
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 24 July 2024
Sec. Pediatric Infectious Diseases
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1407174
 Xinyu Wang1,†
Xinyu Wang1,† Yanhong Lu2,†
Yanhong Lu2,† Feng Chen3
Feng Chen3 Linan Ruan1
Linan Ruan1 Lingtong Gu1
Lingtong Gu1 Ting Wang2
Ting Wang2 Heting Dong2
Heting Dong2 Yuqing Wang2
Yuqing Wang2 Chuangli Hao2
Chuangli Hao2 Li Huang2
Li Huang2 Yongdong Yan2
Yongdong Yan2 Huiming Sun2*‡
Huiming Sun2*‡ Zhengrong Chen2*‡
Zhengrong Chen2*‡
Background: This study aimed to investigate the clinical characteristics of pediatric patients hospitalized with community-acquired pneumonia (CAP) and concomitant cytomegalovirus (CMV) infection.
Methods: This cross-sectional study enrolled consecutive pediatric patients admitted with CAP who tested positive for CMV DNA in bronchoalveolar lavage fluid (BALF). Flexible fiberoptic bronchoscopy was performed when routine treatment for CAP proved ineffective. The study participants were further stratified into two groups based on CMV serological test results: recent CMV infection group and CMV replication group. Clinical characteristics were compared between these two groups.
Results: Among 124 patients aged 1–11 months included in this study, 80 (64.5%) patients were categorized as having recent CMV infection, and 44 (35.5%) tested positive for CMV replication. Co-infection with other pathogens was detected more frequently in the CMV replication group (n = 29, 65.9%) than in the recent CMV infection group (n = 35, 43.7%; P = 0.018). Patients with recent CMV infection were younger and exhibited higher levels of alanine transaminase (ALT) and aspartate aminotransferase compared to those with CMV replication (all P < 0.05). Multivariable regression analysis showed age was independently associated with recent CMV infection (odds ratio [OR], 0.707; 95% confidence interval [CI], 0.586–0.853; P < 0.001). Notably, receiver operating characteristic curve analysis showed that a CMV PCR level of 3,840 copies/ml in blood samples had a sensitivity of 34.7% and specificity of 90.0% for diagnosis of recent CMV infection with an area under the curve (AUC) of 0.625 (95% CI: 0.513–0.736, P = 0.048). A CMV PCR level of 6,375 copies/ml in urine samples had a sensitivity of 77.1% and specificity of 61.5% for diagnosis of recent CMV infection with an AUC of 0.695 (95% CI: 0.531–0.858, P = 0.04). Furthermore, multivariate linear regression analysis revealed that the blood CMV DNA copy number was associated with ALT (B = 0.001; P < 0.001).
Conclusions: The CMV DNA copy numbers in blood and urine could serve as discriminatory markers between recent CMV infection and CMV replication. Measuring CMV DNA levels in blood may be an effective method for monitoring liver function impairment in pediatric patients presenting with CAP and concurrent CMV infection.
Cytomegalovirus (CMV) is common virus, and its prevalence increases with age. It is reported that almost 85% of infants are infected within 1 year after birth (1, 2). After the initial infection, CMV establishes life-long persistence, alternating between latent phases and periods of active infection (3). The interplay between CMV and host immunity may play a role in determining the course of the disease (4, 5).
CMV infection can cause a variety of diseases, including pneumonitis, hepatitis, bacterial superinfection, colitis, encephalitis, and myocarditis (6). Among immunocompetent children hospitalized with community-acquired pneumonia (CAP), CMV is one of the most frequently identified viruses (7). The lungs are an important site for CMV reactivation. Typically, bronchoalveolar lavage fluid (BALF) is collected as a specimen to detect CMV for diagnosis of CMV lung infection (8–10). However, the presence of CMV DNA in BALF may not always be associated with acute CMV pulmonary illness, since CMV can potentially reactivate due to airway inflammation (8, 11). To date, investigations into the clinical characteristics of immunocompetent pediatric CAP patients who test positive for CMV DNA in BALF have been limited.
Currently, there are no anti-CMV treatment guidelines for immunocompetent pediatric patients. CMV IgM positivity is one of the clinical indicators for the diagnosis of CMV pneumonia in immunocompetent patients (12), and patients with severe CMV-related pneumonitis usually need antiviral treatment (13). In addition, establishing a link between CMV detection in BALF, urine, or blood samples and specific symptoms is of great medical importance, as it could also indicate the need for antiviral therapy. In the present study, patients with CMV DNA detected in BALF were categorized into two groups, the recent CMV infection group and CMV replication group, based on CMV serological testing, for comparison of clinical characteristics between these two groups. Additionally, we investigated the association between CMV DNA copy number in different types of samples and the levels of liver enzymes in CAP patients.
This cross-sectional study was conducted at the Children's Hospital of Soochow University, a tertiary teaching hospital in Suzhou, Jiangsu Province, China, from January 2019 to December 2022. During the study period, consecutive immunocompetent pediatric patients under 1 year of age who were admitted with CAP and tested positive for CMV DNA in BALF were screened. This age cutoff was chosen because host immunity is a crucial factor in diagnosing lung CMV infection (14), and young children with immature immune systems may be more susceptible to CMV infection. Flexible fiberoptic bronchoscopy was performed when routine treatment for CAP was ineffective, as the detection of CMV in BALF raised suspicion of it being a possible etiologic agent of CAP. CAP was diagnosed according to the Chinese Society of Pediatrics guidelines (15). The exclusion criteria were: age <28 days, premature birth or birthweight <2,500 g, blood transfusion within the previous 2 weeks, congenital or acquired immunodeficiencies, treatment with certain anti-CMV drugs within the previous 2 months (including ganciclovir, acyclovir, or valganciclovir), rheumatoid arthritis, and history of human herpes virus-6 or Epstein-Barr virus infection. The study was approved by the Ethics Committee of the Children's Hospital of Soochow University (2023CS162).
The following data were collected: (1) demographic data, including sex and age; (2) clinical data, including presence of fever, wheezing, requirement of supplemental oxygen, admission to the pediatric intensive care unit (PICU), requirement of mechanical ventilation, and requirement of Ganciclovir treatment; (3) laboratory data, including peripheral leukocyte count, neutrophil percentage, hemoglobin, platelet count, C-reactive protein, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and BALF cell profile; (4) presence of serum IgM and IgG anti-CMV as determined by magnetic microparticle chemiluminescence method using a CMV antibody detection kit (Autobio Diagnostics Co., Ltd. Zhengzhou, China), following the manufacturer's instructions, with CMV IgM titers ≥8 AU/ml and CMV IgG titers ≥10 AU/ml considered as positive; (5) CMV DNA in BALF, whole blood, and urine as detected by using the CMV Nucleic Acid Test kit (Sansure Biotech Co., Ltd. Hunan, China), following the manufacturer's instructions, with a detection limit >400 copies/ml (16); (6) differential cell counts in BALF as determined using a modified version of Wright-Giemsa staining (Baso Diagnostics Inc., Zhuhai, China), as previously described (16); (7) 11 common respiratory pathogens, including influenza A and influenza B viruses, human bocavirus, adenovirus, human rhinovirus, parainfluenza virus, coronaviruses, respiratory syncytial virus, human metapneumovirus, Mycoplasma pneumoniae and Chlamydophila pneumoniae screened in nasopharyngeal swab samples using a respiratory pathogen multiples detection kit (Health Gene Tech., Ningbo, China) (17); (8) bacteria detection in BALF, with growth >104 cfu/ml considered significant (18); and (9) treatment with ganciclovir therapy (10 mg/kg/day) after admission as decided by the attending physician (19).
The laboratory tests were conducted within 6 h after admission. Detection of the 11 common respiratory pathogens was performed within 24 h after admission. Flexible fiberoptic bronchoscopy was conducted in adherence to recommendations from the China Respiratory Society (15). Whole blood CMV PCR and urine CMV PCR was performed for patients who tested positive for CMV DNA in BALF.
The presence of measurable CMV DNA in BALF combined with a positive CMV IgM titer or a 4-fold increase in the CMV IgG titer was defined as recent CMV infection (19). Conversely, detection of measurable CMV DNA in BALF without a positive CMV IgM titer or a 4-fold increase in the CMV IgG titer was defined as CMV replication (20).
Statistical analyses were conducted using SPSS (version 21). The normality of numerical data was determined by the Shapiro-Wilk test. Non-normally distributed data are presented as median [interquartile range (IQR)]. Between-group comparisons were performed using the Kruskal–Wallis test or Mann–Whitney U-test, as appropriate. The Chi-squared test was used to assess differences in categorical variables. Variables with a P value < 0.05 on univariate analysis were considered for inclusion in the multivariate logistic regression models. The optimal CMV load cutoff for recent CMV infection was determined by receiver operating characteristic (ROC) curve analysis. The correlations between CMV copy number and ALT level were determined using the Spearman correlation analysis. Multiple linear regression analysis was conducted to identify factors independently associated with the ALT level. A value of P < 0.05 was considered statistically significant.
Between January 2019 and December 2022, a total of 124 patients, aged from 1 to 11 months [median, 3.0 months (IQR 2.0–6.0 months)], were admitted with CAP and tested positive for CMV DNA in BALF. Of these patients, 80 (64.5%, 80/124) were categorized as having recent CMV infection, and 44 (35.5%, 44/124) were classified as cases of CMV replication.
In 51.6% of the cases (64/124 patients), a secondary pathogen was detected. Co-infection was more frequently detected in the CMV replication group (n = 29, 29/44) than in the recent CMV infection group (n = 35, 35/80; 65.9% vs. 43.7%, P = 0.018). Respiratory syncytial virus (21.0%, 26/124) and M. pneumoniae (16.1%, 20/124) were the most commonly detected co-infecting pathogens (Figure 1).
The clinical characteristics of patients in the two groups are presented in Table 1. In comparison with the CMV replication group, the recent CMV infection group had a younger age (P < 0.001), lower prevalence of wheezing (P = 0.027), and lower percentage of patients who required supplemental oxygen (P = 0.019). Regarding laboratory findings, patients with recent CMV infection exhibited a lower hemoglobin level but higher ALT and AST levels than patients with CMV replication (all P < 0.05).
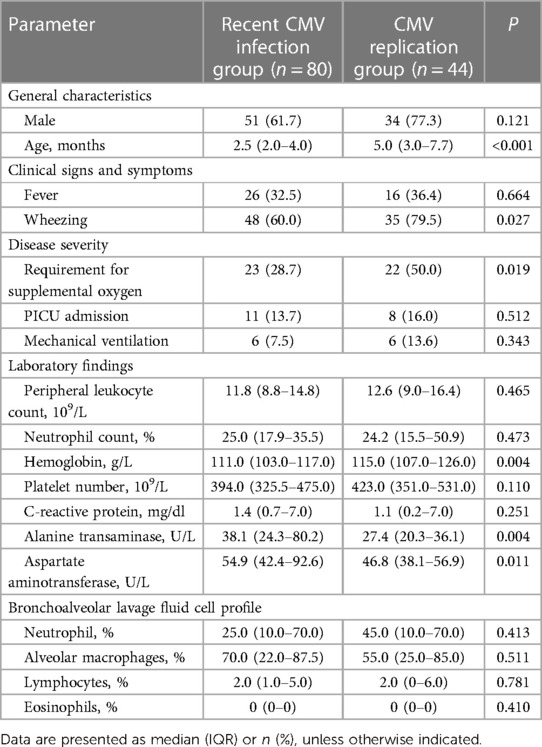
Table 1 Comparison of the clinical characteristics of patients with recent CMV infection and CMV replication.
The following characteristics did not differ significantly between the two groups: gender ratio, rate of fever, rate of PICU admission, mechanical ventilation rate, peripheral leukocyte count, neutrophil percentage, platelet count, level of C-reactive protein, and white blood cell profile (including lymphocyte, alveolar macrophage, eosinophil and neutrophil percentages) in BALF.
We next performed multivariate logistic regression analysis to identify risk factors associated with recent CMV infection. Age (odds ratio [OR], 0.707; 95% confidence interval [CI], 0.586–0.853; P < 0.001) was identified as a significant predictor of recent CMV infection.
No significant difference in the median BALF CMV DNA copy number was detected between the recent CMV infection and CMV replication groups [median 61,300.0 copies/ml (IQR 7,772.5–512,000.0 copies/ml)] vs. 40,950.0 copies/ml [9,507.5–488,750.0 copies/ml], P = 0.728; Figure 2A). Among the 80 patients with recent CMV infection, blood CMV PCR was performed for 72 patients, and 47 (65.3%) tested positive. Similarly, among the 44 patients with CMV replication, blood CMV PCR was performed for 30 patients, and 15 (50.0%) tested positive. The blood CMV PCR positivity rate did not differ significantly between the two groups (65.3% vs. 50.0%, P = 0.150). The median CMV DNA copy number in blood was higher in patients with recent CMV infection than in patients with CMV replication (median 1,065.0 copies/ml [IQR 0–7,223.7 copies/ml] vs. 250.0 copies/ml [0–1,950.0 copies/ml], P = 0.042; Figure 2B).
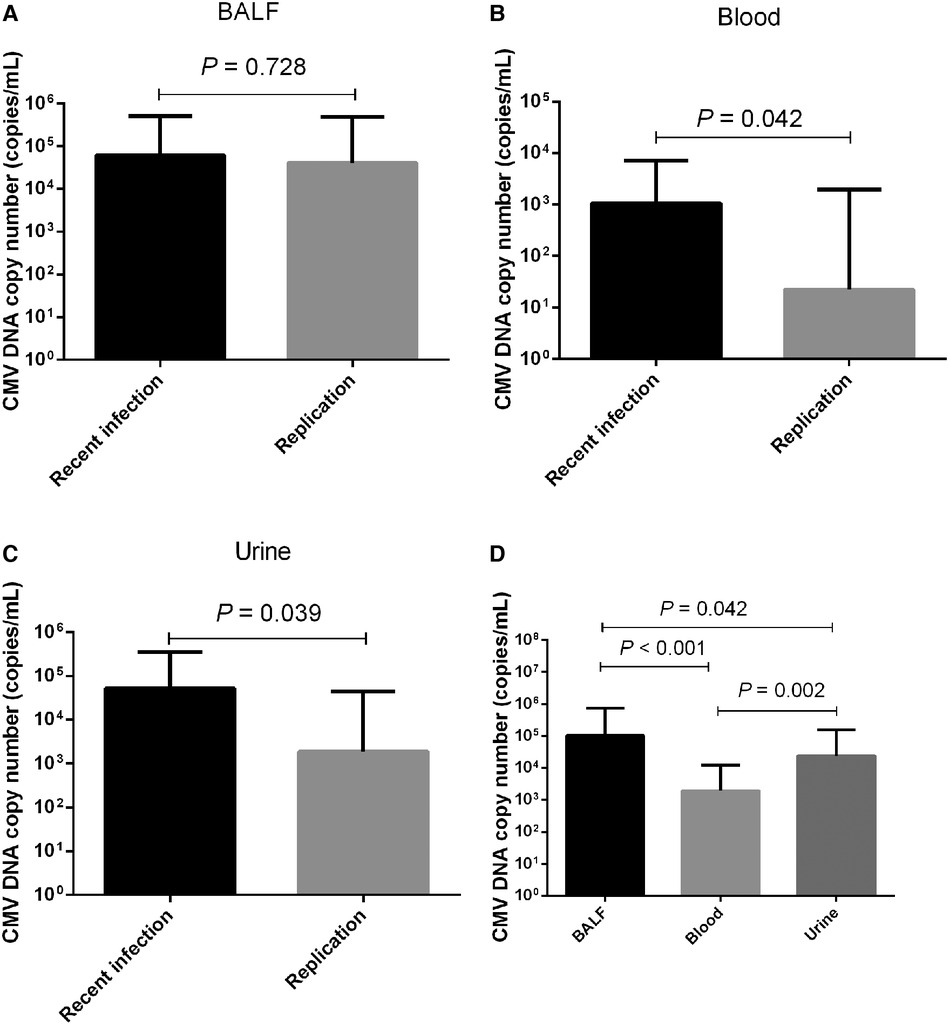
Figure 2 Comparison of CMV DNA copy numbers. Comparison of CMV DNA copy numbers in (A) BALF, (B) blood, and (C) urine between patients with recent CMV infection and CMV replication. (D) CMV DNA copy numbers in BALF, blood, and urine.
Among the 80 patients with recent CMV infection, urine CMV PCR was performed for 35 patients, and 29 (82.8%) tested positive. For the 44 patients with CMV replication, urine CMV PCR was performed for 13 patients, and 9 (69.2%) tested positive. The urine CMV PCR positivity rate did not differ significantly between the two groups (82.8% vs. 69.2%, P = 0.425). The median CMV DNA copy number in urine was higher in patients with recent CMV infection than in patients with CMV replication (median 52,000.0 copies/ml [IQR 7,750.0–353,000.0 copies/ml] vs. 1,870.0 copies/ml [0–45,700.0 copies/ml], P = 0.039; Figure 2C). Out of all 124 CMV-positive patients, BALF, blood, and urine CMV PCR were simultaneously performed for 43 patients. The median CMV DNA copy number in BALF was higher than in urine (median 104,000.0 copies/ml [IQR 8,280.0–35,000.0 copies/ml] vs. 23,900.0 copies/ml [856.0–155,000.0 copies/ml], P = 0.042), as well as higher than in blood (median 104,000.0 copies/ml [IQR 8,280.0–735,000.0 copies/ml] vs. 1,950.0 copies/ml [0–12,200.0 copies/ml], P < 0.001). The median CMV DNA copy number was higher in urine than in blood (median 23,900.0 copies/ml [IQR 856.0–155,000.0 copies/ml] vs. 1,950.0 copies/ml [0–12,200.0 copies/ml], P = 0.002; Figure 2D).
We constructed ROC curves for CMA DNA loads in blood and urine samples to determine thresholds with optimal sensitivity and specificity for recent CMV infection. ROC curve analysis showed that a CMV PCR level of 3,840 copies/ml in blood samples had a sensitivity of 34.7% and specificity of 90.0% for diagnosis of recent CMV infection with an area under the curve (AUC) of 0.625 (95% CI: 0.513–0.736, P = 0.048; Figure 3A). A CMV PCR level of 6,375 copies/ml in urine samples had a sensitivity of 77.1% and specificity of 61.5% for diagnosis of recent CMV infection with an AUC of 0.695 (95% CI: 0.531–0.858, P = 0.04; Figure 3B).
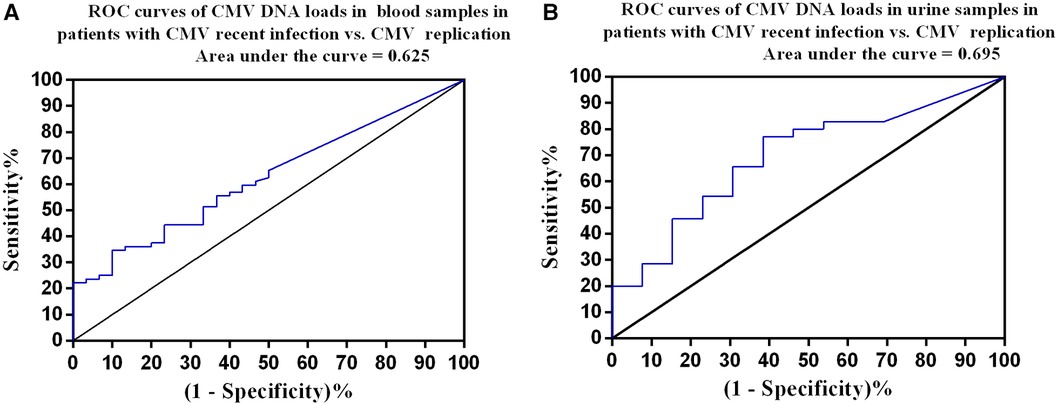
Figure 3 Receiver operating characteristic curves. (A) Patients with recent CMV infection (n = 72) vs. CMV replication (n = 30). The optimal cutoff was 3,840 copies/ml in blood samples, with a sensitivity of 34.7% and specificity of 90%. (B) Patients with recent CMV infection (n = 35) vs. CMV replication (n = 13). The optimal cutoff was 6,375 copies/ml in urine samples, with a sensitivity of 77.1% and specificity of 61.5%.
The associations between the CMV DNA copy number in different samples (BALF, blood, and urine) and liver enzyme levels (ALT, AST) were examined. The results showed that the blood CMV DNA copy number [median 733.5 copies/ml (IQR 0–5,260.0 copies/ml)] exhibited a positive correlation with the ALT level (r = 0.237, P = 0.017; Figure 4A). The urine CMV DNA copy number [median 22,150.0 copies/ml (IQR 1,012.0–142,750.0 copies/ml)] was positively correlated with the ALT level (r = 0.309, P = 0.033; Figure 4B). The BALF CMV DNA copy number [median 50,600.0 copies/ml (IQR 8,265.0–512,000.0 copies/ml)] was not correlated with the ALT level (r = 0.029, P = 0.747).
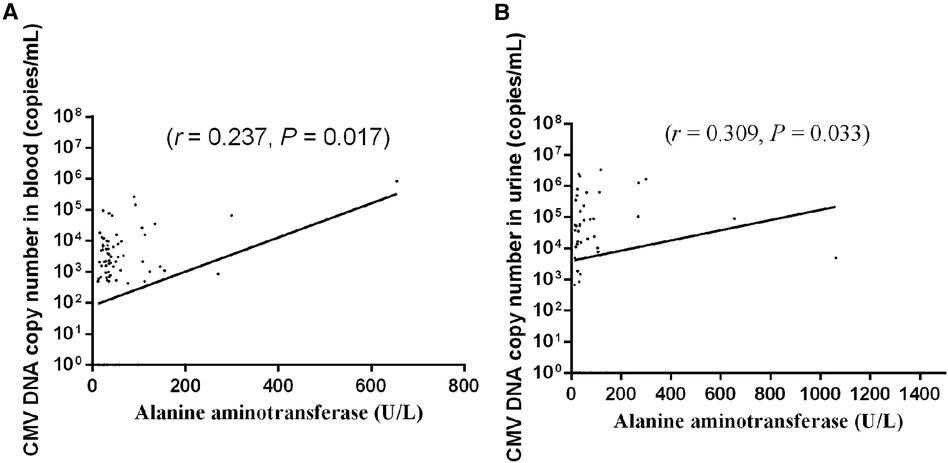
Figure 4 Association between CMV DNA copy number in (A) blood and (B) urine and liver enzyme level. Each dot indicates an individual patient.
In addition, multivariate linear regression analysis revealed that the blood CMV DNA copy number was associated with the ALT level (B = 0.001; P < 0.001).
Among the 80 patients with recent CMV infection, 43 (53.7%) received ganciclovir therapy (10 mg/kg/day). In contrast, of the 44 patients with CMV replication, 10 (22.7%) received ganciclovir therapy (10 mg/kg/day). The utilization rate of ganciclovir was notably higher in patients with recent CMV infection than in those with CMV replication (53.7% vs. 22.7%, P = 0.001). All patients recovered regardless of treatment.
Deciphering the implications of CMV DNA detection in BALF by PCR poses a challenge for pediatric practitioners, and standardized clinical criteria for immunocompetent patients are lacking. In the present study, immunocompetent pediatric patients under 1 year old, who were admitted with CAP and tested positive for CMV DNA in BALF, were further categorized according to the results of CMV serological testing. It was surprising that 64.5% of the patients had CMV recent infection. Age was identified as a significant predictor of recent CMV infection. Consequently, pediatricians should exercise vigilance regarding the possibility of recent CMV infection when managing young patients hospitalized for CAP who have tested positive for CMV DNA in BALF.
In the present study, a secondary pathogen was identified more frequently among cases categorized as CMV replication than among those categorized as recent CMV infection. This observation might not necessarily suggest a causative role for CMV in patients with CMV replication. In addition, the rate of ganciclovir use was higher in patients with recent CMV infection and no detection of a secondary pathogen (n = 30, 30/45) than in those with recent CMV infection and detection of a secondary pathogen (n = 13, 13/35; 66.7% vs. 37.1%, P = 0.009), which might suggest that CMV was usually considered the etiology of cases in which no secondary pathogen was detected. Furthermore, the recent CMV infection group had a lower rate of wheezing than the CMV replication group. This could be attributed to the finding that the most commonly detected pathogens were respiratory syncytial virus and M. pneumoniae, both of which are known to increase susceptibility to wheezing in young children (21, 22).
In our study, the percentage of patients who required supplemental oxygen was lower in the recent CMV infection group than in the CMV replication group. The reason might be that CMV infection in immunocompetent individuals rarely leads to severe illness (23). Furthermore, patients with recent CMV infection had a lower hemoglobin lever than patients with CMV replication, which may be linked to physiologic anemia. Additionally, patients with recent CMV infection had higher ALT and AST levels than patients with CMV replication, indicating that recent CMV infection is more commonly associated with elevation of hepatic aminotransferase levels. However, the ALT and AST levels were not significantly elevated, aligning with the findings from a previous study (24). Notably, the levels of hepatic aminotransferases in cases of CMV infection are generally lower than those in cases of hepatitis caused by hepatitis viruses.
Our results showed that the median BALF CMV DNA copy number did not differ significantly between patients with recent CMV infection and CMV replication. This suggests that the BALF CMV DNA copy number might not be a discriminatory marker for recent CMV infection vs. CMV replication. However, the median CMV DNA copy numbers in blood and urine were higher in patients with recent CMV infection than in patients with CMV replication. ROC curves were constructed and used to determine a cut-off value of 3,840 copies/ml for the CMV PCR level in blood samples (sensitivity: 34.7% and specificity: 90.0%) and a cut-off value of 6,375 copies/ml for the CMV PCR level in urine samples (sensitivity: 77.1% and specificity: 61.5%) for distinguishing recent CMV infection from CMV replication. These results indicate that the CMV DNA copy numbers in blood and urine could serve as discriminators between recent CMV infection and CMV replication. However, both had relatively low AUC values, which may indicate poor diagnostic performance (25). In addition, the median CMV DNA copy number was higher in BALF samples than in blood and urine samples, which might support the concept of a compartmentalization and spill-over effect in the pathogenic response. Thus, having CMV disease in the lungs should theoretically lead to higher CMV loads within the respiratory system, with a spill-over effect extending into the bloodstream when CMV replication cannot be effectively controlled within the lungs (26, 27).
Our results revealed a positive correlation between the blood CMV DNA copy number and ALT level, similar to the results of a previous study (28). This suggests that the level of blood CMV DNA could potentially serve as a monitoring parameter for CMV-associated infantile hepatitis. However, the r value was low, indicating a weak correlation.
This study has several limitations. Firstly, we did not dynamically monitor CMV loads in blood, urine, and BALF samples, which could provide better insight into the correlation between virus load and clinical characteristics and is warranted in further investigations. Secondly, while 10 (22.7%) patients with CMV replication recovered after ganciclovir therapy, the number with recent CMV infection may have been underestimated due to serological testing limitations (23). Future multicenter, randomized clinical trials are needed to determine the indications for ganciclovir as well as its efficacy in immunocompetent patients. Thirdly, our study only included patients over 1 month old, limiting the generalizability of the results to younger infants who require separate evaluation. Fourthly, PCR for CMV in BALF, blood, and urine samples simultaneously was not performed for all patients. Comparison of the clinical characteristics of children with recent CMV infection and CMV replication based on whether blood and urinary CMV tests had been performed showed that patients who underwent urine CMV testing were younger than those who did not among patients with recent CMV infection (P = 0.040), and the percent of lymphocytes in BALF was lower in patients for whom urine CMV testing was performed than in those for whom it was not (P = 0.025) (Supplementary Tables S1–S4). Thus, population bias might exist, and future large-sample clinical studies are needed. Fifthly, we did not set a control group of children with no respiratory symptoms; thus, the percentage of children with recent CMV infection among those with no respiratory symptoms is unknown. However, performing flexible fiberoptic bronchoscopy in children with no respiratory symptoms is unethical. Finally, we did not employ the test for 11 respiratory viruses in BALF due to the potential for nasopharyngeal swabs to partially represent lung pathogens (29), the high cost of the test, and patient reluctance regarding repeat testing. However, we aim to include this analysis in future studies when feasible and permitted by patients' guardians.
In conclusion, it is imperative for pediatricians to maintain a high degree of suspicion for recent CMV infection when managing young patients hospitalized with CAP and a history of CMV DNA detection in BALF. Furthermore, the CMV DNA copy numbers in blood and urine could serve as discriminatory markers between recent CMV infection and active CMV replication. Importantly, testing for CMV DNA levels in blood could prove valuable for monitoring liver function impairment in pediatric patients presenting with CAP and concurrent CMV infection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by this study complied with the declaration of Helsinki and all methods were performed in accordance with the relevant guidelines and regulations of the Ethics Committee of the Children's Hospital of Soochow University (NO. 2023CS162). This study used data collected from patient records while maintaining patient anonymity. Because this study presented no more than minimal risk of harm to patient subjects, the Ethics Committee of the Children's Hospital of Soochow University approved a waiver of patient informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
XW: Writing – original draft. YL: Writing – original draft. FC: Writing – review & editing. LR: Writing – review & editing. LG: Writing – review & editing. TW: Writing – review & editing. HD: Writing – review & editing. YW: Writing – review & editing. CH: Writing – review & editing. LH: Writing – review & editing. YY: Writing – review & editing. HS: Writing – review & editing, Writing – original draft. ZC: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Extracurricular Academic Research Fund for the 25th batch of Soochow University Students (grant numbers KY2023090A, KY2023424B); the Suzhou Livelihood Science and Technology Project (grant number SKJY2021104); the Science and Technology Project of Suzhou City (grant number KJXW2020025); and the National Natural Science Foundation of China (grant number 82100026).
The authors would like to thank Zhiao Du for her assistance in statistical analysis of the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1407174/full#supplementary-material
1. Adland E, Klenerman P, Goulder P, Matthews PC. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front Microbiol. (2015) 6:1016. doi: 10.3389/fmicb.2015.01016
2. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. (2010) 20(4):202–13. doi: 10.1002/rmv.655
3. Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. (2018) 7(1):1–16. doi: 10.1007/s40121-017-0180-z
4. Restrepo-Gualteros SM, Jaramillo-Barberi LE, Gonzalez-Santos M, Rodriguez-Martinez CE, Perez GF, Gutierrez MJ, et al. Characterization of cytomegalovirus lung infection in non-HIV infected children. Viruses. (2014) 6(5):2038–51. doi: 10.3390/v6052038
5. Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. (2008) 197(2):83–96. doi: 10.1007/s00430-008-0082-5
6. Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. (2008) 5:47. doi: 10.1186/1743-422X-5-47
7. Mathew JL, Singhi S, Ray P, Hagel E, Saghafian-Hedengren S, Bansal A, et al. Etiology of community acquired pneumonia among children in India: prospective, cohort study. J Glob Health. (2015) 5(2):050418. doi: 10.7189/jogh.05.020418
8. Burgener EB, Waggoner J, Pinsky BA, Chen SF. Clinical characteristics and outcomes of pediatric patients with CMV DNA detection in bronchoalveolar lavage fluid. Pediatr Pulmonol. (2017) 52(1):112–8. doi: 10.1002/ppul.23494
9. Cook CH, Martin LC, Yenchar JK, Lahm MC, McGuinness B, Davies EA, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. (2003) 31(7):1923–9. doi: 10.1097/01.CCM.0000070222.11325.C4
10. Chilet M, Aguilar G, Benet I, Belda J, Tormo N, Carbonell JA, et al. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. J Med Virol. (2010) 82(8):1384–91. doi: 10.1002/jmv.21825
11. Mansfield S, Griessl M, Gutknecht M, Cook CH. Sepsis and cytomegalovirus: foes or conspirators? Med Microbiol Immunol. (2015) 204(3):431–7. doi: 10.1007/s00430-015-0407-0
12. Grilli E, Galati V, Bordi L, Taglietti F, Petrosillo N. Cytomegalovirus pneumonia in immunocompetent host: case report and literature review. J Clin Virol. (2012) 55(4):356–9. doi: 10.1016/j.jcv.2012.08.010
13. Doan TT, Phung TT, Pham HV, Pham SH, Nguyen LT. Effect of ganciclovir for the treatment of severe cytomegalovirus-associated pneumonia in children without a specific immunocompromised state. BMC Infect Dis. (2013) 13:424. doi: 10.1186/1471-2334-13-424
14. Restrepo-Gualteros SM, Gutierrez MJ, Villamil-Osorio M, Arroyo MA, Nino G. Challenges and clinical implications of the diagnosis of cytomegalovirus lung infection in children. Curr Infect Dis Rep. (2019) 21(7):24. doi: 10.1007/s11908-019-0681-x
15. Subspecialty Group of Respiratory Diseases TSoPCMA, Editorial Board CJoP. Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (I). Zhonghua Er Ke Za Zhi. (2013) 51(10):745–52. doi: 10.3760/cma.j.issn.0578-1310.2013.10.006
16. Sun H, Li S, Yan Y, Chen Z, Wang Y, Hao C, et al. Associations between patient clinical characteristics and the presence of cytomegalovirus DNA in the bronchoalveolar lavage fluid of children with recurrent wheezing. BMC Infect Dis. (2018) 18(1):458. doi: 10.1186/s12879-018-3345-9
17. Wang H, Li X, Zheng Y, Verhagen LM, Gu J, Li L, et al. Concordance in pathogen identification at the upper and lower respiratory tract of children with severe pneumonia. BMC Infect Dis. (2023) 23(1):170. doi: 10.1186/s12879-023-08127-w
18. De Schutter I, De Wachter E, Crokaert F, Verhaegen J, Soetens O, Pierard D, et al. Microbiology of bronchoalveolar lavage fluid in children with acute nonresponding or recurrent community-acquired pneumonia: identification of nontypeable Haemophilus influenzae as a major pathogen. Clin Infect Dis. (2011) 52(12):1437–44. doi: 10.1093/cid/cir235
19. Subspecialty Group of Infectious Diseases TSoPCMA, National Pediatric Clinical Virology Cooperative G, Editorial Board CJoP. A proposal for the diagnosis, treatment and prophylaxis of cytomegalovirus diseases in children. Zhonghua Er Ke Za Zhi. (2012) 50(4):290–2. doi: 10.3760/cma.j.issn.0578-1310.2012.04.013
20. Mansfield S, Dwivedi V, Byrd S, Trgovcich J, Griessl M, Gutknecht M, et al. Broncholaveolar lavage to detect cytomegalovirus infection, latency, and reactivation in immune competent hosts. J Med Virol. (2016) 88(8):1408–16. doi: 10.1002/jmv.24472
21. Sun H, Li S, Wang T, Chen Z. Mycoplasma pneumoniae infection and persistent wheezing in young children: a retrospective case-control study. Front Pediatr. (2022) 10:811086. doi: 10.3389/fped.2022.811086
22. Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol. (2015) 235(2):266–76. doi: 10.1002/path.4462
23. Cunha BA. Cytomegalovirus pneumonia: community-acquired pneumonia in immunocompetent hosts. Infect Dis Clin North Am. (2010) 24(1):147–58. doi: 10.1016/j.idc.2009.10.008
24. Kunno A, Abe M, Yamada M, Murakami K. Clinical and histological features of cytomegalovirus hepatitis in previously healthy adults. Liver. (1997) 17(3):129–32. doi: 10.1111/j.1600-0676.1997.tb00794.x
25. Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. (2022) 75(1):25–36. doi: 10.4097/kja.21209
26. Jeena PM, Govender K, Parboosing R, Adhikari M. The significance of cytomegalovirus in children with pneumonia admitted for mechanical ventilation. Int J Tuberc Lung Dis. (2017) 21(12):1230–6. doi: 10.5588/ijtld.17.0026
27. Tembo J, Kabwe M, Chilukutu L, Chilufya M, Mwaanza N, Chabala C, et al. Prevalence and risk factors for betaherpesvirus DNAemia in children >3 weeks and <years of age admitted to a large referral hospital in sub-Saharan Africa. Clin Infect Dis. (2015) 60(3):423–31. doi: 10.1093/cid/ciu853
28. Funato T, Satou N, Abukawa D, Satou J, Abe Y, Ishii KK, et al. Quantitative evaluation of cytomegalovirus DNA in infantile hepatitis. J Viral Hepat. (2001) 8(3):217–22. doi: 10.1046/j.1365-2893.2001.00277.x
29. Zhu Q, Zhou J, Li F, Shi P, Lu Y, Lin X, et al. Nasopharyngeal aspirates in children with severe community-acquired pneumonia collected within 3 days before bronchoscopy can partially reflect the pathogens in bronchoalveolar lavage fluids. BMC Infect Dis. (2022) 22(1):814. doi: 10.1186/s12879-022-07749-w
Keywords: cytomegalovirus, blood, urine, community-acquired pneumonia, immunocompetent
Citation: Wang X, Lu Y, Chen F, Ruan L, Gu L, Wang T, Dong H, Wang Y, Hao C, Huang L, Yan Y, Sun H and Chen Z (2024) Clinical characteristics of pediatric patients hospitalized with community-acquired pneumonia and cytomegalovirus DNA detected in bronchoalveolar lavage fluid. Front. Pediatr. 12: 1407174. doi: 10.3389/fped.2024.1407174
Received: 26 March 2024; Accepted: 16 July 2024;
Published: 24 July 2024.
Edited by:
Ramos Amador Jose T., Complutense University of Madrid, SpainReviewed by:
Jop Jans, Wilhelmina Children's Hospital, Netherlands© 2024 Wang, Lu, Chen, Ruan, Gu, Wang, Dong, Wang, Hao, Huang, Yan, Sun and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiming Sun, c2htXzE5ODVAMTI2LmNvbQ==; Zhengrong Chen, Y2hlbl96aGVuZ19yb25nQDE2My5jb20=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.