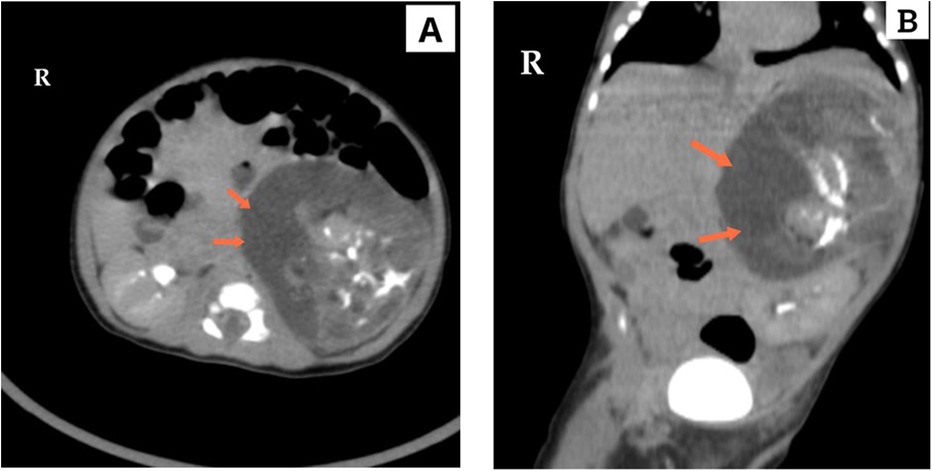
Figure 1. Computed tomography (CT), in (A) axial and (B) coronal section, demonstrates a giant tumor in the left adrenal region with variable tissue components, including calcification, fat, solid areas, and cystic regions. The tumor's significant size is evident as it displaces the left kidney and adjacent structures.
To further assess the cardiac status, an echocardiogram was performed, which revealed normal findings. Based on the suspicion of neuroblastoma, surgical resection of the mass was planned. During the procedure, the mass was completely excised with gross dimensions of 9 cm × 7 cm × 5 cm (Figure 2). The tumor had an irregular shape and was surrounded by a thin capsule. On sectioning, solid and cystic components with bone structures were observed. Notably, the mass was distinct from the adjacent normal adrenal tissue, which was significantly displaced but not entirely subverted. A partial adrenalectomy was performed to preserve adrenal function while ensuring complete removal of the tumor.
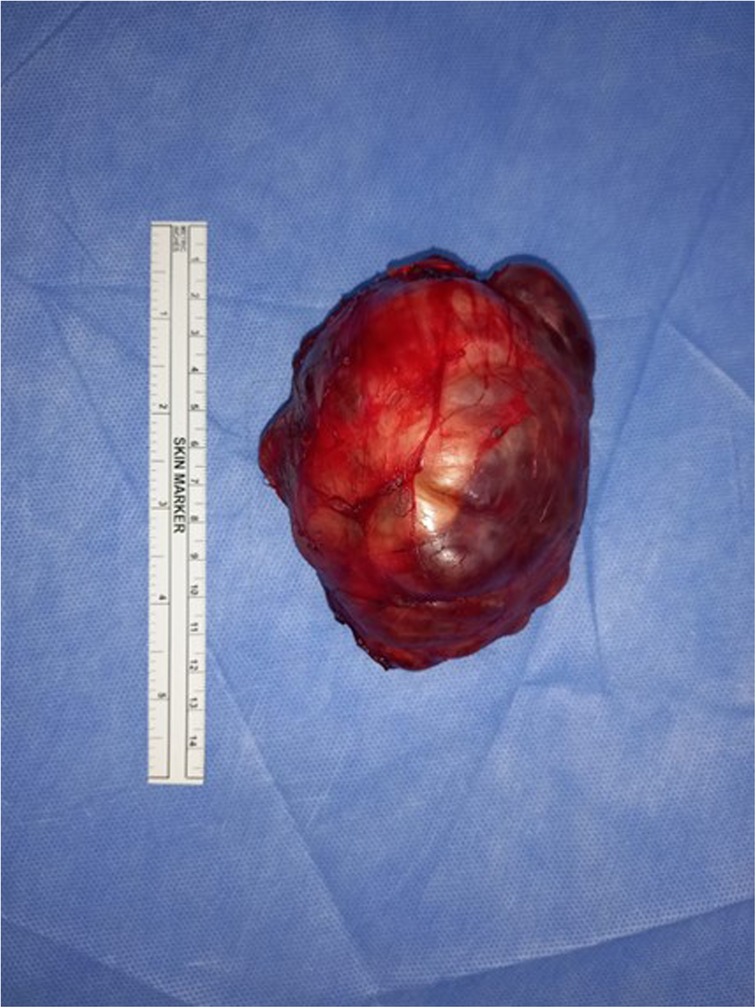
Figure 2. Gross appearance of the restricted tumor showing a large irregular cystic and solid adrenal mass.
Histopathological examination revealed a cystic mature teratoma. Immature elements were present in less than one low-power field and accounted for approximately 2% of the extensively sampled tissues. The mature components include a variety of well-differentiated tissues, such as skin, hair follicles, sebaceous glands, cartilage, and bone. The tumor was composed of 98% mature elements.
The patient has shown excellent progress during the 1-year follow-up period, exhibiting no signs of recurrence based on clinical, radiological, and biochemical evaluations.
Discussion
Teratomas are germ cell tumors that arise from totipotent cells and contain various cell types derived from the three germ layers (ectoderm, mesoderm, and endoderm) (5). During the fourth week of embryonic development, germ cells originating from the yolk sac migrate along the midline of the fetus, specifically through the dorsal mesentery, from the urogenital ridge towards the developing gonads. However, some of these cells fail to complete their migration and instead persist in midline locations such as the pineal gland, anterior mediastinum, retroperitoneum, and sacrococcygeal areas. Germ cells differentiate into extragonadal teratomas in these locations. They can be classified into four types based on their degree of maturation: mature, immature, teratoma with malignant transformation, and monodermal (3, 6). While gonadal teratomas are more common in adults, extragonadal teratomas can occur in various locations such as the mediastinum, retroperitoneum, cranium, sacrococcygeal region, large bowel, and tongue (3).
In pediatric cases, teratomas are commonly found in the sacrococcygeal region (41%), ovaries (28%), and testes (7%) (7). Approximately 24% of teratomas are found in midline structures such as the mediastinum and retroperitoneum. Among these, mediastinal teratomas account for 6%–18% of pediatric mediastinal tumors, with the majority of cases being benign in nature (8). The retroperitoneal location, specifically within the adrenal gland, is extremely rare, comprising only 1%–11% of all primary tumors in the retroperitoneal region (9).
Retroperitoneal mature cystic teratomas exhibit an interesting pattern of incidence, with two distinct peaks observed in the first six months of life and early adulthood (9). Approximately half of all cases are diagnosed within the first year of life, indicating a higher prevalence during infancy (9). Conversely, only 10%–20% of these teratomas occur in adults aged 30 years and older (10). Interestingly, the left suprarenal region, which is the location of the tumor in this patient, appears to be the predominant site for retroperitoneal cystic teratomas (10).
The retroperitoneum provides ample space for these tumors to grow, resulting in a typically substantial size upon presentation. Symptoms may manifest as abdominal or back pain, and in some cases can even cause intestinal obstruction due to compression. Alternatively, some cases may be asymptomatic and may be incidentally discovered. The nonspecific nature of these symptoms presents a challenge for early diagnosis. Although most adrenal teratomas are benign (6), they can give rise to complications. Tumor rupture poses a potential risk, leading to sudden-onset abdominal pain, ascites, hemorrhage, and peritonitis (2, 11, 12).
Diagnosing suprarenal tumors in infants during the antenatal or early neonatal periods poses significant diagnostic challenges. When evaluating a potential adrenal teratoma, it is essential to consider other conditions that may present as suprarenal masses. While neuroblastoma is the most frequently detected suprarenal mass during prenatal ultrasound, it is crucial to include other possibilities, such as adrenal hemorrhage, extrapulmonary sequestration, bronchogenic cyst, ovarian tumors, lymphangioma, and renal dysplasia, in the differential diagnosis (1, 6). Additionally, other adrenal tumors with a fatty component, including myelolipoma, lipoma, angiomyolipoma, and liposarcoma, should be considered when differentiating from adrenal teratomas (13).
The detection of antenatal mature adrenal teratomas is exceedingly rare. These tumors present as complex cystic masses within the fetal abdomen. Diagnostic imaging is crucial for the diagnosis of adrenal teratomas, as laboratory findings are typically nonspecific. Modalities such as plain radiography, abdominal ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) of the abdomen are useful for diagnosing teratomas (14). Radiological features, such as calcification, teeth, and fat, can help in diagnosis. Ultrasonography is often the initial imaging method used in pediatric cases and can differentiate between cystic and solid components (1). CT and MRI are essential for assessing the extent of the tumor in the retroperitoneum and its relationship to major blood vessels, facilitating preoperative planning, and increasing the likelihood of complete tumor removal with minimal iatrogenic damage (1). Internal homogeneity, fat density, cyst formation, and calcification are significant predictors of benign retroperitoneal teratomas on CT. MRI provides superior resolution for soft tissues, allowing the identification of benign and malignant neoplastic features and facilitating tumor staging assessment (15). Histopathological analysis following laparoscopic adrenal tumor resection is often required for a definitive diagnosis.
Most adrenal teratomas are unilateral, with only one documented case of bilateral involvement (16). In patients with bilateral adrenal tumors requiring surgery, subtotal adrenalectomy may be recommended to avoid permanent adrenal insufficiency and steroid dependency, along with its associated risks, such as decreased quality of life and increased morbidity (17, 18). This technique involves removing adrenal pathology while preserving vascularized adrenal tissue to maintain hormone homeostasis. However, it is infrequently performed, and many clinicians may lack familiarity with its considerations; approximately 30% of normal adrenal tissue is needed to ensure adequate steroid levels (18).
Complete surgical resection followed by close monitoring is recommended for mature teratomas and is necessary for definitive diagnosis (1–3). Laparoscopic surgery has emerged as the primary treatment modality for benign adrenal tumors, surpassing open surgery. This shift is attributed to several advantages associated with laparoscopic approaches, including reduced morbidity, faster recovery, and decreased postoperative pain (19). However, in the case of an immature teratoma, adjuvant therapy, such as chemotherapy, radiotherapy, or concurrent chemoradiotherapy, may be required following complete resection of the primary tumor (2). The prognosis of patients with mature adrenal teratomas is generally favorable following complete surgical resection. Long-term follow-up is recommended to monitor for recurrence or metastasis, although these are uncommon (2). Currently, there is a lack of sensitive indicators for effectively monitoring the relapse of adrenal teratomas. However, studies have shown a correlation between alpha-fetoprotein (AFP) levels and the recurrence of teratomas. This suggests that AFP can serve as a predictive index for evaluating the efficacy of treatment and the potential for a cure (20).
Although laparoscopic surgery is advantageous in terms of recovery and reduced morbidity, it has limitations, particularly in very young patients. The huge dimensions of the mass in a newborn often make open access easier and safer (21–23).
Postoperative morbidity is not uncommon and often necessitates an extended hospital stay. Reported complications include heart failure, infections, abdominal fluid collections, and chylous leakage (24). Additionally, the presence of teratomas can lead to unexpected postoperative adrenal insufficiency, particularly if the adrenal tissue is inadvertently damaged during resection (25).
This case emphasizes the importance of a multidisciplinary approach for the evaluation and management of pediatric adrenal masses. It also highlights the challenges in distinguishing between benign and malignant entities based solely on imaging and laboratory findings. Surgical intervention remains a cornerstone of management, providing both diagnostic clarity and therapeutic benefits. Further research is needed to fully understand the pathogenesis and optimal management strategies for these rare tumors.
To the best of our knowledge, there are only a limited number of reported cases in the literature regarding primary mature adrenal teratomas in pediatric patients, as summarized in (Table 1). Most of these cases were either incidentally detected or presented clinically because of abdominal distension. It is exceptionally rare for these tumors to be prenatally diagnosed. To date, only six other cases of antenatally diagnosed adrenal masses have been documented (30, 37, 44, 47, 50).
Conclusion
Primary adrenal teratomas in infants are exceptionally rare tumors. This case report highlights the importance of early detection, accurate diagnosis, and appropriate surgical management to achieve favorable outcomes. The comprehensive documentation of this unique case adds to the limited literature on these elusive tumors and provides valuable insights that can inform future clinical decision-making. Increased awareness and further research are needed to better understand the pathogenesis and optimize the management of these rare pediatric tumors, ultimately improving the care and outcomes of affected patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this Case Report.
Author contributions
AM: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MS: Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. AA: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. WM: Writing – original draft, Writing – review & editing. NM: Writing – original draft, Writing – review & editing. JS: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haddad SE, Hessissen L, Kababri ME, Lamalmi N, Kisra M, Allali N, et al. Primary mature adrenal teratoma in infant. Pan Afr Med J. (2020) 37:27. doi: 10.11604/pamj.2020.37.27.24016
2. Wang X, Li X, Cai H, Xiao W, Su P, Huang X, et al. Rare primary adrenal tumor: a case report of teratomas and literatures review. Front Oncol. (2022) 12:830003. doi: 10.3389/fonc.2022.830003
3. Ciftci I, Cihan T, Koksal Y, Ugras S, Erol C. Giant mature adrenal cystic teratoma in an infant. Acta Inform Med. (2013) 21(2):140. doi: 10.5455/aim.2013.21.140-141
4. Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A. The CARE 2020 guideline: updating consensus surgical case report (CARE) guidelines. Int J Surg. (2020) 84:226–30. doi: 10.1016/j.ijsu.2020.10.034
5. Saba L, Guerriero S, Sulcis R, Virgilio B, Melis G, Mallarini G. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur J Radiol. (2009) 72(3):454–63. doi: 10.1016/j.ejrad.2008.07.044
6. Garg A, Pollak-Christian E, Unnikrishnan N. A rare adrenal mass in a 3-month-old: a case report and literature review. Case Rep Pediatr. (2017) 2017:1–5. doi: 10.1155/2017/4542321
7. Gow KW, Koontz CS, Dickson P, Bannister L, Shehata B. Retropleural teratoma presenting as an abdominal mass in a 9-month-old boy. J Pediatr Surg. (2010) 45(11):e15–8. doi: 10.1016/j.jpedsurg.2010.07.014
8. Billmire D, Vinocur C, Rescorla F, Colombani P, Cushing B, Hawkins E, et al. Malignant mediastinal germ cell tumors: an intergroup study. J Pediatr Surg. (2001) 36(1):18–24. doi: 10.1053/jpsu.2001.19995
9. Gatcombe HG, Assikis V, Kooby D, Johnstone PAS. Primary retroperitoneal teratomas: a review of the literature. J Surg Oncol. (2004) 86(2):107–13. doi: 10.1002/jso.20043
10. Chen JC, Khiyami A, McHenry CR. Retroperitoneal cystic teratoma masquerading as an incidentally discovered adrenal mass. Endocr Pract. (2011) 17(5):e130–4. doi: 10.4158/EP11183.CR
11. Scott AL, Abbassi-Ghadi N, Archer CM, Swamy R, Gupta S. Neuroendocrine carcinoma arising within a retroperitoneal mature teratoma. Ann R Coll Surg Engl. (2010) 92(6):e5–8. doi: 10.1308/147870810X12699662980952
12. Pandya JS, Pai MV, Muchhala S. Retroperitoneal teratoma presenting as acute abdomen in an elderly person. Indian J Gastroenterol. (2000) 19(2):89–90.10812828
13. Lam K-Y, Lo C-Y. Teratoma in the region of adrenal gland: a unique entity masquerading as lipomatous adrenal tumor. Surgery. (1999) 126(1):90–4. doi: 10.1067/msy.1999.98924
14. Ban A, Satapara J, Rathod K, Bahri N. Teratoma involving adrenal gland—a case report and review of literature. Indian J Radiol Imaging. (2019) 29(04):452–6. doi: 10.4103/ijri.IJRI_452_18
15. Zhou L, Pan X, He T, Lai Y, Li W, Hu Y, et al. Primary adrenal teratoma: a case series and review of the literature. Mol Clin Oncol. (2018) 9(4):437–42. doi: 10.3892/mco.2018.1687
16. Xu YE, Jones B, Kimble R. Bilateral adrenal teratomas. J Pediatr Surg Case Rep. (2019) 51:101319. doi: 10.1016/j.epsc.2019.101319
17. Gimm O, Duh Q-Y. Challenges of training in adrenal surgery. Gland Surg. (2019) 8(S1):S3–9. doi: 10.21037/gs.2019.01.08
18. Onuma AE, Sun SH, Miller BS. Partial adrenalectomy: evaluation and management—a clinical practice review. Laparosc Surg. (2023) 7:6–6. doi: 10.21037/ls-22-21
19. Zhong W, Ma R, Cheng S, Tian J, Wang H, Wang T, et al. Clinical characteristics and surgical management of adult adrenal teratoma: a 15-year experience and systematic review of the literature. Urology. (2020) 135:71–5. doi: 10.1016/j.urology.2019.05.032
20. Nam SH, Cho MJ, Kim DY, Kim SC. Half-life of alpha-fetoprotein in neonatal sacrococcygeal teratoma. J Pediatr Surg. (2018) 53(12):2470–4. doi: 10.1016/j.jpedsurg.2018.08.012
21. Li H, Zhao T, Wei Q, Yuan H, Cao D, Shen P, et al. Laparoscopic resection of a huge mature cystic teratoma of the right adrenal gland through retroperitoneal approach: a case report and literature review. World J Surg Oncol. (2015) 13(1):318. doi: 10.1186/s12957-015-0734-z
22. Nicholas R, Cave C, Barrow T, Johncilla M, Dan D. Laparoscopic adrenalectomy for a giant adrenal teratoma: a case report and review of the literature. Int J Surg Case Rep. (2022) 99:107645. doi: 10.1016/j.ijscr.2022.107645
23. Wang J, Zhang J, Xiao C, Fan C. Laparoscopic simultaneous resection of bilateral giant primary mature retroperitoneal teratoma of the adrenal region. Medicine (Baltimore). (2019) 98(44):e17836. doi: 10.1097/MD.0000000000017836
24. Mosbahi S, Sallemi S, Ben Abdejelil N, Mani S, Ben Youssef S, Belhassen S, et al. Excision of giant retroperitoneal immature teratoma with post-operative chylous ascites. J Pediatr Surg Case Rep. (2021) 67:101823. doi: 10.1016/j.epsc.2021.101823
25. Joshi T, Woodford P, Maiti K, Smith R, Gani J, Acharya S. Giant retroperitoneal teratoma associated with unexpected postoperative adrenal insufficiency: CRH and ACTH secretion from teratoma? AACE Clin Case Rep. (2017) 3(1):e8–e11. doi: 10.4158/EP15785.CR
26. Engel RM, Elkins RC, Fletcher BD. Retroperitoneal teratoma. Review of the literature and presentation of an unusual case. Cancer. (1968) 22(5):1068–73. doi: 10.1002/1097-0142(196811)22:5%3C1068::AID-CNCR2820220525%3E3.0.CO;2-3
27. Terada Y, Kato A, Kishi H, Umeda T, Njima T, Yashiro N. Nuclear magnetic resonance imaging of a benign cystic teratoma in the retroperitoneum. J Urol. (1987) 137(1):106–8. doi: 10.1016/S0022-5347(17)43890-0
28. McMillan A, Horwich A. Case report: malignant teratoma presenting with an adrenal mass. Clin Radiol. (1987) 38(3):327–8. doi: 10.1016/S0009-9260(87)80088-0
29. Castillo OA, Vitagliano G, Villeta M, Arellano L, Santis O. Laparoscopic resection of adrenal teratoma. JSLS. (2006) 10(4):522–4.17575773
30. Oguzkurt P, Ince E, Temiz A, Demir S, Akabolat F, Hicsonmez A. Prenatal diagnosis of a mass in the adrenal region that proved to be a teratoma. J Pediatr Hematol Oncol. (2009) 31(5):350–1. doi: 10.1097/MPH.0b013e318190d765
31. Ersoz S, Kucuk H, Mungan S, Turgutalp H, Imamoglu M, Kosucu P. Neurocytoma arising in an adrenal gland mature teratoma. Fetal Pediatr Pathol. (2011) 30(5):275–9. doi: 10.3109/15513815.2011.572955
32. Li Y, Zhong Z, Zhao X. Primary mature teratoma presenting as an adrenal tumor in a child. Urology. (2011) 78(3):689–91. doi: 10.1016/j.urology.2010.12.022
33. Yasui Y, Kohno M, Shironomae T, Kuwahara T, Takahashi S, Oshikiri T. Retroperitoneoscopic resection of a congenital adrenal teratoma in an infant. J Pediatr Surg Case Rep. (2013) 1(11):391–4. doi: 10.1016/j.epsc.2013.10.008
34. Peer S. Mature cystic teratoma of adrenal gland: a case report. Sch J Med Case Rep. (2016) 4(12):926–30. doi: 10.21276/sjmcr.2016.4.12.15
35. Mahzouni P, Sabaghi B, Sanei B, Arjang E. Mature cystic teratoma of adrenal gland (adrenal teratoma). (2016). Available online at: http://crcp.tums.ac.ir (accessed June 27, 2024).
36. Narla S, Jacob S, Kurian A, Parameswaran A. Primary mature cystic teratoma with carcinoid mimicking an adrenal tumor: report of a rare association and review of literature. Indian J Pathol Microbiol. (2016) 59(2):200. doi: 10.4103/0377-4929.182012
37. Singh AP, Tanger R, Ansari M, Gupta AK, Barolia DK. Antenatally diagnosed suprarenal mature cystic teratoma with down syndrome. APSP J Case Rep. (2018) 9(3):15. doi: 10.21699/ajcr.v9i3.44
38. Pandit N, Awale L, Jaiswal LS. Giant calcified retroperitoneal teratoma. Indian J Surg Oncol. (2018) 9(3):436–7. doi: 10.1007/s13193-018-0789-8
39. Kanwal A. Adrenal teratoma: a rare retroperitoneal tumor. MOJ Clin Med Case Rep. (2019) 9(6):154–5. doi: 10.15406/mojcr.2019.09.00327
40. Sharma S, Dawson L, Mandal AK. Primary retroperitoneal teratoma with predominant neurogenic elements masquerading as adrenal tumor. Turk Patoloji Derg. (2016) 35(1):69–73. doi: 10.5146/tjpath.2016.01365
41. Rasul L, Liaqat N, Atta M, Imran R. Javed N. Monster Extracted out of a Seven Year Old Girl: Adrenal Teratoma-A Case Report. (2018).
42. He C, Yang Y, Yang Y, Wang F, Hu J, Zhang J, et al. Teratoma of the adrenal gland: clinical experience and literature review. Gland Surg. (2020) 9(4):1056–64. doi: 10.21037/gs-20-648
43. Banthia R, Yadav P, Bharti A, Lal H. Mature cystic teratoma presenting as suprarenal mass. BMJ Case Rep. (2020) 13(8):e237734. doi: 10.1136/bcr-2020-237734
44. Pace S, Sacks MA, Goodman LF, Tagge EP, Radulescu A. Antenatal diagnosis of retroperitoneal cystic mass: fetiform teratoma or fetus in fetu? A case report. Am J Case Rep. (2021) 22:e929247. doi: 10.12659/AJCR.929247
45. Rey-Rodriguez DE, Hidalgo-Salas JH, Valdes-Gomez JJ, Mora-Flores DM. Excision of giant mature teratoma of the adrenal gland in a 1-year-old girl. J Pediatr Surg Case Rep. (2021) 71:101901. doi: 10.1016/j.epsc.2021.101901
46. Ray R, Dey S, Khatun F, Barman S, Das M, Chatterjee U. Adrenal and mesenteric teratomas in infants: common tumors in uncommon sites. J Indian Assoc Pediatr Surg. (2022) 27(3):354–6. doi: 10.4103/jiaps.JIAPS_26_21
47. Garcia C, Fusi G, Gambart M. Prenatal diagnosis of an adrenal mature teratoma mimicking a neuroblastoma. Arch Clin Cases. (2023) 10(2):66–9. doi: 10.22551/2023.39.1002.10243
48. Zhang X, Ning J. A rare case of fetal retroperitoneal solid mature teratoma. Asian J Surg. (2023) 46(11):4776–7. doi: 10.1016/j.asjsur.2023.05.016
49. Alahdal A, Bahkali M, Abu-Ouf NM, Alturkustani M. A case of Wilms tumor in a primary adrenal mature teratoma. Cureus. (2023) 15(7):e41332. doi: 10.7759/cureus.41332
Keywords: teratoma, adrenal gland, mature teratoma, retroperitoneal, prenatal screening
Citation: Msarweh A, Shehadeh MH, Abualrub AM, Malhes WM, Msarweh N, Sinokrot JK and Aliwisat AH (2024) Case Report: A rare case of antenatally diagnosed mature adrenal teratoma in an infant: insights and literature review. Front. Pediatr. 12:1460251. doi: 10.3389/fped.2024.1460251
Received: 5 July 2024; Accepted: 6 November 2024;
Published: 29 November 2024.
Edited by:
Lisa States, Children's Hospital of Philadelphia, United StatesReviewed by:
Saida Hidouri, University of Monastir, TunisiaGiovanna Riccipetitoni, San Matteo Hospital Foundation (IRCCS), Italy
Copyright: © 2024 Msarweh, Shehadeh, Abualrub, Malhes, Msarweh, Sinokrot and Aliwisat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amar Msarweh, YW1hcm1hc3J3ZUBnbWFpbC5jb20=; Mohammad Hakam Shehadeh, bW9oYW1tYWRoYWthbTBAZ21haWwuY29t
 Amar Msarweh
Amar Msarweh Mohammad Hakam Shehadeh
Mohammad Hakam Shehadeh Ahmad M. Abualrub
Ahmad M. Abualrub Waleed M. Malhes
Waleed M. Malhes Nadeen Msarweh1
Nadeen Msarweh1 Jenan Khaled Sinokrot
Jenan Khaled Sinokrot