- Department of General & Neonatal Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Structural Birth Defect and Reconstruction, Chongqing, China
Purpose: To investigate the clinical features of necrotizing enterocolitis-associated intestinal perforation (NEC-IP) in neonates with different gestational ages (GAs). Furthermore, we also want to identify the risk factors of poor prognosis for these patients.
Methods: The retrospective study of patients with NEC-IP was conducted with basic information, comorbidity, intraoperative findings, related treatment, and prognosis. According to the GA, patients were divided into three groups: early (GA: 28–<32 weeks, Group 1), mid-term (GA: 32–<34 weeks, Group 2), and late (GA: 34–<37 weeks, Group 3). The clinical features of the three groups were analyzed, and risk factors for poor prognosis were identified.
Results: Of the 113 cases, the number of cases in Groups 1 to 3 was 36 (31.9%), 44 (38.9%), and 33 (29.2%), respectively; and the overall proportion of poor prognosis was 19.4% (22/113). For basic information, the birth weight of Group 1 was lower than that of Group 2 and Group 3, while the postnatal day at the time of surgery of NEC and the onset age were higher than that of Group 2 (onset age: G1 12.0[7.00;20.5], G2 9.00[4.00;13.0]; postnatal day at the time of surgery: G1 22.0[13.8;27.2], G2 13.0[8.00;21.0]) (P < 0.016). For comorbidity, the incidence of sepsis, coagulopathy, type of (congenital heart disease) CHD, and hypoproteinemia in Group 1 was higher than that in Group 2 (all P < 0.016), and the incidence of respiratory failure, hypoproteinemia in Group 1 was higher than that in Group 3 (all P < 0.016). For related treatment, the usage rate of vasoactive substances and mechanical ventilation in Group 1 was higher than that of Group 2 and Group 3 (all P < 0.016). By Lasso and Logistic regression analysis, we found that GA (OR: 0.274, 95%CI: 0.078–0.796), sepsis (OR: 7.955, 95%CI: 1.424–65.21), coagulopathy (OR: 19.51, 95%CI: 3.393–179.1), CHD (OR: 6.99, 95%CI: 1.418–54.83) and diseased bowel segment (OR: 2.804, 95%CI: 1.301–7.316) were the independent factors for poor prognosis (all P < 0.05).
Conclusions: The clinical features of NEC-IP patients differ based on GA, particularly in terms of CHD type, postnatal day at the time of surgery, utilization of vasoactive substances, and prognosis. Furthermore, GA, sepsis, coagulopathy, CHD, and diseased bowel segment are independent factors for poor prognosis of patients with NEC-IP.
1 Introduction
Necrotizing enterocolitis (NEC) was one of the most common critical neonatal gastrointestinal emergencies with significant morbidity and mortality (1–3). Approximately 90% of affected patients were preterm infants (4). The study had found that the incidence of NEC in neonates with gestational age (GA) of less than 33 weeks was about 5.1% (1.3%–12.9%), and it increased with the decrease of GA (5). In addition, the mortality rate associated with NEC ranges from 15% to 30% (6), with a significantly higher risk in patients who developed necrotizing enterocolitis-associated intestinal perforation (NEC-IP) (1, 7).
Sharma et al. reported that there were significant differences in the incidence of NEC in early (GA: 28–<32weeks), middle (GA: 32–<34weeks), and late preterm patients (GA: 34–<37weeks) (8). GA was an influencing factor for the prognosis of NEC, that was, low GA was prone to poor prognosis (9). However, the effect of GA on clinical features in patients with NEC-IP and prognosis, remains unclear. Patients with NEC-IP are not uncommon clinically, and the mortality rate is high, especially among preterm patients, making it is of great clinical value to explore the related risk factors. Currently, there are many clinical studies investigating the prognostic factors of NEC, but there is a lack of research to judge the postoperative prognosis of NEC-IP. It is worth noting that the incidence of poor prognosis significantly increased when intestinal perforation (IP) was present in NEC (10). Therefore, more attention should be paid to these NEC-IP patients to improve the overall prognosis.
Therefore, this study aims to investigate the clinical features of NEC-IP in patients with different GAs and identify the risk factors for poor prognosis, providing the theoretical basis for improving the prognosis.
2 Methods
2.1 Study population
A retrospective review was performed on the clinical data of the patients with NEC-IP admitted to our department from January 2014 to March 2019. It was performed after the study protocol was approved by the Institutional Research Ethics Board of Children's Hospital affiliated Chongqing Medical University (Date: 09.28.2021/No: 329). The center operates a Level IV NICU, which provides advanced neonatal care, including specialized treatment for critically ill and premature infants. The inclusion criteria were: (1) patients with GA < 37 weeks; (2) patients diagnosed with NEC, with intraoperative proof of IP; (3) patients with complete clinical data and postoperative follow-up data. The exclusion criteria were as follows: (1) patients with congenital genetic metabolic-related diseases and chromosome diseases; (2) patients with congenital gastrointestinal malformations, such as Hirschsprung's disease, intestinal malrotation, and intestinal atresia; (3) these confirmed as “spontaneous isolated intestinal perforation” during the operation; (4) incomplete relevant variables. Patients were divided into three groups based on the range of GA: early (GA: 28−<32 weeks, Group 1), mid-term (GA: 32−<34 weeks, Group 2) and late (GA: 34−<37 weeks, Group 3).
2.2 Study design
Clinical data of the included patients were retrospectively collected in this study, including (1) basic information: gender, GA, birth weight, age of onset/surgery of NEC, and perinatal data; (2) preoperative comorbidities: hypoproteinemia, respiratory failure, sepsis, shock, liver/renal injury, coagulopathy, congenital heart disease (CHD), hyponatremia; (3) intraoperative findings: location of perforation (recorded as ≥2 segments if perforation site involves two or more of the colon/ileum/ileocecal at the same time); (4) treatment and prognosis. Poor prognosis was defined as NEC-related death during hospitalization or within the 3-month follow-up period after discharge (11).
2.3 Definition
NEC-IP Patients meeting the diagnosis of both NEC and IP were defined as NEC-IP. NEC was diagnosed according to (1) the pathognomonic findings on abdominal radiography, including pneumatosis intestinalis, portal venous gas, fixed dilated loops of bowel, and extraluminal air outside the bowel; and (2) the presence of one or more clinical findings, including intolerance to feeding, abdominal distension, abdominal tenderness, abdominal wall erythema/discoloration or abdominal mass, and bloody stools (12). IP was defined at least one of the following: (1) abdominal radiographs suggesting subphrenic free gas, encapsulated or circumscribed pneumoperitoneum; (2) abdominal ultrasonography indicating abdominal fluid accumulation, disappearance of peristalsis and intestinal necrosis; (3) abdominal puncture confirming the presence of gastrointestinal contents in the abdominal cavity; or (4) perforation found during surgery (4).
CHDs were divided into two groups based on the result of echocardiography: cyanotic CHD (right-to-left intracardiac or extracardiac shunts resulting in hypoxemia, erythrocytosis, and cyanosis), and non-cyanotic CHD (the rest malformations) (13, 14).
Hypoalbuminemia was defined as a serum albumin level below 25 g/L (15). Respiratory Failure in children was characterized by an inability to maintain normal gas exchange, reflected in arterial blood gas analysis (PaO2 < 60 mmHg or PaCO2 > 50 mmHg). Hyponatremia was defined as a serum sodium concentration of less than 135 mmol/L (16). Shock was primarily diagnosed based on clinical manifestations, including hypotension, tachycardia, and compromised peripheral circulation, such as cold extremities and reduced urine output. Liver and Kidney Injury in children were identified by abnormal liver enzymes (for liver injury) or elevated serum creatinine (for kidney injury). Coagulopathy was considerate when one of the disturbs were present: platelet count (PLT) less than 100 (× 109 /L), APTT superior to 45.4 s, and PT-INR superior to 1.3. Sepsis was diagnosed when a pathogen was isolated from either blood or cerebrospinal fluid, and infants exhibiting infectious manifestations were treated with antibiotics for at least five days (17).
2.4 Statistical analysis
Excel 2007 software was used to double-check and enter the recorded data, and Statistical Package for Social Sciences 25.0 software was used for statistical analysis. Continuous data were assessed for normality using the Shapiro–Wilk test. Data following a normal distribution were expressed as the mean ± standard deviation (SD) and analyzed using analysis of variance (ANOVA) for multi-group comparisons. Data that did not follow a normal distribution were presented as the median and interquartile range (IQR) and analyzed using the Kruskal–Wallis test for multi-group comparisons. Categorical data were expressed as n (%), and Fisher's exact test or chi-squared test, as appropriate, was used for comparison. Statistical tests were corrected for multiple comparisons using Bonferroni correction with a corrected P-value of 0.016. Covariate screening was performed using LASSO analysis with the “glmnet” package in R 4.1.3 software, followed by multivariate Logistic regression analysis to identify independent factors associated with poor prognosis in NEC-IP patients. P value < 0.05 was regarded as significant.
3 Results
3.1 General data
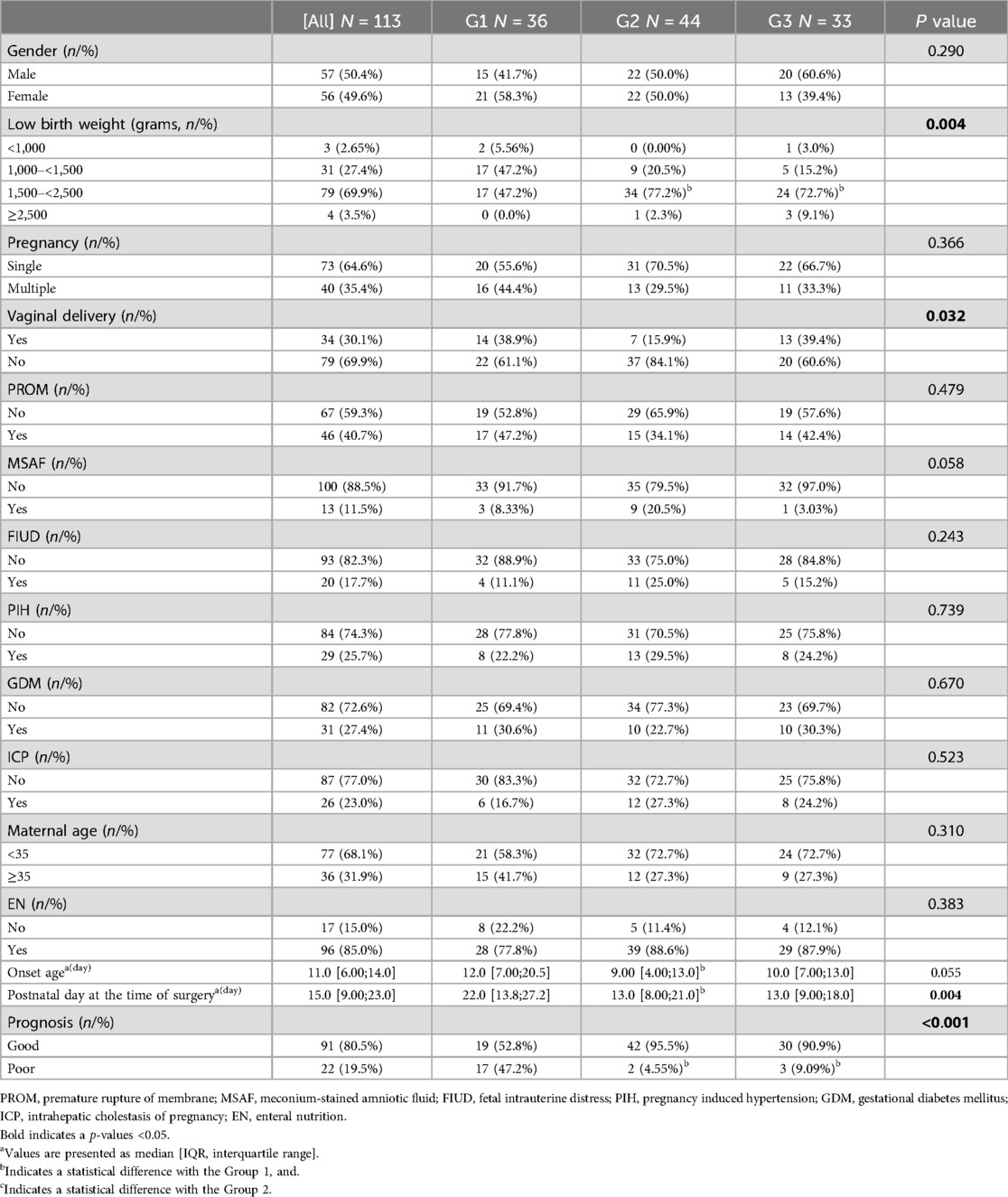
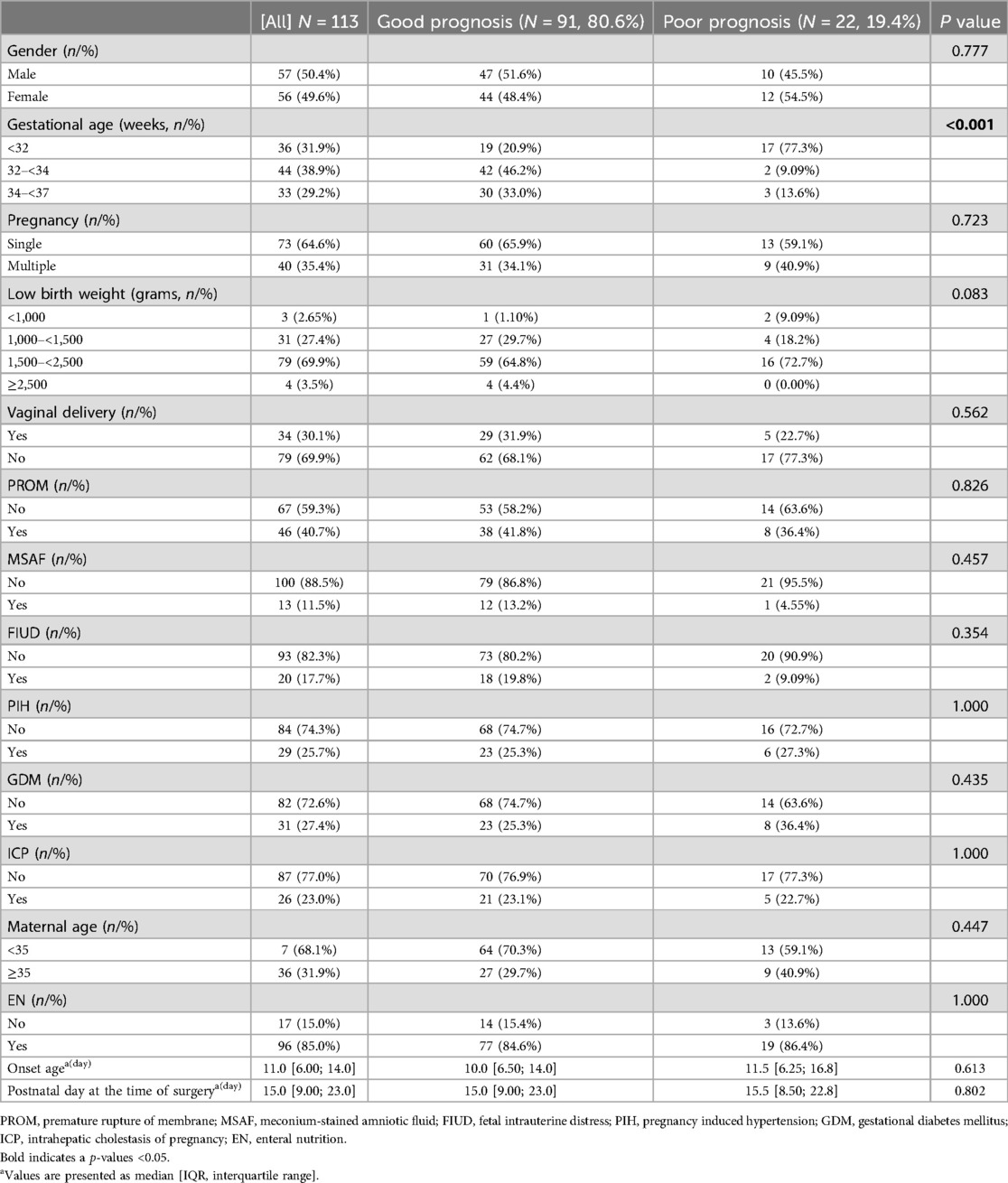
In this study, 129 patients were diagnosed with NEC-IP. Among these cases, 8 had a GA of less than 28 weeks, 5 were associated with intestinal malrotation, and 3 presented with intestinal atresia. Only three cases of IP in full-term NEC patients were identified during retrospective data collection. Due to the small sample size of full-term patients, only preterm NEC patients were analyzed. Ultimately, a total of 113 patients met the inclusion criteria and were enrolled in the study. Out of 113 cases, the average GA and birth weight were 32.29 ± 2.09 weeks, and 1,965.5 ± 542.5 grams, respectively. The incidence was essentially similar in males and females, and the male-to-female ratio was 0.98 (56:57 cases). Additionally, the median onset age was 11 days, and the media postnatal day at the time of surgery was 15 days. During surgery, we found that the most commonly affected segment in IP was the colon (40.7%), followed by the ileum (37.2%), involvement of ≥2 segments (16.8%), and the ileocecal region (5.3%). It should be noted that the overall proportion of poor prognosis was 19.4% (22/113) (Table 1). The all-cause mortality in this study was 30.1% (34/113) and the NEC-related mortality was 19.4 (22/113).
3.2 Clinical features of patients with different GAs
The onset age and postnatal day at the time of surgery in Group 1 were significantly higher than those in Group 2 (median onset age: 12 days vs. 9 days; median postnatal day at surgery: 22 days vs. 13 days, both P < 0.016). However, no statistically significant differences were observed in onset age and postnatal day at surgery between Group 3 and Groups 1/2 (all P > 0.016). As was expected the birth weight in Group 1 was lower than that in Group2 and Group3 (P < 0.016). Furthermore, the incidence of poor prognosis in Group 1 (17 cases, 47.2%) was significantly higher than that in Group 2 (2 cases, 4.55%) and Group 3 (3 cases, 9.09%) (both P < 0.016).
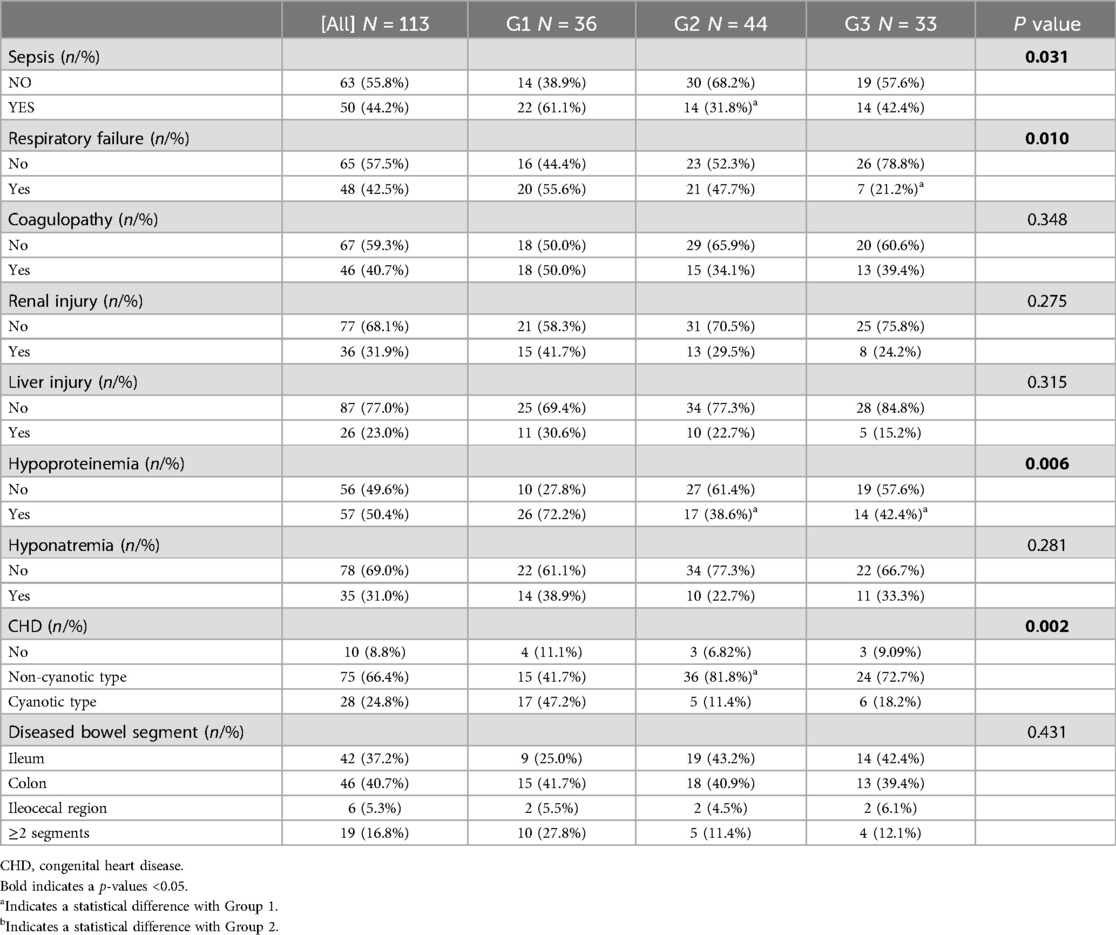
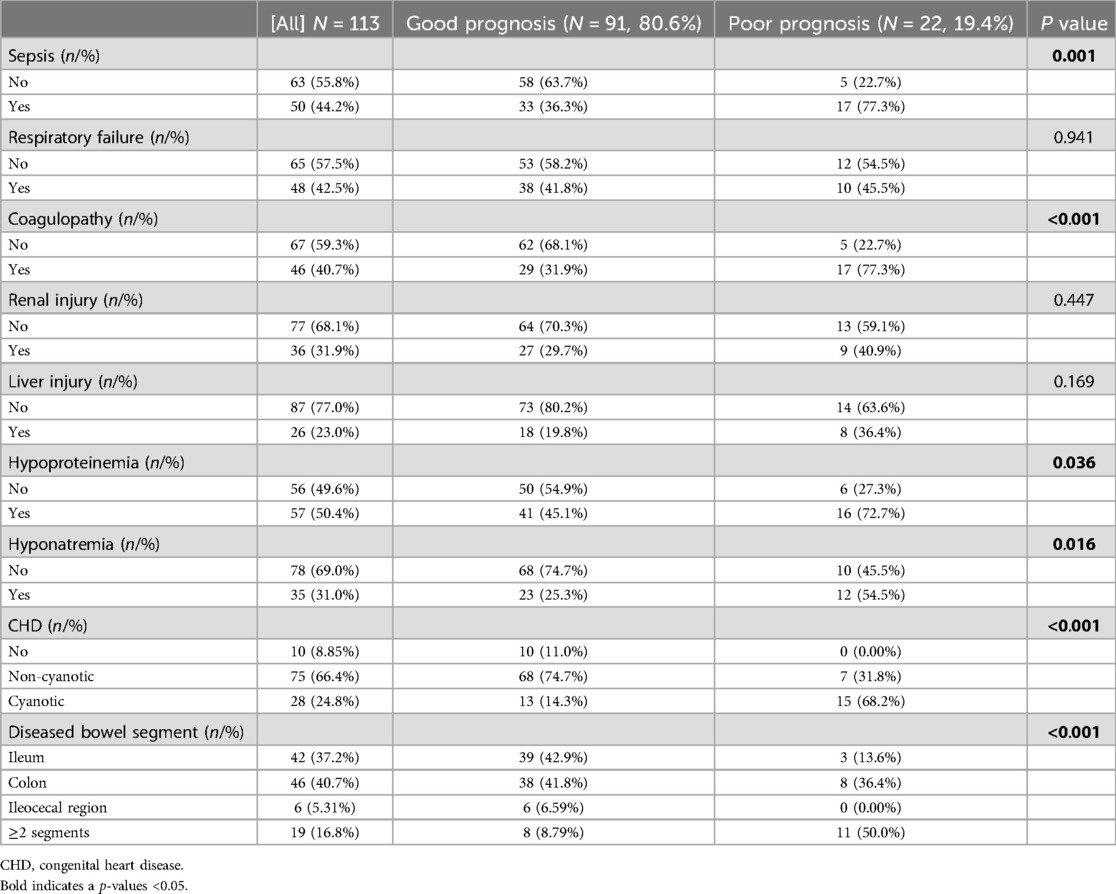
Regarding comorbidities, NEC-IP was most commonly accompanied by CHD (91.2%), followed by hypoproteinemia (50.4%), sepsis (44.2%), coagulopathy (40.7%), and respiratory failure (42.5%) (Table 2). The incidence of respiratory failure, sepsis, renal injury, coagulopathy, and hypoproteinemia were different among different GAs (P < 0.05). Compared with Group 1, the incidence of sepsis (61.1% vs. 31.8%), coagulopathy (50.0% vs. 34.1%), cyanotic CHD (47.2% vs. 11.4%), hypoproteinemia (72.2% vs. 38.6%) was higher than that in Group2, while the incidence of respiratory failure (55.6% vs. 21.2%), hypoproteinemia (72.2% vs. 42.4%) was higher than that in Group3 (all P < 0.016).
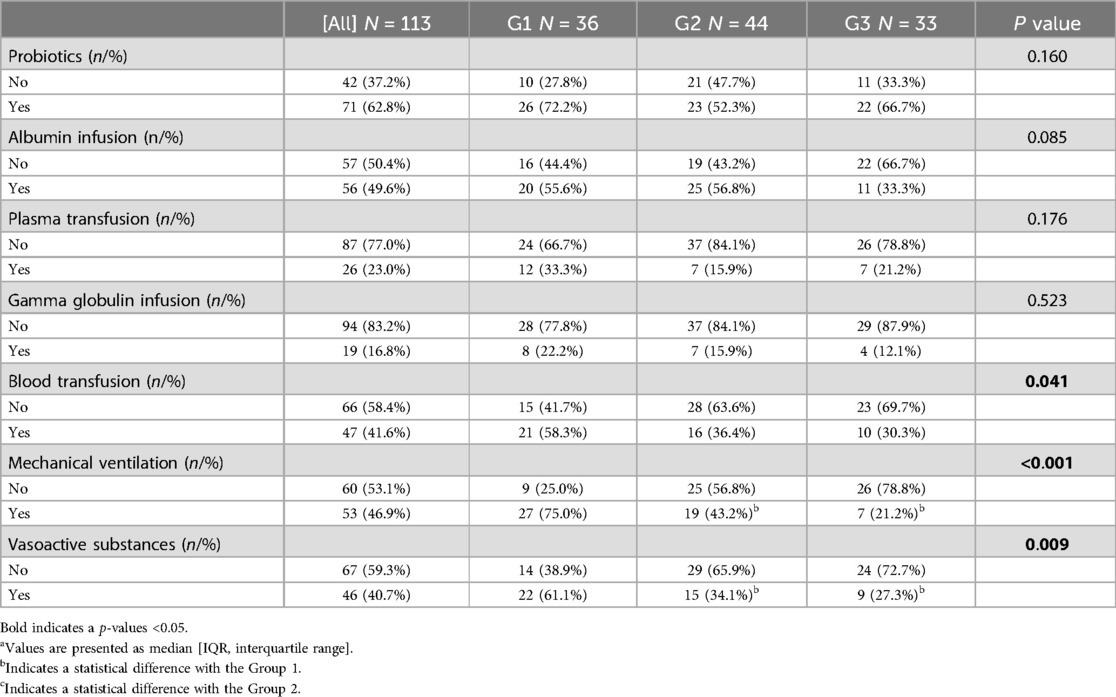
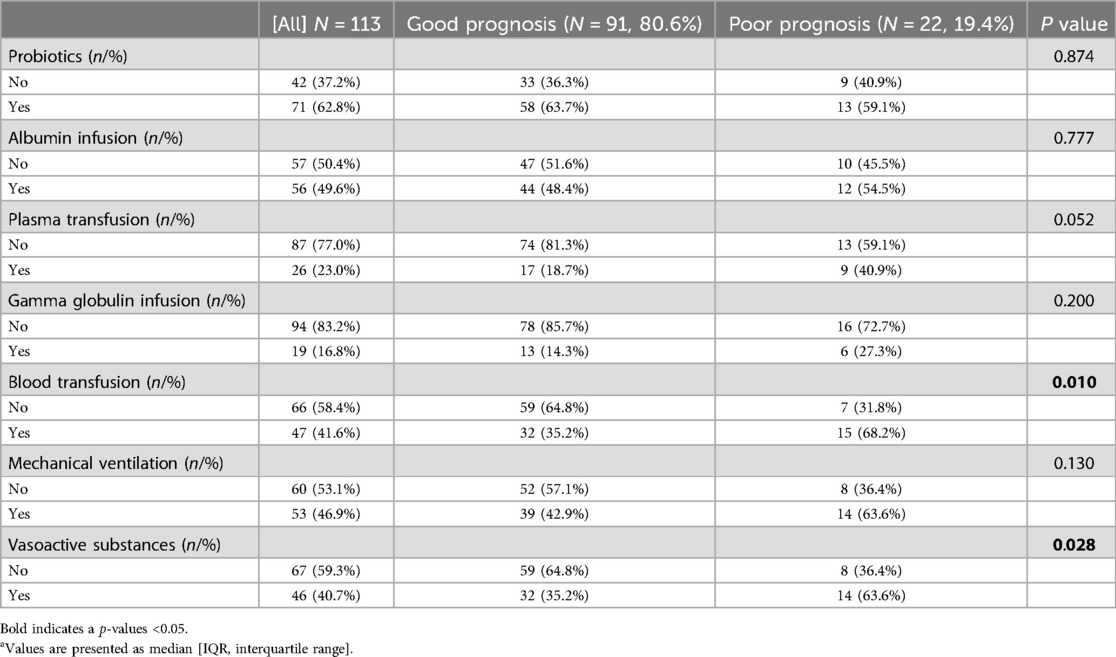
According to the intraoperative findings, we found that there was no significant difference in the diseased bowel segment among patients with different GAs. For related treatment, there were significant differences in the utilization rate of vasoactive substances, mechanical ventilation, and blood transfusion among different GAs (P < 0.05) (Table 3). Furthermore, the utilization rate of vasoactive substances (61.1% vs. 34.1% vs. 27.3%; P < 0.016) and mechanical ventilation (75.0% vs. 43.2% vs. 21.2%; P < 0.016) in Group1 was higher than that in Group2 and Group3, respectively.
3.3 Risk factors for poor prognosis
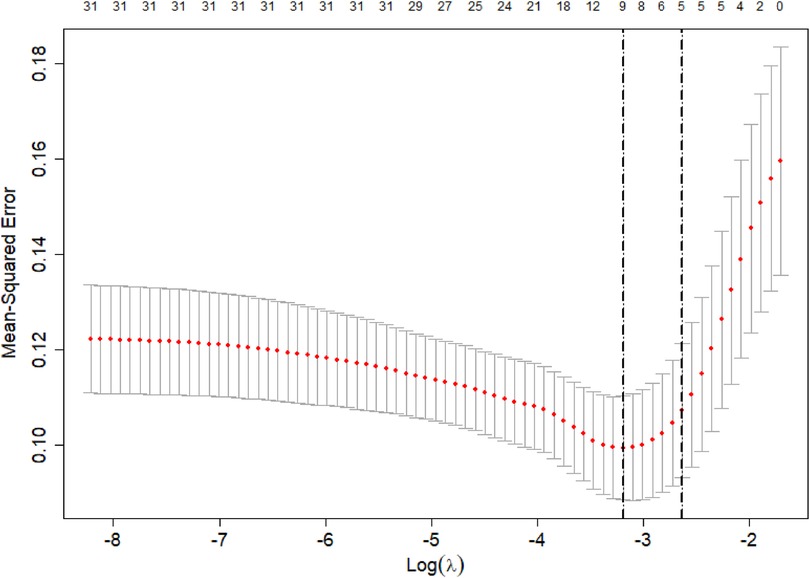
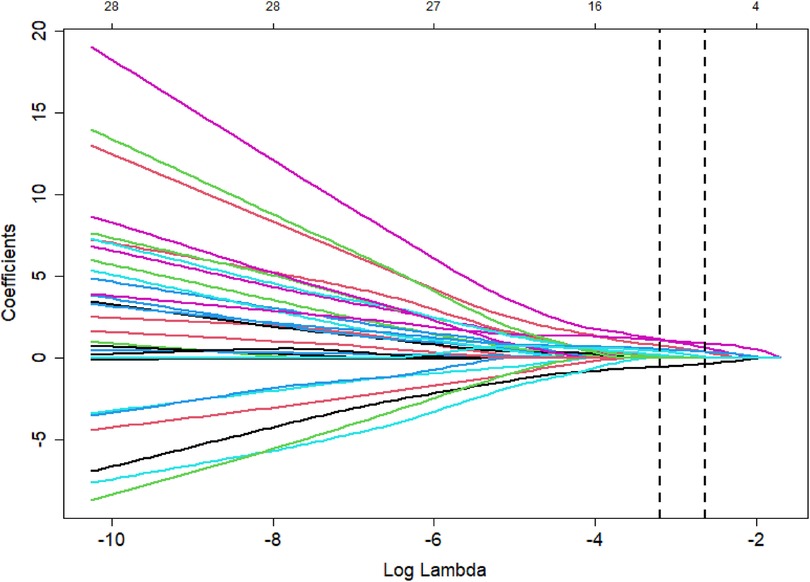
The poor prognosis of NEC-IP patients (assignment: no = 0, yes = 1) was used as the dependent variable, and the influencing factors were screened by LASSO regression (Tables 4–6). The screened influence factors included GA, sepsis, coagulopathy, CHD, diseased bowel segment, and blood transfusion (Figures 1, 2). These factors were used as independent variables for multivariate Logistic regression analysis. The results showed that GA (OR: 0.274, 95%CI: 0.078–0.796O), sepsis (OR: 7.955, 95%CI: 1.424–65.21), coagulopathy (OR: 19.51, 95%CI: 3.393–179.1), CHD (OR: 6.99, 95%CI: 1.418–54.83) and diseased bowel segment (OR: 2.804, 95%CI: 1.301–7.316) were independent factors for poor prognosis (Table 7). The Nagelkerke R-squared and Akaike Information Criterion (AIC) values for the model were 0.726 and 48.789, respectively.
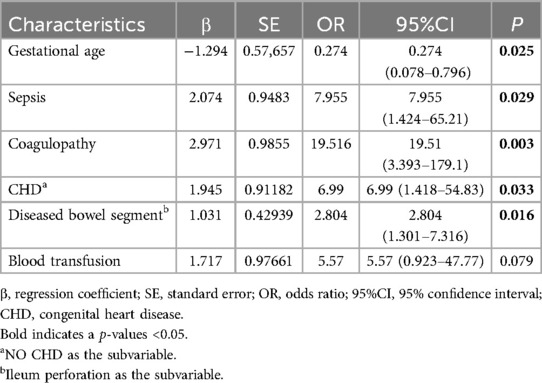
Table 7. Logistic regression screening of independent influencing factors of poor prognosis in NEC-IP patients.
4 Discussion
NEC was typically caused by intestinal inflammation. In the absence of early and effective intervention, the inflammation progressively worsened, extending through the mucosal, submucosal, muscular, and serosal layers of the intestine, ultimately leading to IP (18–20). IP was a life-threatening complication of NEC patients (4), with a mortality rate of nearly 76% (21). However, there were few studies on the poor prognosis of NEC-IP. Although necrotizing enterocolitis with intestinal perforation (NEC-IP) and spontaneous intestinal perforation (SIP) may present with similar clinical features, studies, including the The Necrotizing Enterocolitis Surgery Trial (NEST), identified significant distinctions between the two conditions. These findings suggested that NEC-IP and SIP differ fundamentally in both their pathogenic mechanisms and clinical manifestations (22, 23). This study included only pediatric patients with NEC-IP, all of whom underwent surgical treatment. During surgery, the intestinal pathology was assessed to confirm NEC-IP rather than SIP. GA was known to be a risk factor for NEC progressing to IP (24). The immune function, intestinal maturity, and intestinal colonizing microbiota were different for patients of different GAs (4, 25, 26).
Comparing early preterm patients with middle and late preterm patients, we found that early preterm patients had lower birth weight, later onset age, and later postnatal day at the time of surgery, and were more likely to have ≥2 segments of IPs. In early preterm patients, there was a higher incidence of respiratory failure, sepsis, CHD, and hypoproteinemia. Additionally, the utilization rate of vasoactive substances, mechanical ventilation, and blood transfusion was also higher in this group. However, this difference was not evident in the comparison of middle and late preterm patients. Although kidney and liver failure showed no significant differences between the intermediate and late-stage groups, the incidence of lung failure was notably higher in the intermediate group (47%) compared to the late-stage group (20%). This finding may suggest a higher risk of respiratory system injury, potentially due to bronchopulmonary dysplasia, even among moderately preterm infants.
In this study, the incidence of poor prognosis was 19.4%. Studies have suggested that the risk factors for NEC patients include low birth weight, small GA, mechanical ventilation, premature rupture of membranes, sepsis, shock, pulmonary surfactant drugs, and cesarean section (9, 27–29). By Lasso and Logistic regression analysis, we found that GA, sepsis, coagulopathy, CHD, and diseased bowel segment were the independent factors for poor prognosis in patients with NEC-IP.
As shown in our results, 61.1% of early preterm patients with NEC-IP had sepsis. It was reported that early preterm patients were more susceptible to sepsis than full-term patients (28). Generally, NEC-IP patients exhibited an underdeveloped immune system, an unstable intestinal microbiota, and an increased susceptibility to intestinal barrier damage (27, 30). With sepsis, bacteria multiplied in the blood and produced large amounts of toxins. These toxins acted on immature and damaged intestinal epithelial cells, stimulating the production of TNF-α, IL-8, PAF, and other cytokines, causing a cytokine-mediated inflammatory cascade (6). This further aggravates intestinal damage, resulting in a significant increase in the risk of intestinal necrosis, IP, peritonitis, and even septic shock, which could endanger the patient's life (31, 32). In this study, the incidence of septic shock in patients with NEC-IP was 5.8%. For patients with septic shock, hypoxia further aggravated the degree of intestinal mucosal ischemia and intestinal microcirculation disturbance (33). This led to the deterioration of the patient's intestinal condition and aggravation of the condition, which in turn will affect the prognosis.
Furthermore, coagulopathy was an independent risk factor for patients with NEC-IP. Patients with NEC-IP often exhibited abnormal gene expression of coagulation and anticoagulation proteins, which enhanced coagulation function and damaged the fibrinolytic system, leaving NEC-IP patients in a net pro-coagulation state (34). Moreover, relevant studies had confirmed that coagulopathy and mesenteric thrombosis were common in patients with NEC (35). Both of them aggravated intestinal tissue ischemia and intestinal mucosal epithelial necrosis, increasing the permeability of the intestinal wall, which led to extensive intestinal necrosis and multiple organ dysfunction syndrome (MODS), etc (35). Hutter et al. (36) and Sonntag et al. (37) reported disseminated intravascular coagulation (DIC) in 35% and 28% of patients with NEC, respectively. In our study, we found that 40.7% of patients with NEC-IP had abnormal coagulation, consistent with previous findings. Therefore, it was crucial to regularly assess coagulation function in NEC-IP patients and implement early interventions to correct coagulopathy, aiming to reduce the risk of poor prognosis.
This study found that GA was a risk factor for poor prognosis in patients with NEC-IP. The mortality rate of early preterm patients was 47.2% in this study, which was significantly higher than that of middle and late preterm patients. Small GA patients had a high probability of NEC, neonatal asphyxia, brain injury, and respiratory distress syndrome (38, 39). We found that the smaller the GA patients were, the higher the incidence of respiratory failure, sepsis, CHD, and hypoproteinemia, and the more frequently blood transfusion, ventilator support, and other medical methods were used. The reason may have been that their intestinal tracts were immature and their intestinal flora was not stabilized (2, 6, 40). In addition, the results indicated that the diseased bowel segment was also an influencing factor for the poor prognosis of NEC-IP. The reason may have been that patients with multiple-segment perforation had more severe conditions, more intestinal segments were removed than those with single-segment perforation, and the incidence of short-term and long-term complications was higher.
In this retrospective study, 24.8% of NEC-IP patients had cyanotic congenital heart disease (CHD), with a poor prognosis rate of 68.2%. Patent ductus arteriosus (PDA) accounted for 20.4% (21/103) of the CHD cases. The incidence of poor prognosis was much higher than the NEC patients combined with non-cyanotic type CHD (31.8%) and no CHD (0.00%). Multiple studies had established the association between CHD and an increased risk of NEC (38, 41–44). Specifically, research had shown that the presence of a PDA in NEC patients tripled the risk of severe NEC and increased the risk of mortality by fivefold (45). Patients with NEC-IP combined with cyanotic CHD had a significantly increased risk of poor prognosis, as the study reported, this may be related to CHD whose hemodynamic disorder can lead to intestinal damage. Recently, studies had been conducted on its pathogenesis. The main mechanism was a combination of mesenteric hypoperfusion and hypoxia-induced inflammation (42, 46). The colon and distal ileum were most commonly involved in these patients, which was related to the tendency of hypoperfusion in this area (27, 42, 47). And for these patients, their heart disease was not treated, which meant that after surgery, the blood supply of the intestine would still be affected, leading to intestinal inflammation and nutrient absorption disorders. Hence, it was crucial to ensure effective communication with the guardians of NEC-IP patients with CHD regarding the condition and associated risks before surgery. Furthermore, allocating limited medical resources to those at the highest risk should be prioritized.
5 Limitation
However, there are some limitations to this study. Firstly, considering that our hospital is the largest national clinical research center for child health and disorders in southwest China, the patients were in a relatively serious condition. Secondly, inherent biases were inevitable given the single-center retrospective study with a relatively small sample size. Therefore, further verification is needed for a multi-center, large sample, and multidisciplinary prospective cohort study.
6 Conclusions
We found most NEC-related IP in patients born at GA 32–34. The clinical features of patients with different GAs were different, especially in terms of the type of CHD, onset time of IP, utilization of vasoactive substances, and prognosis. Furthermore, GA, sepsis, coagulopathy, CHD, and diseased bowel segment were independent factors for the prognosis of the patients with NEC-IP. Therefore, recognizing the influencing factors for poor prognosis of NEC-IP facilitates our early identification of those at risk and leads to individualized interventions to improve the clinical outcome.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. WF: Visualization, Writing – review & editing. JH: Validation, Writing – review & editing. XD: Software, Writing – review & editing. ZG: Supervision, Writing – review & editing. YW: Data curation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Center for Clinical Medical Research on Children’s Health and Diseases General Project on Clinical Medical Research [NCRCCHD-2022-GP-04] and Program for Youth Innovation in Future Medicine, Chongqing Medical University [W0125].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. (2006) 368(9543):1271–83. doi: 10.1016/S0140-6736(06)69525-1
2. Yeramilli V, Cheddadi R, Benjamin H, Martin C. The impact of stress, microbial dysbiosis, and inflammation on necrotizing enterocolitis. Microorganisms. (2023) 11(9):2206. doi: 10.3390/microorganisms11092206
3. Kaplina A, Kononova S, Zaikova E, Pervunina T, Petrova N, Sitkin S. Necrotizing enterocolitis: the role of hypoxia, gut microbiome, and microbial metabolites. Int J Mol Sci. (2023) 24(3):2471. doi: 10.3390/ijms24032471
4. Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med. (2009) 60:111–24. doi: 10.1146/annurev.med.60.050207.092824
5. Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. (2012) 129(2):e298–304. doi: 10.1542/peds.2011-2022
6. Duess JW, Sampah ME, Lopez CM, Tsuboi K, Scheese DJ, Sodhi CP, et al. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes. (2023) 15(1):2221470. doi: 10.1080/19490976.2023.2221470
7. Luig M, Lui K. Epidemiology of necrotizing enterocolitis–part II: risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. (2005) 41(4):174–9. doi: 10.1111/j.1440-1754.2005.00583.x
8. Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis. Clin Perinatol. (2013) 40(1):27–51. doi: 10.1016/j.clp.2012.12.012
9. Samuels N, van de Graaf RA, de Jonge R, Reiss I, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. (2017) 17(1):105. doi: 10.1186/s12887-017-0847-3
10. Robinson JR, Rellinger EJ, Hatch LD, Weitkamp JH, Speck KE, Danko M, et al. Surgical necrotizing enterocolitis. Semin Perinatol. (2017) 41(1):70–9. doi: 10.1053/j.semperi.2016.09.020
11. Feng W, Hou J, Die X, Sun J, Guo Z, Liu W, et al. Application of coagulation parameters at the time of necrotizing enterocolitis diagnosis in surgical intervention and prognosis. BMC Pediatr. (2022) 22(1):259. doi: 10.1186/s12887-022-03333-y
12. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364(3):255–64. doi: 10.1056/NEJMra1005408
13. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39(12):1890–900. doi: 10.1016/S0735-1097(02)01886-7
14. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in collaboration with the American society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. (2008) 52(23):e143–263. doi: 10.1016/j.jacc.2008.10.001
15. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. (2012) 7(Suppl 3):193–9. doi: 10.1007/s11739-012-0802-0
16. Palleri E, Frimmel V, Fläring U, Bartocci M, Wester T. Hyponatremia at the onset of necrotizing enterocolitis is associated with intestinal surgery and higher mortality. Eur J Pediatr. (2022) 181(4):1557–65. doi: 10.1007/s00431-021-04339-x
17. Wang ZL, An Y, He Y, Hu XY, Guo L, Li QY, et al. Risk factors of necrotizing enterocolitis in neonates with sepsis: a retrospective case-control study. Int J Immunopathol Pharmacol. (2020) 34:2058738420963818. doi: 10.1177/2058738420963818
18. Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13(10):590–600. doi: 10.1038/nrgastro.2016.119
19. Berrington J, Embleton ND. Discriminating necrotising enterocolitis and focal intestinal perforation. Arch Dis Child Fetal Neonatal Ed. (2022) 107(3):336–9. doi: 10.1136/archdischild-2020-321429
20. Sato TT, Oldham KT. Abdominal drain placement versus laparotomy for necrotizing enterocolitis with perforation. Clin Perinatol. (2004) 31(3):577–89. doi: 10.1016/j.clp.2004.03.017
21. Sakellaris G, Partalis N, Dede O, Alegakis A, Seremeti C, Korakaki E, et al. Gastrointestinal Perforations in Neonatal Period. Pediatr Emerg Care. (2012) 28(9):886–8. doi: 10.1097/PEC.0b013e31826beb0c
22. Dantes G, Keane OA, Do L, Rumbika S, Ellis NH, Dutreuil VL, et al. Clinical predictors of spontaneous intestinal perforation vs necrotizing enterocolitis in extremely and very low birth weight neonates. J Pediatr Surg. (2024) 59(11):161608. doi: 10.1016/j.jpedsurg.2024.06.017
23. Speer AL, Lally KP, Pedroza C, Zhang Y, Poindexter BB, Chwals WJ, et al. Surgical necrotizing enterocolitis and spontaneous intestinal perforation lead to severe growth failure in infants. Ann Surg. (2024) 280(3):432–43. doi: 10.1097/SLA.0000000000006378
24. Yu L, Tian J, Zhao X, Cheng P, Chen X, Yu Y, et al. Bowel perforation in premature infants with necrotizing enterocolitis: risk factors and outcomes. Gastroenterol Res Pract. (2016) 2016:6134187. doi: 10.1155/2016/6134187
25. Suárez-Martínez C, Santaella-Pascual M, Yagüe-Guirao G, Martínez-Graciá C. Infant gut microbiota colonization: influence of prenatal and postnatal factors, focusing on diet. Front Microbiol. (2023) 14:1236254. doi: 10.3389/fmicb.2023.1236254
26. Zhang JY, Greenwald MJ, Rodriguez SH. Gut microbiome and retinopathy of prematurity. Am J Pathol. (2023) 193(11):1683–90. doi: 10.1016/j.ajpath.2023.01.013
27. Bowker RM, Yan X, De Plaen IG. Intestinal microcirculation and necrotizing enterocolitis: the vascular endothelial growth factor system. Semin Fetal Neonatal Med. (2018) 23(6):411–5. doi: 10.1016/j.siny.2018.08.008
28. Pasquier JC, Claris O, Rabilloud M, Ecochard R, Picaud JC, Moret S, et al. Intentional early delivery versus expectant management for preterm premature rupture of membranes at 28–32 weeks’ gestation: a multicentre randomized controlled trial (MICADO STUDY). Eur J Obstet Gynecol Reprod Biol. (2019) 233:30–7. doi: 10.1016/j.ejogrb.2018.11.024
29. Gephart SM, Effken JA, McGrath JM, Reed PG. Expert consensus building using e-delphi for necrotizing enterocolitis risk assessment. J Obstet Gynecol Neonatal Nurs. (2013) 42(3):332–47. doi: 10.1111/1552-6909.12032
30. Golubkova A, Hunter CJ. Development of the neonatal intestinal barrier, microbiome, and susceptibility to NEC. Microorganisms. (2023) 11(5):1247. doi: 10.3390/microorganisms11051247
31. He Y, Cao L, Yu J. Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants. Medicine. (2018) 97(35):e11976. doi: 10.1097/MD.0000000000011976
32. Zhang LP, Lei XP, Luo LJ, Dong WB. Risk factors for necrotizing enterocolitis in very preterm infants: a case-control study in southwest China. J Matern Fetal Neonatal Med. (2019) 32(6):896–901. doi: 10.1080/14767058.2017.1395011
33. Murphy C, Baskind S, Aladangady N, Banerjee J. Measuring gut perfusion and blood flow in neonates using ultrasound doppler of the superior mesenteric artery: a narrative review. Front Pediatr. (2023) 11:1154611. doi: 10.3389/fped.2023.1154611
34. Giuliani S, Tan YW, Zheng D, Petropoulou E, Sohail A, Bradley S, et al. Coagulation gene expression profiling in infants with necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. (2016) 63(6):e169–169e175. doi: 10.1097/MPG.0000000000001215
35. Song R, Subbarao GC, Maheshwari A. Haematological abnormalities in neonatal necrotizing enterocolitis. J Matern Fetal Neonatal Med. (2012) 25(Suppl 4):22–5. doi: 10.3109/14767058.2012.715005
36. Hutter JJ Jr, Hathaway WE, Wayne ER. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr. (1976) 88(6):1026–31. doi: 10.1016/S0022-3476(76)81069-4
37. Sonntag J, Wagner MH, Waldschmidt J, Wit J, Obladen M. Multisystem organ failure and capillary leak syndrome in severe necrotizing enterocolitis of very low birth weight infants. J Pediatr Surg. (1998) 33(3):481–4. doi: 10.1016/S0022-3468(98)90092-6
38. Su Y, Xu RH, Guo LY, Chen XQ, Han WX, Ma JJ, et al. Risk factors for necrotizing enterocolitis in neonates: a meta-analysis. Front Pediatr. (2023) 10:1079894. doi: 10.3389/fped.2022.1079894
39. Zouari M, Ben Ameur H, Ben Saad N, Rhaiem W, Ghariani O, Ben Hamad A, et al. Predictive factors for mortality in pre-term neonates with necrotizing enterocolitis: a retrospective cohort study. Surg Infect. (2023) 24(1):52–7. doi: 10.1089/sur.2022.266
40. Khan A, Mi H, Gao F, Hu Q, Gu X, Ma F, et al. Dynamic changes of the gut microbial colonization in preterm infants with different time points after birth. Front Microbiol. (2023) 14:1078426. doi: 10.3389/fmicb.2023.1078426
41. Velazco CS, Fullerton BS, Hong CR, Morrow KA, Edwards EM, Soll RF, et al. Morbidity and mortality among “big” babies who develop necrotizing enterocolitis: a prospective multicenter cohort analysis. J Pediatr Surg. (2017) S0022–3468(17):30650–4. doi: 10.1016/j.jpedsurg.2017.10.028
42. Burge KY, Gunasekaran A, Makoni MM, Mir AM, Burkhart HM, Chaaban H. Clinical characteristics and potential pathogenesis of cardiac necrotizing enterocolitis in neonates with congenital heart disease: a narrative review. J Clin Med. (2022) 11(14):3987. doi: 10.3390/jcm11143987
43. Kelleher ST, McMahon CJ, James A. Necrotizing enterocolitis in children with congenital heart disease: a literature review. Pediatr Cardiol. (2021) 42(8):1688–99. doi: 10.1007/s00246-021-02691-1
44. Mitra S, de Boode WP, Weisz DE, Shah PS. Interventions for patent ductus arteriosus (PDA) in preterm infants: an overview of cochrane systematic reviews. Cochrane Database Syst Rev. (2023) 4(4):CD013588. doi: 10.1002/14651858.CD013588.pub2
45. Kordasz M, Racine M, Szavay P, Lehner M, Krebs T, Luckert C, et al. Risk factors for mortality in preterm infants with necrotizing enterocolitis: a retrospective multicenter analysis. Eur J Pediatr. (2022) 181(3):933–9. doi: 10.1007/s00431-021-04266-x
46. Carlo WF, Kimball TR, Michelfelder EC, Border WL. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics. (2007) 119(2):330–5. doi: 10.1542/peds.2006-2640
Keywords: gestational age, clinical features, necrotizing enterocolitis, intestinal perforation, prognosis
Citation: Chen M, Feng W, Hou J, Die X, Guo Z and Wang Y (2025) Effect of gestational age on clinical features in necrotizing enterocolitis-associated intestinal perforation. Front. Pediatr. 12:1452207. doi: 10.3389/fped.2024.1452207
Received: 20 June 2024; Accepted: 28 November 2024;
Published: 6 January 2025.
Edited by:
Chhinder Sodhi, Johns Hopkins University, United StatesReviewed by:
Daphne Klerk, University Medical Center Groningen, NetherlandsKoichi Tsuboi, Juntendo University, Japan
Copyright: © 2025 Chen, Feng, Hou, Die, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Wang, d3k3NTczMTFASG90bWFpbC5jb20=
 Minming Chen
Minming Chen Wei Feng
Wei Feng Jinping Hou
Jinping Hou Yi Wang
Yi Wang