- 1Department of Anesthesiology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Division of Anaesthetics, Pain Medicine & Intensive Care, Department of Surgery & Cancer, Faculty of Medicine, Imperial College London, Chelsea and Westminster Hospital, London, United Kingdom
Background: Emergence delirium (ED) is a widely recognized issue that prolongs mechanical ventilation and post-anesthesia care unit (PACU) resuscitation time, consequently increasing hospital costs and mortality. Postoperative disturbance in circadian rhythms, commonly leading to sleep disorders, has been identified as a significant risk factor for ED. However, the influence of surgery timing (morning vs. afternoon) on the incidence of ED in pediatric patients undergoing general anesthesia remains unknown.
Methods: Patients aged 2–6 years who were operated on under general anesthesia with a bispectral index value between 50 and 60 were categorized based on anesthesia start time into either the morning surgery group (Group M, 8:00–12:00) or the afternoon surgery group (Group A, 13:00–17:00). The primary outcome was the post-extubation incidence of ED assessed by the Cornell Assessment of Pediatric Delirium (CAPD) score. Secondary outcomes included extubation time, duration of PACU stay, and adverse postoperative events and complications.
Results: We recruited a total of 560 patients, 280 in group M and 280 in group A. Compared to Group M, Group A exhibited a significantly higher incidence of ED (p < 0.001), elevated CAPD scores (p < 0.001), and prolonged PACU stays (p < 0.001). Notably, there was no significant difference in extubation time and anesthesia-related adverse events or other postoperative complications between the groups.
Conclusion: Our study highlights that the time of surgery significantly affects the incidence of ED, CAPD scores, and PACU stay duration in children. Further validation of these findings may guide future strategies to reduce ED.
Introduction
Emergence delirium (ED) is a complex post-anesthetic perceptual disorder and psychomotor agitation characterized by purposeless resistance movements and is particularly prevalent in children of preschool age following a sevoflurane-based anesthetic (1, 2). Although the incidence of pediatric ED varies, it can be notably high, ranging between 10% and 80% (3). The underlying mechanisms are largely unknown yet identified risk factors of ED include surgery type, age, anesthesia type, preoperative anxiety, preexisting pathological status, and pain (4–6). In general, the ED condition is transient and reversible; however, it is associated with postoperative behavioral issues (7). Children who experience postoperative delirium are at high risk of developing new maladaptive behaviors compared with those without such complications (8). Therefore, identifying ED-related factors and reducing their incidence is important during the perioperative period for young patients.
Circadian rhythms, which are orchestrated by the suprachiasmatic nucleus (SCN) of the hypothalamus, govern our daily physiological and behavioral cycles (9). These rhythms are influenced by various exogenous factors, including light exposure and melatonin secretion (10, 11). Furthermore, the timing of surgery procedures and anesthesia has been shown to exert a significant influence on perioperative circadian rhythms (12–14). Notably, surgeries conducted in the morning, as opposed to those in the afternoon, have been associated with greater disruption of circadian rhythms and sleep patterns in elderly patients, consequently elevating the risk of postoperative delirium, particularly when general anesthesia is administered (15, 16). Despite the widespread use of general anesthesia in pediatric surgeries, the impact of surgery timing on the incidence of ED in children remains insufficiently explored. This observational clinical study aimed to elucidate whether the timing of general anesthesia (morning vs. afternoon) influences the incidence of ED in children, thereby addressing a critical gap in the existing literature.
Materials and methods
Ethical approval and registration
This prospective observational single-center cohort study was conducted in the post-anesthesia care unit (PACU) of Guangzhou Women and Children's Medical Center from May 2022 to May 2023. The study protocol was approved by the ethics committee of the Guangzhou Women and Children's Medical Center [(2022)119A01] and registered with the Chinese Clinical Trials Registry (ChiCTR2200065441). Written consent from all participants’ parents or legal guardians was obtained before the study enrollment.
Patients
Patients who received surgery (including abdominal, head and face, urological, and orthopedic surgeries) under general anesthesia were enrolled in this study. The inclusion criteria were preschool children aged 2–6 years with a physical status graded as I or II according to the American Society of Anesthesia (ASA) classification of physical status who were admitted to the PACU after surgery under general anesthesia. Exclusion criteria were as follows: (1) children undergoing cranial surgery; (2) children with a history of ischemic hypoxic encephalopathy in the past; (3) children with generalized developmental disorders; (4) children with developmental delay or neurological disorders, an abnormal airway such as obstructive sleep apnea, or a history of sedative or psychotropic medications; (5) children with a physical status graded ≥III according to the ASA classification who were transferred to the ICU after surgery; and (6) children whose parents or legal guardians did not understand and agree to the content of the informed consent form.
Perioperative management
All patients received intravenous access the night before surgery, were not premedicated, and fasted for 8, 4, and 2 h for solids, breast milk, and clear fluids, respectively. The participants were divided into the morning group (Group M, 8:00–12:00) and the afternoon group (Group A, 13:00–17:00) based on the start time of the anesthesia. Upon arrival in the operating room, an electrocardiogram, and non-invasive arterial pressure and pulse oxygen saturation monitoring (SpO2) were applied and monitored at 5-min intervals throughout the surgery. End-tidal carbon dioxide and body temperature were monitored after induction, with levels maintained between 35 and 45 mmHg for carbon dioxide and 36°C–37°C for temperature. Anesthesia was induced with phencyclidine hydrochloride (0.01 mg/kg; Nhwa, Jiangsu, China), sufentanil (0.2 μg/kg; Humanwell Healthcare, Hubei, China), cis-atracurium (0.15 mg/kg; Hengrui, Jiangsu, China), and propofol (3 mg/kg; Kelun, Sichuan, China). After laryngeal mask placement or endotracheal intubation, anesthesia was maintained by inhaled sevoflurane (1%–3%) in 50% oxygen, along with intermittent intravenous administration of sufentanil and cis-atracurium. The bispectral index (BIS; Covidien IIc, MA, USA), which has been widely validated in children and recommends a range of 40–60 as in adults (17), was used to monitor the depth of anesthesia. In our study, the BIS levels were maintained between 50 and 60. For urological and abdominal surgeries, an ultrasound-guided transversus abdominis plane (TAP) block with 0.2% ropivacaine at a dose of 1 ml/kg was administered after induction as part of a combined anesthesia approach. After surgery, patients were immediately transferred to the PACU for extubation and recovery in our medical center. In the PACU, anesthesiologists performed extubation once the patients began breathing spontaneously with a tidal volume of at least 6 ml/kg and were able to open their eyes either spontaneously or in response to verbal commands. A team member, the chief anesthesiologist in the PACU, assessed ED using the Cornell Assessment of Pediatric Delirium (CAPD) and evaluated pain intensity with the Face, Legs, Activity, Cry, and Consolability (FLACC) scale 1 min after extubation and every 5 min thereafter. The highest recorded scores during the PACU stay were used for the evaluation. Children received propofol (1 mg/kg) for the management of ED, and sufentanil (0.1 μg/kg) was administered if their FLACC score was ≥4 in the PACU. Patients were discharged from the PACU when their Aldrete score reached 9 or above provided that they showed no signs of agitation or pain.
Blinding
The researchers who conducted the preoperative interviews, including history collection, and physical and cognitive assessments, were blinded to the procedure and anesthesia assignments. The two physicians responsible for assessing postoperative delirium were trained to achieve inter-rater agreement above 75% and were also blinded to the study group assignments.
Study endpoints
The primary outcome was the incidence of ED (during PACU stay) assessed by the CAPD score after extubation. Secondary outcomes included the extubation time, duration of PACU stay (from PACU admission to PACU discharge), the intensity of pain assessed by the FLACC scale, the proportion of patients required rescue propofol for ED during the PACU stay, postoperative adverse events, and other anesthetic-related complications such as vomiting, laryngospasm, and hypoxia (defined as a SpO2 level < 92% maintained for more than 3 min). The duration of monitoring includes the entire PACU stay. CAPD, an adaptation of pediatric anesthesia emergence delirium (PAED), was designed to capture all delirium subtypes, specifically the hypoactive phenotype, and can be used in children of all ages while PAED is applicable to patients aged from 19 months to 6 years (18). The accuracy and efficacy of the Chinese version of CAPD have been validated in pediatric surgical patients with optimal sensitivity (87%) and specificity (98%) when the cut-off point was set at 9 (19). The FLACC score, a validated behavioral observation scale for assessing pain in infants and children aged 2 months to 7 years, assesses pain by rating five behaviors (facial expression, leg position, degree of activity, quality of cry, and consolability) on a scale of 0 to 2 (20). The total score ranges from 0 to 10, with a higher score indicating more severe pain.
Sample size estimation
According to the results of our preliminary study before this study, the incidence of ED was 36.4% (SD 5.1%) in morning surgery and 44.3% (SD 11.9%) in afternoon surgery in our operation center. Assuming a category I error of 0.05 and a shedding rate of 10%, 560 patients were selected as the sample size target with 280 patients in Group M and 280 patients in Group A.
Statistical analysis
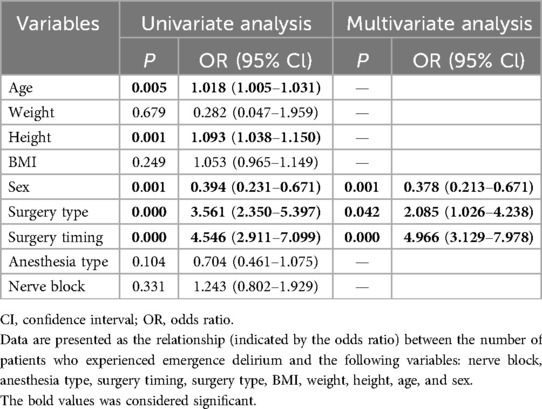
GraphPad Prism 8.0 software and SPSS 20.0 statistical software (SPSS, Chicago, IL, USA) were used for the data analysis. Continuous data were represented as mean (SD) and were analyzed by Student's independent-samples t-test and the Mann–Whitney U-test. The chi-square test was used to analyze differences in the categorical data. A p-value < 0.05 was considered significant. We employed a multivariable logistic regression model to explore the association between surgery timing variations and ED. Confounding factors identified in the literature as potential influencers of ED, such as age, type and duration of surgery, history of anxiety, and proportion of TAP, were included as covariates. The confounders were selected based on their statistical significance in the univariable logistic regression analysis, which was used to identify any potential associations between these variables and ED.
Results
Baseline characteristics
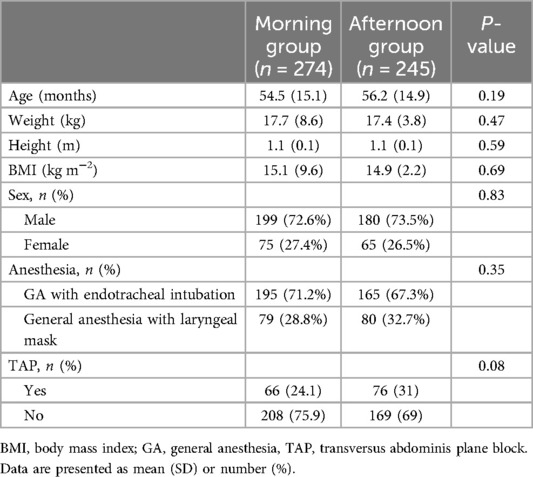
We initially included 622 patients and assessed their eligibility for this study (Figure 1). Of these, 62 were excluded due to not meeting the inclusion criteria. Thus, 560 eligible patients were enrolled in the study and they were assigned to either the morning group (n = 280) or the afternoon group (n = 280) according to the time of anesthesia induction. However, due to further exclusions primarily due to upper respiratory tract infections (21 children) and abnormal laboratory results (12 children), the final analysis included 274 patients in the morning group and 245 patients in the afternoon group. Overall, the two groups were well-matched for baseline variables, including age, weight, height, BMI, sex, anesthesia choice (laryngeal mask or endotracheal intubation), and the use of nerve blocks (P > 0.05; Table 1).
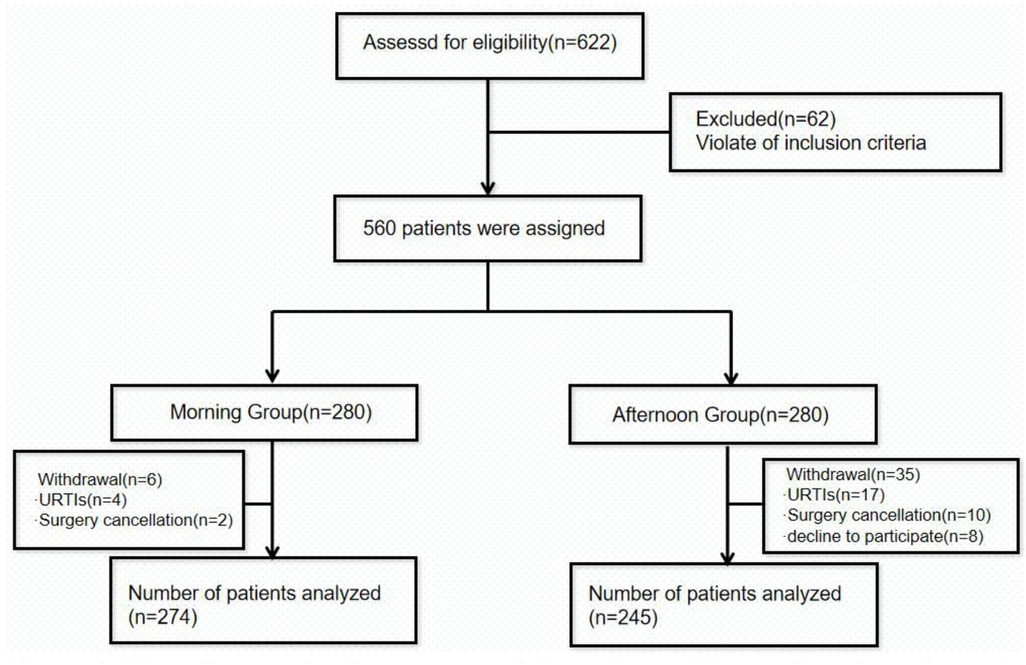
Figure 1. Flow diagram showing the progress of the study participants throughout the study. URTIs, upper respiratory tract infections.
Study outcomes
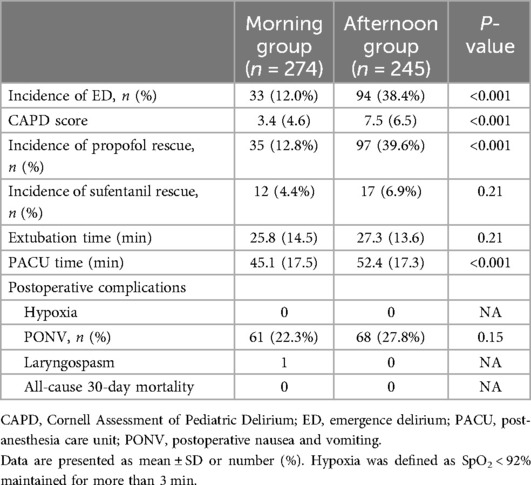
As shown in Table 2, the incidence of ED was significantly higher in Group A compared to Group M (38.4% vs. 12%, P < 0.001, Table 2). Group A also had higher CAPD scores [7.5 (6.5) vs. 3.4 (4.6); P < 0.001, Table 2] and a greater need for rescue propofol during their PACU stay (12.8% vs. 39.6%, P < 0.001, Table 2). However, the postoperative extubation times were similar, with no statistically significant difference between the groups [27.4 (13.6) min vs. 25.8 (14.5) min; P = 0.21, Table 2]. Notably, the duration of PACU stay was significantly longer in Group A compared to Group M [45.1 (17.5) min vs. 52.4 (17.3) min; P < 0.001, Table 2]. There was no statistically significant difference in the use of rescue analgesics (sufentanil) or the occurrence of postoperative adverse events (Table 2). A multivariable logistic regression analysis showed that surgery timing (OR 4.966, 95% CI 3.129–7.978, P < 0.001), surgery type (OR 2.085, 95% CI 1.026–4.238, P < 0.01), and sex (OR 0.378, 95% CI 0.213–0.671, P < 0.01) (Table 3) were associated with an increased risk of ED. A significantly reduced incidence of ED in Group M remained after adjusting for these confounders.
Discussion
In this observational study, we found a significantly higher incidence of ED, elevated total CAPD scores, and increased need for rescue sedatives in the afternoon surgery group compared to the morning group. In addition, afternoon surgeries were associated with longer stays in the PACU. Therefore, our results suggest that conducting surgery in the morning may lead to a lower incidence of ED.
ED is one of the most common complications in pediatric anesthesia, as noted in the evidence-based guidelines from the European Society of Anaesthesiology and Intensive Care. These guidelines underscore various risk factors that contribute to pediatric ED, including preoperative anxiety, age, pain, medications (including inhalation of anesthetics/sedatives, opioids, and anticholinergic agents), and surgical methods (21). After adjusting for these confounders, a significantly reduced incidence of ED was observed in Group M, suggesting that circadian rhythm may serve as an additional risk factor for ED in this population. Previous studies have demonstrated that surgery and anesthesia could cause disturbances in sleep, which is regulated by circadian rhythms (22). For example, Yang et al. (15) reported that morning surgery was associated with fewer sleep disturbances in elderly patients. However, limited research has investigated whether perioperative sleep/circadian rhythm disruption affects the incidence of ED in children. Although our results indicate that morning surgeries significantly decrease the occurrence of ED in children, we did not assess perioperative sleep quality in this observational study. Therefore, further research is needed to determine whether this reduction is due to sleep/circadian rhythm disruption.
Moreover, the interaction between circadian rhythms and general anesthetics may be another important mechanism underlying daytime variation of ED (23). Sevoflurane, one of the most commonly used volatile anesthetics, is one of the proposed risk factors for ED, especially in children (6). Several animal studies have demonstrated that general anesthetics disturb circadian rhythms at multiple levels, suggesting that anesthesia contributes to postoperative circadian disruption and recovery. For example, Kadota et al. (24) found that sevoflurane has a time-dependent effect on the expression of mPer2, the core clock gene in the SCN, with daytime anesthesia having the greatest inhibitory effect on mPer2 expression. Moreover, the expression of mPer2 in the SCN decreases significantly after anesthesia, with normalization after 24 h (25). In addition, circadian rhythms also have an impact on the pharmacodynamics and pharmacokinetics of intravenous anesthetics. It has been suggested that relatively large amounts of anesthetics are required in the morning, which may be related to faster liver and kidney metabolism during that time (13). In animal experiments, the pharmacological effects of propofol can exhibit up to a threefold variation when administered at different times (26). In this study, we also found that compared with the morning surgery group, a higher proportion of children in the afternoon surgery group required intravenous anesthetics in the PACU for postoperative delirium rescue (12.4% vs. 31%).
In addition to anesthetic-related factors, circadian rhythms also exert a regulating effect on the physiological status of children. In this study, we observed a significant difference in the duration of PACU stays between the afternoon group and the morning group, with the former exhibiting a significantly longer PACU stay [52.4 (17.3) min compared to 45.1 (17.5) min in the latter]. First, we postulate that as children often need naps during the noon period, scheduling surgery in the afternoon may disrupt their biological clocks and potentially impact their recovery. In addition, children undergoing surgery in the afternoon may be more susceptible to various physiological challenges, such as an electrolyte imbalance, hypotension, local tissue ischemia/hypoxia, and other symptoms due to a longer fasting time and insufficient preoperative fluid replacement. Such physiological disturbances could lead to delayed awakening and decreased awakening quality. Moreover, Hou et al. (27) found that the level of cortisol in the afternoon surgery group was higher than that in the morning surgery group, suggesting that afternoon surgery may trigger a more pronounced stress response in children, further influencing their post-anesthetic awakening process.
Although our pragmatic, prospective study has many advantages, including a substantial sample size of 560 surgical patients which enabled us to detect significant differences in the primary and some secondary endpoints between the groups, it is important to acknowledge several limitations inherent in our study design: (1) Our study exclusively focused on morning and afternoon groups, omitting an evening group from consideration. This choice was primarily dictated by the fact that nighttime surgeries in our hospital are predominantly emergency procedures, characterized by the patient’s preoperative condition often involving pain, hypoxia, and excessive tension that are uncommon for daytime surgeries. (2) Our study did not incorporate the measurement of dim-light melatonin onset (DLMO), a biomarker for the internal circadian rhythm (28), and did not monitor postoperative changes in body temperature, which exhibits rhythmicity. (3) The inclusion criteria did not consider whether participants had sleep disturbances or circadian rhythm disorders before surgery. These factors could potentially confound the observed effects of circadian rhythms on postoperative outcomes (29). (4) We only collected the perioperative parameters, and we acknowledge the possibility of long-term effects on cognitive function and sleep quality that may be associated with the immediate and reversible circadian rhythm disruption after surgery.
Conclusion
Our study suggests that daytime variation (morning and afternoon) in surgery timing may have an important effect on the incidence of postoperative delirium in children who undergo general anesthesia. Specifically, children scheduled for afternoon surgeries exhibited notably higher rates of delirium, coupled with prolonged stays in the PACU, compared to their counterparts who underwent morning surgeries. This difference may be related to an interaction between circadian rhythms, general anesthetics, and/or surgery and needs further study.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the ethics committee of the Guangzhou Women and Children's Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
WW: Project administration, Resources, Writing – original draft, Writing – review & editing. HX: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. YX: Funding acquisition, Investigation, Resources, Writing – review & editing. JQ: Investigation, Methodology, Writing – original draft. XG: Investigation, Methodology, Software, Supervision, Writing – original draft. XS: Project administration, Resources, Visualization, Writing – review & editing. GY: Conceptualization, Data curation, Formal Analysis, Writing – original draft. NZ: Methodology, Project administration, Supervision, Writing – review & editing. DM: Validation, Visualization, Writing – original draft. YT: Project administration, Resources, Validation, Visualization, Writing – original draft. TZ: Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Guangzhou Municipal Science and Technology Programs (grant numbers 202201020597, 2023A03J0899, 2024A03J1243) and the Guangzhou Science and Technology Projects Key Research and Development Program (grant number 202206010006).
Acknowledgments
We would like to thank Chongyang Duan of the Department of Biostatistics, School of Public Health, Southern Medical University, China, for his statistical analysis guidance during this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moore AD, Anghelescu DL. Emergence delirium in pediatric anesthesia. Paediatr Drugs. (2017) 19(1):11–20. doi: 10.1007/s40272-016-0201-5
2. Mason KP. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. (2017) 118(3):335–43. doi: 10.1093/bja/aew477
3. Vlajkovic GP, Sindjelic RP. Emergence delirium in children: many questions, few answers. Anesth Analg. (2007) 104(1):84–91. doi: 10.1213/01.ane.0000250914.91881.a8
4. Mohkamkar MB, Farhoudi FM, Alam-Sahebpour AM, Mousavi SAM, Khani SP, Shahmohammadi SB. Postanesthetic emergence agitation in pediatric patients under general anesthesia. Iran J Pediatr. (2014) 24(2):184–90.25535538
5. Doerrfuss JI, Kramer S, Tafelski S, Spies CD, Wernecke KD, Frequency NI. Predictive factors and therapy of emergence delirium: data from a large observational clinical trial in a broad spectrum of postoperative pediatric patients. Minerva Anestesiol. (2019) 85(6):617–24. doi: 10.23736/S0375-9393.19.13038-6
6. Costi D, Cyna AM, Ahmed S, Stephens K, Strickland P, Ellwood J, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev. (2014) 9:CD007084. doi: 10.1002/14651858.CD007084.pub2
7. Eckenhoff JE. Relationship of anesthesia to postoperative personality changes in children. AMA Am J Dis Child. (1953) 86(5):587–91. doi: 10.1001/archpedi.1953.02050080600004
8. Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. (2004) 99(6):1648–54. doi: 10.1213/01.ANE.0000136471.36680.97
9. Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. (2018) 19(8):453–69. doi: 10.1038/s41583-018-0026-z
10. Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. (2007) 47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208
11. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. (2005) 437(7063):1257–63. doi: 10.1038/nature04284
12. Wang D, Huang Y, Wang X, Chen X, Li J, Zhang S, et al. Circadian differences in emergence from volatile anaesthesia in mice: involvement of the locus coeruleus noradrenergic system. Br J Anaesth. (2020) 125(4):548–59. doi: 10.1016/j.bja.2020.07.012
13. Potts AL, Cheeseman JF, Warman GR. Circadian rhythms and their development in children: implications for pharmacokinetics and pharmacodynamics in anesthesia. Paediatr Anaesth. (2011) 21(3):238–46. doi: 10.1111/j.1460-9592.2010.03343.x
14. Orts-Sebastian A, Ludin NM, Pawley MDM, Cheeseman JF, Warman GR. Impact of anaesthesia on circadian rhythms and implications for laboratory experiments. Exp Neurol. (2019) 311:318–22. doi: 10.1016/j.expneurol.2018.09.017
15. Yang R, Xu XX, Liu H, Dai W, Zhang ZQ, Wang TT, et al. The impact of morning surgery or afternoon surgery on postoperative sleep quality and melatonin levels of elderly patients: a prospective, randomized study. Nat Sci Sleep. (2022) 14:1677–86. doi: 10.2147/NSS.S377209
16. Song Y, Liu Y, Yuan Y, Jia X, Zhang W, Wang G, et al. Effects of general versus subarachnoid anaesthesia on circadian melatonin rhythm and postoperative delirium in elderly patients undergoing hip fracture surgery: a prospective cohort clinical trial. EBioMedicine. (2021) 70:103490. doi: 10.1016/j.ebiom.2021.103490
17. Sadhasivam S, Ganesh A, Robison A, Kaye R, Watcha MF. Validation of the bispectral index monitor for measuring the depth of sedation in children. Anesth Analg. (2006) 102(2):383–8. doi: 10.1213/01.ANE.0000184115.57837.30
18. Silver G, Traube C, Kearney J, Kelly D, Yoon MJ, Nash Moyal W, et al. Detecting pediatric delirium: development of a rapid observational assessment tool. Intensive Care Med. (2012) 38(6):1025–31. doi: 10.1007/s00134-012-2518-z
19. Hong H, Guo C, Liu ZH, Wang BJ, Zhou SZ, Mu DL, et al. The diagnostic threshold of Cornell Assessment of Pediatric Delirium in detection of postoperative delirium in pediatric surgical patients. BMC Pediatr. (2021) 21(1):87. doi: 10.1186/s12887-021-02538-x
20. Crellin DJ, Harrison D, Santamaria N, Babl FE. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. (2015) 156(11):2132–51. doi: 10.1097/j.pain.0000000000000305
21. Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. (2017) 34(4):192–214. doi: 10.1097/EJA.0000000000000594
22. O'Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. (2021) 135(6):1132–52. doi: 10.1097/ALN.0000000000004046
23. Turek FW, Pinto LH, Vitaterna MH, Penev PD, Zee PC, Takahashi JS. Pharmacological and genetic approaches for the study of circadian rhythms in mammals. Front Neuroendocrinol. (1995) 16(3):191–223. doi: 10.1006/frne.1995.1007
24. Kadota K, Iijima N, Ohe-Hayashi Y, Takumi K, Higo S, Sakamoto A, et al. Time-dependent repression of Mper2 expression in the suprachiasmatic nucleus by inhalation anesthesia with sevoflurane. Neurosci Lett. (2012) 528(2):153–8. doi: 10.1016/j.neulet.2012.07.061
25. Mizuno T, Higo S, Kamei N, Mori K, Sakamoto A, Ozawa H. Effects of general anesthesia on behavioral circadian rhythms and clock-gene expression in the suprachiasmatic nucleus in rats. Histochem Cell Biol. (2022) 158(2):149–58. doi: 10.1007/s00418-022-02113-0
26. Sato Y, Seo N, Kobahashi E. The dosing-time dependent effects of intravenous hypnotics in mice. Anesth Analg. (2005) 101(6):1706–8. doi: 10.1213/01.ANE.0000184127.67866.2E
27. Hou H, Wu S, Qiu Y, Song F, Deng L. The effects of morning/afternoon surgeries on the early postoperative sleep quality of patients undergoing general anesthesia. BMC Anesthesiol. (2022) 22(1):286. doi: 10.1186/s12871-022-01828-w
28. St Hilaire MA, Lockley SW. Measuring dim light melatonin onset in humans. Methods Mol Biol. (2022) 2550:13–20. doi: 10.1007/978-1-0716-2593-4_3
Keywords: emergence delirium, diurnal variations, circadian rhythms, pediatric, general anesthesia
Citation: Wei W, Xie H, Xu Y, Qin J, Guo X, Song X, Yu G, Zhang N, Ma D, Tan Y and Zhao T (2024) The impact of diurnal variations on emergence delirium following general anesthesia and surgery in children. Front. Pediatr. 12:1437460. doi: 10.3389/fped.2024.1437460
Received: 23 May 2024; Accepted: 25 September 2024;
Published: 16 October 2024.
Edited by:
Nikolaos Zavras, University General Hospital Attikon, GreeceReviewed by:
Maksim Parfyonov, Cleveland Clinic, United StatesDong-Xin Wang, Peking University First Hospital, China
Copyright: © 2024 Wei, Xie, Xu, Qin, Guo, Song, Yu, Zhang, Ma, Tan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianyun Zhao, d2VueWFuXzQwNEB0b20uY29t; Yonghong Tan, eW9uZ2hvbmd0YW5kb2N0b3JAeWVhaC5uZXQ=
†These authors have contributed equally to this work
 Wei Wei1,†
Wei Wei1,† Haihang Xie
Haihang Xie Jingwen Qin
Jingwen Qin Xinying Guo
Xinying Guo Xingrong Song
Xingrong Song Gaofeng Yu
Gaofeng Yu Na Zhang
Na Zhang Daqing Ma
Daqing Ma Yonghong Tan
Yonghong Tan Tianyun Zhao
Tianyun Zhao