- 1Department of Radiology, Shaoxing Central Hospital, Shaoxing, China
- 2Imaging Center, Tengzhou Central People’s Hospital, Tengzhou, China
- 3Department of Pathology, Tengzhou Central People’s Hospital, Tengzhou, China
Wandering spleen (WS) concurrent with splenic pedicle torsion and infarction has been described rarely. We reported our experience in diagnosing and treating such a condition in a 16-year-old girl with acute abdominal pain. A plain CT scan showed the wandering of the spleen from the left upper quadrant. Contrast-enhanced CT indicated dilatation and distortion in the splenic vein, a counterclockwise “whirl sign” in the splenic pedicle, pancreatic tail torsion, and splenic infarction. The patient was diagnosed with WS combined with splenic pedicle torsion and splenic infarction and underwent splenectomy for treatment. She showed a satisfactory outcome during the follow-up. To enhance our understanding of it, we performed a comprehensive literature research to summarize the clinical manifestations, treatment options, and outcomes among adolescent patients.
1 Introduction
Wandering spleen (WS), also known as ectopic spleen, is a rare clinical condition in which the spleen migrates to an abnormal position within the abdomen or pelvis (1). It mainly affects children aged between 3 months and 10 years old and women of childbearing age (2). A major complication of WS is the torsion of the splenic pedicle, which can subsequently cause splenic infarction and rupture (3). In clinical practice, WS combined with acute splenic torsion is considered a life-threatening emergency (4). However, the diagnosis of this condition remains challenging due to its asymptomatic progression.
Currently, the diagnosis of WS mainly depends on clinical and imaging features, especially contrast-enhanced CT. This imaging technique can reveal the location, size, vascular condition of the spleen, and presence of splenic pedicle torsion. To date, less attention has been paid to the WS and the splenic torsion due to the rarity of cases. In this study, we present a case of a 16-year-old girl who presented with acute abdominal pain and was eventually diagnosed with WS, pedicle torsion, and splenic infarction.
2 Case presentation
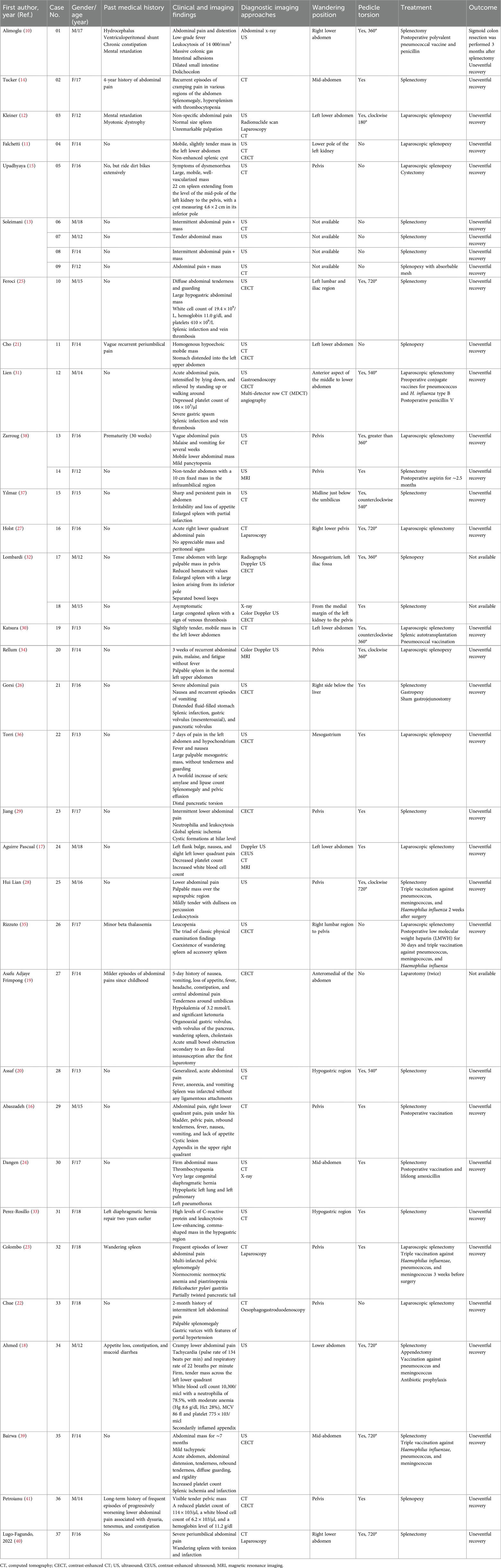
A 16-year-old girl presented to our hospital’s emergency department with progressive aggravation of abdominal pain for at least 24 h. Physical examination revealed a migrating mass with tenderness in the left lower quadrant. A plain CT scan showed the spleen absent from the left upper quadrant (Figure 1, Plate 1A). In other areas of the left abdomen, a soft tissue density shadow in an elliptic shape was observed. The visualization of hyperdense splenic vessels suggested thrombosis of the splenic vein (Figure 1, Plate 1B). A contrast-enhanced CT scan showed a counterclockwise “whirl sign” in the splenic pedicle, along with pancreatic tail torsion and splenic infarction (Figure 1, Plate 1C). Furthermore, the visualized splenic veins showed dilatation and distortion (Figure 1, Plate 1D). Vascular reconstruction showed an elongated splenic pedicle vessel (Figure 1, Plate 2). The CT scan revealed an enlarged spleen with no enhancement in the arterial and venous phase, suggesting splenic ischemia and infarction (Figure 1, Plate 3).
Figure 1. Plate 1, contrast-enhanced computed tomography (CECT) images. (A) Absence of the spleen in the left upper abdomen after plain CT scan. (B) CT scan indicated an elliptic soft tissue density shadow (yellow arrow) in the left upper quadrant. The hyperdense shadows in splenic veins suggested splenic vein thrombosis (red arrow). (C) The axial section showed a “whirl sign” (red arrow) of the splenic pedicle vessels counterclockwise, together with pancreatic tail torsion (green arrow). The white arrow indicated the absence of obvious enhancement in the spleen, showing a possibility of splenic infarction. (D) The visualized splenic veins showed dilatation and tortuosity (yellow arrow). Plate 2, computed tomography (CT) image showing elongated splenic pedicle vessels (yellow arrow). Plate 3, coronal and sagittal CT images. (A) Contrast-enhanced CT scan indicated splenomegaly. There was pelvic fluid induced by splenic infarction. (B) There was a filling of contrast agent in the upper segment of the vessels (yellow arrow), while there was no filling in the lower segment (red arrow). This indicated a block of the splenic artery.
The patient was finally diagnosed with WS combined with splenic pedicle torsion and splenic infarction. Laparoscopy was then performed in a supine position after anesthesia. A 10 mm trocar was inserted through an arc-shaped incision below the umbilicus. Carbon dioxide was injected to establish pneumoperitoneum, with pressure set to 14 mmHg. The laparoscope showed a black and infarcted spleen without ligamentous fixation that was merely suspended by the vascular pedicles in the left lower abdomen. In addition, the splenic pedicle was twisted 720° counterclockwise, along with pancreatic tail torsion.
For the treatment, splenectomy (Figure 2) was performed since the splenic ischemia showed no improvement even after splenic repositioning. Three trocars (i.e., 10 mm, 5 mm, and 5 mm) were placed in the right lower abdomen, right umbilicus, and left upper abdomen, respectively. The spleen was repositioned to the splenic fossa, where it showed no adhesion to the surrounding structure and omentum. The splenic pedicle vessels were double-ligated at the pancreatic tail, and the splenic artery and vein branches were separated and cut using Hem-o-lock clips. The spleen was placed in a retrieval bag and was cut into pieces, and then the pieces were removed through the umbilical incision. The abdominal cavity was flushed, and the splenic blood vessels were ligated. A drainage tube was placed in the left upper abdomen and exited through the left trocar hole. The surgical time was 200 min, and the intraoperative bleeding volume was ∼30 ml. Postoperative histopathological analysis confirmed ischemic necrosis. During the 10-month follow-up, the patient showed normal conditions with no recurrence of abdominal pain.
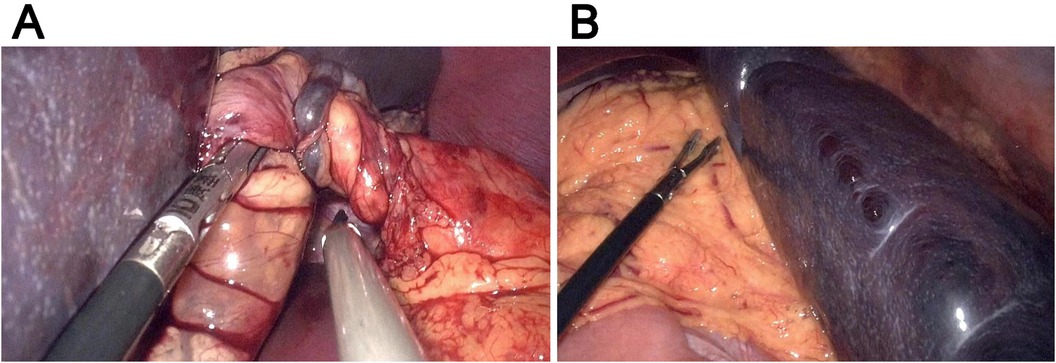
Figure 2. Representative intraoperative images. (A) The twisted splenic pedicle. (B) The splenic tissues of the spleen.
3 Discussion
WS with torsion of the splenic pedicle is an extremely rare condition with an incidence rate of <0.2% (5). There is no sex difference under the age of 10 years; however, after the first decade, females have a higher prevalence of WS than that in males, with a ratio of 7:1 (6). Nevertheless, the diagnosis of WS combined with splenic pedicle torsion in adolescents remains challenging due to the rarity of cases. In this study, we report a 16-year-old girl with WS accompanied by splenic pedicle torsion and splenic infarction. Meanwhile, a comprehensive literature review was undertaken to fully enhance our understanding of this condition.
Anatomically, the spleen is fixed by six primary suspensory ligaments (i.e., gastrosplenic, splenorenal, splenophrenic, splenocolic, splenopancreatic, and presplenic fold ligaments) and two ligaments (pancreaticocolic and phrenicocolic ligaments) in an indirect association. The gastrosplenic, splenorenal, and phrenicocolic ligaments are usually loose or absent in the etiology of WS (2). Congenitally, WS is caused by the absence or deficits of ligaments resulting from the failure of the dorsal mesentery to fuse with the posterior abdominal wall during embryogenesis, which can be accompanied by developmental abnormalities such as congenital diaphragmatic hernia or intestinal malrotation (7). Additionally, the laxity of the supporting ligaments has been identified as an acquired cause of WS. This condition was mainly presented in women of childbearing age, secondary to multiple pregnancies and hormonal changes (8). Other acquired causes included malaria-, infectious mononucleosis- or lymphoma-induced chronic splenomegaly, abdominal trauma, and surgery (9).
Congenital WS is more common in adolescents than in adults. Cases are generally asymptomatic or show mild symptoms in childhood as the WS does not twist in the early years. The risk of WS increases with age and exercise. In adulthood, the prevalence of WS increases, with many patients developing acquired WS. In this study, we present a case of a 16-year-old girl with acute abdominal pain due to WS and splenic pedicle torsion. Therefore, we focused on the literature search on this condition in adolescents rather than adults. Herein, we identified 32 (10–41) previous case reports and case series published from 2003 to 2023 involving 37 WS cases (female, 25; male, 12) aged 12–18 years, as presented in Table 1. There was no difference in the sex ratio under the age of 10 years, but females showed a higher prevalence of WS than that in males (6).
The diagnosis of WS is usually a challenge in clinical practice. Its symptoms vary according to the size and location of the spleen. For example, in our literature review, some cases were completely asymptomatic in the early stage (11). Along with disease progression, migration of the spleen predisposes to the torsion of the lengthy and loosening of the splenic pedicle. In the presence of mild torsion, the thin-walled splenic vein and its tributaries were first affected, resulting in congestive splenomegaly, abdominal discomfort or intermittent abdominal pain (13), and enlarged palpable mass with tenderness. Regional portal hypertension and gastric varices bleeding may occur in a long-term incomplete torsion (22). Moreover, the chronic splenomegaly can induce complete torsion. On this basis, the splenic artery blood flow will be blocked, and the spleen will be infarcted. The clinical symptoms usually include acute abdominal pain, hypotension, and even shock, accompanied by peritoneal irritation (31). The additional signs include fever, nausea, vomiting, loss of appetite, and a palpable mass in the mid-lower abdomen or pelvic cavity. Our case showed acute abdominal pain when presenting to our department. Indeed, attention should be paid to the differential diagnosis of WS from other diseases with acute abdominal pain as the initial symptom.
Laboratory tests for WS patients are mostly normal and non-specific, and occasionally, some patients may present with leukocytosis (10, 17, 28, 29, 33), thrombocytopenia (14, 17, 24, 31, 41), and neutrophilia (18, 29). Additionally, two patients (i.e., Patients 13 and 17) (32, 38) exhibited mild pancytopenia. One patient (i.e., Patient 22) (36) showed a twofold increase in serum amylase and lipase counts. Another patient (i.e., Patient 31) (33) showed an increased C-reactive protein level. Our case showed normal laboratory indicators, which implied that these laboratory indices may somehow contribute to the diagnosis of WS, but with low efficiency.
WS is usually confirmed by imaging methods such as plain x-ray (42), Doppler ultrasonography (USG) (43), CT (44), and magnetic resonance imaging (MRI) (39). In addition to these methods, one study (17) applied contrast-enhanced ultrasound (CEUS) for the diagnosis of WS. The images were characterized by the absence of the spleen in the left upper quadrant and the presence of a soft tissue mass elsewhere in the abdomen or pelvis. Nevertheless, CT is still the modality of choice for the final diagnosis of WS (45) as it shows multiple advantages such as high sensitivity (46) for the identification of splenic pedicle torsion. In our literature review, 30/37 (81%) adolescents were diagnosed with WS by CT. For the incomplete torsion, CT showed congestive enlargement of the spleen, together with dilated and twisted splenic veins. For complete torsion, splenic artery blood flow blockage was observed after the CT scan, and some or all non-enhancement areas appeared in the spleen. This indicated partial or total splenic infarction. Splenic pedicle torsion is characterized by splenomegaly and abnormal orientation of the splenic hilum, and a “whirl sign” can be used to predict parenchymal organ torsion (44). The hyperdense splenic pedicle is indicative of the thrombosed splenic vein (47). Capsule sign and peritoneal effusion can indirectly reflect splenic infarction. The VR reconstruction would visualize the position of the torsion and the elongated splenic pedicle. Our case presented with WS, combined with complete torsion of the splenic pedicle and splenic infarction. Contrast-enhanced CT showed the absence of mass enhancement in the left lower quadrant, and it was necessary to differentiate mesenteric cysts, lymphangiocysts, and adnexal tumors. Additionally, pancreatic volvulus was also reported in Patients 21 (26), 22 (36), 27 (19), and 32 (23). There may be rare symptoms such as pancreatic tail necrosis and pancreatitis under the simultaneous torsion of the WS and the pancreatic tail. Our case showed torsion of the pancreatic tail. The splenic artery was a terminal artery without communication and was also the terminal arterial circulation. Therefore, the incidence of splenic infarction was higher than that of other organs. Theoretically, any disease that can form an embolism can lead to splenic infarction. In addition to splenic pedicle torsion caused by WS, the other common causes of splenic infarction in children included infection, blood system diseases, and autoimmune diseases, such as infective endocarditis, malaria, sickle cell anemia, infectious mononucleosis, chronic myeloid leukemia, lymphoma, antiphospholipid syndrome, and arteritis (48). In the future, attention should be paid to the exclusion of in situ splenic infarction before considering the possibility of WS with splenic pedicle torsion.
Splenopexy and splenectomy have been used for the treatment of WS. In our literature review, 27 (73%) patients underwent splenectomy. Among these patients, 22 (81%) cases presented with different degrees of splenic torsion (Table 2). Splenopexy is the preferred treatment option for WS when there is no splenic torsion or when there is normal circulation in the distorted splenic vessels or normal blood supply after repositioning the splenic torsion. It is worth noting that a long-term follow-up is necessary given the likelihood of postoperative recurrence of splenic torsion. Splenectomy is required for treating WS accompanied by complications such as splenomegaly, splenic infarction, rupture, and thrombosis (6, 49). However, there is a high possibility of septicemia and severe infection after splenectomy. In our literature review, Patient 1 (10) received polyvalent pneumococcal vaccine and penicillin after splenectomy. Patient 12 (31) received conjugate vaccines against pneumococcus and Haemophilus influenza type B before laparoscopic splenectomy, as well as postoperative penicillin V. Patient 14 (38) received aspirin for about 2.5 months after splenectomy. Three patients (i.e., Patients 25, 26, and 39) (28, 35, 39) received triple vaccination against pneumococcus, meningococcus, and Haemophilus influenza after splenectomy, while one patient (i.e., Patient 32) (23) received the same vaccine before splenectomy. Patient 30 (24) received lifelong amoxicillin. Therefore, it is recommended to use antibiotics and capsular bacterial vaccines (e.g., Haemophilus influenzae, meningococcal, and pneumococcal vaccines).
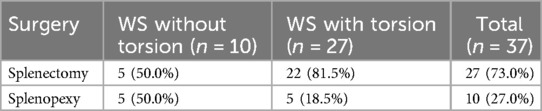
Table 2. Summary of surgical procedures for wandering spleen with and without torsion in the literature review.
4 Conclusion
We reported a rare case of WS combined with splenic pedicle torsion and infarction with acute abdominal pain as the initial symptom. WS with splenic pedicle torsion should be considered in the differential diagnosis in children who present with recurrent abdominal pain and a palpable abdominal mass. CECT is the first choice for early diagnosis of WS, which may improve the possibility of spleen preservation and prevent the occurrence of complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethical Committee of Shaoxing Central Hospital (No. 2023-018). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
QC: Data curation, Formal Analysis, Investigation, Resources, Software, Writing – original draft. XX: Data curation, Formal Analysis, Visualization, Writing – original draft. CL: Data curation, Formal Analysis, Methodology, Writing – original draft. LT: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reisner DC, Burgan CM. Wandering spleen: an overview. Curr Probl Diagn Radiol. (2018) 47(1):68–70. doi: 10.1067/j.cpradiol.2017.02.007
2. Puranik AK, Mehra R, Chauhan S, Pandey R. Wandering spleen: a surgical enigma. Gastroenterol Rep (Oxf). (2017) 5(3):241–3. doi: 10.1093/gastro/gov034
3. Ely AB, Zissin R, Copel L, Vasserman M, Hertz M, Gottlieb P, et al. The wandering spleen: CT findings and possible pitfalls in diagnosis. Clin Radiol. (2006) 61(11):954–8. doi: 10.1016/j.crad.2006.06.007
4. Viana C, Cristino H, Veiga C, Leão P. Splenic torsion, a challenging diagnosis: case report and review of literature. Int J Surg Case Rep. (2018) 44:212–6. doi: 10.1016/j.ijscr.2018.02.032
5. Memari M, Nikzad M, Nikzad H, Taherian A. Wandering spleen in an adult man associated with the horseshoe kidney. Arch Trauma Res. (2013) 2(3):129–32. doi: 10.5812/atr.9332
6. Nastiti NA, Niam MS, Khoo PJ. Emergency laparoscopic splenectomy for torsion of wandering spleen in a geriatric patient: a case report. Int J Surg Case Rep. (2019) 61:91–5. doi: 10.1016/j.ijscr.2019.07.021
7. Brown CV, Virgilio GR, Vazquez WD. Wandering spleen and its complications in children: a case series and review of the literature. J Pediatr Surg. (2003) 38(11):1676–9. doi: 10.1016/s0022-3468(03)00582-7
8. Romero JR, Barksdale EM Jr. Wandering spleen: a rare cause of abdominal pain. Pediatr Emerg Care. (2003) 19(6):412–4. doi: 10.1097/01.pec.0000101582.65509.cc
9. Mehta A, Vana PG, Glynn L. Splenic torsion after congenital diaphragmatic hernia repair: case report and review of the literature. J Pediatr Surg. (2013) 48(3):e29–31. doi: 10.1016/j.jpedsurg.2013.01.003
10. Alimoglu O, Sahin M, Akdag M. Torsion of a wandering spleen presenting with acute abdomen: a case report. Acta Chir Belg. (2004) 104(2):221–3. doi: 10.1080/00015458.2004.11679541
11. Falchetti D, Torri F, Dughi S, Porto C, Manciana A, Boroni G, et al. Splenic cyst in a wandering spleen: laparoscopic treatment with preservation of splenic function. J Pediatr Surg. (2007) 42(8):1457–9. doi: 10.1016/j.jpedsurg.2007.03.063
12. Kleiner O, Newman N, Cohen Z. Pediatric wandering spleen successfully treated by laparoscopic splenopexy. J Laparoendosc Adv Surg Tech A. (2006) 16(3):328–30. doi: 10.1089/lap.2006.16.328
13. Soleimani M, Mehrabi A, Kashfi A, Fonouni H, Büchler MW, Kraus TW. Surgical treatment of patients with wandering spleen: report of six cases with a review of the literature. Surg Today. (2007) 37(3):261–9. doi: 10.1007/s00595-006-3389-0
14. Tucker ON, Smith J, Fenlon HM, McEntee GP. Recurrent torsion of a wandering spleen. Am J Surg. (2004) 188(1):96–7. doi: 10.1016/j.amjsurg.2003.11.042
15. Upadhyaya P, St Peter SD, Holcomb GW 3rd. Laparoscopic splenopexy and cystectomy for an enlarged wandering spleen and splenic cyst. J Pediatr Surg. (2007) 42(5):E23–7. doi: 10.1016/j.jpedsurg.2007.03.001
16. Abaszadeh F, Taebi M, Nikzad Jamnani H. Torsion of wandering spleen attached to the omentum: a rare case report from Iran. Int J Gen Med. (2020) 13:333–6. doi: 10.2147/ijgm.S248259
17. Pascual EA, Fontanilla T, Pérez Í, Muñoz B, Carmona MS, Minaya J. Wandering spleen torsion-use of contrast-enhanced ultrasound. BJR Case Rep. (2017) 3(1):20150342. doi: 10.1259/bjrcr.20150342
18. Ahmed M, Nasir M, Negash A, Haile K. Wandering spleen with splenic torsion: unusual cause of acute abdomen. Int Med Case Rep J. (2022) 15:625–30. doi: 10.2147/imcrj.S388271
19. Frimpong GAA, Aboagye E, Ayisi-Boateng NK, Antwi K, Bawuah KA, Coleman NE, et al. Concurrent occurrence of a wandering spleen, organoaxial gastric volvulus, pancreatic volvulus, and cholestasis - a rare cause of an acute abdomen. Radiol Case Rep. (2019) 14(8):946–51. doi: 10.1016/j.radcr.2019.05.018
20. Assaf R, Shebli B, Alzahran A, Rahmeh AR, Mansour A, Hamza R, et al. Acute abdomen due to an infarction of wandering spleen: case report. J Surg Case Rep. (2020) 2020(2):rjz378. doi: 10.1093/jscr/rjz378
21. Cho SA, Choh NA, Dar I, Jehangir M, Yousuf R. Wandering spleen presenting as recurrent abdominal pain in a young female. Indian J Pediatr. (2008) 75(11):1181–2. doi: 10.1007/s12098-008-0181-8
22. Chue KM, Tan JKH, Pang NQ, Kow AWC. Laparoscopic splenectomy for a wandering spleen with resultant splenomegaly and gastric varices. ANZ J Surg. (2020) 90(10):2124–5. doi: 10.1111/ans.15737
23. Colombo F, D'Amore P, Crespi M, Sampietro G, Foschi D. Torsion of wandering spleen involving the pancreatic tail. Ann Med Surg (Lond). (2020) 50:10–3. doi: 10.1016/j.amsu.2019.12.001
24. Dangen J, Lau S, Abbas S. Treatment of a congenital diaphragmatic hernia with associated wandering spleen: case report of a 17-year-old girl. Int J Surg Case Rep. (2020) 77:32–5. doi: 10.1016/j.ijscr.2020.10.049
25. Feroci F, Miranda E, Moraldi L, Moretti R. The torsion of a wandering pelvic spleen: a case report. Cases J. (2008) 1(1):149. doi: 10.1186/1757-1626-1-149
26. Gorsi U, Bhatia A, Gupta R, Bharathi S, Khandelwal N. Pancreatic Volvulus with wandering spleen and gastric Volvulus: an unusual triad for acute abdomen in a surgical emergency. Saudi J Gastroenterol. (2014) 20(3):195–8. doi: 10.4103/1319-3767.133026
27. Holst JM. Acute abdominal pain in the pediatric emergency department: a case of right lower quadrant wandering spleen that acutely torsed. J Emerg Med. (2013) 44(6):e395–6. doi: 10.1016/j.jemermed.2012.11.063
28. Lian HH, Hayati F, Ali AA, Azizan N, Ani MFC, Suhaili MA, et al. Wandering spleen: a unique cause of acute abdomen. Folia Morphol (Warsz). (2018) 77(2):400–2. doi: 10.5603/FM.a2017.0097
29. Jiang M, Chen P, Ruan X, Ye X, Huang Q. Acute torsion of wandering spleen in a 17-year-old girl. Int J Clin Exp Med. (2015) 8(7):11621–3.26379994
30. Katsura S, Kawamura D, Harada E, Enoki T, Hamano K. Single-incision laparoscopic splenectomy and splenic autotransplantation for an enlarged wandering spleen with torsion. Eur J Pediatr Surg Rep. (2014) 2(1):23–5. doi: 10.1055/s-0033-1357262
31. Lien CH, Lee HC, Yeung CY, Chan WT, Wang NL. Acute torsion of wandering spleen: report of one case. Pediatr Neonatol. (2009) 50(4):177–80. doi: 10.1016/s1875-9572(09)60059-0
32. Lombardi R, Menchini L, Corneli T, Magistrelli A, Accinni A, Monti L, et al. Wandering spleen in children: a report of 3 cases and a brief literature review underlining the importance of diagnostic imaging. Pediatr Radiol. (2014) 44(3):279–88. doi: 10.1007/s00247-013-2851-6
33. Perez-Rosillo MA, Gomez-Huertas M, Salmeron-Ruiz A, Lainez-Ramos-Bossini AJ. Acute abdomen secondary to torsion and infarction of a wandering spleen. Gastroenterol Hepatol. (2021) 44(8):585–6. doi: 10.1016/j.gastrohep.2020.05.013
34. Rellum R, Risseeuw G, Blaauw I, Lequin M. Splenorenal collaterals as hallmark for a twisted wandering spleen in a 14-year-old girl with abdominal pain: a case report. Eur J Pediatr Surg Rep. (2014) 2(1):26–8. doi: 10.1055/s-0034-1370776
35. Rizzuto A, Di Saverio S. Laparoscopic splenectomy for a simultaneous wandering spleen along with an ectopic accessory spleen. Case report and review of the literature. Int J Surg Case Rep. (2018) 43:36–40. doi: 10.1016/j.ijscr.2018.01.017
36. Torri F, Parolini F, Vanzetti E, Milianti S, Cheli M, Alberti D. Urgent laparoscopic mesh splenopexy for torsion of wandering spleen and distal pancreas: a case report. Asian J Endosc Surg. (2015) 8(3):350–3. doi: 10.1111/ases.12188
37. Yılmaz Ö, Bayrak V, Daştan E, Kotan Ç. Torsion of wandering spleen as a rare reason for acute abdomen: a presentation of two cases. Ulus Cerrahi Derg. (2013) 29(4):200–2. doi: 10.5152/ucd.2013.47
38. Zarroug AE, Hashim Y, El-Youssef M, Zeidan MM, Moir CR. Wandering spleen as a cause of mesenteric and portal varices: a new etiology? J Pediatr Surg. (2013) 48(3):e1–4. doi: 10.1016/j.jpedsurg.2012.12.042
39. Bairwa BL, Gupta S, Singh AK, Gupta P. Wandering spleen with torsion: a rare cause of acute abdomen in a 14-year-old girl. Arch Clin Cases. (2022) 9(2):56–61. doi: 10.22551/2022.35.0902.10204
40. Lugo-Fagundo E, Fishman EK. Wandering spleen with torsion and infarction: a case report. Radiol Case Rep. (2022) 17(9):3377–9. doi: 10.1016/j.radcr.2022.06.073
41. Petroianu A, Sabino KR. Wandering splenomegaly reduction after splenopexy. Int J Surg Case Rep. (2021) 85:106273. doi: 10.1016/j.ijscr.2021.106273
42. Gordon DH, Burrell MI, Levin DC, Mueller CF, Becker JA. Wandering spleen–the radiological and clinical Spectrum. Radiology. (1977) 125(1):39–46. doi: 10.1148/125.1.39
43. Nemcek AA Jr, Miller FH, Fitzgerald SW. Acute torsion of a wandering spleen: diagnosis by CT and duplex Doppler and color flow sonography. AJR Am J Roentgenol. (1991) 157(2):307–9. doi: 10.2214/ajr.157.2.1853811
44. Blázquez MJP, Vargas DR, Ferrer MG, González JT, Serrano BV. Torsion of wandering spleen: radiological findings. Emerg Radiol. (2020) 27(5):555–60. doi: 10.1007/s10140-020-01786-1
45. Bough GM, Gargan KE, Cleeve SJ, Farrell S. Diagnosis, management and outcome of splenic torsion; a systematic review of published studies. Surgeon. (2022) 20(5):e296–305. doi: 10.1016/j.surge.2021.08.006
46. Nel D, Kloppers C, Panieri E. Laparoscopic splenopexy for wandering spleen with absorbable mesh fixation. S Afr J Surg. (2021) 59(1):30a-b. doi: 10.17159/2078-5151/2021/v59n1a353
47. Chu J, Li Z, Luo B, Yang J. Wandering spleen with torsion and complete infarction. Acta Radiol. (2011) 52(8):911–3. doi: 10.1258/ar.2011.100504
48. Estevão-Costa J, Correia-Pinto J, Fragoso A, Soares-Oliveira M, Carvalho JL. Neonatal splenic necrosis not related to wandering spleen. Eur J Pediatr Surg. (2003) 13(5):344–6. doi: 10.1055/s-2003-43579
Keywords: wandering spleen, torsion, infarction, contrast-enhanced computed tomography, acute
Citation: Cui Q, Xu X, Li C and Tang L (2024) Wandering spleen combined with pedicle torsion and splenic infarction: a rare case report and literature review. Front. Pediatr. 12:1429490. doi: 10.3389/fped.2024.1429490
Received: 8 May 2024; Accepted: 12 August 2024;
Published: 16 September 2024.
Edited by:
Anna Maria Musolino, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Rahul Gupta, Synergy Institute of Medical Sciences, IndiaJosé Estevão-Costa, University of Porto, Portugal
Copyright: © 2024 Cui, Xu, Li and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihao Tang, bmVkdmVkMTk4N0AxNjMuY29t
†These authors have contributed equally to this work
 Qian Cui1
Qian Cui1 Lihao Tang
Lihao Tang