- Department of Rheumatology and Immunology, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
Background: Kawasaki disease (KD), an acute self-limiting vasculitis, is the main cause of acquired heart disease. Timely diagnosis and treatment can mitigate the occurrence of complications and improve patient prognosis. Facial nerve paralysis (FNP) and ptosis are rare complications of KD and are rarely reported, but FNP is considered a high risk factor for coronary aneurysms. If giant coronary artery aneurysms are formed, clinicians should be vigilant when diagnosing the formation of systemic artery aneurysms (SAAs).
Patient presentation: A 10-month-old girl with fever, diffuse rash, and conjunctival congestion was hospitalized locally, diagnosed with KD, and treated with intravenous infusion of gamma globulin (IVIG). She had fever again after 1 week of temperature stability, accompanied by conjunctival congestion, and was treated with a second dose of IVIG, but she still experienced recurrent fever. The day prior to admission, she developed facial asymmetry, left FNP, diffuse erythema and membranous peeling of the fingers of both hands. The patient's body temperature normalized after treatment with 20 mg/kg methylprednisolone, but cardiac ultrasound revealed progressive enlargement of the coronary artery aneurysms. On day 37of the illness, transient eyelid ptosis developed; fortunately, the left FNP and eyelid ptosis finally resolved, leaving no sequelae. Two years and eight months after onset, the patient developed bilateral humeral aneurysm.
Conclusion: This is the first KD patient involving two neurological complications combined with giant coronary artery aneurysms and SAAs. KD needs to be considered in infants with unexplained recurrent fever who present with FNP or ptosis. FNP secondary to KD is a high risk factor for coronary artery aneurysms, so it is necessary to perform cardiac ultrasound for accurate diagnosis. KDs combined with giant coronary aneurysms require careful physical examination and noninvasive angiography during follow-up to detect SAA formation.
Introduction
Kawasaki disease (KD) is an acute self-limiting vasculitis disease that primarily affects children under 5 years of age, and the pathogenesis of KD has remained unclear until now. Coronary artery lesions (CALs) are serious complications of KD that can cause sudden death, myocardial infarction and ischemic heart disease in children and are now the leading cause of acquired heart disease in children. Timely diagnosis and treatment with intravenous infusion of gamma globulin (IVIG) reduce the probability of CALs from 25% to approximately 4%. The extent of coronary artery involvement is a crucial factor affecting the long-term prognosis of KD patients (1, 2). It is well known that KD causes systemic inflammation in all medium-sized arteries, and the incidence of systemic artery aneurysms (SAAs), including those of the subclavian, brachial, axillary, and iliac arteries, is approximately 0.8%–2.0% (3). In addition to affecting the cardiovascular system, KD can also cause complications in other organs and tissues. The incidence of neurological complications is approximately 1%–30% (4, 5), including headache, epilepsy, lethargy, irritability, aseptic meningitis, bulging fontanel, facial nerve paralysis (FNP) and sensorineural hearing loss (6). The incidence of KD combined with FNP is only 0.9%–1.3% (7). KD combined with bilateral ptosis is even rarer, with only 6 cases reported thus far (8–13). We report a patient with KD with FNP, transient bilateral ptosis and giant coronary artery aneurysms. Bilateral brachial artery aneurysms were found after 2 years and 8 months of follow-up.
Case
This was a retrospective study approved by the Institutional Review Board of Wuhan Children's Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2021R074-E02).
A 10-month-old female infant who was diagnosed with KD at a local hospital after infection was ruled out and given IVIG at a dose of 2 g/kg and aspirin (30 mg/kg/day) treatment due to fever for 1 week accompanied by generalized erythema, conjunctival hyperemia and chapped lips. After stabilizing her temperature for one week, her fever recurred with bilateral conjunctival congestion, without rash, and without symptoms related to infection such as cough or rhinorrhea, and she subsequently received a second dose of 2 g/kg IVIG at the same local hospital, but the fever still recurred. On the 20th day of recurrent fever, facial asymmetry appeared, the left nasolabial sulcus was shallowed, and the left eye could not be completely closed; therefore, he was admitted to our hospital.
Physical examination on admission, which revealed bilateral bulbar conjunctival congestion, strawberry tongue, fissuring of the lips, swollen lymph nodes in the neck, erythematous rash scattered throughout the body, incomplete closure of the left eyelid, corners of the mouth inclined to the right, shallow left nasolabial furrows, and membranous peeling on the fingers of both hands. Thus, IVIG-resistant KD was diagnosed.
Cephalic magnetic resonance and electroencephalogram results were normal. Electromyography suggested a lesion in the left peripheral facial nerve (Figure 1). Her white blood cell count was 14.00 × 109/L, hemoglobin level was 93 g/L, platelet count was 629 × 109/L, C-reactive protein level was 16.80 mg/L, and her interleukin-6 level was 164.95 pg/mL. Testing for antibodies to EB virus, cytomegalovirus, herpes simplex virus type I, and herpes simplex virus type II has ruled out infection. Routine analysis of cerebrospinal fluid revealed a white blood cell count of 21 × 106/L (reference range 0–15 × 106/L), dominant mononuclear cells, a cerebrospinal fluid protein level of 0.50 (reference range 0.05–0.45 g/L), and a cerebrospinal fluid glucose level of 4.09 (2.2–3.9 mmol/L). After treatment with methylprednisolone (20 mg/kg) for 3 days, aspirin (50 mg/kg/day) and mecobalamin were administered. After the infusion of methylprednisolone, her temperature normalized. The remaining FNP was alleviated after 1 week. After discharge, prednisone, clopidogrel, warfarin, and aspirin were continued. Her fever reappeared on the 32th day with conjunctival congestion, and she was admitted to our department for treatment again. Her temperature returned to normal after IVIG (2 kg/kg) treatment, but on the 37th day after disease progression, this child presented with bilateral ptosis, but muscle strength level 5, and the knee-jerk reflex can be normally elicited. Unlike myasthenia gravis, this child's bilateral ptosis was obvious in the morning, relatively relieved in the afternoon, and persisted for 5 days, and this phenomenon was largely relieved. Ganglioside antibody testing revealed positivity for anti-GD2 IgM and anti-GM2 IgM. After a follow-up period of 2 years and 8 months, the parents discovered masses in both elbows, while bilateral brachial aneurysms were indicated by cardiac CT angiography. A progress chart presenting historical findings and treatment over time in Figure 2 (14).

Figure 1. Electromyogram: facial nerve conduction velocity. Normal conduction velocity of the right facial nerve. No definitive compound muscle action potential was evoked in the left facial nerve motor conduction study with recording from the orbicularis oris muscle. (A) Left facial nerve - orbicularis oris muscle. (B) Right facial nerve - orbicularis oris muscle. (C) Left facial nerve - orbicularis oculi. (D) Right facial nerve - orbicularis oculi.
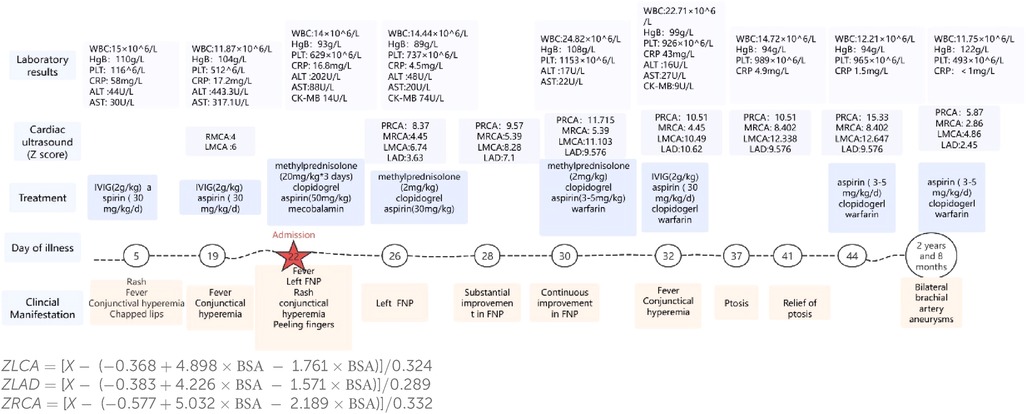
Figure 2. A progress chart presenting historical findings and treatment over time. PRCA: proximal segment of the right coronary artery. MRCA: middle segment of the right coronary artery. RMCA, left main coronary artery; LMCA, left main coronary artery; LAD, left anterior descending; WBC, white blood cell; HgB, hemoglobin; PLT, platelet; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK-MB, creatine kinase-MB.
Discussion
KD is a unique syndrome characterized by persistent fever, conjunctival congestion, fissured lips or strawberries, erythema or edema of the hands and feet, cervical lymphadenopathy and polymorphous rash (15, 16). CALs are serious complications of KD, and the risk of coronary artery aneurysm in infants less than 1 year old is significantly increased (17). In addition, IVIG resistance is also a risk factor for coronary aneurysms. A single-center retrospective study in Italy revealed that approximately 50% of children under 1 year of age with KD had coronary artery lesions and increased IVIG resistance (18).
Although the incidence of neurological complications associated with KD is not high, neurological complications such as irritability, aseptic meningitis, facial nerve paralysis, and sensorineural hearing loss have been recognized as neurological manifestations of KD (1). To date, there have been a few cases of KD combined with FNP. This is the first patient with unilateral FNP and bilateral ptosis accompanied by bilateral giant coronary aneurysms and bilateral brachial aneurysms during follow-up. We summarized the clinical features and prognosis of 19 patients with KD complicated with FNPs since 2018 (Table 1). Seventy-nine percent of the patients were under 1 year old, twelve patients had left FNP, the median onset time of facial nerve palsy was the 11th day of illness, 18 out of 19 patients had coronary artery lesions, and the proportion of coronary artery aneurysms was 68%. According to the article published by Stowe, FNP secondary to KD mainly affects infants under 1 year of age (63.9%), and the onset time is 16 days in the course of the disease. Left FNP is more common, and coronary artery aneurysms are observed in 24 out of 36 KD patients with FNP (28). Our two research conclusions are basically consistent. Therefore, FNP in KD patients may be a high risk factor for severe coronary artery aneurysm (20). Histopathological examination of the nervous system in children with KD revealed ganglionitis and neuritis of cranial and peripheral nerves, so it is believed that ischemic vasculitis supplying the facial nerve is the cause of FNP (4). In addition, we found that in 6 patients, including our infants, FNP occurred following the administration of IVIG, and all of these FNPs were associated with CALs. Therefore, we believe that an excessive inflammatory response is also involved in the occurrence of FNP (21, 27). However, the prognosis of FNP secondary to KD is not bad, and it can be completely alleviated except for 2 patients with bilateral nerve paralysis (23, 29).
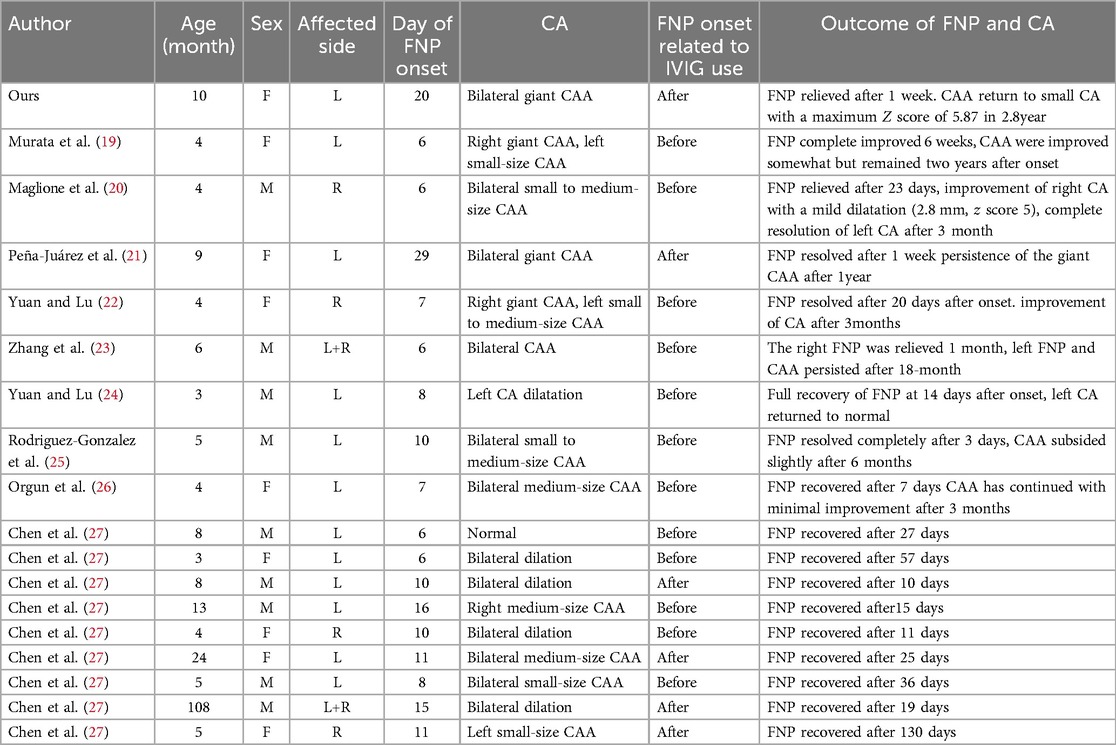
Table 1. The clinical features and prognosis of 19 patients with KD complicated with FNPs since 2018.
Ptosis secondary to KD was rarer, with only 6 cases in the early stage, none of which were complicated with CALs. Regardless of whether it occurs in the acute or subacute phase of the disease course, the symptoms of ptosis can be completely relieved (9–12). The mechanism of ptosis in KD remains unclear, and Falcini et al. suggested that it may be caused by ischemic vasculitis supplying the elevator muscles of the palpebra (9). Sanchez Marcos's study revealed positivity for anti-acetylcholine receptor antibodies (12), but laboratory tests in our child revealed the presence of ganglioside antibodies. IgM anti-GM2 or GD2 antibodies are rare types of antiganglioside antibodies that can be detected in Guillain-Barré syndrome (GBS). GBS is also an immune-mediated acute polyradiculoneuropathy. Studies have indicated that IgM anti-GM2 antibodies are associated with facial weakness in patients with GBS (30), in our case, the patient presented with bilateral ptosis. Therefore, we hypothesized that this KD child also had a variant of GBS. Regrettably, we did not repeat the examination of cerebrospinal fluid, electromyography, and cranial magnetic resonance imaging to confirm.
There are few studies on the risk factors, incidence and prognosis of SAAs in KD patients. Zhao et al. suggested that the incidence of KD combined with SAA is approximately 2%, and this group of patients all have medium-sized coronary aneurysms with a Z value >8, 73.9% (17 of 23) of which are giant CAAs; in addition, the occurrence of SAAs is generally symmetrical (31). Similar to the prognosis of coronary aneurysms, the regression rate of SAAs is related to artery diameter, and an SAA diameter >10 mm in the acute stage indicates that stenosis may occur in the later stage (32). The patient we reported had a palpable mass in both elbows during physical examination and bilateral brachial aneurysms with a diameter of 12 mm by cardiac CT angiography at 2 years and 8 months of follow-up after disease onset (Figure 3). Therefore, noninvasive imaging modalities should be used to screen for SAAs within 2 months of onset in children with giant coronary aneurysms. During the recovery phase, patients are followed up by peripheral angiography. The duration of follow-up was determined by the extent of coronary artery damage (31).
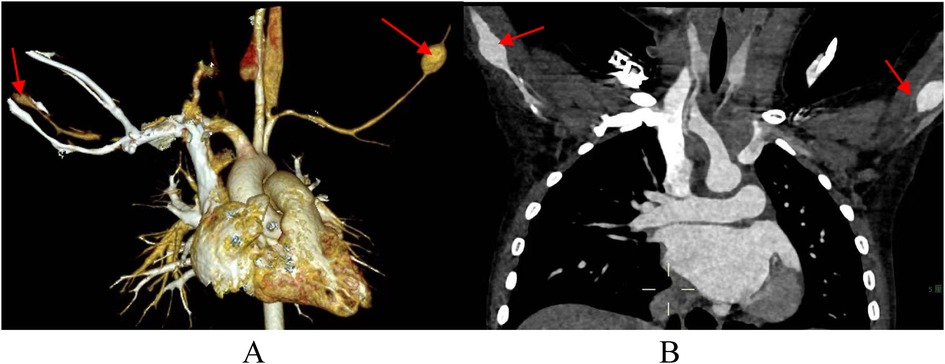
Figure 3. Cardiac CT angiography at 2 years and 8 months of follow-up. (A,B) arrows indicate bilateral brachial aneurysms.
Conclusion
Both FNP and bilateral ptosis are rare complications of KD, so the presence of FNP or transient ptosis in children with unexplained prolonged fever should be considered for KD, especially in infants under 1 year of age. Different from previous cases, our patient developed unilateral FNP and transient ptosis in a short period of time, with progressive enlargement of the coronary aneurysm. After these two symptoms are basically relieved, the coronary aneurysm also began to gradually recover. Therefore, we believe that the more neurological complications associated with Kawasaki disease, the more intense the inflammatory response, and long-term follow-up is necessary to be vigilant for the formation of SAA. Fortunately, these two neurological complications have good prognoses and few sequelae.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Wuhan Children's Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
WY: Writing – original draft, Writing – review & editing. YW: Supervision, Writing – review & editing. SW: Supervision, Writing – review & editing. HT: Supervision, Writing – review & editing. YD: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
KD, Kawasaki disease; CALs, coronary artery lesions; FNP, facial nerve paralysis; SAA, systemic artery aneurysm; IVIG, intravenous infusion of gamma globulin; GBS, Guillain-Barré syndrome.
References
1. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135(17):e927–99. doi: 10.1161/CIR.0000000000000484
2. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Circulation. (2004) 110(17):2747–71. doi: 10.1161/01.CIR.0000145143.19711.78
3. Seki M, Minami T. Kawasaki disease: pathology, risks, and management. Vasc Health Risk Manag. (2022) 18:407–16. doi: 10.2147/VHRM.S291762
4. Amano S, Hazama F. Neutral involvement in Kawasaki disease. Acta Pathol Jpn. (1980) 30(3):365–73. doi: 10.1111/j.1440-1827.1980.tb01331.x
5. Terasawa K, Ichinose E, Matsuishi T, Kato H. Neurological complications in Kawasaki disease. Brain Dev. (1983) 5(4):371–4. doi: 10.1016/S0387-7604(83)80041-2
6. Liu X, Zhou K, Hua Y, Wu M, Liu L, Shao S, et al. Neurological involvement in Kawasaki disease: a retrospective study. Pediatr Rheumatol Online J. (2020) 18(1):61. doi: 10.1186/s12969-020-00452-7
7. Alves NR, Magalhães CM, Almeida Rde F, Santos RC, Gandolfi L, Pratesi R. Prospective study of Kawasaki disease complications: review of 115 cases. Rev Assoc Med Bras (1992). (2011) 57(3):295–300. doi: 10.1016/S0104-4230(11)70062-8
8. Zhao SH, et al. Ptosis of both palpebra superiors caused by Kawasaki disease in a child. Zhongguo Dang Dai Er Ke Za Zhi. (2007) 9(1):83.17306090
9. Falcini F, La Torre F, Conti G, Vitale A, Messina MF, Calcagno G. Intermittent bilateral superior palpebra ptosis in a 20-month-old infant. Clin Exp Rheumatol. (2011) 29(2):360.21385559
10. Hameed A, Alshara H, Schleussinger T. Ptosis as a complication of Kawasaki disease. BMJ Case Rep. (2017) 2017:bcr2017219687. doi: 10.1136/bcr-2017-219687
11. Lin Y, Wang L, Li A, Zhang H, Shi L. Eyelid ptosis and muscle weakness in a child with Kawasaki disease: a case report. BMC Pediatr. (2021) 21(1):526. doi: 10.1186/s12887-021-02979-4
12. Sánchez Marcos E, Flores Perez P, Jimenez García R. Refusal to walk and ptosis as an atypical presentation of Kawasaki disease. Pediatr Infect Dis J. (2022) 41(8):e342–3. doi: 10.1097/INF.0000000000003551
13. Giri P, Pal P, Ghosh A. Kawasaki disease with bilateral ptosis-a case report. Ann Paediatr Rheumatol. (2013) 2:168–70. doi: 10.5455/apr.101620132056
14. Liu WQ, Xia B, Fan W, Yu Z, Lin WL, Chen L, et al. Analysis of 2 diagnostic criteria of echocardiography for coronary artery aneurysm in Kawasaki disease. Zhonghua Er Ke Za Zhi. (2022) 60(6):588–93. doi: 10.3760/cma.j.cn112140-20220316-00205
15. Kobayashi T, Ayusawa M, Suzuki H, Abe J, Ito S, Kato T, et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr Int. (2020) 62(10):1135–8. doi: 10.1111/ped.14326
16. Sundel RP. Kawasaki disease. Rheum Dis Clin North Am. (2015) 41(1):63–73, viii. doi: 10.1016/j.rdc.2014.09.010
17. Belay ED, Maddox RA, Holman RC, Curns AT, Ballah K, Schonberger LB. Kawasaki syndrome and risk factors for coronary artery abnormalities: United States, 1994–2003. Pediatr Infect Dis J. (2006) 25(3):245–9. doi: 10.1097/01.inf.0000202068.30956.16
18. Mastrangelo G, Cimaz R, Calabri GB, Simonini G, Lasagni D, Resti M, et al. Kawasaki disease in infants less than one year of age: an Italian cohort from a single center. BMC Pediatr. (2019) 19(1):321. doi: 10.1186/s12887-019-1695-0
19. Murata Y, Masuda H, Moroka S, Yotani N, Morimoto N, Kubota M, et al. Kawasaki disease with facial nerve palsy. Indian J Pediatr. (2024) 91(1):101. doi: 10.1007/s12098-023-04835-y
20. Maglione M, Barlabà A, Grieco M, Cosimi R, Di Nardo G, Di Marco GM, et al. Incomplete Kawasaki disease with peripheral facial nerve palsy and lung nodules: a case report and literature review. Children (Basel). (2023) 10(4):679. doi: 10.3390/children10040679
21. Peña-Juárez A, Medina-Andrade MA, Olivares IER, Colín-Ortíz JL, Yamazaki-Nakashimada MA, Garrido-Garcia LM. Multiresistant Kawasaki disease complicated with facial nerve palsy, bilateral giant coronary artery aneurysms, and stenosis of the right coronary artery in an infant. J Clin Rheumatol. (2021) 27(8s):S351–4. doi: 10.1097/RHU.0000000000001586
22. Yuan Y, Lu N. Coronary artery aneurysm and facial drooping in a infant with Kawasaki disease. Cardiol Young. (2020) 30(12):1957–9. doi: 10.1017/S104795112000298X
23. Zhang B, Hao Y, Zhang Y, Yang N, Li H, Liang J. Kawasaki disease manifesting as bilateral facial nerve palsy and meningitis: a case report and literature review. J Int Med Res. (2019) 47(8):4014–8. doi: 10.1177/0300060519854287
24. Yuan Y, Lu N. Facial nerve palsy presenting as rare neurological complication of Kawasaki disease: a case report. Medicine (Baltimore). (2019) 98(34):e16888. doi: 10.1097/MD.0000000000016888
25. Rodriguez-Gonzalez M, Castellano-Martinez A, Perez-Reviriego AA. Atypical presentation of incomplete Kawasaki disease: a peripheral facial nerve palsy. J Emerg Med. (2018) 55(1):118–20. doi: 10.1016/j.jemermed.2018.04.013
26. Orgun A, Karagöl C, Pamuk U, Gürsu HA, Çetin İ. A rare cause of facial nerve palsy in a young infant: Kawasaki disease. Turk J Pediatr. (2018) 60(4):433–5. doi: 10.24953/turkjped.2018.04.013
27. Chen J, Liu P, Hu W, Xu Y, Deng J. Facial nerve palsy may indicate coronary artery lesions in Kawasaki disease. Clin Rheumatol. (2021) 40(10):4191–7. doi: 10.1007/s10067-021-05791-8
28. Stowe RC. Facial nerve palsy, Kawasaki disease, and coronary artery aneurysm. Eur J Paediatr Neurol. (2015) 19(5):607–9. doi: 10.1016/j.ejpn.2015.05.010
29. Yu X, Liu X, Wang Y, Lu N, Wang M, Sun L. Kawasaki disease complicating bilateral facial nerve palsy and giant coronary artery aneurysms: a case report. Medicine (Baltimore). (2019) 98(7):e14395. doi: 10.1097/MD.0000000000014395
30. Kusunoki S, Willison HJ, Jacobs BC. Antiglycolipid antibodies in Guillain-Barré and Fisher syndromes: discovery, current status and future perspective. J Neurol Neurosurg Psychiatry. (2021) 92(3):311–8. doi: 10.1136/jnnp-2020-325053
31. Zhao QM, Chu C, Wu L, Liang XC, Sun SN, He L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. (2019) 144(6):e20192254. doi: 10.1542/peds.2019-2254
Keywords: Kawasaki disease, coronary artery lesions, facial nerve paralysis, ptosis, systemic artery aneurysms
Citation: Yin W, Wu Y, Wang S, Tang H and Ding Y (2024) Kawasaki disease involving both the nervous system and cardiovascular system: a case report and literature review. Front. Pediatr. 12:1459143. doi: 10.3389/fped.2024.1459143
Received: 3 July 2024; Accepted: 12 November 2024;
Published: 2 December 2024.
Edited by:
Elizabeth Secord, Wayne State University, United StatesReviewed by:
Takamichi Ishikawa, Hamamatsu University School of Medicine, JapanSatoru Iwashima, Chutoen General Medical Center, Japan
Copyright: © 2024 Yin, Wu, Wang, Tang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxia Tang, dGFuZ2hvbmd4aWE1MTNAMTI2LmNvbQ==; Yan Ding, ZGluZ3lhbm14QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Wen Yin
Wen Yin Yali Wu†
Yali Wu† Hongxia Tang
Hongxia Tang Yan Ding
Yan Ding