- 1Department of Respiratory Medicine, The Affiliated Wuxi People's Hospital, Wuxi Children's Hospital of Nanjing Medical University, Wuxi, Jiangsu, China
- 2Department of Respiratory Medicine & Clinical Allergy Center, Affiliated Children's Hospital of Jiangnan University (Wuxi Children's Hospital), Wuxi, China
- 3Department of Respiratory Medicine, Zhumadian Central Hospital, Zhumadian, Henan, China
- 4Department of Respiratory Medicine, Children’s Hospital of Nanjing Medical University, Nanjing, China
Background: Mycoplasma pneumoniae pneumonia (MPP) is a common disease of childhood pneumonia, and atelectasis is a serious comorbidity. Traditional diagnostic methods for MPP are limited by low accuracy, emphasizing the need for improved diagnostic approaches. This study aimed to establish a predictive scoring model for early detection of MPP complicated with atelectasis following standardized treatment.
Methods: A total of 572 children were retrospectively enrolled, including 40 patients with MPP complicated by atelectasis despite standardized treatment and 532 patients in the non-atelectasis group. Clinical, laboratory, and imaging data within 24 h of admission were collected, including demographic information and various biomarkers. Multivariate logistic regression analysis was employed to identify risk factors and construct a predictive model, evaluated using receiver operating characteristic (ROC) curve analysis.
Results: Significant differences were observed between the MPP complicated with atelectasis group and the non-atelectasis group in terms of age, hospital admission time, fever duration, neutrophil percentage and count, CRP, ALT, and LDH levels (P < 0.05). According to the multivariate logistic regression analysis, length of fever, neutrophil ratio, platelet count, ALT, LDH, age were incorporated into the nomogram. The predictive model exhibited a sensitivity of 87.97% and specificity of 77.50% according to the ROC curve.
Conclusion: Our study presents a preliminary risk association model incorporating clinical indicators such as fever duration, neutrophil ratio, platelet count, ALT value, LDH value, and age to aid in the early prediction of atelectasis in children with MPP. Given the methodological limitations, the generalizability of our findings is constrained, and this model should be viewed as an initial framework for clinical assessment rather than a definitive tool.
Introduction
Mycoplasma pneumoniae (MP) is the smallest microorganism known to survive independently. Among children aged approximately 5 years, Mycoplasma pneumoniae pneumonia (MPP) constitutes the most prevalent form of community-acquired pneumonia (CAP) (1, 2). MP infection is prevalent worldwide, accounting for 15%–50% of CAP pathogens. School-age children are particularly susceptible to infections. The epidemic cycle is generally 4–7 years, and the infection rate can be as high as 30%–50% (3–6). However, in 2023, a global outbreak of MP infection occurred, with China experiencing a significant surge, reaching an infection rate as high as 61.1% (7, 8). This outbreak led to a large number of MPP cases accompanied by complications. Children with severe or refractory MPP can develop pleural effusion, atelectasis, or necrotizing pneumonia. Some children become critically ill and die (9–11). Furthermore, severe MP infection can also leave sequelae such as chronic pulmonary interstitial fibrosis, bronchiolitis obliterans, unilateral pulmonary abnormal light syndrome, and reduced lung diffusion function, seriously affecting children's physical and mental health and increasing family and social burdens (12, 13).
Atelectasis represents a severe pulmonary complication associated with MPP, potentially affecting one or more pulmonary segments and reducing lung volume or air content (14). Regarding clinical features, children with MPP complicated with atelectasis often have higher C-reactive protein, neutrophil-to-lymphocyte ratio, and LDH lactate dehydrogenase levels and more complications, including erythema, liver damage, pleural effusion, and hypercoagulability. Regarding clinical outcomes, children with MPP complicated with atelectasis have a higher proportion of refractory cases, ICU admissions, and patients undergoing oxygen therapy; significantly longer total fever duration and hospital stay; greater hospital expenses; and are more prone to necrotizing pneumonia (15). These complications make atelectasis clinically intractable. Despite treatment, some children with atelectasis persist, and lung fibrosis was observed with concomitant local repeated infections, necessitating lung resection intervention (16, 17).
Therefore, early detection of atelectasis in children with MPP and the implementation of proactive treatment measures based on early prediction to mitigate pulmonary inflammation progression are crucial. Identifying and diagnosing MPP complicated by atelectasis in its early stages is challenging, especially for children undergoing standardized treatment, as physicians may not frequently perform imaging examinations, leading to underdiagnosis of atelectasis. By the time it is discovered in later stages, the optimal treatment window may have been missed. Traditional diagnosis heavily relies on physician judgment, which can be subjective and influenced by clinical experience and diagnostic biases. Establishing straightforward, sensitive, and specific diagnostic scoring scale is therefore paramount for optimizing clinical diagnosis, tailoring treatment, and assessing potential adverse prognostic risks. In this study, we retrospectively analyzed the clinical characteristics and laboratory and imaging data of children with MPP who developed atelectasis despite standardized treatment, alongside a non-atelectasis group. Additionally, we developed a nomogram association model to forecast the occurrence of atelectasis in children with MPP, aiming to facilitate early diagnosis and intervention for this comorbidity.
Materials and methods
Ethics approval and informed consent
This study was approved by the Medical Ethics Committee of Wuxi Children's Hospital (no. WXCH2020-02-005) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2000038742). All the methods used in this study were conducted following the Declaration of Helsinki. All children enrolled in the study provided informed consent from their parents or legal guardians.
Study population
The inclusion criteria were as follows: 572 children hospitalized for the first time in the Department of Respiratory Medicine of Wuxi Children's Hospital or Nanjing Children's Hospital were included. All children met the diagnostic criteria of the MPP guidelines (18). The clinical manifestations included fever, irritating dry cough, shortness of breath, and other systemic manifestations. Among the 572 children included in the study, 287 underwent only chest x-ray, 33 underwent only chest CT scans, and 252 underwent both chest x-ray and chest CT scans (including chest CT done on an outpatient basis or in other hospitals), and chest x-ray or chest CT findings suggestive of inflammatory infiltrates. The etiological test showed that the MP-immunoglobulin M (IgM) was positive, the antibody titer was more than 1:160, or the polymerase chain reaction result for M. pneumoniae is positive (18, 19). The diagnosis of atelectasis was based on imaging manifestations included increased opacity of affected lobe, bronchovascular crowding, narrowing of the ipsilateral intercostal spaces, compensatory hyperinflation, compensatory shift of adjoining structures and/or diaphragm, hilar displacement, ipsilateral hemithoracic contraction (in massive collapse), and silhouette sign affecting contiguous mediastinal structures (20). Exclusion criteria: 1. pulmonary infection with other pathogens or chronic pulmonary inflammation; 2. basic diseases or malignant tumors of the liver, kidney, heart, brain, and other systems; 3. without informed consent or incomplete data collection.
The group was divided into an Atelectasis group and a Control group according to the presence or absence of radiographic signs of atelectasis following 7 days of conventional treatment. This treatment regimen included macrolide antibiotics, corticosteroids, expectorants, and various other symptomatic therapies.
Data collection
We collected the following common variables for hospitalized children, including the following: 1. Continuous variables: age, hospitalization days, fever days before admission, blood routine within 24 h of admission: white blood cell count (WBC), neutrophil percentage, hemoglobin (HB), platelet count (PLT), C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase isoenzyme (CK -MB); 2. Binary categorical variables: sex, presence or absence of pleural effusion at admission, whether there was large-area lung consolidation on chest imaging, and whether there were related underlying diseases; 3. Nominal variables: antibodies against common respiratory tract pathogens, serum mycoplasma antibody titer, blood culture, sputum culture, and alveolar lavage fluid culture.
Statistical analysis
R × 64 3.6.0 and RStudio for Windows (Auckland, New Zealand) were used for data processing. Statistical significance was set at P < 0.05. The t-test was used for normally distributed continuous data, and the median and interquartile range (IQR) for non-normally distributed data were calculated. Categorical variables were analyzed using the chi-squared test. A multivariate analysis was performed using a logistic regression model to determine the risk factors related to the occurrence of atelectasis complication in MPP patients following standardized treatment. The final regression model was transformed into a nomogram using the R software. Taking sensitivity as the ordinate and 1-specificity as the abscissa, a receiver operating characteristic (ROC) curve was drawn, the regression model of atelectasis MPP was predicted, and the specificity and sensitivity of the prediction scale were calculated.
Results
The research group retrospectively evaluated 4,745 children with pneumonia admitted to Wuxi Children's Hospital and Nanjing Children's Hospital from June to December 2020. After statistical analysis, 4,127 cases of infection with other pathogens, co-infections, or co-extrapulmonary diseases were excluded. Additionally, 618 cases of mycoplasma pneumonia consistent with simple mycoplasma pneumonia, of which 46 cases had atelectasis on admission and were excluded. Finally, 572 children were included in the MPP group, and the children were classified into atelectasis and non-atelectasis groups according to lung imaging findings after 7 days of conventional treatment, which included macrolide antibiotics, corticosteroids, expectorants, and other symptomatic treatments. Among them, 40 cases were complicated with atelectasis after treatment, while 532 cases were not (Figure 1).
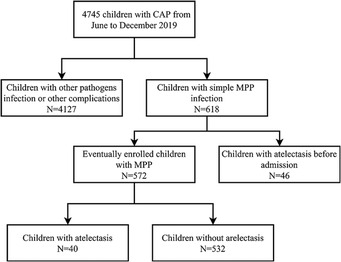
Figure 1. Research flow chart: patient recruitment and exclusion. CAP, community-acquired pneumonia; MPP, M. pneumoniae pneumonia.
A total of 572 patients with confirmed MPP were included in this study. The clinical, laboratory, and imaging data of children with MPP were collected within 24 h of admission, and additional imaging data were collected after 7 days of conventional treatment. The clinical features, laboratory findings, and imaging findings of the two groups were compared.
There was no statistically significant difference in sex distribution between the atelectasis and non-atelectasis groups. However, the mean age of onset in the atelectasis group was significantly higher than in the non-atelectasis group (5.25 vs. 3.17, P < 0.05). Additionally, the length of hospital days (13.00 vs. 8.00, P < 0.05) and the number of days with fever (8.00 vs. 4.00, P < 0.05) were significantly higher in the atelectasis group than in the non-atelectasis group.
Compared to the non-atelectasis group, the percentage of neutrophils (67.09% vs. 56.35%, P < 0.05), neutrophil count (6.24 × 109 vs. 4.52 × 109, P < 0.05), CRP levels (24.00 ng/ml vs. 8.00 ng/ml, P < 0.05), ALT levels (34.00 U/L vs. 15.00 U/L, P < 0.05), and LDH levels (592.00 U/L vs. 318.50 U/L, P < 0.05) in the atelectasis group were significantly increased.
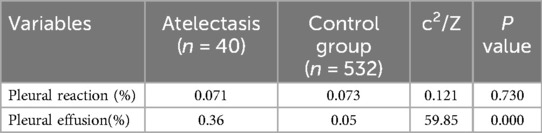
Furthermore, the two groups had no statistically significant differences in the other laboratory results (Table 1). The presence or absence of a pleural response was not statistically different between the two groups (7.1% vs. 7.3%, P > 0.05); however, imaging results showed more pleural effusion in the atelectasis group (36% vs. 5%, P < 0.05) (Table 2).
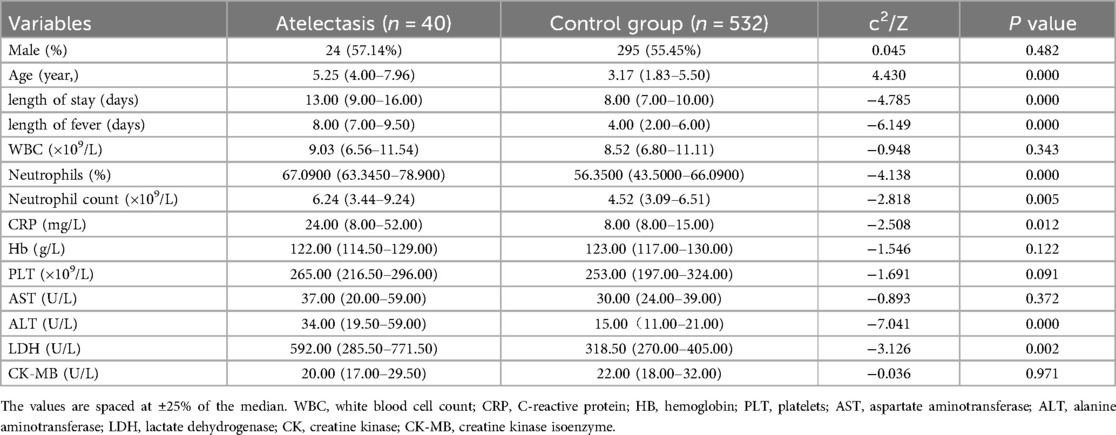
Table 1. Admission information of children with Mycoplasma pneumoniae pneumonia and atelectasis and control group.
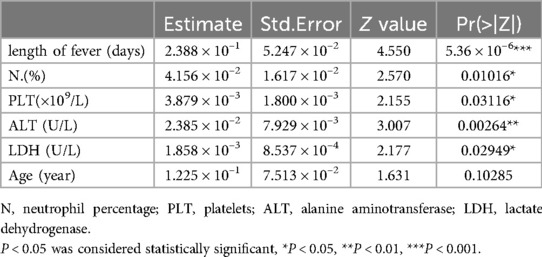
Multivariate logistic regression analysis was conducted to determine the weight of each index in predicting the occurrence of atelectasis. The weights of the indices, listed in descending order, were as follows: duration of fever, ALT levels, neutrophil percentage, LDH levels, PLT, and age. Duration of fever, ALT levels, neutrophil percentage, LDH levels, PLT were independent factors influencing the occurrence of atelectasis in MPP patients following standardized treatment (Table 3).
Variables including days of fever, neutrophil percentage, ALT, LDH values, and PLT were statistically significant. Although children's age did not exhibit significant differences in the multivariate analysis, it had been significant in previous studies (21, 22). The nomogram assigns a weighted score to each independent risk factor, with a maximum of 100 points per factor. The overall risk of atelectasis, ranging from 0.001 to 0.95, is calculated by summing the scores for all high-risk factors. A higher cumulative score corresponds to an increased likelihood of atelectasis occurrence.
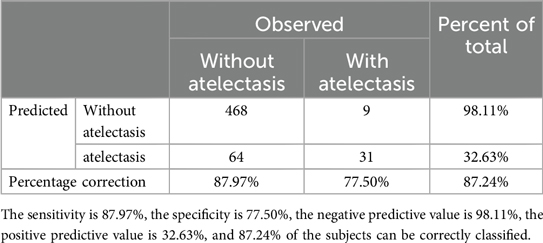
ROC curves were drawn to determine the predictive model based on the best critical probability using a confusion matrix to validate the model internally (Figure 2). The sensitivity and specificity of the evaluation model were 87.97% and 77.50%, respectively, and the model correctly classified 87.24% of the study subjects (Table 4). These results suggested that the early association model can be used for the early prediction of atelectasis in children with MPP.
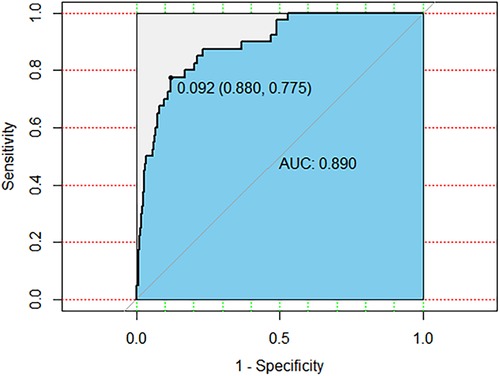
Figure 2. The ROC curve is drawn based on the clinical prediction model of MPP with atelectasis. The AUC is 0.890, the most the best critical probability is 0.092.
To facilitate clinical application, a nomogram based on the logistic regression model was developed, enabling clinicians to quickly calculate a risk score based on clinical symptoms and laboratory tests. The nomogram allows for the assessment of atelectasis risk scores based on the following metrics (Figure 3).
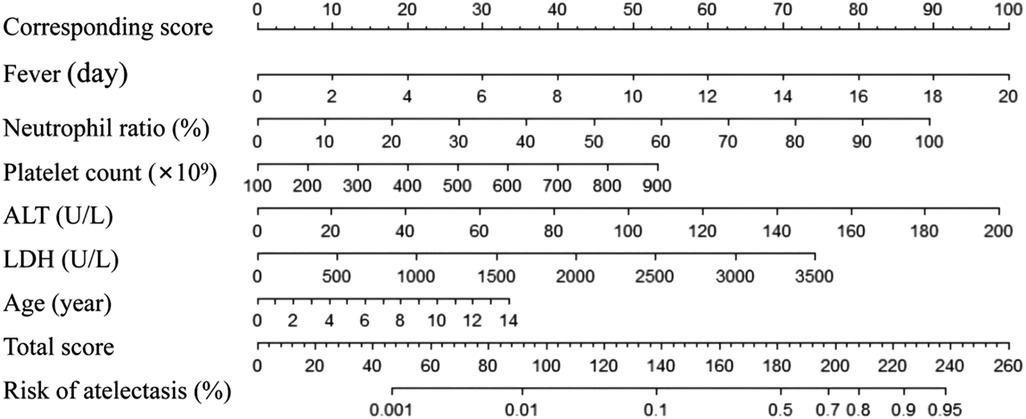
Figure 3. Nomogram MPP atelectasis clinical prediction assessment scale. The score in the first row corresponds to the value of each of the following indicator, and the score is calculated based on the value of each variable. Evaluate the risk probability of MPP atelectasis based on the total score. How to use the table: Enlarge and print it on A4 paper according to the scale, use a ruler and other measuring tools to calculate the corresponding score.
Discussion
MP is a common pathogen associated with respiratory tract infections in children. MP infection often cause multi-system damage inside and outside the lung through cell adhesion, toxin invasion, and excessive immune damage (5, 6, 23–27). Recently, the number of children with severe or refractory MPP appears to have increased with the emergence of drug resistance. This increase may be accompanied by a rise in complications such as atelectasis, pulmonary fibrosis, and bronchiolitis obliterans, which potentially pose serious threats to the safety and quality of life of affected children. Therefore, despite the critical need for early identification of severe or refractory MPP (2, 28, 29), there remains a lack of comprehensive risk association models, particularly for MPP complicated with atelectasis. This gap in knowledge makes it challenging for clinical pediatricians to effectively guide patient management strategies.
The radiographic signs of atelectasis and lung consolidation are different. Direct signs of atelectasis on chest films include fissure deviation, parenchymal opacification with unbroken linear borders, and vascular displacement (20). Pulmonary consolidation refers to the replacement of alveolar air by pathological fluids, cells, or tissues and manifests an increase in the density of the lung parenchyma, resulting in obscuring the margins of vessels and airway walls (30). Additionally, the air bronchogram may be present.
To predict the risk of MPP complicated with atelectasis and potentially reduce the occurrence of atelectasis complications, we have identified six indicators, including fever days, neutrophil percentage, PLT, ALT, LDH, and age, as predictors of pulmonary atelectasis. In this study, the number of days of fever was analyzed, and it was observed that the fever duration in the MPP combined with the atelectasis group was longer than that in the non-atelectasis group. Jang (31) suggested that fever is a risk factor for bronchial mucus obstruction in children with MPP, which has also been reported previously (32). The neutrophil percentage is a common clinical indicator of infection. Several studies suggest that the extracellular traps of neutrophils may play a crucial bactericidal role in infectious diseases such as CAP (33, 34). An increase in the proportion of neutrophils indicates that the infection is worsening (14, 35). Furthermore, it's noteworthy that these indicators were observed in children who still developed atelectasis despite standardized treatment protocols. This underscores the importance of early recognition and intervention strategies even in cases where conventional treatment has been administered.
Some studies suggest that MP infection can potentially lead to the release of several inflammatory factors, resulting in endothelial cell damage and platelet aggregation, leading to a hypercoagulable state and thrombosis (36). Furthermore, Ling (37) reported the severe clinical status of patients with lower respiratory tract infections and thrombocytosis. This suggests a potential link between MP infection and thromboembolic events, although further research is needed to elucidate the underlying mechanisms.
Additionally, elevated levels of ALT and LDH, as observed in our study, may indicate extrapulmonary damage associated with MP infection. Previous studies have reported that MP infection can cause M. pneumonia-associated hepatitis and elevated liver enzymes (38, 39). However, elevated LDH levels are thought to reflect generalized tissue damage or cellular necrosis. Previous studies have also suggested a potential association between elevated LDH levels and epithelial damage in the lungs (40, 41), these findings underscore the systemic impact of MP infection and highlight the importance of monitoring LDH levels as a marker of tissue injury in affected patients. Importantly, our study further demonstrates the significance of LDH in the early identification of atelectasis complicating MPP.
In this retrospective study, we found that the median age at onset in the MPP combined with atelectasis group was higher than in the non-atelectasis group. Studies have shown that the main pathogenic mechanism of MPP is related to an excessive immune response in the body, which in turn is related to the relative immaturity of the immune mechanism in young children. Excessive inflammation tends not to be produced in young children; however, the opposite is true in older children. This may be one of the causes of MPP complicated with atelectasis in older children (26, 42, 43). While excessive inflammation is less commonly observed in young children, the opposite tends to be true in older children. This age-related difference in immune response may contribute to the increased risk of developing atelectasis complicating MPP in older children.
There have been previous studies on predictive scales related to MPP and refractory MPP and studies on MPP and the risk prediction model of bronchiolitis obliterans (22, 44). However, there are no studies on the risk model of MPP combined with atelectasis. Risk association models incorporate fever duration, neutrophil ratio, PLT, ALT, LDH, and age into clinical association models.
The ROC curve was drawn for each predictor; the area under the curve (AUC) was 0.890, and the optimal critical probability was 0.092, suggesting high specificity and sensitivity. These results demonstrate a high level of specificity and sensitivity in predicting the risk of MPP complicated with atelectasis.
An increasing body of evidence suggest that an excessive host immune response plays an important role in the pathogenesis of atelectasis (37, 45, 46). As a result, early administration of glucocorticoids may be a promising treatment option for children with MPP complicated by atelectasis (45).
This predictive model potentially offers several significant advantages. Firstly, it could enhances the ability of healthcare providers to promptly recognize and stratify patients at high risk of developing atelectasis, allowing for timely and targeted interventions to mitigate potential complications and improve patient outcomes. Secondly, the model's simplicity and ease of use might make it suitable for implementation across a wide range of healthcare settings, from primary care clinics to tertiary hospitals, facilitating its widespread adoption and integration into routine clinical practice. Additionally, the high specificity and sensitivity of the model, as indicated by the robust performance metrics such as the area under the ROC curve, underscore its reliability and effectiveness in accurately predicting the risk of atelectasis in children with MPP.
By potentially enabling early identification and intervention, our predictive model may significantly reduce the morbidity and mortality associated with MPP complicated by atelectasis, thereby alleviating the burden on healthcare systems and improving the overall quality of care for pediatric patients with respiratory infections. Furthermore, as the incidence of MPP continues to rise globally, particularly in regions experiencing outbreaks such as China, the need for effective risk association models to guide clinical management becomes increasingly imperative. In this context, our study represents a crucial step forward in advancing our understanding of MPP-related complications and improving clinical outcomes for affected children.
However, our study has several limitations that should be acknowledged. Firstly, as a retrospective study, it lacks prospective data collection and external validation. Therefore, the utility and generalizability of our experimental findings and nomogram require validation across multiple centers. Additionally, the relatively small sample size of patients with MPP and atelectasis in our study may limit the robustness and generalizability, which may limit the robustness and generalizability of our findings. As such, further data collection and external multicenter validation are warranted to confirm the reliability and applicability of our predictive model in diverse clinical settings.
Conclusion
In conclusion, while our study provides a preliminary risk association model incorporating clinical indicators such as fever duration, neutrophil ratio, platelet count, ALT value, LDH value, and age to support early prediction of atelectasis in children with MPP, we acknowledge that methodological constraints limit the generalizability of our findings. As a retrospective analysis with a relatively small sample size and limited external validation, this model should be interpreted with caution and further validated through prospective, multicenter studies. The proposed model, therefore, serves as an initial framework for clinical assessment, rather than a definitive tool, underscoring the need for additional research to refine and confirm these predictive associations in diverse clinical settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Wuxi Children's Hospital (no. WXCH2020-02-005). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
MX: Data curation, Writing – original draft. MF: Methodology, Writing – original draft. HW: Writing – original draft. JQ: Supervision, Writing – review & editing. YJ: Data curation, Writing – original draft. YZ: Writing – review & editing. DZ: Writing – review & editing. FL: Methodology, Writing – review & editing. YG: Writing – review & editing. LL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Wuxi Taihu Lake Talent Plan(Grant No.DJTD202304), the Natural Science Foundation of Jiangsu Province (Grants No BK20230189), Wuxi Municipal Bureau on Science and Technology (grant number: K20221033), the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (grant number: BJ2023089) and the Wuxi Maternal and Child Health Research Project (grant number: FYKY202003).
Acknowledgments
The authors would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guo D, Hu W, Wei R, Wang H, Xu B, Zhou W, et al. Epidemiology and mechanism of drug resistance of mycoplasma pneumoniae in Beijing, China: a multi-center study. Bosnian J Basic Med. (2019) 19:288–96. doi: 10.17305/bjbms.2019.4053
2. Xing Y, Wang D, Sheng K, Xiao X, Wei H, Liu L, et al. Dynamic change of mycoplasma pneumoniae pneumonia in hospitalized children in a general hospital: a 3-year retrospective analysis. Transl Pediatr. (2020) 9:522–31. doi: 10.21037/tp-20-149
3. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. Children. New Engl J Med. (2015) 372:835–45. doi: 10.1056/NEJMoa1405870
4. Oumei H, Xuefeng W, Jianping L, Kunling S, Rong M, Zhenze C, et al. Etiology of community-acquired pneumonia in 1500 hospitalized children. J Med Virol. (2018) 90:421–8. doi: 10.1002/jmv.24963
5. Kutty PK, Jain S, Taylor TH, Bramley AM, Diaz MH, Ampofo K, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. (2019) 68:5–12. doi: 10.1093/cid/ciy419
6. Beeton ML, Zhang XS, Uldum SA, Bebear C, Dumke R, Gullsby K, et al. Mycoplasma pneumoniae infections, 11 countries in Europe and Israel, 2011 to 2016. Eurosurveillance. (2020) 25(2):1900112. doi: 10.2807/1560-7917.ES.2020.25.2.1900112
7. Yan C, Xue GH, Zhao HQ, Feng YL, Cui JH, Yuan J. Current status of mycoplasma pneumoniae infection in China. World J Pediatr. (2024) 20:1–4. doi: 10.1007/s12519-023-00783-x
8. Meyer SP, Beeton ML. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. (2024) 5:e100–1. doi: 10.1016/S2666-5247(23)00344-0
9. Kim SH, Lee E, Song ES, Lee YY. Clinical significance of pleural effusion in mycoplasma pneumoniae pneumonia in children. Pathogens. (2021) 10(9):1075. doi: 10.3390/pathogens10091075
10. Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. (2017) 30:747–809. doi: 10.1128/CMR.00114-16
11. Chen Q, Hu T, Wu L, Chen L. Clinical features and biomarkers for early prediction of refractory mycoplasma Pneumoniae pneumonia in children. Emerg Med Int. (2024) 2024:9328177. doi: 10.1155/2024/9328177
12. Su DQ, Li JF, Zhuo ZQ. Clinical analysis of 122 cases with mycoplasma pneumonia complicated with atelectasis: a retrospective study. Adv Ther. (2020) 37:265–71. doi: 10.1007/s12325-019-01129-8
13. Yang B, Zhang W, Gu W, Zhang X, Wang M, Huang L, et al. Differences of clinical features and prognosis between mycoplasma pneumoniae necrotizing pneumonia and non-mycoplasma pneumoniae necrotizing pneumonia in children. BMC Infect Dis. (2021) 21:797. doi: 10.1186/s12879-021-06469-x
14. Luo Z, Luo J, Liu E, Xu X, Liu Y, Zeng F, et al. Effects of prednisolone on refractory mycoplasma pneumoniae pneumonia in children. Pediatr Pulm. (2014) 49:377–80. doi: 10.1002/ppul.22752
15. Huang X, Gu H, Wu R, Chen L, Lv T, Jiang X, et al. Chest imaging classification in mycoplasma pneumoniae pneumonia is associated with its clinical features and outcomes. Resp Med. (2024) 221:107480. doi: 10.1016/j.rmed.2023.107480
16. Wang X, Zhong LJ, Chen ZM, Zhou YL, Ye B, Zhang YY. Necrotizing pneumonia caused by refractory mycoplasma pneumonia pneumonia in children. World J Pediatr. (2018) 14:344–9. doi: 10.1007/s12519-018-0162-6
17. Jiang Z, Li S, Zhu C, Zhou R, Leung P. Mycoplasma pneumoniae infections: pathogenesis and vaccine development. Pathogens. (2021) 10(2):119. doi: 10.3390/pathogens10020119
18. Association RBOC, Pediatrics EBOC. Expert consensus on diagnosis and treatment of mycoplasma pneumoniae pneumonia in children (2015). Chin J Appl Clin Pediatr. (2015) 30:1304–8. doi: 10.3760/cma.j.issn.2095-428X.2015.17.006
19. Meyer SP, Unger W, van Rossum A, Berger C. The art and science of diagnosing mycoplasma pneumoniae infection. Pediatr Infect Dis J. (2018) 37:1192–5. doi: 10.1097/INF.0000000000002171
21. Chen D, Yang XL, Shen ZB, Sun XM, Guo Q, Ren YH, et al. Significance of neutrophil extracellular trap and its markers in the early diagnosis of community-acquired pneumonia in children. Zhongguo Dang Dai Er Ke Za Zhi. (2019) 21:868–75. doi: 10.7499/j.issn.1008-8830.2019.09.005
22. Li L, Guo R, Zou Y, Wang X, Wang Y, Zhang S, et al. Construction and validation of a nomogram model to predict the severity of mycoplasma pneumoniae pneumonia in children. J Inflamm Res. (2024) 17:1183–91. doi: 10.2147/JIR.S447569
23. Meyer SP, Theiler M, Buettcher M, Seiler M, Weibel L, Berger C. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. (2020) 156:144–50. doi: 10.1001/jamadermatol.2019.3602
24. Vizarraga D, Kawamoto A, Matsumoto U, Illanes R, Perez-Luque R, Martin J, et al. Immunodominant proteins P1 and P40/P90 from human pathogen mycoplasma pneumoniae. Nat Commun. (2020) 11:5188. doi: 10.1038/s41467-020-18777-y
25. Becker A, Kannan TR, Taylor AB, Pakhomova ON, Zhang Y, Somarajan SR, et al. Structure of CARDS toxin, a unique ADP-ribosylating and vacuolating cytotoxin from mycoplasma pneumoniae. Proc Natl Acad Sci USA. (2015) 112:5165–70. doi: 10.1073/pnas.1420308112
26. Shi S, Zhang X, Zhou Y, Tang H, Zhao D, Liu F. Immunosuppression reduces lung injury caused by mycoplasma pneumoniae infection. Sci Rep-UK. (2019) 9:7147. doi: 10.1038/s41598-019-43451-9
27. Zhao C, Liu J, Yang H, Xiang L, Zhao S. Mycoplasma pneumoniae-associated bronchiolitis obliterans following acute bronchiolitis. Sci Rep-UK. (2017) 7:8478. doi: 10.1038/s41598-017-08861-7
28. Hubert D, Dumke R, Weichert S, Welker S, Tenenbaum T, Schroten H. Emergence of macrolide-resistant mycoplasma pneumoniae during an outbreak in a primary school: clinical characterization of hospitalized children. Pathogens. (2021) 10(3):328. doi: 10.3390/pathogens10030328
29. Chen S, Ding Y, Vinturache A, Gu H, Lu M, Ding G. Pulmonary embolism associated with mycoplasma in a child. Lancet Infect Dis. (2020) 20:1347. doi: 10.1016/S1473-3099(20)30253-X
30. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. (2020) 30:4381–9. doi: 10.1007/s00330-020-06801-0
31. Jang MS, Kim BG, Kim J. Prediction model for prolonged fever in patients with mycoplasma pneumoniae pneumonia: a retrospective study of 716 pediatric patients. BMC Pulm Med. (2021) 21:168. doi: 10.1186/s12890-021-01534-2
32. Xu Q, Zhang L, Hao C, Jiang W, Tao H, Sun H, et al. Prediction of bronchial mucus plugs formation in patients with refractory mycoplasma pneumoniae pneumonia. J Trop Pediatrics. (2017) 63:148–54. doi: 10.1093/tropej/fmw064
33. Monteith AJ, Miller JM, Maxwell CN, Chazin WJ, Skaar EP. Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens. Sci Adv. (2021) 7:eabj2101. doi: 10.1126/sciadv.abj2101
34. Cagnina RE, Michels KR, Bettina AM, Burdick MD, Scindia Y, Zhang Z, et al. Neutrophil-derived tumor necrosis factor drives fungal acute lung injury in chronic granulomatous disease. J Infect Dis. (2021) 224:1225–35. doi: 10.1093/infdis/jiab188
35. Zheng XX, Lin JL, Dai JH. Value of lactate dehydrogenase in predicting refractory mycoplasma pneumoniae pneumonia in children: an evaluation based on decision curve analysis and dose-response analysis. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22:112–7. doi: 10.7499/j.issn.1008-8830.2020.02.006
36. Shimizu T, Kida Y, Kuwano K. Cytoadherence-dependent induction of inflammatory responses by mycoplasma pneumoniae. Immunology. (2011) 133:51–61. doi: 10.1111/j.1365-2567.2011.03408.x
37. Ling Y, Ning J, Xu Y. Explore the predictive value of peripheral blood cell parameters in refractory mycoplasma pneumoniae pneumonia in children over 6 years old. Front Pediatr. (2021) 9:659677. doi: 10.3389/fped.2021.659677
38. Jujaray D, Juan LZ, Shrestha S, Ballgobin A. Pattern and significance of asymptomatic elevation of liver enzymes in mycoplasma pneumonia in children. Clin Pediatr. (2018) 57:57–61. doi: 10.1177/0009922816688737
39. Song WJ, Kang B, Lee HP, Cho J, Lee HJ, Choe YH. Pediatric mycoplasma pneumoniae infection presenting with acute cholestatic hepatitis and other extrapulmonary manifestations in the absence of pneumonia. Pediatr Gastroenterol Hepatol Nutr. (2017) 20:124–9. doi: 10.5223/pghn.2017.20.2.124
40. Luo Y, Wang Y. Risk prediction model for necrotizing pneumonia in children with mycoplasma pneumoniae pneumonia. J Inflamm Res. (2023) 16:2079–87. doi: 10.2147/JIR.S413161
41. Fan F, Lv J, Yang Q, Jiang F. Clinical characteristics and serum inflammatory markers of community-acquired mycoplasma pneumonia in children. Clin Respir J. (2023) 17:607–17. doi: 10.1111/crj.13620
42. Zhang Y, Zhou Y, Li S, Yang D, Wu X, Chen Z. The clinical characteristics and predictors of refractory mycoplasma pneumoniae pneumonia in children. PLoS One. (2016) 11:e0156465. doi: 10.1371/journal.pone.0156465
43. Wang M, Wang Y, Yan Y, Zhu C, Huang L, Shao X, et al. Clinical and laboratory profiles of refractory mycoplasma pneumoniae pneumonia in children. Int J Infect Dis. (2014) 29:18–23. doi: 10.1016/j.ijid.2014.07.020
44. Cheng Q, Zhang H, Shang Y, Zhao Y, Zhang Y, Zhuang D, et al. Clinical features and risk factors analysis of bronchitis obliterans due to refractory mycoplasma pneumoniae pneumonia in children: a nomogram prediction model. BMC Infect Dis. (2021) 21:1085. doi: 10.1186/s12879-021-06783-4
45. Zhu Z, Zhang T, Guo W, Ling Y, Tian J, Xu Y. Clinical characteristics of refractory mycoplasma pneumoniae pneumonia in children treated with glucocorticoid pulse therapy. BMC Infect Dis. (2021) 21:126. doi: 10.1186/s12879-021-05830-4
Keywords: children, M. pneumoniae, M. pneumoniae pneumonia, atelectasis, association model
Citation: Xu M, Fan M, Wang H, Qian J, Jiang Y, Zhu Y, Zhao D, Liu F, Guo Y and Li L (2024) Risk association model for atelectasis complication in Mycoplasma pneumoniae pneumonia patients following standardized treatment. Front. Pediatr. 12:1422074. doi: 10.3389/fped.2024.1422074
Received: 23 April 2024; Accepted: 12 November 2024;
Published: 28 November 2024.
Edited by:
Janet R. Hume, University of Minnesota Medical Center, United StatesReviewed by:
Zhiqiang Zhuo, Xiamen Children's Hospital, ChinaYongdong Yan, Soochow University, China
Albert Martin Li, The Chinese University of Hong Kong, China
Copyright: © 2024 Xu, Fan, Wang, Qian, Jiang, Zhu, Zhao, Liu, Guo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Guo, Z3VveXVuQGppYW5nbmFuLmVkdS5jbg==; Ling Li, TGlsaW5nQG5qbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Mingyi Xu
Mingyi Xu Minhao Fan2,†
Minhao Fan2,† Huixia Wang
Huixia Wang Yifan Zhu
Yifan Zhu Deyu Zhao
Deyu Zhao Feng Liu
Feng Liu Ling Li
Ling Li