- Department of Pediatrics, The First Hospital of Quanzhou Affiliated to Fujian Medical University, Quanzhou, Fujian, China
Background: Nephrotic syndrome (NS) is a prevalent kidney disease in children. Acute kidney injury (AKI) is a severe complication of NS and has the potential to be life-threatening.
Objective: The aim of this study was to analyze the prevalence and risk factors of AKI in children with NS, and to provide an evidence-based medical basis for the early identification of high-risk children in the clinic.
Methods: A comprehensive search was conducted in publicly available databases, namely PubMed, Embase, Web of Science, Scopus, and the Cochrane Library, covering the period from the inception of each database until May 2024. The analysis involved examining basic characteristics (age, sex), the concomitant diseases (hypertension, infections), NS disease characteristics (steroid susceptibility classification, pathologic classification), laboratory test (e.g., serum albumin), and the use of nephrotoxic drugs. Traditional and network meta-analyses were performed for analysis.
Results: A total of 11 studies were included in the analysis, revealing an incidence of AKI of 29% (95% CI: 23%–37%). The analysis of factors indicated that the age of NS onset [standardized mean difference (SMD): 0.31; 95% confidence interval (CI): 0.08, 0.54; p = 0.009], sex [odds ratio (OR): 1.49; 95% CI: 1.03, 2.16; p = 0.035], serum albumin level (SMD: −0.43; 95% CI: −0.85, −0.02; p = 0.041), response to steroid treatment (OR: 0.52; 95% CI: 0.33, 0.80; p = 0.003), infection (OR: 3.60; 95% CI: 1.91, 6.78; p < 0.001), hypertension (OR: 4.02; 95% CI: 2.94, 5.51; p < 0.001), and nephrotoxic drug application (OR: 4.43; 95% CI: 1.86, 10.53; p = 0.001), were all significantly associated with the incidence of AKI. Furthermore, the results of the network meta-analysis suggested that the pathologic type of minor glomerular abnormalities (MGA)/diffuse mesangial proliferation (DMP), the type of infrequent relapses (IFRNS)/steroid-sensitive NS (SSNS), and the use of diuretic medications were associated with a relatively low risk of AKI occurrence.
Conclusion: Factors upon admission of children with NS are associated with the onset of AKI. Emphasis should be placed on populations with a heightened risk of AKI in clinical practice. Further research is warranted to confirm the findings due to the limitations of this study.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024571170, PROSPERO (CRD42024571170).
1 Introduction
Nephrotic syndrome (NS) is one of the most common types of kidney disease in children, and it is characterized by significant proteinuria, hypoalbuminemia, generalized edema, and hyperlipidemia (1). The global incidence of NS is relatively stable, with approximately 2.92 (range 2–7) new cases per 100,000 children per year, and a prevalence of approximately 16 cases per 100,000 children (2, 3). The main complications of NS include infection, acute kidney injury (AKI), and thromboembolism (TE). Failure to timely diagnose and treat these complications may pose a threat to the patient's life (2).
AKI is a severe complication of nephrotic syndrome (NS), and its risk factors are intertwined with NS, resulting in increased hospitalization and mortality rates. Based on the diversity of diagnostic criteria for AKI, the incidence of AKI among hospitalized children with NS ranges from 3.3% to 58.6% (4, 5). Research also indicates that the onset of AKI significantly increases the patient's risk of long-term development of chronic kidney disease (CKD), end-stage renal disease (ESKD), and death (6). The causes of AKI in children with NS are multifaceted. Possible etiologies encompass both direct and indirect factors, include intravascular volume deficiency, acute tubular necrosis, interstitial nephritis, nephrotoxic drug usage, and bilateral renal vein thrombosis et al. However, in clinical practice, only a few methods have been proven effective in preventing or reducing the occurrence of AKI (7–9). Therefore, having a deep understanding of the progression pattern of kidney disease after the onset of AKI is particularly important. The purpose of this study was to analyze the incidence and risk factors of AKI in children with NS, and provide an evidence-based medical basis for the early identification of high-risk children in clinical practice.
2 Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.
2.1 Literature search
Relevant studies were searched in databases including PubMed, Embase, Web of Science, Scopus, and the Cochrane Library, from database inception to May 2024. The literature search formula was performed using medical subject headings (MeSH), keywords and Boolean, as (“Children” OR “paediatric” OR “pediatric”) AND (“Acute Kidney Injury”) AND (“nephrotic syndrome”) without any language restrictions. Furthermore, we screened the references of relevant reviews to avoid omission.
The inclusion criteria for studies were as follows: (1) they were observational or cohort studies involving children with nephrotic syndrome; (2) they utilized clear definitions of AKI to classify children; and (3) they provided data on risk factors for AKI in NS patients. The predefined data included basic patient characteristics (age, sex), presence of concomitant diseases (hypertension, infection), NS disease characteristics (steroids-sensitivity classification, pathological classification), laboratory test results (such as serum albumin), and details of nephrotoxic drug usage. However, the time point of renal function tests was not fully stated in the included studies. Since there was a significant difference between the results of renal function tests at admission and when AKI occurred, the differences in renal function results were not analyzed in this study.
Studies were excluded based on the following criteria: (1) studies involving adult NS patients; (2) studies that lacked information on the diagnosis, classification, or characteristics of AKI patients; (3) studies that did not report the characteristics of the non-AKI group within the NS population; (4) studies that used Propensity Score Matching to select the control group (as it could result in inappropriate matching of key risk factors between groups); (5) studies that did not report the aforementioned risk factors of interest; (6) studies that were duplicates or had unextractable data. Furthermore, reviews, comments, and case reports were also excluded.
2.2 Literature screening and data extraction
Two authors rigorously screened all retrieved literature independently, adhering to the predefined inclusion and exclusion criteria. Initially, a preliminary screening was conducted by reviewing the titles and abstracts of all retrieved studies. Following the exclusion of duplicate studies and those not meeting the inclusion criteria, the remaining studies were identified through a comprehensive reading of the full text. The information such as the name of first author, country, year of publication, study design, sample size, AKI incidence, and reported risk factors was extracted from each eligible study. The outcome encompassed the original information on the risk factors between the AKI and no-AKI groups, as well as the unadjusted and multivariate-adjusted odds ratios (ORs) predicting the incidence of AKI. Any disputes that arose during the literature screening and data extraction process were resolved through discussion. In cases where consensus could not be reached, a third researcher (corresponding author) was consulted, and the final decision was determined by the corresponding author.
2.3 Design quality assessment
The risk of bias in the included studies was evaluated using the Newcastle-Ottawa Scale (NOS). This scale comprises three components: the representativeness of the exposed cohort, the comparability of the groups based on design or analysis, and the reported outcomes. Each study was assigned a total score out of 9 points, with ratings of 0–3, 4–6, and 7–9 indicating low-quality, moderate-quality, and high-quality studies, respectively. To ensure reliability, two independent reviewers conducted the quality assessments.
2.4 Statistical analysis
Meta-analysis was performed using R software (version 4.3.2). First, the incidence of AKI in each included study was pooled and analyzed. And then, the association between risk factors and the incidence of AKI was examined. For the pooled analysis, the original dichotomous and continuous outcome data were combined as odds ratios (OR) or standardized mean differences (SMD) with 95% confidence intervals (CI). Additionally, adjusted or unadjusted odds ratios (OR) values obtained from regression analysis were also utilized. The level of heterogeneity was assessed using the I2 statistic. If there was statistical heterogeneity (p < 0.05 or I2 ≥ 50%), a random effects model was employed for the pooled effect size analysis. Conversely, if there was no statistical heterogeneity (p ≥ 0.05 and I2 < 50%), a fixed effects model was used. Furthermore, we employed the formula proposed by Luo et al. (2018) to convert the median and interquartile range (IQR) of continuous data into mean and standard deviation (SD). To assess the influence of individual studies on the combined results, sensitivity analysis was conducted. Publication bias was examined through a visual funnel plot and evaluated using Begg's test, and Egger's test (for OR results) or Pustejovsky's test (for SMD results) (10). A significance level of p < 0.05 was considered indicative of a statistically significant difference. While network meta-analysis is primarily designed for indirect comparisons among high-quality studies, in this study, we also attempted to compare multiple categories in single risk factor type using the frequentist network meta-analysis. The risk of AKI in different risk factor arms was ranked using Surface Under the Cumulative Ranking curve (SCURA).
3 Results
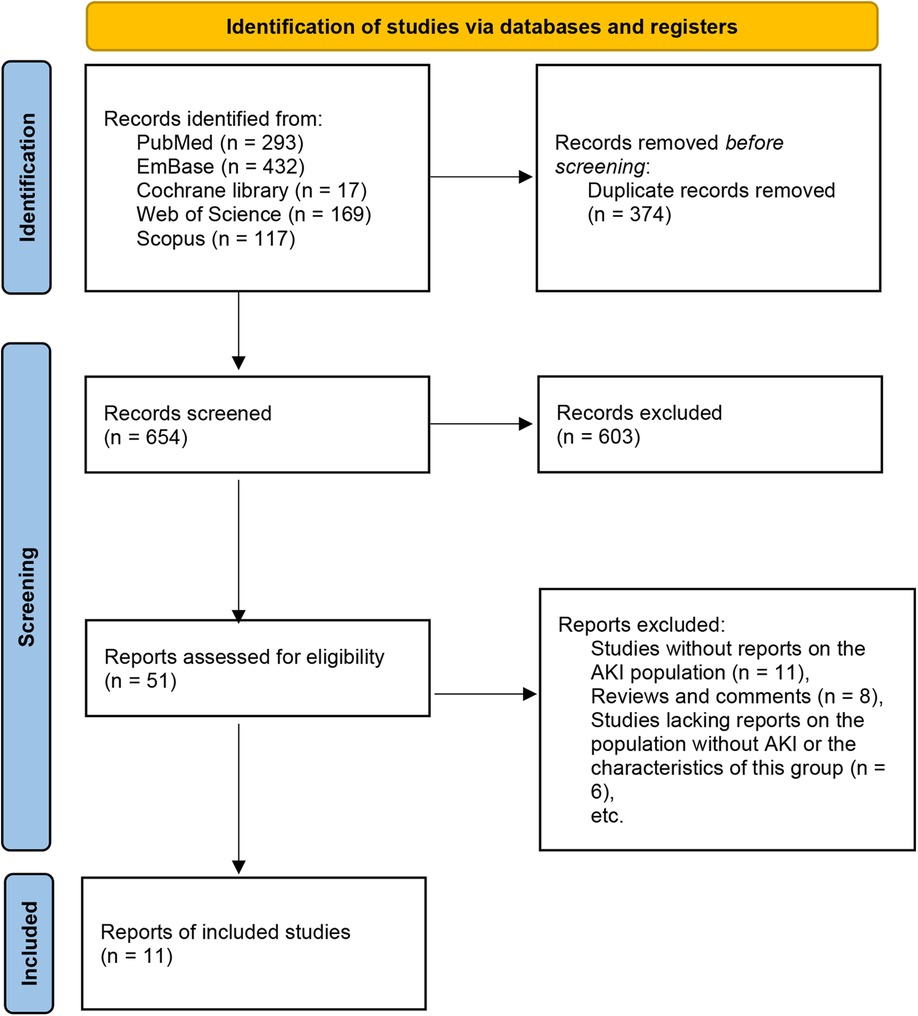
A total of 1,028 articles were initially retrieved. After removing duplicates, 654 articles remained. Following the screening of titles and abstracts, 603 irrelevant studies were excluded. Subsequently, 51 studies underwent full-text review. The following articles were excluded due to: studies without reports on the AKI population (n = 11), reviews and comments (n = 8), studies lacking reports on the population without AKI or the characteristics of this group (n = 6), studies involving adult patients (n = 5), studies that included other types of patients without separate report on NS patients (n = 5), and case reports (n = 5). Ultimately, 11 studies were included for analysis (6, 11–20) (Figure 1, Table 1).
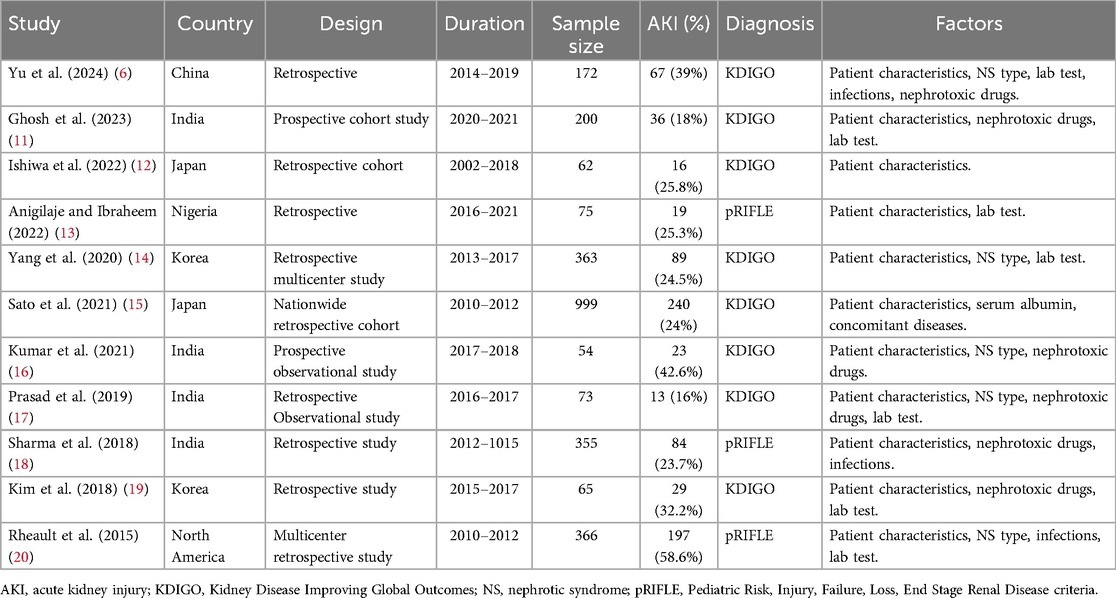
The included studies spanned from 2015 to 2024 and primarily focused on research conducted in Asia, specifically China, Japan, South Korea, and India. Additionally, two studies were conducted in Nigeria and North America (13, 20). Out of the total studies, two were prospective, while the remaining were retrospective. One study had an unclear type but leaned towards being retrospective based on its research content (17). The duration of the studies ranged mostly from 1 to 5 years. In total, 2,784 children with NS were included, with 813 of them having AKI. Only 8 children with secondary NS were included from the study by Anigilaje and Ibraheem (2022) (13), representing 0.3% of the sample, with the remaining 2,776 children having idiopathic NS. It is worth noting that some studies did not provide the exact number of patients with AKI but instead reported the frequency of AKI during hospitalizations. The quality of the included studies may have been influenced by certain factors, such as an unclear follow-up duration and the use of questionnaires instead of clinical records to gather information on AKI patients. However, the overall design quality of the studies was high (Supplementary Table 1).
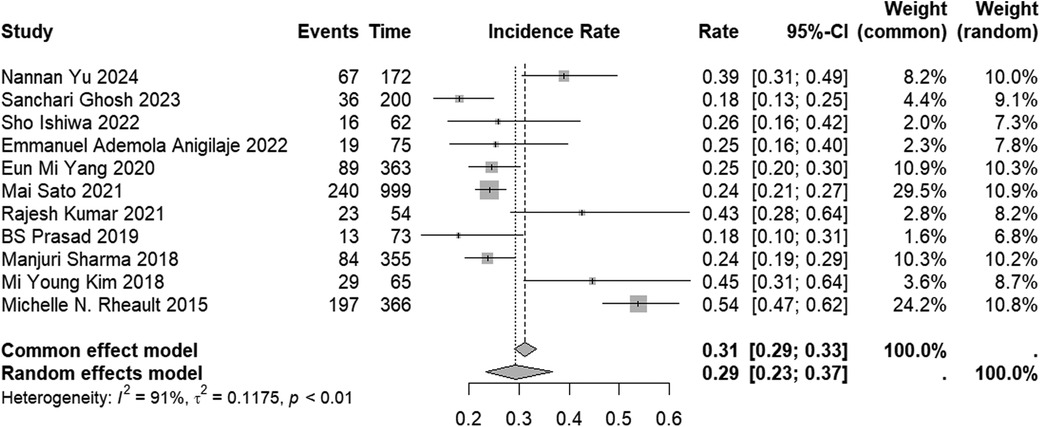
The incidence of AKI in the included studies varied from 18% to 54%. When the results were combined, the overall incidence of AKI was found to be 29% (95% CI: 23%–37%) (Figure 2). However, studies that solely reported the incidence without providing information on the characteristics of risk factors for AKI occurrence were excluded. Therefore, it is important to interpret this result with caution. The subgroup analyses of AKI incidence across all subtype populations are presented in Supplementary Table 2.
For age, the analysis involved two aspects: the patient's admission age and the patient's age at the onset of NS. In the combined analysis of children's admission results using a random effects model, no significant difference in admission age was found between the AKI and non-AKI groups (SMD: 0.44; 95% CI: −0.04, 0.91; p = 0.071) (Figure 3A). Sensitivity analysis indicated that the findings reported by RajeshKumar 2021 significantly influenced the statistical difference in the combined results (Supplementary Figure 1A). Furthermore, the analysis of publication bias revealed no evidence of bias (Begg's test, p = 0.805; Pustejovsky's test, p = 0.997) (Supplementary Figure 1B).
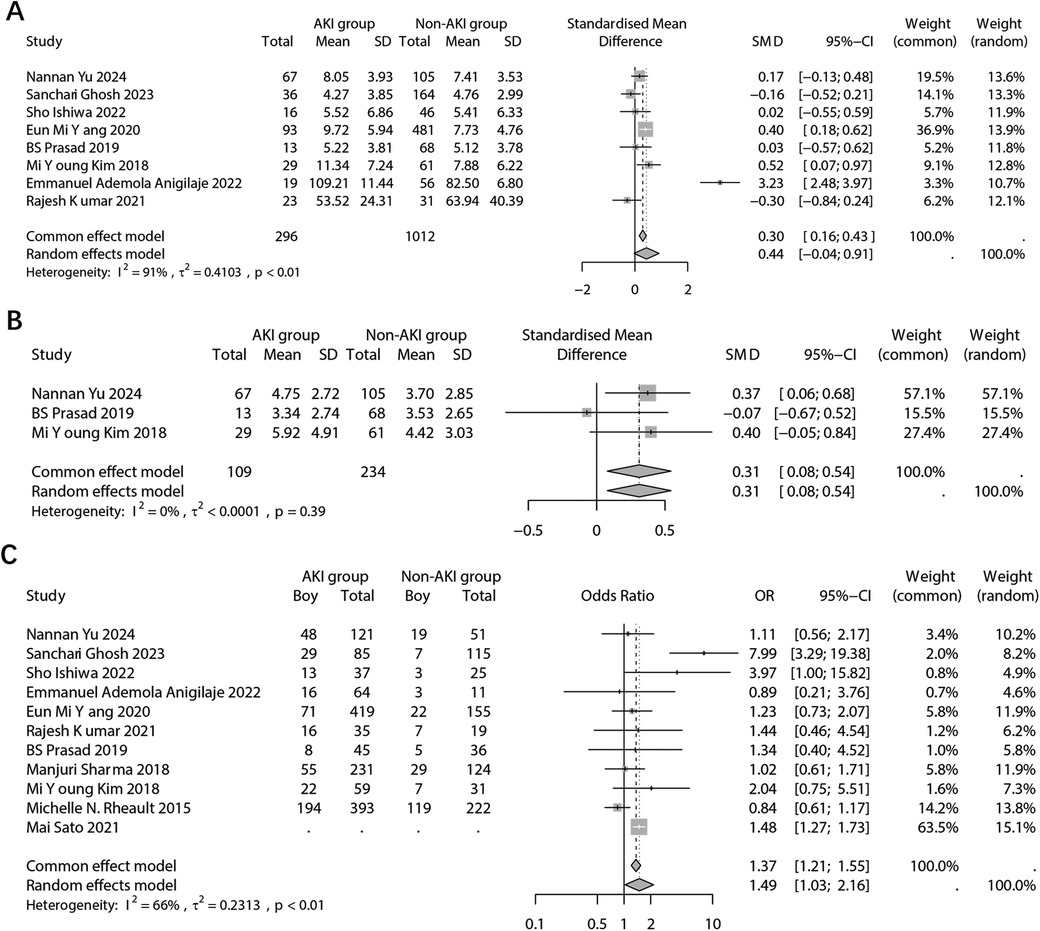
Figure 3. Forest plots showing the pooled results of age in admission (A), age of NS onset (B), and sex (C) between the AKI group and the non-AKI group.
A pooled analysis was also conducted on the age of onset of NS. The results, indicated a significant difference in the age of onset of NS between the two groups based on fixed effects model (SMD: 0.31; 95% CI: 0.08, 0.54; p = 0.009) (Figure 3B). The age of onset of NS in children with AKI was found to be significantly older. However, due to the limited number of included studies, sensitivity and publication bias analyses were not performed.
For children's sex. the results, using a random effects model, revealed a significant difference in sex distribution between the two groups (OR: 1.49; 95% CI: 1.03, 2.16; p = 0.035) (Figure 3C). Specifically, the proportion of boy was significantly higher in the group with AKI. It is important to note that the Mai 2021 study did not report the original frequency data but the OR value. Sensitivity analysis indicated that multiple studies may have influenced the final pooled results (Supplementary Figure 2A). Furthermore, the analysis of publication bias showed no evidence of potential bias (Begg's p = 0.484; Egger's p = 0.664) (Supplementary Figure 2B).
In the laboratory test results, the analysis focused on the difference in serum albumin levels between the two groups. The combined results indicated a significantly lower serum albumin level in the population with AKI (SMD: −0.43; 95% CI: −0.85, −0.02; p = 0.041) (Figure 4A). Sensitivity analysis revealed that multiple studies had an impact on the pooled results (Supplementary Figure 3A). Furthermore, no potential publication bias was detected (Begg's p = 1.00; Pustejovsky's p = 0.984) (Supplementary Figure 3B).
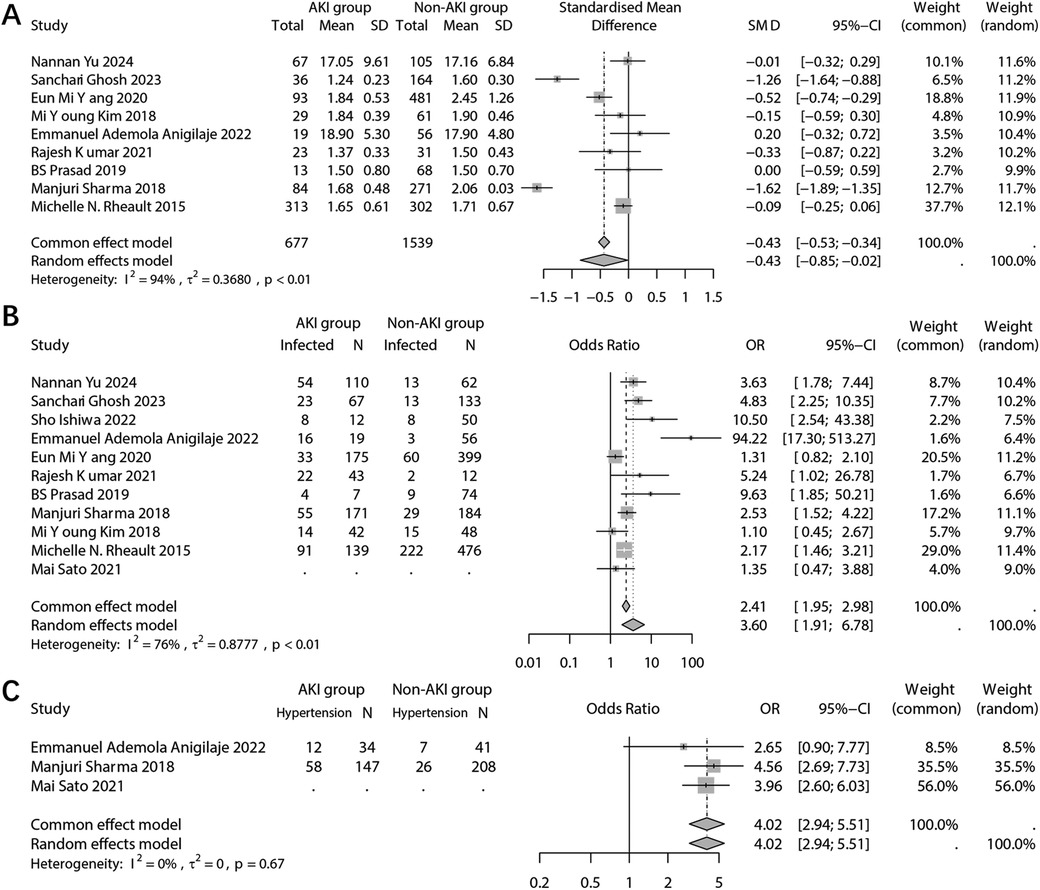
Figure 4. Forest plots showing the pooled results of serum albumin level (A), infection (B), and hypertension (C) between the AKI group and the non-AKI group.
The analysis examined the relationship between infection and the occurrence of AKI. It was found that infected patients had a significantly higher incidence of AKI (OR: 3.60; 95% CI: 1.91, 6.78; p < 0.001) (Figure 4B). The sensitivity analysis confirmed the robustness of the pooled results (Supplementary Figure 4A). However, there was a possibility of publication bias (Begg's test p = 0.036; Egger's test p = 0.041) (Supplementary Figure 4B). And trim and fill method was employed to add potentially missing studies. After adjustments, the random effects model failed to detect a significant association between infection and AKI (OR: 2.12; 95% CI: 0.97, 4.64; p = 0.061).
The combined analysis revealed a significant association between comorbid hypertension and the incidence of incidence of AKI (OR: 4.02; 95% CI: 2.94, 5.51; p < 0.001) (Figure 4C). However, due to the limited number of studies included in the analysis, sensitivity analysis and publication bias analysis were not conducted.
In the analysis of NS characteristics, the risk of AKI was examined based on pathological findings and steroid sensitivity types. The pathological results included focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), minor glomerular abnormalities (MGA)/diffuse mesangial proliferation (DMP), C1q nephropathy (C1q), mesangial proliferative glomerulonephritis (MesPGN)/Others, and cases where pathological examination was not conducted (Figure 5A). Using the not done population as a reference, there was no significant difference in the risk of AKI among the other pathological types, except for the FSGS group. According to the SUCRA ranking, FSGS may be considered the pathological type with the highest risk of AKI (SUCRA = 0.07), while the pathological type MGA/DMP exhibited a relatively lower risk of AKI (SUCRA = 0.87) (Figure 5B).
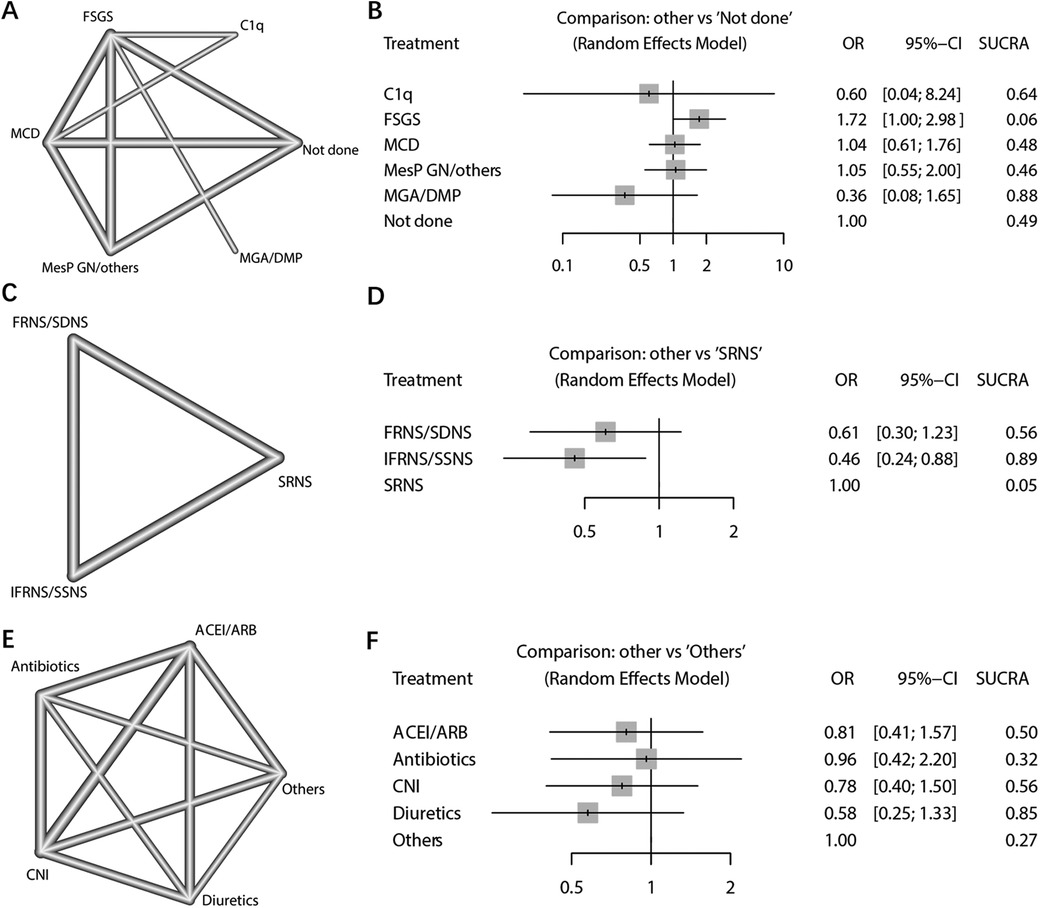
Figure 5. Network meta-analysis of NS pathological types, steroid-response NS types, and nephrotoxic drug types. (A) Network plot of pathological results; (B) Network Forest plot of pathological results; (C) Network plot of steroid response type results; (D) Network Forest plot of steroid response type results; (E) Network plot of types of nephrotoxic drugs; (F) Network Forest plot of types of nephrotoxic drugs.
The analysis focused on the type of NS in response to steroid treatment. The dichotomous analysis revealed a higher incidence of AKI in individuals who exhibited resistance to steroid treatment (OR: 0.52; 95% CI: 0.33, 0.80; p = 0.003) (Figure 6A). Sensitivity analyses indicated that the study conducted by Michelle in 2015 had a significant impact on the final combined results (Supplementary Figure 5A). No evidence of potential publication bias was found (Begg's test p = 0.458; Egger's test p = 0.436) (Supplementary Figure 5B). Furthermore, the NS types were further subdivided into frequent relapses NS (FRNS)/steroid-dependent NS (SDNS), infrequent relapses (IFRNS)/steroid-sensitive NS (SSNS), and steroid-resistant NS (SRNS) (Figure 5C). When set SRNS as reference, the IFRNS/SSNS type was found to have a significantly lower incidence of AKI (OR: 0.46; 95% CI: 0.24, 0.88) and a higher SUCRA rank (0.89) (Figure 5D).
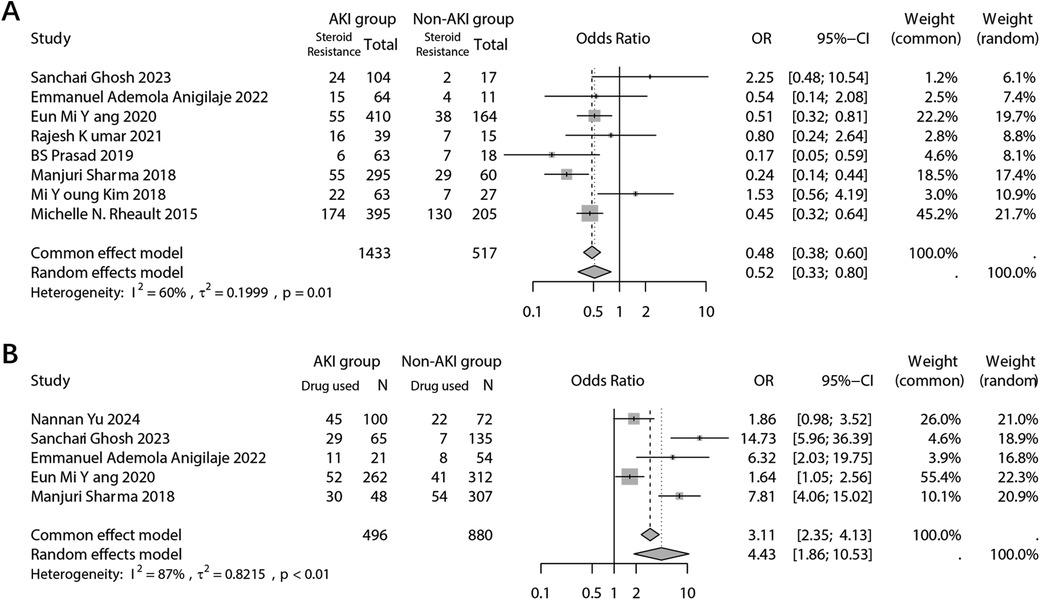
Figure 6. Forest plots showing the pooled results of steroid-resistance NS type (A) and use of nephrotoxic drugs (B) between the AKI group and the non-AKI group.
The combined analysis demonstrated a significant association between the use of nephrotoxic drugs and the incidence of AKI (OR: 4.43; 95% CI: 1.86, 10.53; p = 0.001) (Figure 6B). The sensitivity analysis confirmed the robustness of the combined outcome (Supplementary Figure 6A). Despite the inclusion of only five studies, a publication bias analysis was conducted. The results indicated no potential publication bias (Begg's test p = 0.142; Egger's test p = 0.149) (Supplementary Figure 6B). A network analysis was performed to examine different types of nephrotoxic drugs, including Angiotensin-Converting Enzyme Inhibitors (ACEI)/Angiotensin Receptor Blockers (ARB), antibiotics, calcineurin inhibitors (CNI), diuretics, and others (e.g., contrast agents, hypovolemia-inducing agents, ionotropes, etc.) (Figure 5E). When using the “others” arm as a reference, no other type of drug showed a significantly lower risk of AKI occurrence. Among the nephrotoxic drugs, diuretics exhibited a relatively high SUCRA score of 0.85, suggesting a relate low risk of AKI compared to other types of nephrotoxic drugs (Figure 5F).
4 Discussion
This study evaluated the incidence and risk factors for developing AKI in children with NS through a meta-analysis, firstly. The findings revealed that the overall risk of developing AKI in children with NS was 29%. The analysis of risk factors indicated that the age of NS onset, rather than the age of admission, sex, serum albumin level, response to steroid treatment, infection, hypertension, and nephrotoxic drug application, were all significantly associated with the incidence of AKI. Furthermore, the results of the network meta-analysis suggested that the pathologic type of MGA/DMP, the type of IFRNS/SSNS, and the use of diuretic medications were associated with a relatively low risk of AKI occurrence.
In the results of this study, the proportion of AKI following NS was higher in boys than in girls, and this proportion was statistically significant. Therefore, this conclusion is primarily drawn from the statistical findings of the population analysis. In terms of mechanism, sexual dimorphism exists in the gene expression of proximal tubules and endothelial cells in mice with ischemic AKI. This suggests that different sexes exhibit sex-specific gene expression patterns in response to ischemia. For instance, the upregulation of injury-associated genes lipocalin-2 (Lcn2), hepatitis A virus cellular receptor 1 (Havcr1), and keratin 18 (Krt18) is less pronounced in female proximal tubules compared to males. Conversely, adhesion molecules and cytokines/chemokines are upregulated in males but not in females (21). Additionally, variations in sex hormone production alter the susceptibility of different sexes to ischemic kidney injury (22). Estrogen may exert a protective effect in ischemic AKI, and this protective mechanism may involve gene targets such as estrogen sulfotransferase (SULT1E1/EST) and Sirtuin-3 (23, 24).
Children with NS often experience infections, which are a significant contributing factor to the development of AKI. Abnormalities in the immune system, characterized by a significant loss of immunoglobulins and complement regulators in the urine, increase the susceptibility to infections in NS patients. Furthermore, the use of immunosuppressive therapy further elevates the risk of infection. Following an infection, viruses and bacteria directly damage the renal tubular epithelial cells (25, 26). Additionally, the heightened systemic inflammation resulting from the infection, as indicated by elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), has been found to correlate with increased levels of creatinine and urea nitrogen, thus being associated with the development of AKI (27). Inflammatory related factors such as soluble intercellular adhesion molecule-1 (ICAM-1), thrombomodulin, von Willebrand factor (vWF), and selectins have also been associated with the risk of AKI (28). Clinical studies have demonstrated that AKI is a common complication of sepsis, further highlighting the significant role of dysregulated inflammatory and immune responses in the development of AKI (29). The combined results of this study strongly suggest a substantial correlation between infection and the development of AKI in children with NS. However, it is important to note that the methodological analysis indicates the possibility of publication bias influencing the combined results.
Children with NS are more likely to be exposed to nephrotoxic drugs, and the risk of AKI increases with each additional nephrotoxic drug. The duration and intensity of nephrotoxic drug exposure are associated with the occurrence of AKI. Renin-angiotensin-aldosterone system (RAAS) inhibitors, for example, can cause vasodilation of small arteries and decrease intraglomerular pressure, leading to ischemia, increased cell membrane permeability, tubular injury, and deterioration of renal function (30). And then, the use of diuretics combined with hypovolemia due to urinary protein loss can further worsen renal tissue damage, leading to the development of thrombosis and AKI (31). The findings of this meta-analysis indicate a significant association between the use of nephrotoxic drugs and the occurrence of AKI. However, among the various types of nephrotoxic drugs, diuretics are relatively less likely to contribute to the occurrence of AKI compared to other nephrotoxic drugs. Nevertheless, caution should still be exercised when using diuretics, and they should only be administered after correcting the most severe forms of edema and hypovolemia (32). In conclusion, the administration of nephrotoxic drugs should be individualized based on the patient's glomerular filtration rate, with close monitoring of adverse drug reactions and regular assessment of renal function to minimize the risk of AKI.
Non-response to steroid therapy in children with NS is often indicative of a poor prognosis (33). In such cases, second-line immunosuppressive agents, primarily calcineurin inhibitors, are frequently employed. However, these drugs are considered nephrotoxic, which may contribute to the increased incidence of AKI. The progression of SRNS to multidrug-resistant NS can also lead to renal failure. Among cases of multidrug-resistant NS, FSGS is a commonly observed pathology type (34). Interestingly, the results of this study indicate that FSGS has the lowest SUCRA value among the pathology classifications, suggesting a relatively high risk of AKI development in these cases. The results of this study revealed a significant association between concomitant hypertension and an increased incidence of AKI.
A considerable proportion of children with NS exhibit hypertension (35, 36). Hypothyroidism, which can be observed in patients with NS, can contribute to hypertension due to the excessive excretion of thyroid hormones and binding globulins through the urine (37). Furthermore, hyperlipidemia, which can also lead to renal vein embolism, may be associated with the development of hypertension and AKI in NS patients (38). Moreover, medications such as prednisone, methylprednisolone, RAAS inhibitors, and CNIs have been implicated in raising blood pressure levels and increase the risk of developing hypertension (39). Taken together, these factors suggest a potential link between the use of immunosuppressive drugs, the severity of proteinuria, hypertension resulting from renal vein embolism, and the development of AKI. Further research is warranted to fully elucidate the underlying mechanisms and explore potential interventions to mitigate these risks.
The focus of this study was primarily on analyzing the association between relevant factors and the occurrence of AKI, rather than constructing risk prediction models. Specifically, the study aimed to identify differences between the AKI and non-AKI groups. Among the comparative results, p-values below 0.001 were observed for infection, use of nephrotoxic drugs, and hypertension. These factors can be given attention in the process of constructing a risk prediction model. On the other hand, risk factors such as age of NS onset, sex, and albumin level had p-values greater than 0.01. This suggests that their importance in the prediction model may be relatively lower.
This study has several research limitations that should be acknowledged. Firstly, there was a high degree of heterogeneity among the results of the included studies. This heterogeneity may have arisen from differences in the criteria used to assess AKI. Secondly, the design characteristics of the included studies were primarily prospective or retrospective cohort studies. These types of studies generally have a lower level of evidence compared to randomized controlled trials (RCTs). This is particularly relevant when examining the association between nephrotoxic drugs and the occurrence of AKI, as RCTs provide a higher level of evidence in establishing causal relationships. Furthermore, this study only explored the correlation between risk factors and the occurrence of AKI. Although, the selected risk factors were non-interventional, and there was a time lag between the recording of the risk factors and the occurrence of AKI (such as admission recording and AKI occurrence during hospitalization), this study is unable to draw definitive conclusions about the causal relationship between these risk factors and AKI. In fact, there may be intricate and intrinsic links between different risk factors, such as serum albumin level, infection, and hypertension, which could contribute to the development of AKI. Lastly, most of the studies included in this analysis were based on Asian populations and had relatively small sample sizes. Therefore, further well-designed studies with larger and more diverse populations are needed to validate and refine the results of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CC: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. BQ: Conceptualization, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. JW: Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. LY: Data curation, Software, Writing – original draft, Writing – review & editing. YH: Formal Analysis, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1452568/full#supplementary-material
References
1. McKay AM, Parekh RS, Noone D. Therapeutic trials in difficult to treat steroid sensitive nephrotic syndrome: challenges and future directions. Pediatr Nephrol. (2023) 38(1):17–34. doi: 10.1007/s00467-022-05520-6
2. Hilmanto D, Mawardi F, Lestari AS, Widiasta A. Disease-associated systemic complications in childhood nephrotic syndrome: a systematic review. Int J Nephrol Renovasc Dis. (2022) 15:53–62. doi: 10.2147/IJNRD.S351053
3. Vivarelli M, Gibson K, Sinha A, Boyer O. Childhood nephrotic syndrome. Lancet. (2023) 402(10404):809–24. doi: 10.1016/S0140-6736(23)01051-6
4. Menon S. Acute kidney injury in nephrotic syndrome. Front Pediatr. (2018) 6:428. doi: 10.3389/fped.2018.00428
5. Ping Z. Research progress of nephrotic syndrome in children with acute kidney injury. Chin J Difficult Complicat Case. (2023) 22(7):762–6; 771. doi: 10.3969/j.issn.1671-6450.2023.07.019
6. Yu N, Ouyang X, Li J, Gao J, Zeng S, Zhuang H, et al. Risk factors and renal outcomes of AKI in children with secondary steroid-resistant nephrotic syndrome. Ren Fail. (2024) 46(1):2314637. doi: 10.1080/0886022X.2024.2314637
7. Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. (2016) 27(3):687–97. doi: 10.1681/ASN.2015030309
8. Yang Y, Song M, Liu Y, Liu H, Sun L, Peng Y, et al. Renoprotective approaches and strategies in acute kidney injury. Pharmacol Ther. (2016) 163:58–73. doi: 10.1016/j.pharmthera.2016.03.015
9. Deng YH, Yan P, Zhang NY, Luo XQ, Wang XF, Duan SB. Acute kidney disease in hospitalized pediatric patients with acute kidney injury in China. Front Pediatr. (2022) 10:885055. doi: 10.3389/fped.2022.885055
10. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27(6):1785–805. doi: 10.1177/0962280216669183
11. Ghosh S, Akhtar S, Pradhan SK, Sarkar S, Dasgupta D, Parween R, et al. Incidence and risk factors of acute kidney injury among childhood nephrotic syndrome: a prospective cohort study. Eur J Pediatr. (2023) 182(5):2443–51. doi: 10.1007/s00431-023-04903-7
12. Ishiwa S, Sato M, Kamei K, Nishi K, Kanamori T, Okutsu M, et al. Risks and renal outcomes of severe acute kidney injury in children with steroid-resistant nephrotic syndrome. Clin Exp Nephrol. (2022) 26(7):700–8. doi: 10.1007/s10157-022-02198-w
13. Anigilaje EA, Ibraheem I. Acute kidney injury in children with nephrotic syndrome at the university of Abuja teaching hospital, Abuja, Nigeria: 2016 to 2021. AIMS Med Sci. (2022) 9(1):18–31. doi: 10.3934/medsci.2022004
14. Yang EM, Yoo KH, Ahn YH, Kim SH, Lee JW, Chung WY, et al. Lower albumin level and longer disease duration are risk factors of acute kidney injury in hospitalized children with nephrotic syndrome. Pediatr Nephrol. (2021) 36(3):701–9. doi: 10.1007/s00467-020-04740-y
15. Sato M, Ishikura K, Ando T, Kikunaga K, Terano C, Hamada R, et al. Japanese pediatric survey holding information of nephrotic syndrome study of the Japanese study group of renal disease in C. Prognosis and acute complications at the first onset of idiopathic nephrotic syndrome in children: a nationwide survey in Japan (JP-SHINE study). Nephrol Dial Transplant. (2021) 36(3):475–81. doi: 10.1093/ndt/gfz185
16. Kumar R, Agrwal S, Mantan M, Yadav S. Acute kidney injury in children hospitalized with a relapse of nephrotic syndrome: a short-term outcome study. Saudi J Kidney Dis Transpl. (2021) 32(2):437–44. doi: 10.4103/1319-2442.335456
17. Prasad BS, Kumar M, Dabas A, Mishra K. Profile of acute kidney injury in hospitalized children with idiopathic nephrotic syndrome. Indian Pediatr. (2019) 56(2):119–22. doi: 10.1007/s13312-019-1483-9
18. Sharma M, Mahanta A, Barman AK, Mahanta PJ. Acute kidney injury in children with nephrotic syndrome: a single-center study. Clin Kidney J. (2018) 11(5):655–8. doi: 10.1093/ckj/sfy024
19. Kim MY, Cho MH, Kim JH, Ahn YH, Choi HJ, Ha IS, et al. Acute kidney injury in childhood-onset nephrotic syndrome: incidence and risk factors in hospitalized patients. Kidney Res Clin Pract. (2018) 37(4):347–55. doi: 10.23876/j.krcp.18.0098
20. Rheault MN, Zhang L, Selewski DT, Kallash M, Tran CL, Seamon M, et al. Midwest pediatric nephrology C. AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol. (2015) 10(12):2110–8. doi: 10.2215/CJN.06620615
21. Vinas JL, Porter CJ, Douvris A, Spence M, Gutsol A, Zimpelmann JA, et al. Sex diversity in proximal tubule and endothelial gene expression in mice with ischemic acute kidney injury. Clin Sci. (2020) 134(14):1887–909. doi: 10.1042/CS20200168
22. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. (2013) 24(1):37–42. doi: 10.1681/ASN.2012080800
23. Silva Barbosa AC, Zhou D, Xie Y, Choi YJ, Tung HC, Chen X, et al. Inhibition of estrogen sulfotransferase (SULT1E1/EST) ameliorates ischemic acute kidney injury in mice. J Am Soc Nephrol. (2020) 31(7):1496–508. doi: 10.1681/ASN.2019080767
24. Shen H, Holliday M, Sheikh-Hamad D, Li Q, Tong Q, Hamad CD, et al. Sirtuin-3 mediates sex differences in kidney ischemia-reperfusion injury. Transl Res. (2021) 235:15–31. doi: 10.1016/j.trsl.2021.03.015
25. Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, et al. COVID-19 and kidney injury: pathophysiology and molecular mechanisms. Rev Med Virol. (2021) 31(3):e2176. doi: 10.1002/rmv.2176
26. Huang H, Ding R, Chen Z, Yi Z, Wang H, Lv Y, et al. Goose nephritic astrovirus infection increases autophagy, destroys intercellular junctions in renal tubular epithelial cells, and damages podocytes in the kidneys of infected goslings. Vet Microbiol. (2021) 263:109244. doi: 10.1016/j.vetmic.2021.109244
27. Wang J, Yang X, Li Y, Huang JA, Jiang J, Su N. Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction. Virol J. (2021) 18(1):117. doi: 10.1186/s12985-021-01588-y
28. Reilly JP, Anderson BJ, Mangalmurti NS, Nguyen TD, Holena DN, Wu Q, et al. The ABO histo-blood group and AKI in critically ill patients with trauma or sepsis. Clin J Am Soc Nephrol. (2015) 10(11):1911–20. doi: 10.2215/CJN.12201214
29. Odum JD, Wong HR, Stanski NL. A precision medicine approach to biomarker utilization in pediatric sepsis-associated acute kidney injury. Front Pediatr. (2021) 9:632248. doi: 10.3389/fped.2021.632248
30. Garcia-de Los Rios C, Roa-Chamorro R, Torres-Quintero L, Gonzalez-Bustos P. Hypertension secondary to abdominal mass. Hipertens Riesgo Vasc. (2022) 39(1):46–8. doi: 10.1016/j.hipert.2021.07.002
31. Ostermann M, Liu K, Kashani K. Fluid management in acute kidney injury. Chest. (2019) 156(3):594–603. doi: 10.1016/j.chest.2019.04.004
32. Boyer O, Baudouin V, Berard E, Dossier C, Audard V, Guigonis V, et al. Idiopathic nephrotic syndrome. Arch Pediatr. (2017) 24(12):1338–43. doi: 10.1016/j.arcped.2017.09.022; Aspects cliniques du syndrome nephrotique idiopathique de l'enfant.29169714
33. Kamei K, Ishikura K, Sako M, Ito S, Nozu K, Iijima K. Rituximab therapy for refractory steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. (2020) 35(1):17–24. doi: 10.1007/s00467-018-4166-1
34. Ying D, Liu W, Chen L, Rong L, Lin Z, Wen S, et al. Long-term outcome of secondary steroid-resistant nephrotic syndrome in Chinese children. Kidney Int Rep. (2021) 6(8):2144–50. doi: 10.1016/j.ekir.2021.05.001
35. Politano SA, Colbert GB, Hamiduzzaman N. Nephrotic syndrome. Prim Care. (2020) 47(4):597–613. doi: 10.1016/j.pop.2020.08.002
36. Jaber BA, Azat NFA, Al-Daffaie AA. Complications of nephrotic syndrome in children. Wiad Lek. (2022) 75:2226–32. doi: 10.36740/WLek202209209
37. Fukata S, Ito M, Nishikawa M, Kasahara T, Nishihara E, Akamiuzu T, et al. Hypothyroidism due to nephrotic syndrome: a notable clinical entity. Endocr J. (2022) 69(3):307–11. doi: 10.1507/endocrj.EJ21-0387
38. Barbano B, Gigante A, Amoroso A, Cianci R. Thrombosis in nephrotic syndrome. Semin Thromb Hemost. (2013) 39(5):469–76. doi: 10.1055/s-0033-1343887
Keywords: nephrotic syndrome, acute kidney injury, incidence, risk factors, meta-analysis
Citation: Chen C, Qiu B, Wang J, Yang L and Huang Y (2024) Incidence and risk factors for acute kidney injury in children with nephrotic syndrome: a meta-analysis. Front. Pediatr. 12:1452568. doi: 10.3389/fped.2024.1452568
Received: 21 June 2024; Accepted: 11 November 2024;
Published: 20 December 2024.
Edited by:
Ana Cristina Simões E. Silva, Federal University of Minas Gerais, BrazilReviewed by:
O. P. Mishra, Heritage Institute of Medical Sciences, IndiaDaniel Turudic, University Hospital Center Zagreb, Croatia
Copyright: © 2024 Chen, Qiu, Wang, Yang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingbing Qiu, cWl1YmluZ2JpbmcxMzZAYWxpeXVuLmNvbQ==
 Changdi Chen
Changdi Chen Bingbing Qiu
Bingbing Qiu