
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 10 September 2024
Sec. Children and Health
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1400110
Introduction: Autism spectrum disorder (ASD) is a neurodevelopmental condition that significantly impacts the mental, emotional, and social development of children. Early screening for ASD typically involves the use of a series of questionnaires. With answers to these questionnaires, healthcare professionals can identify whether a child is at risk for developing ASD and refer them for further evaluation and diagnosis. CHAT-23 is an effective and widely used screening test in China for the early screening of ASD, which contains 23 different kinds of questions.
Methods: We have collected clinical data from Wuxi, China. All the questions of CHAT-23 are regarded as different kinds of features for building machine learning models. We introduce machine learning methods into ASD screening, using the Max-Relevance and Min-Redundancy (mRMR) feature selection method to analyze the most important questions among all 23 from the collected CHAT-23 questionnaires. Seven mainstream supervised machine learning models were built and experiments were conducted.
Results: Among the seven supervised machine learning models evaluated, the best-performing model achieved a sensitivity of 0.909 and a specificity of 0.922 when the number of features was reduced to 9. This demonstrates the model's ability to accurately identify children for ASD with high precision, even with a more concise set of features.
Discussion: Our study focuses on the health of Chinese children, introducing machine learning methods to provide more accurate and effective early screening tests for autism. This approach not only enhances the early detection of ASD but also helps in refining the CHAT-23 questionnaire by identifying the most relevant questions for the diagnosis process.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder usually manifested by behavioral and social deficits (1). The prevalence of autism has been increasing over the years, with varying estimates depending on the region and population studied. Statistics indicate that over 1.5% of children in the United States, or approximately one in every 54 children, are diagnosed with ASD. However, the prevalence of autism in China is estimated to be lower than 1%. This difference may be due to the underrepresentation of the mainstream school population in the statistical data and the need for more up-to-date research to assess the prevalence of autism in China accurately (2, 3). Matson et al. conducted research on cross-cultural autism behavior and also showed that children with autism have different behavioral characteristics and severity due to regional differences and cultural backgrounds (4).
Infancy is a critical period of plasticity for brain development (5, 6). The primary objective of early intervention is to make full use of brain plasticity during infancy, enhance brain development induce behavioral changes, and ultimately reduce the impact of the disorder in children with ASD. Based on the characteristics of ASD in 8-year-olds, it has been found that the diagnostic accuracy of children under 36 months of age exceeds that of children over 36 months of age. Some studies use different age groups as controls, finding that younger children, compared to adolescents, provide more stable and accurate results for early diagnosis (7, 8). Those findings highlight the importance of early screening for autism. Early screening allows for timely intervention to help children receive treatment for autism (9). There are two main lines for early screening of autism, including Scale-based methods, and machine learning-based methods.
Scale-based early screening tests for ASD can be classified into two levels: the level 1 ASD screening test and the level 2 ASD screening test. Examples include the Modified Checklist for Autism in Toddlers (M-CHAT) (10), the Quantitative Checklist for Autism in Toddlers (Q-CHAT) (11), and the Checklist for Autism in Toddlers (CHAT-23) (12). On the other hand, Level 2 ASD screening tests are intended for children at high risk for ASD, such as the Infant-Toddler Checklist (ITC) (13), the Baby and Infant Screen for Children with aUtIsm Traits (BISCUIT) (14), and the Systematic Observation of Red Flags (SORF) (15). Among various screening tests, the CHAT-23 serves as a Level 1 screening test for ASD used in Chinese children. CHAT-23 is a scale screening test consisting of a parental questionnaire and a physician’s observation of the children’s behaviors. This test has significant value in early screening as it assesses a child’s social and behavioral performance, enabling the recognition of potential signs of autism (16). For example, an online screening system utilizing telemedicine technology was developed for ASD by using CHAT-23 (17).
Machine learning based ASD methods require training on data, which can be in the form of questionnaires or image data. Machine learning is a technique that automatically analyzes data to obtain patterns and uses them to make predictions about unknown data (18). Benefiting from the ability to learn from existing data, machine learning has become a popular way for ASD (19). Depending on the type of data used for machine learning, there are two main types of machine learning based early screening methods, one is the Scale-based method, and the other is image-based method. Scale-based methods have solid practical experience from experts and can provide strong evidence for the machine learning model. For example, based on the scale information collected using the Q-CHAT, a range of machine learning models including Logistic Regression, Support Vector Machine, Naive Bayes, Random Forest, and K-Nearest Neighbors have been used to predict autism in toddlers (20). Using logistic regression and decision tree algorithm, Duda et al. found that 7 key items were selected from all items in the Autism Diagnostic Interview-Revised (ADI-R) to build an accurate model for classifying children with autism (21). Multiple machine learning methods have been effectively demonstrated to differentiate between Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) (22). Some unsupervised clustering machine learning methods also have been employed for modeling data related to Autism Spectrum Disorder (ASD), and their results have proven to help facilitate autism diagnosis (23). Siddiqui et al. achieved a high classification accuracy for children with ASD by utilizing monitoring devices to acquire behavioral gesture information. They employed models such as K-Nearest Neighbors and Random Forest to perform the classification (24). Biomarkers based on brain imaging as input features for machine learning techniques can provide objective evidence for autism classification. Functional magnetic resonance imaging (fMRI) data combined with machine learning models has also been utilized to assist in diagnosing ASD (25). There also exists mixed data used for modeling machine learning. Abbas et al. create low-cost and accurate autism screening tests using parent report questionnaires and home videos of children (26).
Several studies show that ASD is closely connected with other diseases. Precenzano et al. demonstrated that electroencephalographic abnormalities are typically associated with more severe forms of ASD (27). Rossi et al. reported that nearly half of children with ASD, indicated by Subclinical Electroencephalographic Abnormalities, even without epilepsy, show abnormal development within the first year, with epilepsy and intellectual disability (28). Early diagnosis of ASD can be beneficial, as it allows for treatment before the condition worsens, potentially reducing costs and promoting family life. Operto et al.’s report showed that ASD can be treated at an earlier stage by means of cheaper intervention, such as speech therapy, psychomotor therapy, occupational therapy, etc. As grow older, treatment may have to consider drug therapy, and the amount of drugs used increases with age (29). Therefore, considering the importance of early screening, we believe that highly efficient machine learning-based methods for early autism screening should be considered. Several machine learning based methods for early screening validate their effectiveness on American children (26, 30, 31). However, there is still a vacant study on Chinese children. Thus, we first collected questionnaire data from age-appropriate children and processed the raw data through a series of preprocessing steps to make it suitable for modeling. We then built seven different machine learning models using above data. From all the models, we identified the most appropriate one to assist in diagnosing ASD. In conclusion, our work and main contributions include:
(1) We built machine learning models by applying data from Chinese children.
(2) We applied mRMR feature engineering to automatically acquire a set of features for better construction of machine learning models.
(3) We modeled seven different machine learning models, and compared the prediction performance of these models based on collected question data, identifying the best-performing machine learning model.
(4) We performed feature engineering upon the original 23-dimensional question features, and rank these questions according to their importance. Finally, the LGBM model achieved the highest specificity and sensitivity of 0.922 and 0.909.
This study uses data collected from the Affiliated Hospital of Jiangnan University, ranging from January 4th, 2022 to September 28th, 2022. Participants selection flow is as Figure 1A shows. According to the practice of CHAT-23, the applicable age is 18 to 24 months, we exclude those data whose month age does not meet this range. We also exclude those questionnaires with missing information. This participant selection flow obtained 371 copies of the children’s CHAT-23 scale information. According to statistics, the average age of children is (20.27 2.68) months. As shown in Figure 1B, in all cases, 48 people were diagnosed as autism positive and 323 were negative, which is an important basis for our research.
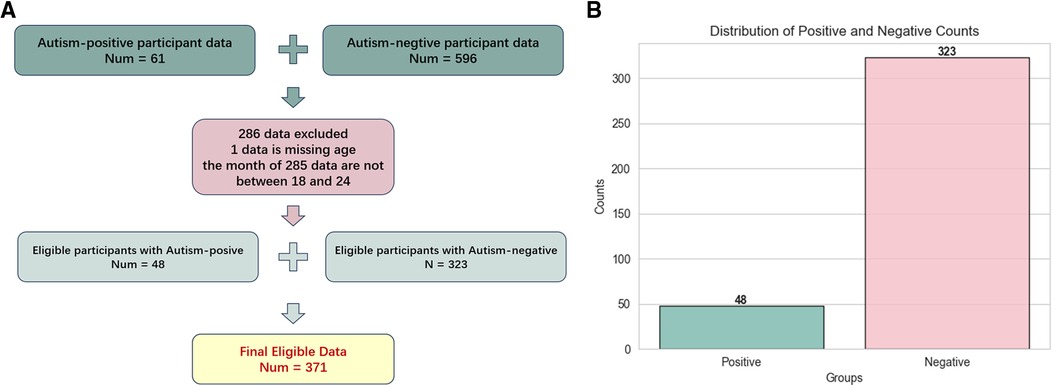
Figure 1. Figure 1A is the flow chart of participants selection, and Figure 1B is the distribution of positive and negative results for autism in 371 eligible questionnaires.
After we got eligible data, three main processes were divided into five minor steps designed to process and screen important data. The overall workflow is as Figure 2 shows. The three main processes are preprocessing, feature engineering, and machine learning model building, each focusing respectively on data, features, and models. The first step is data encoding. In this step, we encode the text information collected in the questionnaire into a digital format, so machine learning models can process that. Next step, we split the dataset into training and testing sets. As the negative and positive samples are imbalanced for the training set, we will use resampling techniques to balance the numbers of samples from each class, to mitigate the impact of data imbalance on model construction. In the third step, we conduct feature filtering based on statistical methods to reduce the original feature dimension. The next step is feature selection, in which we used mRMR to further find out the most relevant features to help the model build. In the last step, we build seven machine learning models to compare and evaluate the experimental results.
To encode the text data from the CHAT-23 questionnaires, we follow the experience introduced in Vakadakar’s work (32). Since the collected questionnaire data is in text form, for each CHAT-23 question section, we set four different frequency words to record the test subject’s information, which is never, occasionally, sometimes, and often. We encode them as 0, 1, 2, 3 respectively. For the diagnosis results, we use 0 to represent negative, and we use 1 to represent positive respectively. In the end, we get the normalized 23-question characteristics.
We found there exists a clear imbalance in the experimental data, with 323 negatives vs. 48 positives. Since there are significantly more negative samples than positive samples, if we do not process this phenomenon, the machine learning based model may prefer to remember the characteristics of the negative samples but despise the positive sample features. The data imbalance would result in the model having a high prediction success rate for negative samples, while the positive sample prediction effect is poor. This will lead to a catastrophic decrease in sensitivity.
To balance the impact of negative and positive input data in the model, we use a combination of up-and-down sampling techniques. We first use down-sampling technology, which randomly deletes negative samples to reduce the number of negative samples. We set the parameters of the sampling strategy to 0.3, the negative sample data is randomly retained at 160. However, if we use too aggressive down-sampling strategy, we are likely to lose the information of negative samples, so we use a combination of up-sampling technology. We copy some positive samples, and set the parameters of the up-sampling strategy to 0.5, so we can get 80 positive samples. The resampled data is fed into the model, which balances the model’s ability to learn from both positive and negative samples.
Not all question features from CHAT-23 are useful for machine learning models to predict outcomes correctly, so we use statistical-based filtering to find and remove these less important features. We use variance and -value as two criteria for filtering.
First, we calculate the variance value of each question feature of all instances. The larger the variance, the greater the change in the value range of the feature in the sample, which means that the feature has a stronger ability to distinguish whether the target is finally positive. In this way, the characteristic is more conducive to building a model. Among them, those features with small variance are similar to most people, regardless of negative or positive testers, and their values on these features are similar, so this feature is relatively secondary to the discriminant result. There are two features with variance below 0.1, which correspond to Q12 and Q16. Through the above steps, we removed the features with variance below 0.1 from the original 23 question features, while retaining the remaining 21 features.
Next, we utilize the chi-square method to calculate the -value for each feature, taking into consideration the correlation between the feature and the diagnosis (4). We regard features with a p-value less than 0.05 as features that are important to the diagnosis result. Thus, we remove all features with a p-value greater than 0.05 and finally retain 15 features, and the results are listed in Table 1. The corresponding questions to removed features include Q1, Q3, Q4, Q10, Q11, Q18.
A suitable feature selection not only improves the accuracy of machine learning methods’ predictions but also reflects which features are more important for predicting negative and positive outcomes. mRMR can discover those features that are most relevant to the diagnostic results among all features and have the least redundancy between each feature. Thus, we use mRMR to select features sequentially. In mRMR, the MIQ parameter calculates the correlation between two features through mutual information and measures the degree of confusion between multiple feature groups through information entropy. After setting different weights for the calculated correlation and difference, we can get the sequence of features, and the higher the feature is considered as the more important feature. The sequence of this feature will be incrementally used as input to the model to train different machine-learning models. The feature sequences discovered by the mRMR method are displayed in Table 2.
We choose 7 widely used supervised machine learning models to perform primary screening for autism, including two non-ensemble learning models: Support Vector Machine (SVM), and Decision Tree (DT), as well as four ensemble learning models: Gradient Boosting Decision Tree (GBDT), Light Gradient Boosting Machine (LGBM), Random Forest (RF), and eXtreme Gradient Boosting (XGB), and one boosting algorithm: Adaptive Boosting (AdaBoost). We selected the “rbf” kernel function for SVM and set its regularization parameter “C” to 1. For DT and RF models, we used the “gini” criterion to measure the quality of each split. The number of base learners was controlled by setting the “n_estimators” parameter for GBDT, RF, AdaBoost, XGB, and LGBM, which were set to 100, 100, 50, 800, and 100, respectively. Before training, we performed feature selection to determine the most important combination of features to use as input for the machine learning models. Subsequently, we obtained predictions for all test data using the trained models.
We evaluated the performance of our machine learning model using sensitivity and specificity. Sensitivity measures the detection ability of the model when screening positive cases, which is the proportion of true positive results to all actual positive results. The closer the sensitivity is to 1, the lower the rate of missed diagnoses for true positives. Specificity measures the ability of the model to exclude non-patients, which is the proportion of true negative objects that the model can identify when the detection target does not have autism. The closer the specificity is to 1, the lower the misdiagnosis rate. Both sensitivity and specificity are important metrics for evaluating the performance of a machine-learning model. All data analysis, model training, and evaluation were performed using Python (version 3.9.13) and Scikit-learn (version 1.2.2).
To compare the performance of seven different models in predicting the diagnosis of ASD and identify the model with the best classification performance, we trained various models using the same set of features. We use a total of 15 features as inputs for the 7 models we built. These features are listed in Table 3. During model training, we ensured that each model used the same amount of data for both training and testing. Among the models tested, LGBM exhibited significantly better specificity which is 0.933 than SVM, DT, AdaBoost, RF, and XGB, compared to GBDT 0.778.
To determine and select the optimal number of important features for training, we gradually increased the number of input features from 1 to 15, adding them in order of the mRMR feature selection method described in the feature selection section. Because LGBM shows a comparative performance in Table 3, we used the LGBM model as the base classifier for this experiment.
Based on the result presented in Table 4, we observed that when using the first 9 features as inputs, the model achieved its highest sensitivity value of 0.909, while maintaining a relatively high specificity level of 0.922. As we gradually increased the number of features to 9, we noticed an overall upward trend in specificity. However, it did not continue to increase with additional features. Meanwhile, when the number of features was limited to 2 and 5, although sensitivity was high, reaching 0.900, specificity remained relatively low, failing to exceed 0.820. Furthermore, after surpassing 9 features, sensitivity could not reach above 0.900. Based on these findings, we identified the 9 best features for constructing our LGBM model, namely Q21, Q6, Q9, Q13, Q7, Q14, Q5, Q15, and Q17. Detailed descriptions of these question features are provided in the Table 5.
We employed seven commonly used machine learning techniques to model early screening models for Autism. The experimental results demonstrated that the LGBM model outperformed the other models, exhibiting superior specificity and sensitivity. Notably, the LGBM model achieved its highest sensitivity value of 0.909 when the number of features reached 9 while maintaining a consistently high specificity level of 0.922. We analyzed our autistic patients and individual patient explanations using SHAP technology. Figure 3 presents SHAP summary plots of the top 15 clinical features in the contribution of ML models to the prediction of autism development in our study. The implication is that the model’s interpretation is consistent with the feature rank ordering we obtain via mRMR. Figure 4 shows a patient at low risk of having autism.
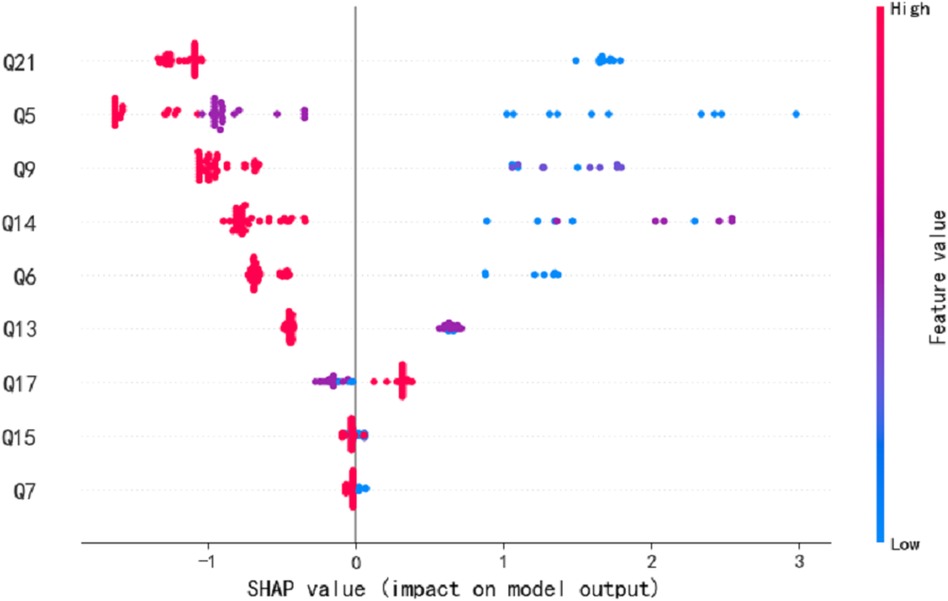
Figure 3. The SHAP summary plots for the LGBM model. This depicts the 9 most effective features on prediction.
After optimizing the feature for convenience in clinical practice, the time required is reduced, making it easier for parents to complete and for professionals to assess. The consistency in clinical presentation is evident in the construction of 9 items based on the MRMR model. Among these, questions 5, 7, 9, 13, and 15 are core components of the CHAT-23 clinical scale. These items cover areas such as pretend play, pointing skills, initiating sharing, imitation ability, and joint attention. The lack or delay in these specific skills reflects the clinical characteristics of social aspects in children with autism spectrum disorders.
While building machine learning models can provide significant assistance for early autism detection, there are still some limitations regarding practical application. Firstly, our machine learning models are specifically tailored to data from Chinese children, meaning they perform best within this demographic. Exploring the development of more universally applicable models might be a worthwhile direction for future research. Additionally, once the models are built, their parameters remain static, making them outdated over time. Currently, our best model uses at least nine features, further reducing the number of features used in building machine learning models could make them more efficient in practical applications.
Our future goal is to combine the model with an online questionnaire format and deploy it on a web platform. By using fewer questions, we can quickly provide accurate autism assessment results to parents via mobile phones or other online tests, enabling them to obtain efficient and reliable references for their children. This approach not only improves efficiency but also reduces the workload of medical personnel.
In this study, we have developed a machine learning approach based on the Chinese children’s scale data, which was obtained by filling in the CHAT-23 questionnaire questions. Then, we conducted feature engineering by using statistical methods and mRMR feature selection on the 23 questions of the CHAT-23 scale. Through the above process, we ranked these questions according to their importance, and the final 9 questions were selected, specifically Q21, Q6, Q9, Q13, Q7, Q14, Q5, Q15, and Q17. These selected questions were then applied to an LGBM model, which demonstrated superior performance compared to the other popular machine learning models (SVM, DT, GBDT, ADA, XGB, RF). By utilizing this machine learning model with only 9 questions, we achieved comparable results, which use all 23 questions as features for computer-aided autism diagnosis.
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Hospital Committee of Jiangnan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
HL: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. HZ: Data curation, Formal Analysis, Funding acquisition, Resources, Validation, Writing – review & editing. YZ: Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. X-YM: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. M-FZ: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. TQ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by Top Talent Support Program for Young and Middle-aged People of Wuxi Health Committee (HB2023083; BJ2023081), New Technology Project of Affiliated Women's Hospital of Jiangnan University (Grant No. 202217), and Maternal and Child Health Research Project of Jiangsu Province Health Committee (F202308).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. A. P. Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, USA: American Psychiatric Association (2013).
2. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morb Mortal Wkly Rep Surveill Summ. (2018) 67:1–23. doi: 10.15585/mmwr.ss6706a1
3. Wang F, Lu L, Wang SB, Zhang L, Ng CH, Ungvari GS, et al. The prevalence of autism spectrum disorders in China: a comprehensive meta-analysis. Int J Biol Sci. (2018) 14:717–25. doi: 10.7150/ijbs.24063
4. Matson JL, Matheis M, Burns CO, Esposito G, Venuti P, Pisula E, et al. Examining cross-cultural differences in autism spectrum disorder: a multinational comparison from Greece, Italy, Japan, Poland, and the United States. Eur Psychiatry. (2017) 42:70–6. doi: 10.1016/j.eurpsy.2016.10.007
5. Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. (2008) 20:775–803. doi: 10.1017/S0954579408000370
6. Sullivan K, Stone WL, Dawson G. Potential neural mechanisms underlying the effectiveness of early intervention for children with autism spectrum disorder. Res Dev Disabil. (2014) 35:2921–32. doi: 10.1016/j.ridd.2014.07.027
7. Bala M, Ali MH, Satu MS, Hasan KF, Moni MA. Efficient machine learning models for early stage detection of autism spectrum disorder. Algorithms. (2022) 15:166. doi: 10.3390/a15050166
8. Pierce K, Gazestani VH, Bacon E, Barnes CC, Cha D, Nalabolu S, et al. Evaluation of the diagnostic stability of the early autism spectrum disorder phenotype in the general population starting at 12 months. JAMA Pediatr. (2019) 173:578–87. doi: 10.1001/jamapediatrics.2019.0624
9. Wergeland GJH, Posserud M-B, Fjermestad K, Njardvik U, Öst L-G. Early behavioral interventions for children and adolescents with autism spectrum disorder in routine clinical care: a systematic review and meta-analysis. Clin Psychol Sci Pract. (2022) 29:400–14. doi: 10.1037/cps0000106
10. Robins DL, Fein D, Barton ML, Green JA. The modified checklist for autism in toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. (2001) 31:131–44. doi: 10.1023/A:1010738829569
11. Allison C, Matthews FE, Ruta L, Pasco G, Soufer R, Brayne C, et al. Quantitative checklist for autism in toddlers (q-chat). a population screening study with follow-up: the case for multiple time-point screening for autism. BMJ Paediatr Open. (2021) 5:e000700. doi: 10.1136/bmjpo-2020-000700
12. Fang-yan W, Xiu X, Jing L, Jing-wei X, Li-juan C. Study on the application of autism screening scale (CHAT-23). Chin J Child Health Care. (2010) 18:288–91.
13. Wetherby AM, Prizant BM. Communication and Symbolic Behavior Scales: Developmental Profile. Baltimore, Maryland: Paul H Brookes Publishing Co. (2002).
14. Matson JL, Wilkins J, Sevin JA, Knight C, Boisjoli JA, Sharp B. Reliability and item content of the baby and infant screen for children with autism traits (biscuit): parts 1–3. Res Autism Spectr Disord. (2009) 3:336–44. doi: 10.1016/j.rasd.2008.08.001
15. Choueiri R, Wagner S. A new interactive screening test for autism spectrum disorders in toddlers. J Pediatr. (2015) 167:460–6. doi: 10.1016/j.jpeds.2015.05.029
16. Wong V, Hui L-HS, Lee W-C, Leung L-SJ, Ho P-KP, Lau W-LC, et al. A modified screening tool for autism (checklist for autism in toddlers [CHAT-23]) for Chinese children. Pediatrics. (2004) 114:e166–76. doi: 10.1542/peds.114.2.e166
17. Qiu T, Zhang H, Zhou C, Tang Q, Wang L, Ke X. Application of telemedicine for preliminary screening of autism spectrum disorder. Front Pediatr. (2022) 9:745597. doi: 10.3389/fped.2021.745597
18. Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. (2015) 349:255–60. doi: 10.1126/science.aaa8415
19. Das S, Zomorrodi R, Mirjalili M, Kirkovski M, Blumberger DM, Rajji TK, et al. Machine learning approaches for electroencephalography and magnetoencephalography analyses in autism spectrum disorder: a systematic review. Prog Neuro-Psychopharmacol Biol Psychiatry. (2023) 123:110705. doi: 10.1016/j.pnpbp.2022.110705
20. Tartarisco G, Cicceri G, Di Pietro D, Leonardi E, Aiello S, Marino F, et al. Use of machine learning to investigate the quantitative checklist for autism in toddlers (q-chat) towards early autism screening. Diagnostics. (2021) 11:574. doi: 10.3390/diagnostics11030574
21. Duda M, Daniels J, Wall DP. Clinical evaluation of a novel and mobile autism risk assessment. J Autism Dev Disord. (2016) 46:1953–61. doi: 10.1007/s10803-016-2718-4
22. Eslami T, Almuqhim F, Raiker JS, Saeed F. Machine learning methods for diagnosing autism spectrum disorder and attention- deficit/hyperactivity disorder using functional and structural MRI: a survey. Front Neuroinform. (2021) 14:575999. doi: 10.3389/fninf.2020.575999
23. Parlett-Pelleriti M, Dixon D, Linstead EJ. Applications of unsupervised machine learning in autism spectrum disorder research: a review. Rev J Autism Dev Disord. (2023) 10:406–21. doi: 10.1007/s40489-021-00299-y
24. Siddiqui UA, Ullah F, Iqbal A, Khan A, Ullah R, Paracha S, et al. Wearable-sensors-based platform for gesture recognition of autism spectrum disorder children using machine learning algorithms. Sensors. (2021) 21:3319. doi: 10.3390/s21103319
25. Liu M, Li B, Hu D. Autism spectrum disorder studies using fMRI data and machine learning: a review. Front Neurosci. (2021) 15:697870. doi: 10.3389/fnins.2021.697870
26. Abbas H, Garberson F, Glover E, Wall DP. Machine learning approach for early detection of autism by combining questionnaire and home video screening. J Am Med Inform Assoc. (2018) 25:1000–7. doi: 10.1093/jamia/ocy039
27. Precenzano F, Parisi L, Lanzara V, Vetri L, Operto FF, Pastorino GMG, et al. Electroencephalographic abnormalities in autism spectrum disorder: characteristics and therapeutic implications. Medicina. (2020) 56:419. doi: 10.3390/medicina56090419
28. Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P. EEG features and epilepsy in patients with autism. Brain Dev. (1995) 17:169–74. doi: 10.1016/0387-7604(95)00019-8
29. Operto FF, Smirni D, Scuoppo C, Padovano C, Vivenzio V, Quatrosi G, et al. Neuropsychological profile, emotional/behavioral problems, and parental stress in children with neurodevelopmental disorders. Brain Sci. (2021) 11:584. doi: 10.3390/brainsci11050584
30. Heinsfeld AS, Franco AR, Craddock RC, Buchweitz A, Meneguzzi F. Identification of autism spectrum disorder using deep learning and the abide dataset. Neuroimage Clin. (2018) 17:16–23. doi: 10.1016/j.nicl.2017.08.017
31. Thabtah F, Abdelhamid N, Peebles D. A machine learning autism classification based on logistic regression analysis. Health Inf Sci Syst. (2019) 7:12. doi: 10.1007/s13755-019-0073-5
Keywords: autism spectrum disorder, CHAT-23, early screening, feature engineering, machine learning, Chinese children
Citation: Lu H, Zhang H, Zhong Y, Meng X-Y, Zhang M-F and Qiu T (2024) A machine learning model based on CHAT-23 for early screening of autism in Chinese children. Front. Pediatr. 12:1400110. doi: 10.3389/fped.2024.1400110
Received: 13 March 2024; Accepted: 31 July 2024;
Published: 10 September 2024.
Edited by:
Giorgia Bussu, Uppsala University, SwedenReviewed by:
Francesca Felicia Operto, University of Salerno, ItalyCopyright: © 2024 Lu, Zhang, Zhong, Meng, Zhang and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Qiu, cWl1dGluZzA4MkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.