- 1Department of Rheumatology and Immunology, Children’s Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Ultrasonography, Children’s Hospital of Nanjing Medical University, Nanjing, China
Objective: It has been recognized that there is a nexus among Trisomy 8 (T8), Behcet's disease (BD), and myelodysplastic syndrome (MDS). We reported a series of inflammatory features in 2 children with T8 without hematological involvement.
Methods: 2 children with trisomy 8 who were excluded from MDS were retrospectively collected from the Department of Rheumatology and Immunology, Children's Hospital of Nanjing Medical University, Nanjing.
Results: Patients developed a range of inflammatory manifestations before a diagnosis of T8. The clinical manifestations of T8 patients vary from normal to severely disabled. Glucocorticoids and thalidomide can effectively relieve inflammation in patients with T8.
Conclusion: The early clinical manifestations of T8 in children lack specificity, and the diagnosis is mainly based on karyotype analysis, gastrointestinal endoscopy and bone marrow aspiration findings. Active and effective immunoregulatory therapy and long-term follow-up can improve the prognosis of patients with T8.
Introduction
T8 is a rare chromosomal abnormality disorder. T8 may be an acquired abnormality or a congenital abnormality that mainly occurs in the form of a chimera. However, complete T8 often results in miscarriage or embryo termination in the first trimester (1). T8, as an acquired condition, is found in haematological disorders, notably in myelodysplasia (MDS) and acute myeloid leukaemia (AML), and is restricted to the malignant cells. These arise in the bone marrow and may also be found in the peripheral blood (2). The phenotype of T8 varies from normal to severely disabled, characterized by mental retardation, facial deformities, skeletal deformities, delayed psychomotor development, urogenital and cardiovascular malformations, and developing neoplastic disorders (3). There is a strong link between MDS and T8 (+8-MDS) and autoinflammatory diseases, particularly Behcet's disease (BD) (4). Recent studies have focused on the autoimmune and autoinflammatory characteristics of T8-MDS while ignoring abnormal karyotypes without myelodysplastic abnormalities (5). BD is a chronic recurrent systemic inflammatory disease with unknown etiology that is mainly characterized by oral ulcers, genital ulcers, and ocular inflammation. Fever is a common manifestation of various diseases in children. In addition to infection-related causes, recurrent fever in children may also be caused by non-infective factors such as autoinflammation, thermic dysregulation, single gene mutations, and chromosome abnormalities. Relevant studies have shown that T8 is an important cause of inflammatory fever, which often causes periodic fever and Behcet's-like disease (5).
Case presentation
Case 1
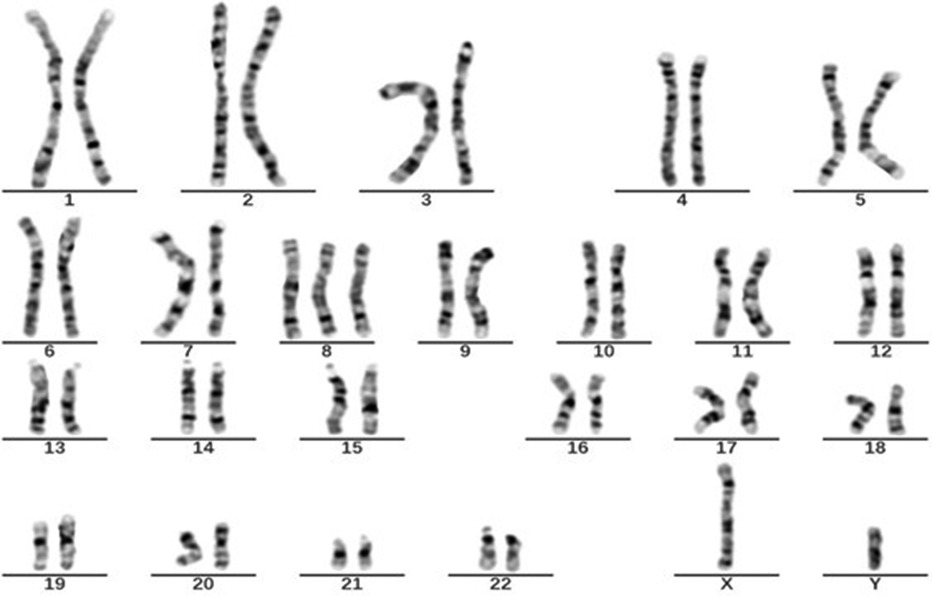
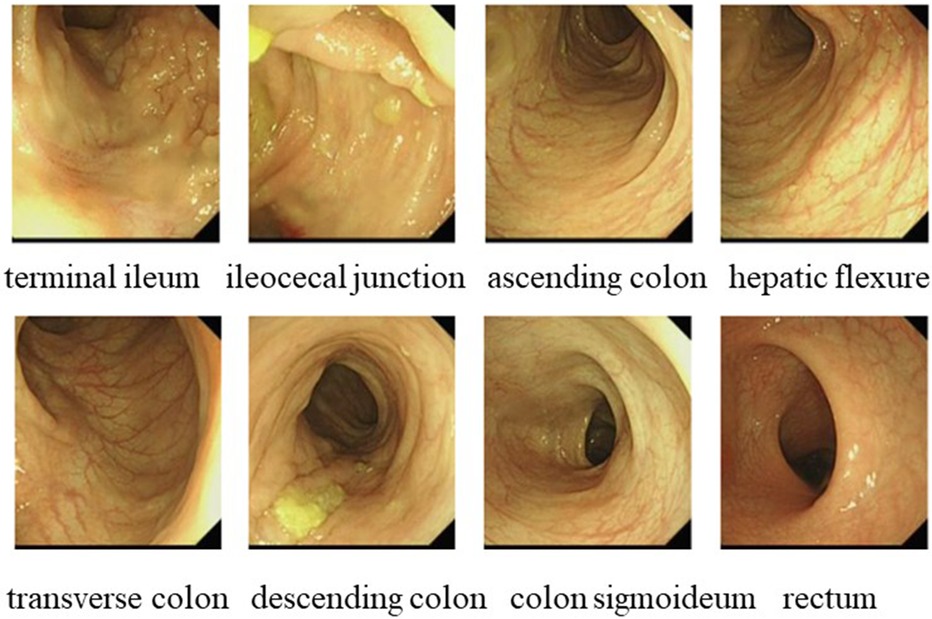
In October 2021, a 4-year-old boy who presented to our hospital with recurrent fever and oral ulcers lasting for 6 months was suspected of having PFAPA syndrome due to recurrent regular fever (every month with a duration of 4–5 days), oral ulcers, tonsillitis, and elevated inflammatory markers, the absence of rash, and the ineffectiveness of antibiotic treatment. His C-reactive protein (CRP) (35.81 mg/L), erythrocyte sedimentation rate (ESR) (30 mm/h), procalcitonin (PCT) (1.57 ng/ml), and heparin-binding protein (85.5 ng/ml) levels were high. Etiological examinations, such as blood culture and urine culture, were both negative. The possibility of infection with Epstein–Barr virus, cytomegalovirus, hepatitis B virus, or Mycobacterium tuberculosis was excluded after pathogen antibody and DNA detection. IgA levels (1.51 g/L) were elevated, while IgM (0.799 g/L) levels were decreased. Tests for both autoinflammatory factors and anti-nuclear antibodies were negative. Fever was controlled in the patient treated with 17.5 mg of prednisone, an anti-inflammatory therapy. To determine the etiology, karyotype analysis was performed, and the patient was diagnosed with a duplication of chromosome 8 (Figure 1). In March 2023, the patient was readmitted for further investigation via gastrointestinal endoscopy (Figure 2) and bone marrow aspiration, both of which revealed no abnormalities. The child started receiving treatment with 25 mg of thalidomide once a night on March 9, 2023. Four months after the drug treatment, the patient's CRP (2.15 mg/L) and ESR (15 mm/h) were within the normal range. And during follow-up, the patient's symptoms were well controlled.
Case 2
In April 2021, a 7-year-old boy who presented to our hospital with “recurrent oral ulcers and fever for 5 years” and who was newly diagnosed with incomplete BD was diagnosed with fever and multiple refractory ulcers in the mouth, ileum, cecum, transverse colon, descending colon, and perianal region (Figure 3). There were no detectable skin lesions or ocular manifestations of BD, and the skin prick test was negative. The ESR (21 mm/h) and fecal calprotectin (197.7 ug/g) concentration were elevated. IgA (3.22 g/L) and IgG (13.1 g/L) levels were elevated, while IgM (0.755 g/L) levels were decreased. Tests for both autoinflammatory factors and anti-nuclear antibodies were negative. The patient was treated with a combination of mesalamine, sulfasalazine, and prednisone. In June 2021, chromosome karyotype analysis revealed a duplication on chromosome 8 and a mutation in the CYBB gene (Figure 4). Then, the patient underwent the first BM examination, which revealed that the morphology of bone marrow cells was normal. After 4 months and 2 years of drug treatment, the patient was re-examined via gastroenteroscopy, and no intestinal ulcers were observed in either examination (Figure 3). In addition, the patient's CRP (6.79 mg/L), ESR (8 mm/h), and ferritin (76.7 ng/ml) levels were all within normal limits upon re-examination. During the reduction of GCs, the patient's symptoms did not recur, and her blood cells did not decrease. Currently, the child is receiving oral administration of thalidomide for maintenance therapy and has undergone regular follow-up.
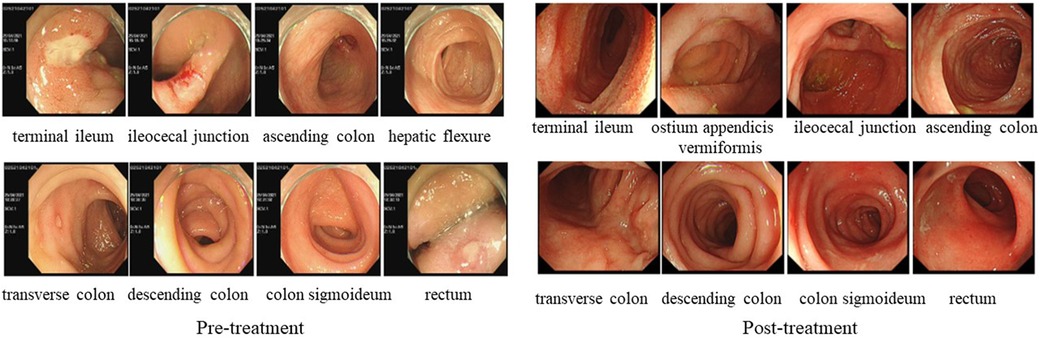
Figure 3. LEFT: gastrointestinal endoscopy in case 2 before treatment showed multiple ulcers throughout the distal ileum, ileocecal part, and colon. RIGHT: gastrointestinal endoscopy in case 2 after treatment showed multiple intestinal ulcers have recovered.
Discussion and conclusion
Herein, we report two patients with T8 disease without myelodysplasia. These patients had autoinflammatory features, such as recurrent fever, rash, gastrointestinal involvement, and elevated inflammatory marker levels, without abnormalities in hematology, such as anemia and multilineage cytopenia. In Patient 1, periodic fever was the main manifestation. In Patient 2, the patient had gastrointestinal involvement. Studies of multiple patients have shown that almost all BD-like diseases occurring in +8-MDS patients are different from classical BD, with fewer eye lesions but more inflammation of the gut (mainly ileocecal). The BD-like manifestations of Patient 2 were consistent with this feature. Both of our patients experienced major symptom relief when treated with corticosteroids and thalidomide. In addition, corticosteroid reduction was successful, with no adverse events during follow-up.
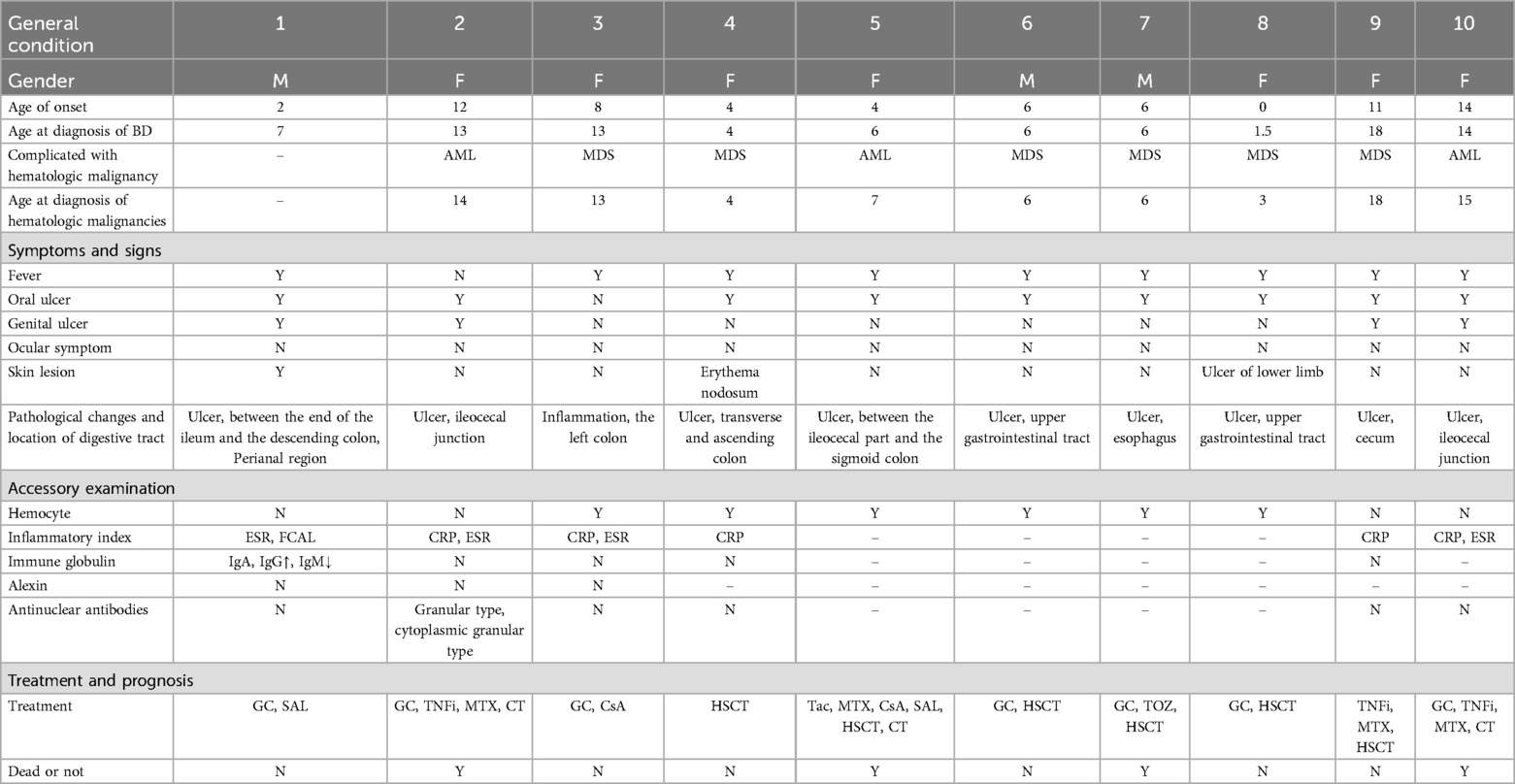
T8 associated with Behçet's-like disease is less reported in children than in adults. We searched the literature on T8 with BD in children published in PubMed using keywords including “Trisomy 8” and “Behçet's disease”, and selected patients under the age of 18 with gastrointestinal involvement. In addition, we listed 2 cases with gastrointestinal involvement reported by ZHAO Wanwen et al. in a Chinese literature that is not indexed in PubMed in Table 1. We found a total of 9 children with T8 who have gastrointestinal manifestations, excluding our two patients. Almost all of these patients presented with gastrointestinal ulcers, of whom 4 patients had ileocecal involvement, and several patients had ulcers in the esophagus, upper digestive tract, ascending colon, transverse colon, descending colon, and sigmoid colon. Most patients had recurrent oral and genital ulcerations. However, skin lesions were present in only 2 of the 9 patients, and there were no eye lesions in any of the patients. Gastrointestinal involvement without eye lesions is one of the characteristic clinical findings in BD associated with T8. Our case 2 is consistent with this conclusion. In addition, all 9 patients had hematological system involvement, mainly manifested as AML and MDS, which occurred after the diagnosis of BD. In terms of treatment, glucocorticoids are used in more than ordinary cases and effectively alleviate the inflammatory manifestations in patients. Four patients were treated with tumor necrosis factor inhibitors (TNFi), thalidomide, and tacrolimus, which can effectively relieve gastrointestinal symptoms. Only 1 patient still experienced recurrent abdominal pain and intestinal ulcers after TNFi treatment, which were relieved after surgical treatment (6–10).
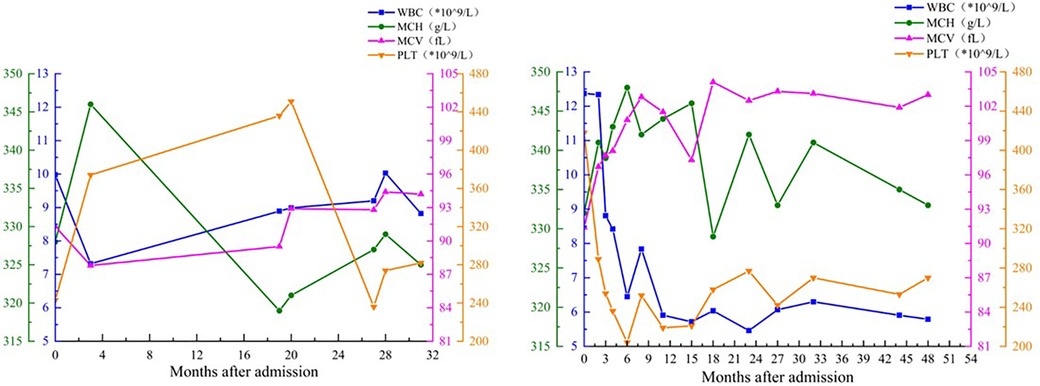
In cases of cytogenetic abnormalities, T8 is the only chromosomal aberration in 5% of AML patients and 10% of MDS patients (11). However, in the absence of morphological features to diagnose MDS, T8 is not sufficient to diagnose MDS; in other words, T8 alone is not sufficient to induce the occurrence of hematological diseases. Significantly, patients with T8 disease are at increased risk of developing bone marrow malignancies, and patients with T8 disease without MDS may develop MDS over time (4). Whether and how long children's disease progresses to MDS is unknown and may vary depending on factors such as disease severity and treatment response. Therefore, for these two T8 patients, continuous follow-up of the blood system is highly important, even though no significant abnormalities have been observed in the peripheral blood in these two children at present (Figure 5).
Chromosome 8 contains genes that regulate immune and inflammatory responses, and abnormal chromosome expression causes elevated levels of interleukin (IL)-1β, IL-6, and other inflammatory factors. Repeatedly high levels of inflammatory factors can activate neutrophils and promote their production of reactive oxygen species, leading to the occurrence and development of BD (1). However, only a minority of T8 patients exhibit BD-like disease, suggesting that T8 is also insufficient to cause autoinflammatory or BD-like phenotype (11). A retrospective analysis of MDS patients and BD patients revealed that the occurrence of intestinal ulcers was closely related to MDS and T8, while fever seemed to be mainly related to T8 (12). Furthermore, in previous reports, T8 was the most frequently detected chromosomal abnormality in cases of inflammatory fever, and it often leads to periodic fever in children (4). Therefore, for children with BD and unexplained fever, the possibility of T8 should be considered when there are atypical manifestations, such as developmental abnormalities, special facial features, joint malformations, atypical endoscopic intestinal lesions, or signs of hematological disease (1). A thorough physical examination revealed that patient 1 had finger deformities (claw hands), a spherical nose, and a slightly wider cleft palate. Patient 2 had an occult penis. Therefore, at the first admission of these two patients, we performed a chromosome karyotype examination for each patient and detected +8 as early as possible, which is highly important for disease control and prognosis in children.
At present, there are no guidelines for the diagnosis and treatment of T8 children with BD, fever, or other related inflammatory manifestations, and it is necessary to develop an individualized treatment plan according to clinical symptoms and immunological phenotypes. Glucocorticoids are widely used in T8 patients and can effectively control T8-related symptoms, such as fever, abdominal pain, and oral ulcers, and inflammation indicators are significantly reduced. However, the side effects of glucocorticoids cannot be ignored, especially for growing children. Timely and safe reduction of glucocorticoids is essential for alleviating side effects, but adverse reactions such as fever and elevated inflammatory indicators may occur during the process of reduction (4), which is also a difficult challenge in the treatment process. Therefore, the use of immunosuppressants and biologics is crucial for promoting glucocorticoid reduction and improving systemic inflammation. These two patients and five nonhematological T8 children in the study by Zhao et al. had a good prognosis and no deaths after aggressive treatment. However, if the blood system of a child with T8 disease is affected, the patient may not respond to conventional drug therapy, which may lead to adverse outcomes, and early bone marrow or peripheral blood stem cell transplantation may be able to completely control the disease (1).
Thalidomide (THD) is an immunomodulatory drug broadly applied in BD treatment. A large number of clinical cases have demonstrated that THD can rapidly improve oral and genital lesions in patients with Behcet's disease, prolong the recurrence time, and reduce the severity of symptoms (13). TNF-α is a new cytopathogenic factor of BD and MDS that can induce programmed cell death by inhibiting normal hematopoietic cells and subsequently accelerating the disease progression of BD or MDS (14). Thalidomide exerts antivascular effects by inhibiting the secretion of TNF-α by white blood cells and reducing the production of vascular endothelial growth factor (VEGF). In addition, thalidomide is more affordable than other biologics (14). Patients with gastrointestinal involvement of Behçet's syndrome who were refractory to the conventional therapy can often get clinical and endoscopic remission with TNF-alpha antagonists and/or thalidomide (15). Based on clinical experience and literature reports, THD was also used in our 2 patients, and satisfactory results were obtained.
However, our study is only a single-center report from our institution and not a large multicenter study; thus, the results are merely a preliminary summary of the clinical characteristics of such patients, and the specific mechanisms require further investigation. Additionally, there is currently a lack of reference guidelines for the treatment and clinical management of T8-BD children, particularly regarding high inflammatory manifestations and gastrointestinal involvement. Besides, the risk of future hematological disorders in these children is unknown.
In conclusion, children with T8 can have a wide variety of phenotypes that vary in severity. We focus on the fact that the early clinical manifestations of T8 in children lack specificity. Therefore, for children with BD, fever of unknown origin, recurrent oral ulcers, and unexplained gastrointestinal ulcers, perfecting chromosome karyotype analysis, gastrointestinal endoscopy, and bone marrow aspiration as soon as possible is highly important for the diagnosis, treatment, and prognosis of patients. Both of our cases treated with THD achieved favorable therapeutic effects, which may provide clinical physicians with treatment experience for T8 associated with gastrointestinal involvement. Active and effective immunoregulatory therapy and long-term follow-up can improve the prognosis of patients with T8.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the studies involving human participants were reviewed and approved by Children's Hospital of Nanjing medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Writing – original draft, Writing – review & editing, Conceptualization. YZ: Conceptualization, Writing – review & editing. YP: Data curation, Formal Analysis, Methodology, Writing – review & editing. JJ: Writing – review & editing, Data curation, Formal Analysis, Methodology. ZF: Writing – review & editing, Funding acquisition, Project administration, Supervision, Writing – original draft. HY: Writing – review & editing, Funding acquisition, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the National Key R&D Program of China (grant number: 2021YFC2702000), the National Natural Science Foundation of China (grant number: 81771762 and 82271838) and Project of the Jiangsu Provincial Health Commission (grant number: M2022018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wanwen Z, Haimei L, Tianyu Z, Sirui Y, Li S, Hongmei S. Clinical features of childhood Behcet ’s disease like trisomy 8: a summary of 19 cases. Med J Peking Union Med Coll Hosp. (2023) 14(02):299–305. doi: 10.12290/xhyxzz.2022-0683
2. Fitchettt LMS-WAM. Constitutional and acquired trisomy 8. Leuk Res. (1995) 19:737–40. doi: 10.1016/0145-2126(95)00051-o
3. Laursen AC, Sandahl JD, Kjeldsen E, Abrahamsson J, Asdahl P, Ha SY, et al. Trisomy 8 in pediatric acute myeloid leukemia: a NOPHO-AML study. Genes Chromosomes Cancer. (2016) 55(9):719–26. doi: 10.1002/gcc.22373
4. Fu Y, Wu W, Chen Z, Gu L, Wang X, Ye S. Trisomy 8 associated clonal cytopenia featured with acquired auto-inflammation and its response to JAK inhibitors. Front Med (Lausanne). (2022) 9:895965. doi: 10.3389/fmed.2022.895965
5. Sun B, Yang M, Hou J, Wang W, Ying W, Hui X, et al. Chromosomal abnormalities related to fever of unknown origin in a Chinese pediatric cohort and literature review. Orphanet J Rare Dis. (2022) 17(1):292. doi: 10.1186/s13023-022-02444-0
6. Shigemura T, Agematsu K, Yamazaki T, Sakashita K, Nakayama Y, Higuchi Y, et al. A case of Behcet’s disease associated with myelodysplastic syndrome involving trisomy 8 and a gain-of-function mutation in SHP-2. Rheumatology (Oxford). (2011) 50(7):1342–4. doi: 10.1093/rheumatology/ker137
7. Kanamitsu K, Shimada A, Nishiuchi R, Shigemura T, Nakazawa Y, Koike K, et al. Pediatric intestinal Behçet disease complicated by myeloid malignancies. Int J Hematol. (2017) 105(3):377–82. doi: 10.1007/s12185-016-2127-7
8. Yanir AD, Krauss A, Stein J, Steinberg-Shemer O, Gilad O, Lotan SN, et al. Pediatric myelodysplastic syndrome with inflammatory manifestations: diagnosis, genetics, treatment, and outcome. Pediatr Blood Cancer. (2021) 68(10):e29138. doi: 10.1002/pbc.29138
9. Asano T, Sato S, Furuya MY, Takahashi H, Shichishima-Nakamura A, Ohkawara H, et al. Intestinal Behçet disease associated with myelodysplastic syndrome accompanying trisomy 8 successfully treated with abdominal surgery followed by hematopoietic stem cell transplantation: a case report. Medicine (Baltimore). (2019) 98(46):e17979. doi: 10.1097/md.0000000000017979
10. Udayakumar AM, Al-Kindy A. Constitutional trisomy 8 mosaicism syndrome: case report and review. J Pediatr Genet. (2013) 2(4):197–201. doi: 10.3233/pge-13069
11. Paulsson K, Johansson B. Trisomy 8 as the sole chromosomal aberration in acute myeloid leukemia and myelodysplastic syndromes. Pathol Biol (Paris). (2007) 55(1):37–48. doi: 10.1016/j.patbio.2006.04.007
12. Esatoglu SN, Hatemi G, Salihoglu A, Hatemi I, Soysal T, Celik AF. A reappraisal of the association between Behçet’s disease, myelodysplastic syndrome and the presence of trisomy 8: a systematic literature review. Clin Exp Rheumatol. (2015) 33(6 Suppl 94):S145–51.25664843
13. Huang MX, Wang CY, Guo JY, Li JH, Li XH, Zhang JA, et al. Pharmacotherapy for Behçet’s disease and the risk of malignancy. Front Pharmacol. (2021) 12:661150. doi: 10.3389/fphar.2021.661150
14. Wei Q, Zhang X, Peng Y, Chen K, Xu S, Huang X, et al. Successful treatment by thalidomide therapy of intestinal Behçet’s disease associated with trisomy 8 myelodysplastic syndrome. Rheumatology (Oxford). (2021) 60(6):e200–2. doi: 10.1093/rheumatology/keaa848
15. Hatemi I, Hatemi G, Pamuk ON, Erzin Y, Celik AF. TNF-alpha antagonists and thalidomide for the management of gastrointestinal Behçet’s syndrome refractory to the conventional treatment modalities: a case series and review of the literature. Clin Exp Rheumatol. (2015) 33(6 Suppl 94):S129–37. doi: 10.1111/j.1365-4632.2011.04871.x
Keywords: trisomy 8, Behcet’s disease, myelodysplastic syndrome, thalidomide, gastrointestinal involvement
Citation: Zhang X, Zhao Y, Pan Y, Jin J, Fan Z and Yu H (2024) Trisomy 8 presentation by inflammatory manifestations and its response to thalidomide: two case reports and narrative review. Front. Pediatr. 12:1431511. doi: 10.3389/fped.2024.1431511
Received: 22 May 2024; Accepted: 2 August 2024;
Published: 20 August 2024.
Edited by:
Vahid Ziaee, Tehran University of Medical Sciences, IranReviewed by:
Yakai Fu, Shanghai Jiao Tong University, ChinaFarhad Salehzadeh, Ardabil University of Medical Sciences, Iran
Copyright: © 2024 Zhang, Zhao, Pan, Jin, Fan and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidan Fan, emhpZGFuZmFuQG5qbXUuZWR1LmNu; Haiguo Yu, aGFpZ3VvX3l1QG5qbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xiaohua Zhang
Xiaohua Zhang Yan Zhao2,†
Yan Zhao2,† Haiguo Yu
Haiguo Yu