- 1School of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2School of Nursing, Shandong Academy of Medical Sciences, Shandong First Medical University, Taian, Shandong, China
- 3Department of Nursing, The First Affiliated Hospital of Shandong First Medical University, Jinan, Shandong, China
Background: The rising incidence of drug abuse among pregnant women has rendered neonatal opioid withdrawal syndrome a significant global health concern.
Methods: Databases including PubMed, Web of Science, the Cochrane Library, Embase, Elton B. Stephens. Company (EBSCO), China National Knowledge Infrastructure (CNKI), and Wanfang were searched for comparative studies of the Eat, Sleep, Console model vs. traditional assessment tools for neonatal opioid withdrawal syndrome. Two reviewers conducted literature searches, screened according to the inclusion criteria, extracted data, and independently verified accuracy. All meta-analyses were conducted using Review Manager Version 5.4.
Results: In total, 18 studies involving 4,639 neonates were included in the meta-analysis. The Eat, Sleep, Console model demonstrated superior outcomes in assessing neonatal opioid withdrawal syndrome, significantly reducing the need for pharmacological treatment [risk ratio = 0.44, 95% confidence interval (CI) = 0.34–0.56, P < 0.001], decreasing the length of hospital stay [standard mean difference (SMD) = −2.10, 95% CI = −3.43 to −0.78, P = 0.002], and shortening the duration of opioid treatment (SMD = −1.33, 95% CI = −2.22 to −0.45, P = 0.003) compared to the Finnegan Neonatal Abstinence Scoring System.
Conclusions: The Eat, Sleep, Console model is more effective than the Finnegan Neonatal Abstinence Scoring System in improving the assessment and management of neonatal opioid withdrawal syndrome.
1 Introduction
Neonatal opioid withdrawal syndrome (NOWS) occurs in over 55% of infants born to mothers who are addicted to or treated with opioids during pregnancy (1, 2). The clinical symptoms of NOWS typically manifest within the first few days post-birth and include gastrointestinal disturbances, irritability, hypertonia, and seizures (2, 3). With the rising prevalence of drug abuse among pregnant women, the severity of NOWS has increased. This syndrome is now a global concern, causing extended hospital stays, heightened demand for drugs, and increased hospitalization costs, particularly in the United States and Canada (1, 4–6), thus exacerbating the economic burden (7). Since 2014, management strategies for NOWS have evolved to include pharmacological and non-pharmacological interventions, as well as streamlined clinical evaluations (8). However, due to regional and institutional variations, between 50% and 80% of affected newborns require pharmacological treatment (9).
In the United States, the Finnegan Neonatal Abstinence Scoring System (FNASS), devised in 1975, remains the predominant method for assessing NOWS (10, 11). The FNASS, which is based on the observed severity of 21 withdrawal symptoms, involves scoring neonates every 2–6 h by pediatricians or nurses, with pharmacological treatment initiated if scores reach ≥12 or if three consecutive scores of ≥8 are recorded (12). Despite the development of new objective scoring methods, the FNASS is still employed in over 52% of cases involving newborns with NOWS (13). To enhance the reliability of the FNASS in clinical settings, healthcare providers must participate in initial and ongoing training (14). However, studies consistently show that the FNASS may lead to unnecessary pharmacological treatments, resulting in prolonged hospital stays. Extended stays, coupled with less-than-ideal conditions, hinder family members from engaging in the care for infants (15, 16).
In 2017, Grossman et al. introduced a novel model for assessing NOWS, termed the Eat, Sleep, Console (ESC) model (15). This model evaluates crucial physiological functions, such as eating and sleeping. Under the ESC model, pharmacological interventions are withheld if these functions remain unaffected, even if FNASS scores exceed 8 (17). Unlike the FNASS, the ESC model promotes family involvement in the care of infants with NOWS and encourages active participation in managing symptoms (18). In addition, by prioritizing non-pharmacological interventions that increase family engagement, both the need for pharmacological treatment and the duration of hospital stays are significantly reduced (19, 20). Although numerous studies have compared the ESC model with the FNASS, no meta-analysis like this one has previously been conducted. This review aims to vividly illustrate the differences between these two assessment tools and determine if the ESC model provides better outcomes for managing NOWS than traditional methods. The findings are expected to offer practical and theoretical support for selecting an evaluation approach for NOWS in the future.
2 Methods
2.1 Review protocol
Studies utilizing the ESC model for assessing NOWS were reviewed. Exposure to prenatal opioids included heroin, prescription opioids, over-the-counter opioids, and prescription or illegal opioid replacement therapies. Individuals who used multiple substances were excluded from this analysis. The inclusion criteria are listed in Table 1. Pharmacological treatment requirement was designated as the primary outcome, and hospital stay duration and opioid treatment duration were the secondary outcomes.
2.2 Search strategy
This meta-analysis was performed according to the guidelines of Preferred Reporting Items for Meta-Analyses (PRISMA) (21). We searched the PubMed, ScienceNet, Cochrane Library, Embase, Elton B. Stephens. Company (EBSCO), China National Knowledge Infrastructure (CNKI), and Wanfang databases to collect comparative studies of the Eat, Sleep, Console model vs. traditional assessment tools for NOWS up to January 2024, using the following words: prenatal opioid exposure OR neonatal passive addiction OR neonatal opioid withdrawal syndrome OR neonatal withdrawal syndrome OR neonatal opioid withdrawal syndrome, Eat, Sleep, Console model OR ESC, assessment. The retrieval strategy adopted was based on PubMed as an example is as follows:
1# “prenatal opioid exposure”[TW] OR “neonatal passive addiction”[TW] OR “neonatal opioid withdrawal”[TW] OR “neonatal opioid withdrawal syndrome”[TW]
2# “neonatal abstinence syndrome”[MeSH] OR “neonatal abstinence syndrome”[TW] OR “neonatal withdrawal syndrome”[TW] OR neonatal[TIAB] OR abstinence[TIAB] OR withdrawal[TIAB]
3# 1# OR 2#
4# “Infant, Newborn”[Mesh] OR “newborn infant”[TIAB] OR infant[TIAB] OR newborn[TIAB]
5# “Analgesics, Opioid”[Mesh] OR “Analgesics, Opioid” [Pharmacological Action] OR “opioid analgesics”[TIAB] OR opioid[TIAB]
6# “eat, sleep, console”[TW] OR eat[TW] OR sleep[TW] OR console[TW] OR comfort[TW] OR ESC[TW]
7# 3# OR 4# AND 5# AND 6#
2.3 Study selection and data collection
The literature searches and screenings were independently conducted by two reviewers. After eliminating duplicates, titles and abstracts were examined. A detailed review of the full texts established eligibility. Discrepancies were resolved through discussion with the corresponding author. Following the selection of studies, detailed data extraction was performed by the reviewers. From the studies included, a matrix was constructed detailing the author's name, year of study, study design, sample characteristics, intervention comparisons, outcomes, and outcome data.
2.4 Statistical methods
Statistical analysis was conducted using Review Manager (version 5.4; Cochrane Informatics & Technology Services) software, considering a P-value <0.05 as statistically significant. Discontinuous variables were analyzed using the risk ratio (RR) and 95% confidence interval (CI), while continuous outcomes utilized the standard mean difference (SMD) and 95% CI. Heterogeneity was assessed using the I2 statistic. Values of I2 between 0% and 40% indicate minimal heterogeneity, while 75%–100% suggest high heterogeneity. Moderate heterogeneity was indicated by I2 values between 30% and 60%, and significant heterogeneity by 50%–90%. For I2 ≥50, a random-effects model was applied; for I2 <50, a fixed-effects model was utilized. Potential sources of heterogeneity, including differences in health education, non-pharmacological treatment methods, literature quality, and gestational age, prompted the use of a random-effects model. In addition, a sensitivity analysis was performed by excluding each study individually to identify the sources of high heterogeneity.
2.5 Quality assessment
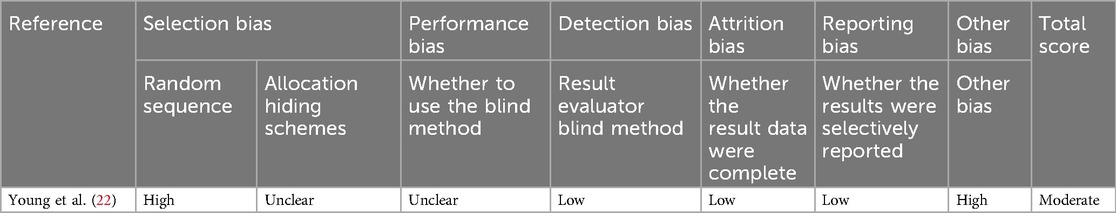
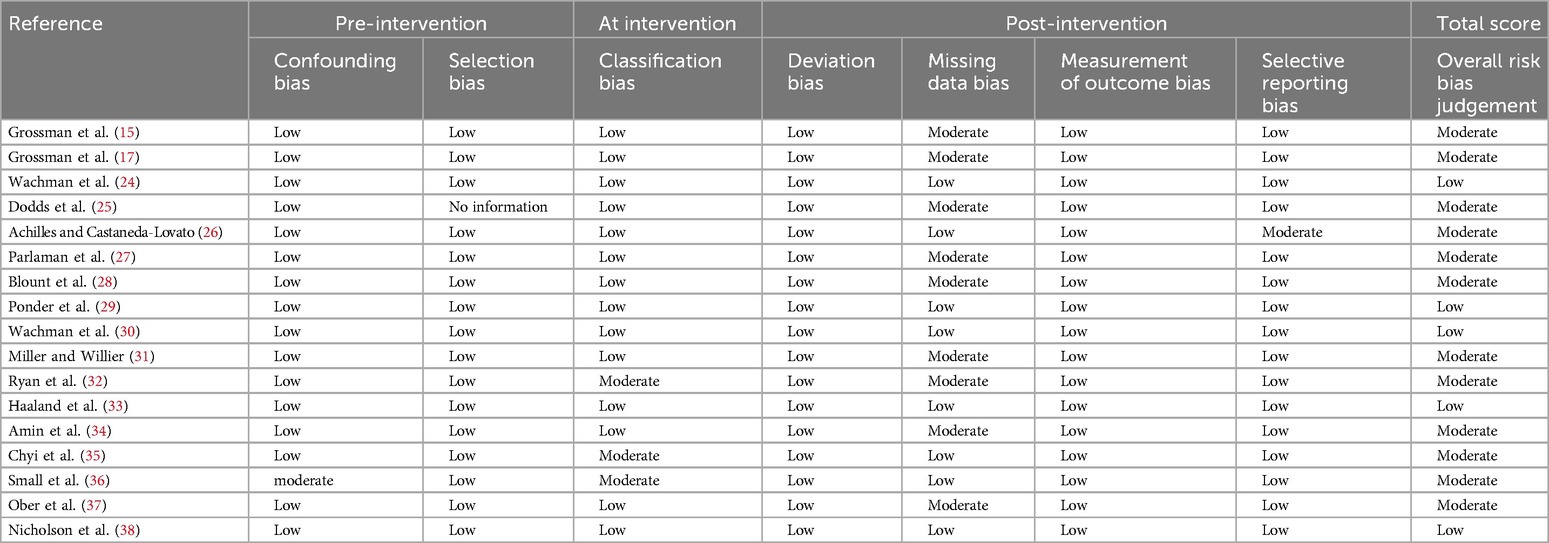
The quality of included randomized controlled trials was assessed using the Cochrane Handbook's recommended tool. This tool evaluates seven bias-related aspects, including selection, performance, and detection bias, categorizing the risk of bias into three levels: low, high, and unclear (Table 2). The risk of bias in non-randomized studies was assessed using the ROBINS-I tool (23), which evaluates four levels of bias risk: low, moderate, serious, and no information (Table 3). Bias risk assessments were independently conducted by two authors, with discrepancies resolved by consulting the corresponding author.
3 Results
3.1 Study selection and characteristics
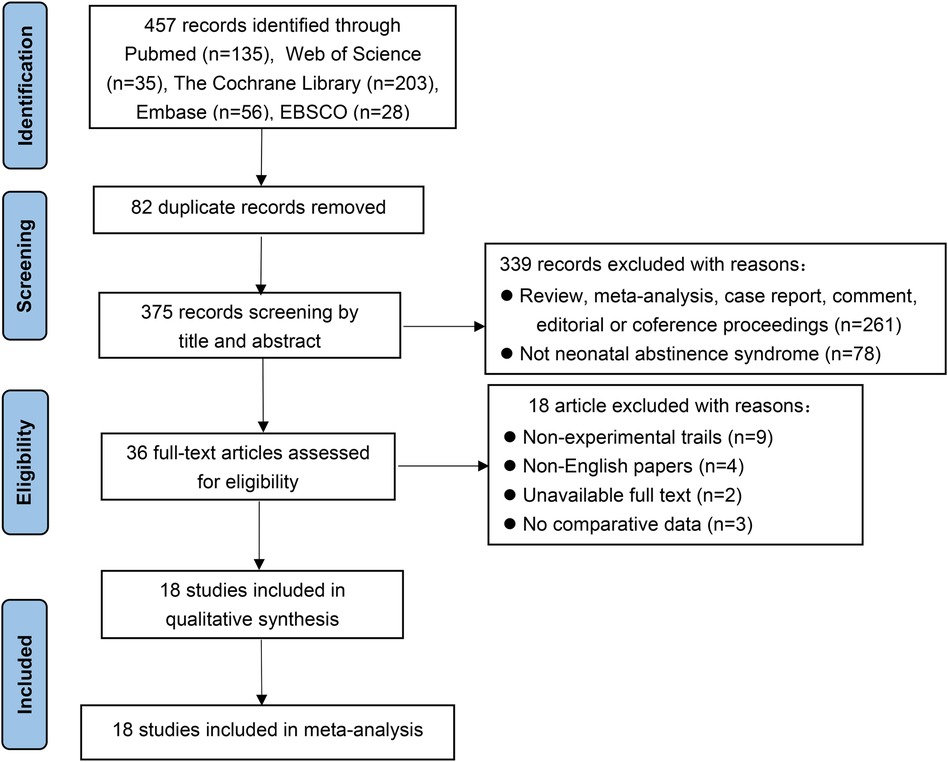
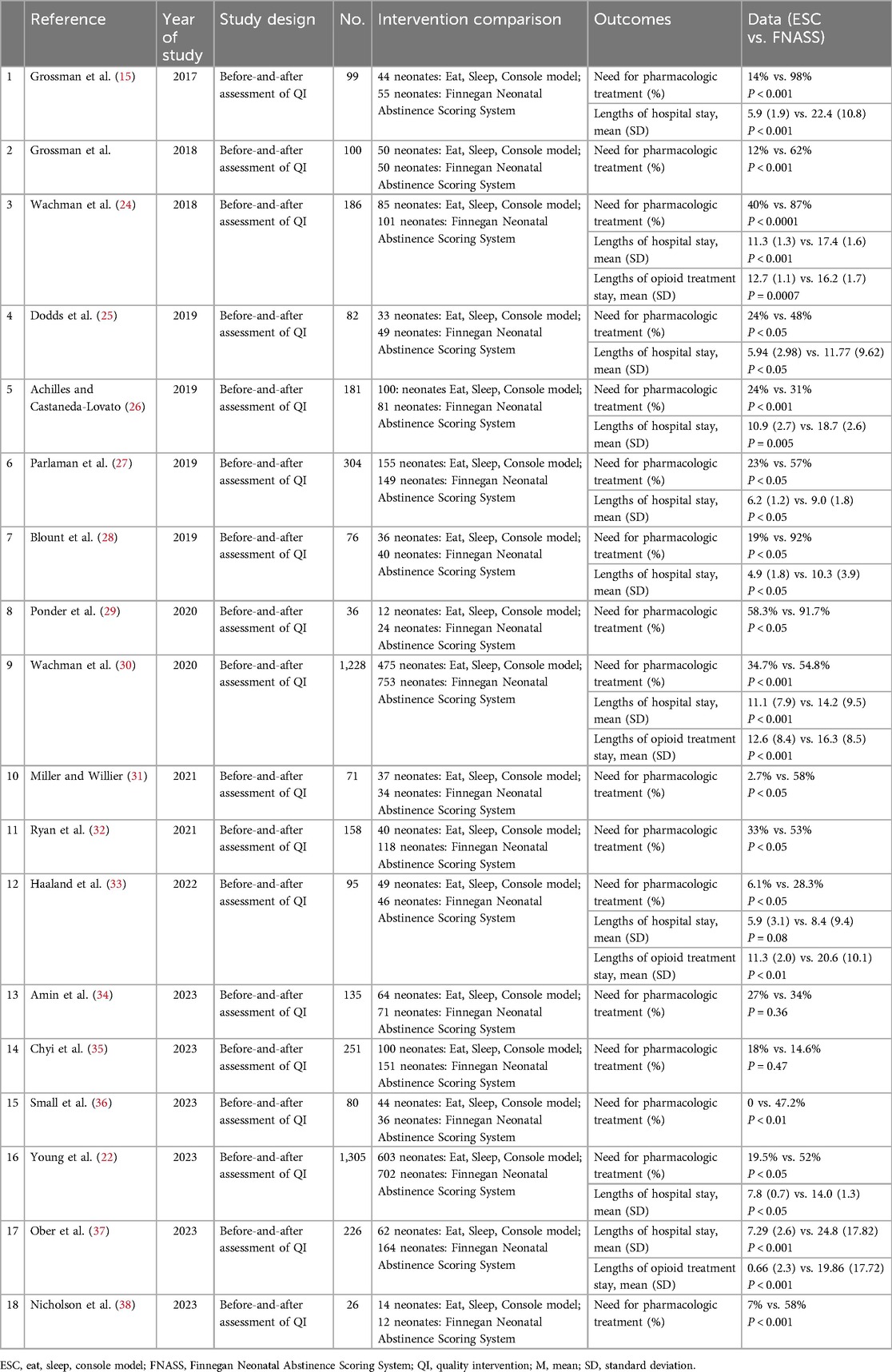
Of the 457 studies reviewed, 18 (15, 17, 22, 24–38) were included in this meta-analysis. The literature search process and outcomes are illustrated in Figure 1. A total of 4,639 patients were analyzed, with 2,003 (43.2%) assessed using the ESC model and 2,636 (56.8%) using the FNASS. These studies, conducted between 2017 and 2023, varied in sample size, geographic location, and clinical setting (Table 4).
3.2 Requirement for pharmacotherapy
Seventeen studies (15, 17, 22, 24–36, 38) demonstrated that infants with NOWS were less likely to require pharmacological treatment under the ESC model compared to the FNASS. As depicted in Figure 2, the ESC model showed superior outcomes (RR = 0.44, 95% CI = 0.34–0.56, P < 0.001). Significant heterogeneity was noted (P < 0.001, I2 = 80%), potentially due to variations in health education, non-pharmacological treatment methods, literature quality, and gestational age.
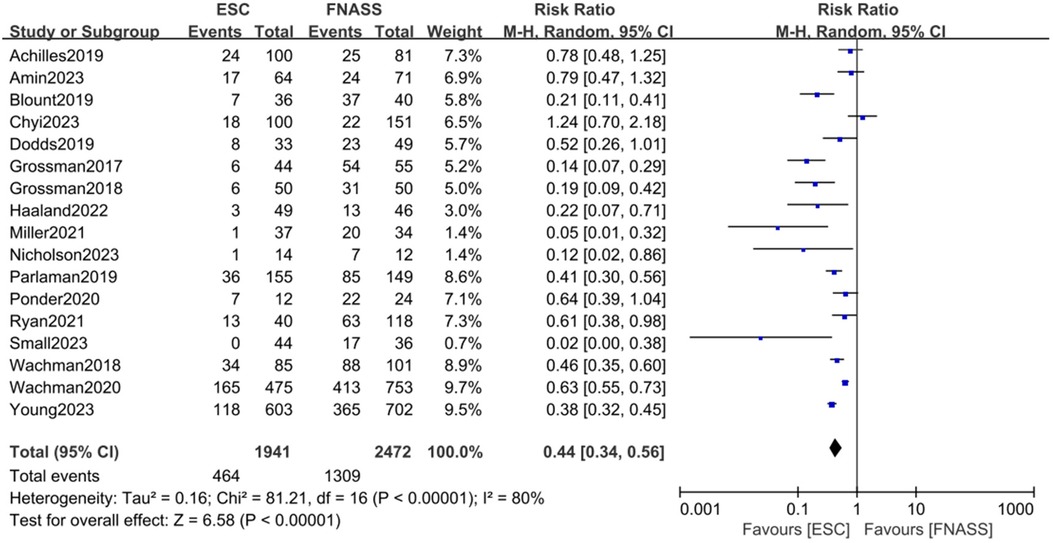
Figure 2. Eat, Sleep, Console model vs. Finnegan Neonatal Abstinence Scoring System on the requirement for pharmacotherapy. ESC, Eat, Sleep, Console model; FNASS, Finnegan Neonatal Abstinence Scoring System; CI, confidence interval; M-H, Mantel–Haenszel.
3.2.1 Subgroup analysis
3.2.1.1 Overview
Health education and non-pharmacological treatment interventions (e.g., demand feeding, promotion of breastfeeding, parental bedside presence, skin-to-skin contact, swaddling, environmental stimulation reduction, and caloric enhancement of formula) are commonly implemented alongside the ESC model. Given that the included studies originated from various hospitals across different regions, notable similarities and differences in the approaches to health education and non-pharmacological interventions were observed. Consequently, subgroup analyses were precluded. Instead, analyses were conducted based on literature quality and gestational age, with results presented in Figures 3, 4.

Figure 3. Subgroup analysis on the outcome of assessment categorized by literature quality (group 1: high quality; group 2: moderate quality). ESC, Eat, Sleep, Console model; FNASS, Finnegan Neonatal Abstinence Scoring System; M-H, Mantel–Haenszel; CI, confidence interval.
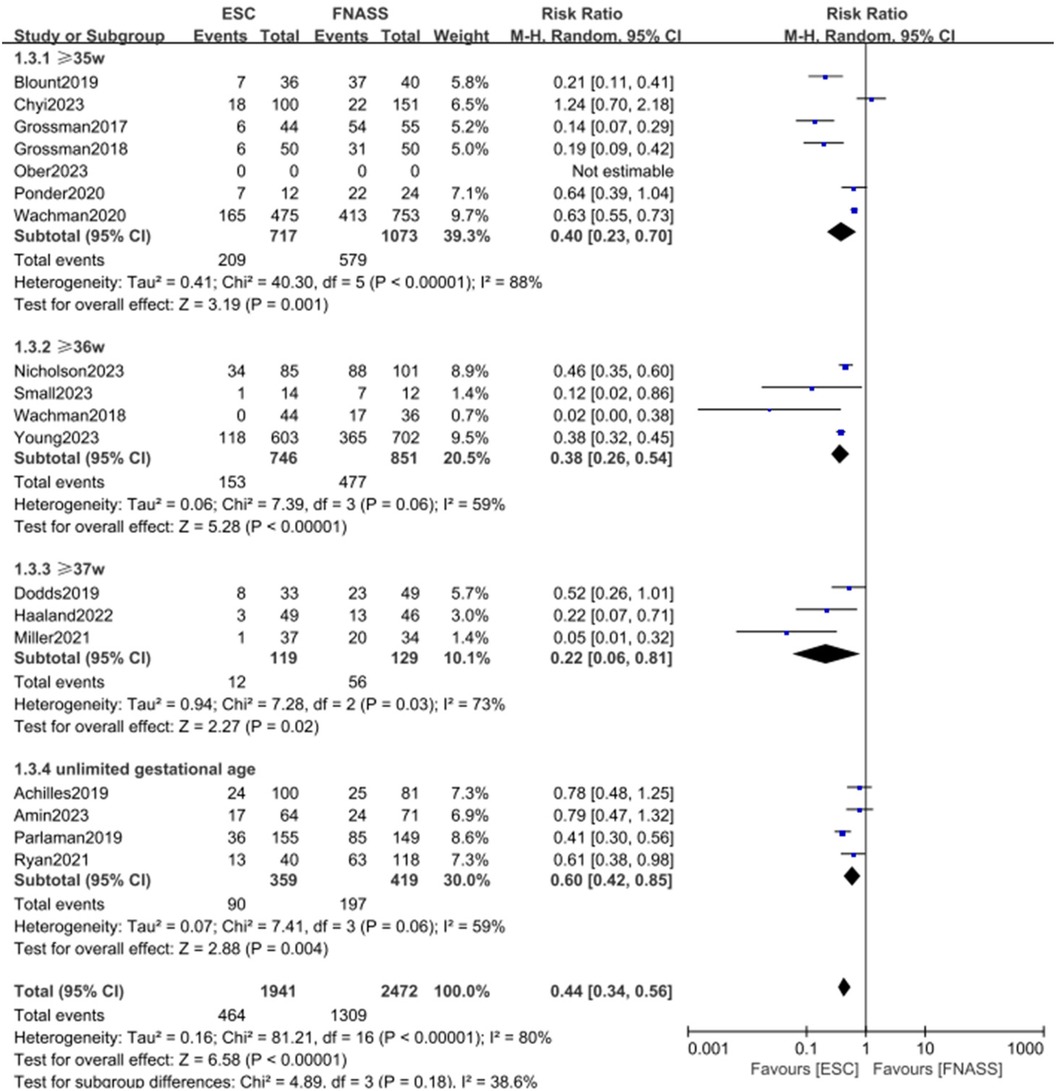
Figure 4. Subgroup analysis on the outcome of assessment categorized by gestational age (group1: ≥35 weeks; group 2: ≥36 weeks; group 3: ≥37 weeks; group 4: unlimited gestational age). ESC, Eat, Sleep, Console model; FNASS, Finnegan Neonatal Abstinence Scoring System; M-H, Mantel–Haenszel; CI, confidence interval.
3.2.1.2 First subgroup categorized by literature quality
The subgroup analysis, based on literature quality, categorized studies into high- and moderate-quality groups. As depicted in Figure 3, outcomes were more favorable in the ESC model (subgroup 1: RR = 0.52, 95% CI = 0.39–0.70; P < 0.001; subgroup 2: RR = 0.40, 95% CI = 0.28–0.57; P < 0.001). Both groups exhibited some heterogeneity (P = 0.05, I2 = 58%, vs. P < 0.001, I2 = 81%), with observable subgroup differences (P = 0.27, I2 = 17.7%). In addition, the analysis indicated a reduced requirement for pharmacotherapy in the ESC model (RR = 0.44, 95% CI = 0.34–0.56; P < 0.001).
3.2.1.3 Second subgroup categorized by gestational age
The second subgroup analysis was based on gestational age, including categories of ≥35 weeks, ≥36 weeks, ≥37 weeks, and undefined gestational age. Figure 4 shows that outcomes were similarly more favorable in the ESC model (subgroup 1: RR = 0.40, 95% CI = 0.23–0.70, P = 0.001; subgroup 2: RR = 0.38, 95% CI = 0.26–0.54, P < 0.001; subgroup 3: RR = 0.22, 95% CI = 0.06–0.81; P = 0.02; subgroup 4: RR = 0.60, 95% CI = 0.42–0.85; P = 0.004). All groups displayed considerable heterogeneity (subgroup 1: P < 0.001, I2 = 88%; subgroup 2: P = 0.06, I 2 = 59%; subgroup 3: P = 0.03, I2 = 73%; subgroup 4: P = 0.06, I2 = 59%), with observed subgroup differences (P = 0.18, I2 = 38.6%). Moreover, the ESC model was associated with a significant overall benefit (RR = 0.44, 95% CI = 0.34–0.56; P < 0.001).
3.3 Length of hospital stay
Ten studies (15, 22, 24–28, 30, 33, 37) found that infants with NOWS who underwent ESC model assessment experienced significantly shorter hospital stays compared to those who underwent FNASS assessment. According to the meta-analysis presented in Figure 5, the ESC model yielded better outcomes (SMD = −2.10, 95% CI = −3.43 to −0.78, P = 0.002). Significant heterogeneity was observed (P < 0.001, I2 = 99%), potentially due to variations in health education and non-drug treatment interventions. A sensitivity analysis was conducted by excluding each study individually, revealing no substantial changes in the results, which affirmed the reliability of the meta-analysis.
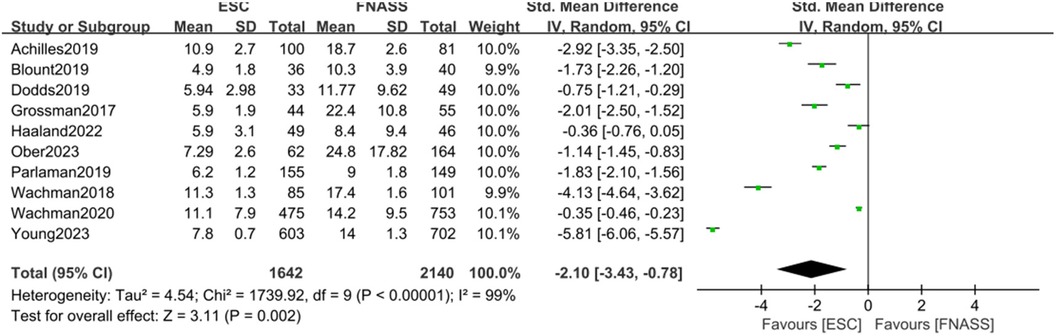
Figure 5. Eat, Sleep, Console model vs. Finnegan Neonatal Abstinence Scoring System on the length of hospital stay. SD, standard deviation; IV, inverse variance; CI, confidence interval; ESC, Eat, Sleep, Console model; FNASS, Finnegan Neonatal Abstinence Scoring System.
3.4 Length of opioid treatment stay
Four studies (24, 30, 33, 37) indicated that infants with NOWS under ESC model had significantly shorter opioid treatment stays than those under the FNASS. The meta-analysis, depicted in Figure 6A, demonstrated superior results for the ESC model (SMD = −1.33, 95% CI = −2.22 to −0.45, P = 0.003). Sensitivity analysis identified two studies (24, 30) as sources of high heterogeneity (P < 0.001, I2 = 97%), but upon their exclusion, heterogeneity markedly decreased (P = 0.93, I2 = 0%; Figure 6B). Given that the excluded studies were conducted by the same research team, it is plausible that the observed heterogeneity stemmed from differences in non-pharmacological treatment intervention methods.
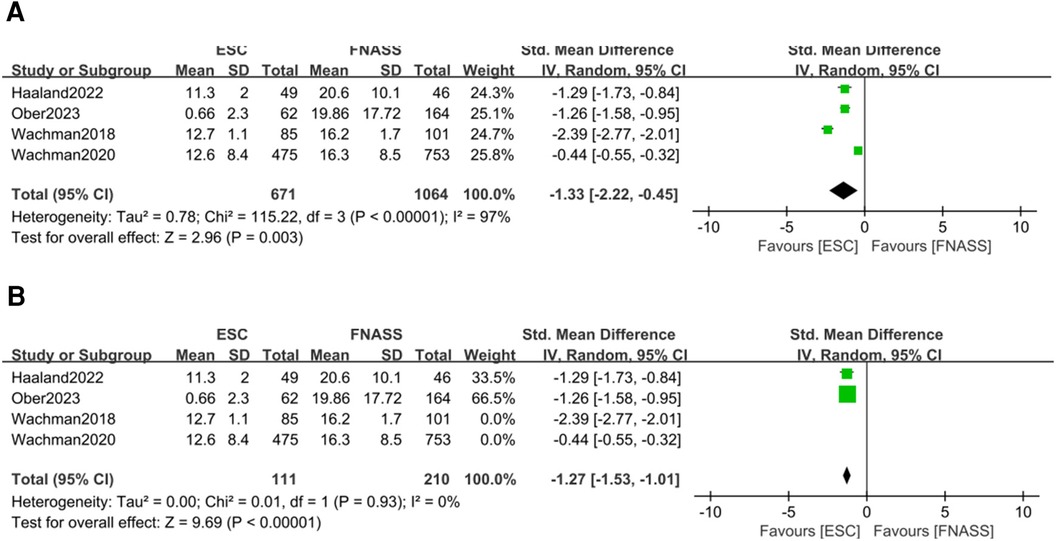
Figure 6. Eat, Sleep, Console model vs. Finnegan Neonatal Abstinence Scoring System for assessment on the length of opioid treatment stay. (A) Before sensitivity analysis. (B) After sensitivity analysis. SD, standard deviation; IV, inverse variance; CI, confidence interval; ESC, Eat, Sleep, Console model; FNASS, Finnegan Neonatal Abstinence Scoring System.
4 Discussion
NOWS is characterized by symptoms that emerge following the abrupt cessation of maternally transferred opioids during the postpartum period (24, 39). Some studies have developed a novel, non-intrusive method for assessing newborns with NOWS, focusing on the infant's function rather than the severity of withdrawal symptoms (40). This approach enhances parental involvement by increasing opportunities for non-pharmacological therapy. Numerous studies (15, 17, 22, 24–38) have demonstrated that the use of the ESC model can significantly reduce both the length of hospital stays and the need for pharmacological treatment in infants with NOWS, thereby also diminishing postnatal pharmacological interventions (20). As hospitalization durations decrease, newborns can transition to the home environment more rapidly, facilitating their progression through normal developmental milestones such as sleep–wake cycles and tummy time.
As the ESC model gains prominence as a tool for evaluating NOWS, the body of related literature continues to grow. In this meta-analysis, all studies were retrospective and of medium to high quality, displaying consistent results across the dataset (Tables 2, 3). The risk of bias was considered low, given that all included studies adhered to a standardized definition of the ESC model and yielded objective results, such as the requirements for drug treatment, and the lengths of hospital and opioid treatment stays. However, the ESC model was not implemented in isolation. The included studies featured various co-interventions during the assessment, including changes in scoring practices that may account for some observed improvements. While it is possible that factors such as pharmacological treatment, skin-to-skin contact, and enhanced parental involvement could confound the results, we consider these not as confounding but as mediating factors that contribute to the benefits of the ESC model.
Although the ESC model is a relatively new approach for treating NOWS compared to the FNASS and conventional opioids, it has demonstrated significant reductions in pharmacological interventions and advantages in the evaluation and treatment of NOWS (15). The internal reliability of the FNASS necessitates repeated training, and errors or subjective assessments by scorers may lead to medical management decisions based on potentially inaccurate scores (14). The ESC model simplifies the evaluation process by prioritizing newborn function as the main measurement index, thus minimizing subjective influences (15, 17). When parameters are exceeded, the ESC model will administer opioids as needed but will not extend the treatment duration beyond a set period (18). Due to its novelty and the limited number of studies, further research is required to substantiate the ESC model's benefits. Despite advancements in evaluation and treatment methods, there is a scarcity of research on the long-term neurodevelopmental and behavioral outcomes of NOWS in all newborns. This gap may stem from stigma, as mothers, fearing the discovery of problems, might avoid developmental follow-up appointments, believing their child to be normal. This issue may also arise from several factors such as social barriers, communication, challenges, judgments by follow-up clinic staff, or loss of follow-up due to placement in child safety (15, 17, 22, 24–38). Regardless of the cause, this area of research warrants a comprehensive review of the various approaches for evaluating and managing NOWS.
5 Limitations
Some limitations of this study must be acknowledged. First, due to variations in health education, non-pharmacological treatment interventions, and data collection methods, a certain level of heterogeneity was present in this meta-analysis. Second, although genetic variation in opioid metabolism may influence infants’ responses to NOWS therapy, the impact of genetic factors remains unclear (41, 42). Third, this review did not address the short- and long-term consequences of inadequate treatment of neonatal neuromodulation dysfunction in the ESC model (43, 44). Furthermore, studies were included in the meta-analysis only if they provided the means and standard deviations of the relevant variables. Finally, since all research articles included in this review were in English, potential language bias may exist. Despite these limitations, our findings offer valuable insights for clinical practice.
6 Conclusions
Consistent evidence supports the ESC model as an effective tool for assessing and managing NOWS, significantly reducing the need for pharmacological therapy, the length of hospital stays, and the duration of opioid treatment. The ESC model enhances parental involvement in newborn care by increasing opportunities for non-pharmacological therapy, thereby facilitating a quicker transition of the newborn to the family environment. All studies included in this meta-analysis were retrospective, exhibiting low to moderate bias, and the findings were consistent across the studies. The ESC model is recommended as the preferred method for evaluating infants with NOWS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. CX: Data curation, Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dumbhare O, Taksande A. Neonatal abstinence syndrome: an insight over impact of maternal substance use. Cureus. (2023) 15(10):e47980. doi: 10.7759/cureus.47980
2. Khan L. Neonatal abstinence syndrome. Pediatr Ann. (2020) 49(1):e3–7. doi: 10.3928/19382359-20191211-01
3. Buczkowski A, Avidan O, Cox D, Craig A. The parental experience of newborns with neonatal abstinence syndrome across inpatient care settings: a qualitative study. J Addict Med. (2020) 14(5):e183–7. doi: 10.1097/ADM.0000000000000624
4. Jilani SM, Frey MT, Pepin D, Jewell T, Jordan M, Miller AM, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome—six states, 2013–2017. MMWR Morb Mortal Wkly Rep. (2019) 68(1):6–10. doi: 10.15585/mmwr.mm6801a2
5. MacMillan KDL. Neonatal abstinence syndrome: review of epidemiology, care models, and current understanding of outcomes. Clin Perinatol. (2019) 46(4):817–32. doi: 10.1016/j.clp.2019.08.012
6. Lisonkova S, Richter LL, Ting J, Muraca GM, Wen Q, Mehrabadi A, et al. Neonatal abstinence syndrome and associated neonatal and maternal mortality and morbidity. Pediatrics. (2019) 144(2):e20183664. doi: 10.1542/peds.2018-3664
7. Rees P, Carter B, Gale C, Petrou S, Botting B, Sutcliffe AG. Cost of neonatal abstinence syndrome: an economic analysis of English national data held in the national neonatal research database. Arch Dis Child Fetal Neonatal Ed. (2021) 106(5):494–500. doi: 10.1136/archdischild-2020-319213
8. Filteau J. Trends in incidence of neonatal abstinence syndrome in Canada and associated healthcare resource utilization. Drug Alcohol Depend. (2018) 185:313–21. doi: 10.1016/j.drugalcdep.2017.12.019
9. Raffaeli G, Cavallaro G, Allegaert K, Wildschut ED, Fumagalli M, Agosti M, et al. Neonatal abstinence syndrome: update on diagnostic and therapeutic strategies. Pharmacotherapy. (2017) 37(7):814–23. doi: 10.1002/phar.1954
10. Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int J Clin Pharmacol Biopharm. (1975) 12(1–2):19–32.1100537
11. Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. (1975) 2(1–2):141–58.1163358
12. Gomez Pomar E, Finnegan LP, Devlin L, Bada H, Concina VA, Ibonia KT, et al. Simplification of the Finnegan neonatal abstinence scoring system: retrospective study of two institutions in the USA. BMJ Open. (2017) 7(9):e016176. doi: 10.1136/bmjopen-2017-016176
13. Casavant SG, Meegan T, Fleming M, Hussain N, Gork S, Cong X. Integrated review of the assessment of newborns with neonatal abstinence syndrome. J Obstet Gynecol Neonatal Nurs. (2021) 50(5):539–48. doi: 10.1016/j.jogn.2021.04.014
14. Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. (2010) 10:CD002059. doi: 10.1002/14651858.CD002059.pub3
15. Grossman MR, Berkwitt AK, Osborn RR, Xu Y, Esserman DA, Shapiro ED, et al. An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Pediatrics. (2017) 139(6):e20163360. doi: 10.1542/peds.2016-3360
16. Boucher AM. Nonopioid management of neonatal abstinence syndrome. Adv Neonatal Care. (2017) 17(2):84–90. doi: 10.1097/ANC.0000000000000371
17. Grossman MR, Lipshaw MJ, Osborn RR, Berkwitt AK. A novel approach to assessing infants with neonatal abstinence syndrome. Hosp Pediatr. (2018) 8(1):1–6. doi: 10.1542/hpeds.2017-0128
18. Nicholson S, Waskosky A. The eat, sleep, console method: a literature review. Neonatal Netw. (2022) 41(6):333–40. doi: 10.1891/NN-2021-0003
19. Taylor K, Maguire D. A review of feeding practices in infants with neonatal abstinence syndrome. Adv Neonatal Care. (2020) 20(6):430–9. doi: 10.1097/ANC.0000000000000780
20. Devlin LA, Hu Z, Merhar SL, Ounpraseuth ST, Simon AE, Lee JY, et al. Influence of eat, sleep, and console on infants pharmacologically treated for opioid withdrawal: a post hoc subgroup analysis of the ESC-NOW randomized clinical trial. JAMA Pediatr. (2024) 178(6):525. doi: 10.1001/jamapediatrics.2024.0544
21. Moher D, Alessandro L. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Syst Rev. (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
22. Young LW, Ounpraseuth ST, Merhar SL, Hu Z, Simon AE, Bremer AA, et al. Eat, sleep, console approach or usual care for neonatal opioid withdrawal. N Engl J Med. (2023) 388(25):2326–37. doi: 10.1056/NEJMoa2214470
23. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
24. Wachman EM, Grossman M, Schiff DM, Philipp BL, Minear S, Hutton E, Saia K, et al. Quality improvement initiative to improve inpatient outcomes for neonatal abstinence syndrome. J Perinatol. (2018) 38(8):1114–22. doi: 10.1038/s41372-018-0109-8
25. Dodds D, Koch K, Buitrago-Mogollon T, Horstmann S. Successful implementation of the eat sleep console model of care for infants with NAS in a community hospital. Hosp Pediatr. (2019) 9(8):632–8. doi: 10.1542/hpeds.2019-0086
26. Achilles JS, Castaneda-Lovato J. A quality improvement initiative to improve the care of infants born exposed to opioids by implementing the eat, sleep, console assessment tool. Hosp Pediatr. (2019) 9(8):624–31. doi: 10.1542/hpeds.2019-0144
27. Parlaman J, Deodhar P, Sanders V, Jerome J, McDaniel C. Improving care for infants with neonatal abstinence syndrome: a multicenter, community hospital–based study. Hosp Pediatr. (2019) 9(8):608–14. doi: 10.1542/hpeds.2019-0083
28. Blount T, Painter A, Freeman E, Grossman M, Sutton AG. Reduction in length of stay and morphine use for NAS with the “eat, sleep, console” method. Hosp Pediatr. (2019) 9(8):615–23. doi: 10.1542/hpeds.2018-0238
29. Ponder KL, Egesdal C, Kuller J, Joe P. Project console: a quality improvement initiative for neonatal abstinence syndrome in a children’s hospital level IV neonatal intensive care unit. BMJ Open Qual. (2021) 10(2):e001079. doi: 10.1136/bmjoq-2020-001079
30. Wachman EM, Houghton M, Melvin P, Isley BC, Murzycki J, Singh R, et al. A quality improvement initiative to implement the eat, sleep, console neonatal opioid withdrawal syndrome care tool in Massachusetts’ PNQIN collaborative. J Perinatol. (2020) 40(10):1560–9. doi: 10.1038/s41372-020-0733-y
31. Miller PA, Willier T. Baby STRENGTH: eat, sleep, console for infants with neonatal abstinence syndrome. Adv Neonatal Care. (2021) 21(2):99–106. doi: 10.1097/ANC.0000000000000840
32. Ryan K, Moyer A, Glait M, Yan K, Dasgupta M, Saudek K, et al. Correlating scores but contrasting outcomes for eat sleep console versus modified Finnegan. Hosp Pediatr. (2021) 11(4):350–7. doi: 10.1542/hpeds.2020-003665
33. Haaland G, Kunkel M, Nguyen CM, Wonder AH. Using the eat sleep console model to promote optimal care and outcomes for infants with neonatal abstinence syndrome: a nurse-driven, multidisciplinary initiative. Adv Neonatal Care. (2022) 23(4):320–9. doi: 10.1097/ANC.0000000000001028
34. Amin A, Frazie M, Thompson S, Patel A. Assessing the eat, sleep, console model for neonatal abstinence syndrome management at a regional referral center. J Perinatol. (2023) 43(7):916–22. doi: 10.1038/s41372-023-01666-9
35. Chyi LJ, Li S, Lee C, Walsh EM, Kuzniewicz MW. Independent impact of eat, sleep, console assessment on neonatal opioid withdrawal syndrome. Clin Pediatr (Phila). (2024) 63(8):1097–105. doi: 10.1177/00099228231204448
36. Small S, Pham R, Turbenson M, Coleman Z, Manuel V, Muniraman H. Implementation of ESC QI initiative in neonatal unit setting and adaptation during the pandemic. Hosp Pediatr. (2023) 13(7):597–606. doi: 10.1542/hpeds.2022-006806
37. Ober C, Bloom L, Obiri N. Implementation of the eat, sleep, and console model of care: a quality improvement project. Neonatal Netw. (2023) 42(6):320–8. doi: 10.1891/NN-2023-0037
38. Nicholson S, Waskosky A, Moon D. Improving outcomes in infants with neonatal abstinence syndrome with the eat, sleep, console method. Adv Neonatal Care. (2023) 23(6):509–15. doi: 10.1097/ANC.0000000000001103
39. MacVicar S, Kelly LE. Systematic mixed-study review of nonpharmacological management of neonatal abstinence syndrome. Birth. (2019) 46(3):428–38. doi: 10.1111/birt.12427
40. Grisham LM, Stephen MM, Coykendall MR, Kane MF, Maurer JA, Bader MY. Eat, sleep, console approach: a family-centered model for the treatment of neonatal abstinence syndrome. Adv Neonatal Care. (2019) 19(2):138–44. doi: 10.1097/ANC.0000000000000581
41. Ceccanti M, Blum K, Bowirrat A, Dennen CA, Braverman ER, Baron D, et al. Future newborns with opioid-induced neonatal abstinence syndrome (NAS) could be assessed with the genetic addiction risk severity (GARS) test and potentially treated using precision amino-acid enkephalinase inhibition therapy (KB220) as a frontline modality instead of potent opioids. J Pers Med. (2022) 12(12):2015. doi: 10.3390/jpm12122015
42. Wachman EM, Farrer LA. The genetics and epigenetics of neonatal abstinence syndrome. Semin Fetal Neonatal Med. (2019) 24(2):105–10. doi: 10.1016/j.siny.2019.01.002
43. Camerota M, Davis JM, Dansereau LM, Oliveira EL, Padbury JF, Lester BM. Effects of pharmacologic treatment for neonatal abstinence syndrome on DNA methylation and neurobehavior: a prospective cohort study. J Pediatr. (2022) 243:21–6. doi: 10.1016/j.jpeds.2021.12.057
Keywords: Eat, Sleep, Console model, Finnegan Neonatal Abstinence Scoring System, neonatal opioid withdrawal syndrome, assessment, meta-analysis
Citation: Chu L, Liu X and Xu C (2024) Eat, Sleep, Console model for neonatal opioid withdrawal syndrome: a meta-analysis. Front. Pediatr. 12:1416383. doi: 10.3389/fped.2024.1416383
Received: 12 April 2024; Accepted: 26 July 2024;
Published: 16 August 2024.
Edited by:
Enrique Gomez-Pomar, University of Kentucky, United StatesReviewed by:
Nathan M. Money, The University of Utah, United StatesBryanne Colvin, Washington University in St. Louis, United States
Copyright: © 2024 Chu, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuiping Xu, eHVjdWlwaW5nNzc1QHNvaHUuY29t
†These authors have contributed equally to this work and share first authorship
 Liangliang Chu1,†
Liangliang Chu1,† Xiaoyi Liu
Xiaoyi Liu