- 1The Eighth Clinical Medical College, Guangzhou University of Chinese Medicine, Foshan, Guangdong, China
- 2Foshan Hospital of Traditional Chinese Medicine, Guangzhou University of Chinese Medicine, Foshan, Guangdong, China
Background: The understanding of the prevalence and early predictive factors of scoliosis in children and adolescents is limited, which poses challenges to developing preventative strategies. This systematic review and meta-analysis aimed to clarify the prevalence and predictors of scoliosis among children and adolescents.
Methods: We conducted a comprehensive search in PubMed, Cochrane, Embase, and Web of Science through October 2023. The quality of included studies was evaluated using the Joanna Briggs Institute scale or the Newcastle-Ottawa Scale. Subgroup analyses were performed to examine different types of scoliosis and specific demographic groups.
Results: From 32 studies encompassing 55,635,351 children and adolescents, we identified 284,114 cases of scoliosis, resulting in a prevalence rate of 3.1% (95% CI: 1.5%–5.2%). This rate varied by gender, degrees of scoliosis severity, and between idiopathic vs. congenital forms. Notable predictors included gender, age, Body Mass Index (BMI), race, environmental factors, and lifestyle choices.
Conclusion: Scoliosis is a significant condition affecting a minority of children and adolescents, particularly adolescent girls and individuals who are overweight. It is recommended that guardians and schools enhance educational efforts towards its prevention.
Systematic Review Registration: https://www.crd.york.ac.uk/, Identifier CRD42023476498.
1 Introduction
Scoliosis is a three-dimensional (3D) spinal deformation characterized by a lateral curvature across one or more segments of the spine, coupled with vertebral rotation, resulting in core deviation and sagittal progression (1). Severe scoliosis can result in significant health complications, such as cardiovascular issues, reduced pulmonary function, chronic pain, and psychological distress (2).
Scoliosis can be categorized etiologically into idiopathic, congenital, and neuromuscular types. Adolescent idiopathic scoliosis (AIS), the most common form, has an unknown cause, which is reflected in the term “idiopathic” (3, 4). Depending on when it manifests, idiopathic scoliosis may be classified by age brackets: Infantile (ages 0–3 years), Juvenile (ages 3–10 years), Adolescent (ages 10–18 years), and Adult (ages above 18 years) (5). AIS is typically detected during childhood and adolescence, especially during rapid growth periods. Treatment options include observation, wearing orthotic braces, and surgery in more severe cases. Congenital scoliosis is caused by abnormal development of the spine during the embryonic stage, which may involve vertebral non-segmentation, abnormal shape, or abnormal quantity. This type of scoliosis is often associated with genetic factors and may coexist with other congenital abnormalities, so it is usually identified at birth or in early infancy (6). Treatment methods primarily involve surgical correction, especially in cases of severe or rapidly progressing curvature.
Currently, there is limited research available regarding the worldwide prevalence of scoliosis in children and adolescents, and a comprehensive investigation of associated risk factors is lacking. Consequently, the development of preventive strategies for children and adolescents faces considerable challenges. Therefore, the objective of this systematic review and meta-analysis was to provide a detailed description of the prevalence and early predictors of scoliosis in adolescents globally, and to offer evidence-based guidance for the detection and prevention of scoliosis.
2 Methods
2.1 Study registration
This meta-analysis was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (The PRISMA 2020) guidelines, and our protocol was registered with PROSPERO (ID: CRD42023476498).
2.2 Eligibility criteria
For our systematic review, we strictly included cohort or cross-sectional studies, while expressly excluding the following studies: (1) Studies that were based solely on subject self-reporting of disease diagnosis or assessments derived from specific scales that lacked clinical validation; (2) Case reports, meta-analyses, reviews, and guidelines; (3) Studies that had a sample size of fewer than 20 cases.
2.3 Data sources and search strategy
We systematically searched databases including PubMed, Cochrane Library, Embase, and Web of Science. The search was conducted from the inception of each database up to October 2023, without geographical restrictions. Details of the search are presented in Supplementary Table S1.
2.4 Study selection
All identified literature was imported into EndNote, where duplicate publications were initially filtered out automatically and manually. Studies relevant to our topic were selected by reviewing titles and abstracts, and then the full-texts of potentially relevant articles were downloaded and read. Finally, original research articles that met the inclusion criteria were selected upon full-text review. The literature screening process was independently carried out by two researchers. In the event of any discrepancies, a third researcher was consulted to discuss and make a final decision.
2.5 Data extraction
Before initiating data extraction, we devised a standardized template to systematically collect relevant data. The details gathered included DOI/PMID, first author, year of publication, type of study, author's nationality, patient demographics, period of sample collection, age group included, scoliosis categories, scoliosis diagnostic criteria, total participant count, number of scoliosis cases, along with age, gender, and independent correlation factors.
Data extraction was independently carried out by two reviewers. In instances of disagreement, a third reviewer was consulted to contribute to the resolution process.
2.6 Risk of bias in studies
The original studies encompassed in this systematic review were either cohort or cross-sectional studies. For the cohort studies, we evaluated their quality using the Newcastle-Ottawa Scale (NOS), a renowned assessment tool designed to judge the quality of non-randomized studies in meta-analyses (7). The NOS examines three domains through eight items, allocating up to one point each for most questions, with the exception of the comparability category, which has a potential for 2 points. Studies achieving a score between 7 and 9 are considered high quality, whereas scores from 4 to 6 signify moderate quality. For cross-sectional studies, the appraisal of quality was conducted using the Joanna Briggs Institute (JBI) scale (8).
The risk of bias in these studies was assessed independently by two researchers. In cases of discrepancy, a third researcher was enlisted to reach a consensus.
2.7 Synthesis methods
Data analysis for this meta-analysis was conducted using R software (version 4.2.2). Prior to performing the meta-analysis of prevalence, data underwent transformation based on predefined criteria: (1) No transformation was necessary if the average prevalence rate across all samples was between 20% and 80%; (2) Logit transformation was applied when the rate was under 20% or exceeds 80%; and (3) The double arcsine transformation method was applied in cases with a significant number of extreme values (0% or/and 100%) (9). The choice of model was guided by the level of heterogeneity, which was determined by the I2 index. A random-effects model was employed when I2 exceeded 50%; otherwise, a fixed-effect model was used. In circumstances of substantial heterogeneity, sensitivity and subgroup analyses were conducted to investigate potential sources of variability. Funnel plots were constructed to visually inspect for publication bias across the studies, complemented by statistical assessment via Egger's test. In instances where publication bias was detected, the trim-and-fill method was applied to evaluate its influence on the meta-analysis outcomes. A P-value of less than 0.05 was considered indicative of statistical significance.
3 Results
3.1 Study selection
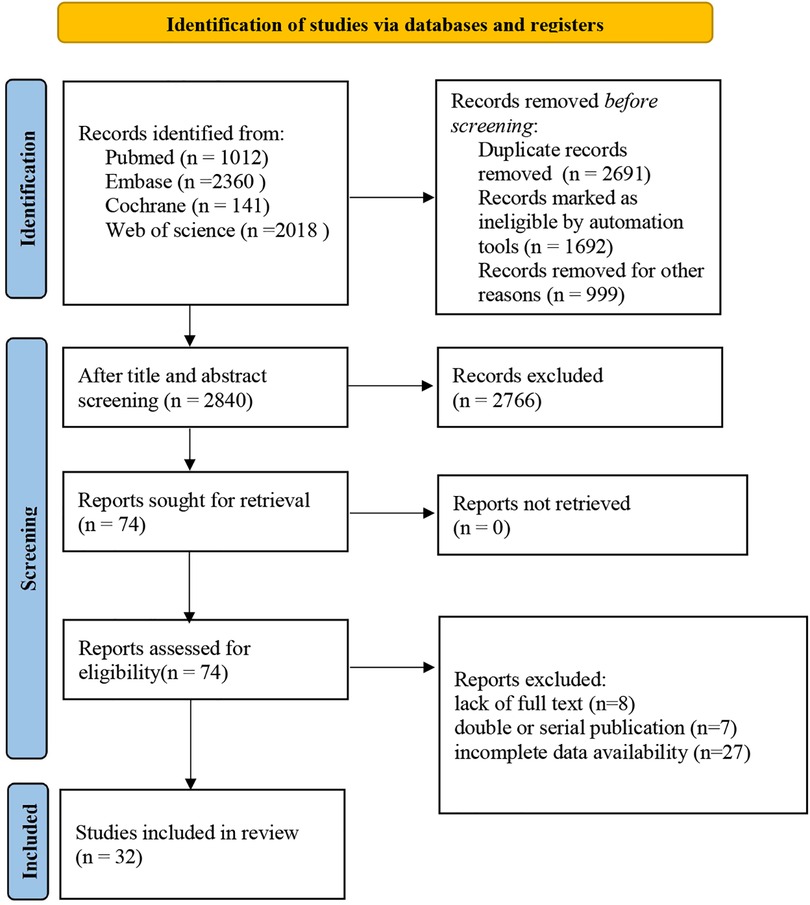
We retrieved a total of 5,531 records. Following the elimination of duplicates, 2,840 records were screened by their titles and abstracts. Of these, 2,766 records were excluded for not meeting our inclusion criteria. A thorough assessment of the full texts for the remaining 74 articles resulted in further exclusion of 42 articles for various reasons including unavailability of full text (n = 8), duplicate or serial publications (n = 7), and incomplete data (n = 27). Consequently, 32 studies were included in our meta-analysis (8–39) (Figure 1).
3.2 Study characteristics
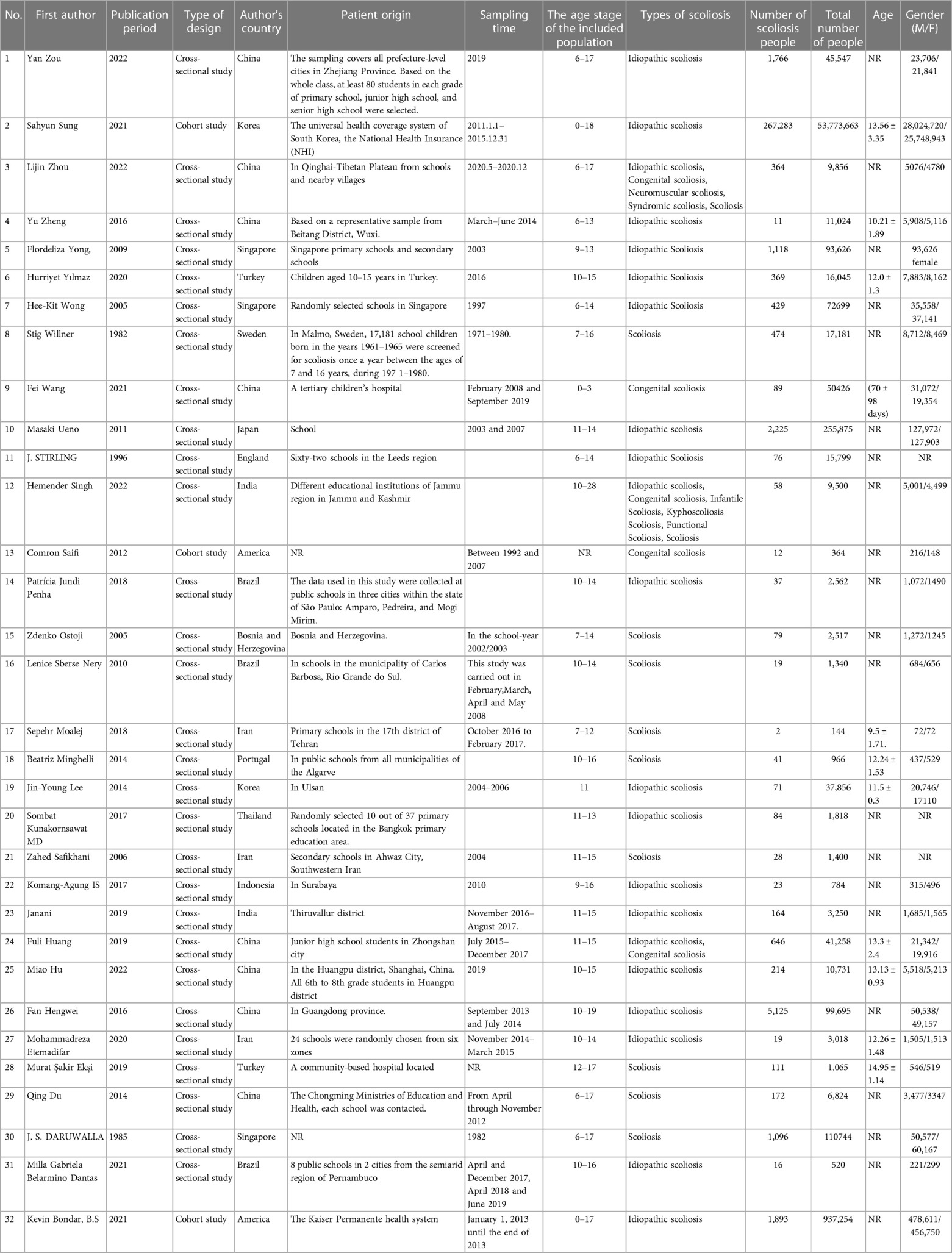
This analysis incorporated 32 epidemiological investigations conducted from 1982 to 2022, involving a total of 55,635,351 children and adolescents. Among these, there were 29 cross-sectional studies (8–17, 19–21, 23–26, 39) and 3 cohort studies (18, 22, 29). The research spanned across different regions, with 21 studies (9, 12, 15–17, 23–29, 31–33, 39) conducted in Asia, including China, South Korea, Thailand, Japan, India, Singapore, Indonesia, and Iran; 6 studies (8, 10, 13, 14, 19, 30) in Europe from countries such as Sweden, England, Bosnia and Herzegovina, Portugal, and Turkey; 3 studies (11, 20, 21) in Brazil in South America; and 2 studies (18, 22) in the United States in North America. Most participants were 10–15 years old. All studies shared a common standard for diagnosing scoliosis: a positive Adam's Forward Bending Test (FBT) and a Cobb angle greater than 10°. Of these studies, 21 (19–33, 39) reported on the prevalence of idiopathic scoliosis, 5 (17, 18, 24, 31, 36) on congenital scoliosis, and the remaining 9 studies (8–16) did not specify the type of scoliosis (Table 1).
3.3 Risk of bias in studies
Due to the inclusion of both cross-sectional and cohort studies, we utilized the NOS and JBI to assess their quality, respectively. The NOS revealed that the three cohort studies scored between 7 and 9, indicating that they are of high quality. The JBI demonstrated that the cross-sectional studies had no significant risk of bias (Supplementary Table S2).
3.4 Meta-analysis
3.4.1 Scoliosis
(1) Synthesized results
In this analysis, 11 studies did not categorize scoliosis. A random-effects model was used for data analysis, and the pooled prevalence rate of scoliosis was found to be 3.1% (95% CI: 1.5%–5.2%) (Figure 2).
(2) Subgroup analysis
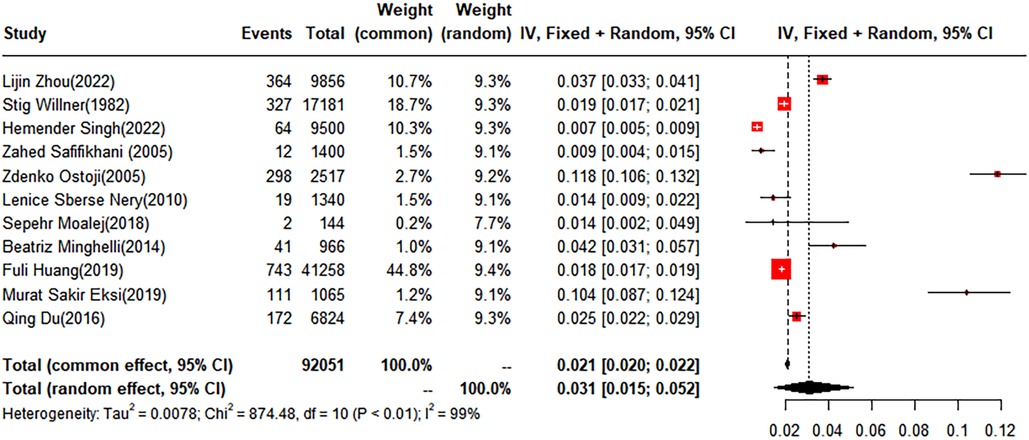
Figure 2 Forest plot for the meta-analysis of the prevalence of scoliosis in children and adolescents.
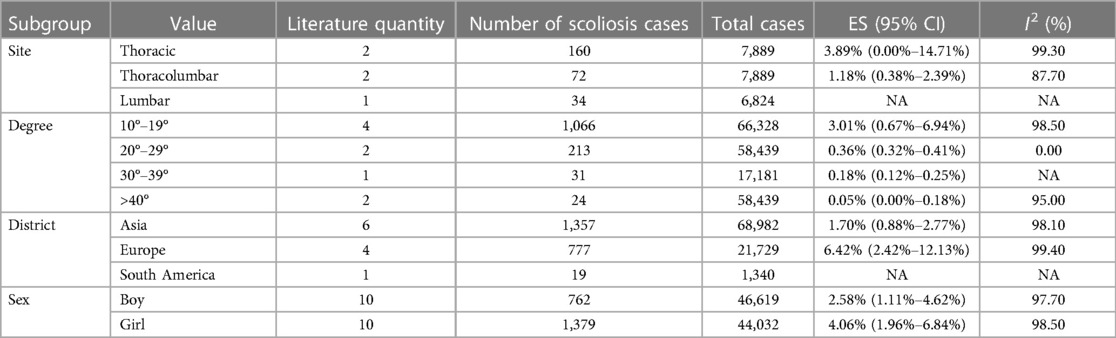
The pooled prevalence of scoliosis was found to be 2.58% (95% CI: 1.11%–4.62%) in males and 4.06% (95% CI: 1.96%–6.48%) in females. When classified by degree, the prevalence rates for scoliosis within the subgroups of 10–19 degrees, 20–29°, and over 40° were 3.01% (95% CI: 0.67%–6.94%), 0.36% (95% CI: 0.32%–0.41%), and 0.05% (95% CI: 0%–0.18%), respectively. Geographically, the children and adolescents included in the study were from four regions: Asia, Europe, South America, and North America. The prevalence rates for scoliosis in the Asian and European groups were 1.70% (95% CI: 0.88%–2.77%) and 6.42% (95% CI: 2.42%–12.13%), respectively. No studies conducted in North America met our inclusion criteria. Of the included studies, five conducted a subgroup analysis based on anatomical location, dividing cases into thoracic, thoracolumbar, and lumbar. The prevalence rates for the thoracic and thoracolumbar groups were 3.89% (95% CI: 0%–14.71%) and 1.18% (95% CI: 0.38%–2.39%), respectively (Table 2).
(3) Sensitivity analysis and publication bias
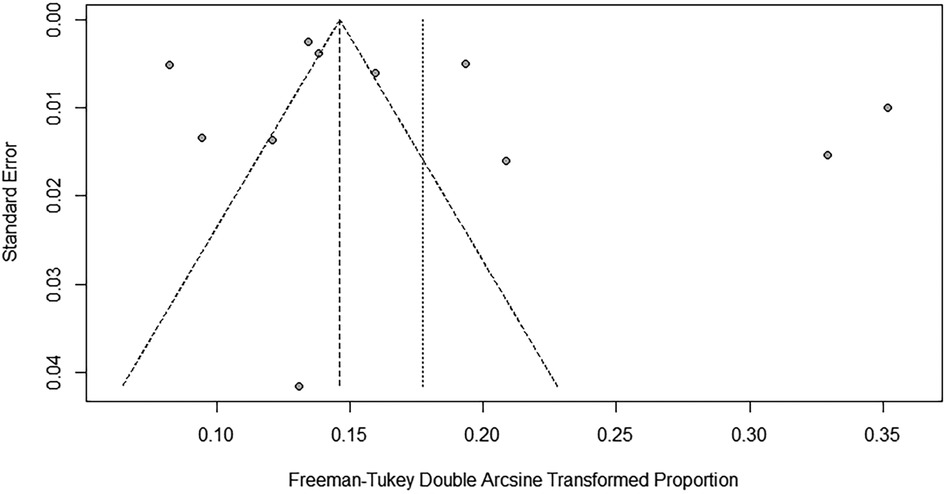
To evaluate the robustness and reliability of the overall findings of the meta-analysis, a sensitivity analysis was performed. This technique involved sequentially omitting individual studies and recalculating the meta-analysis with the remaining studies. We investigated whether this exclusion led to significant deviations in the outcomes, thereby confirming the sturdiness of our conclusions. Funnel plots were used to assess potential publication bias, and the results indicated the presence of publication bias. Consequently, the trim-and-fill method was implemented. When two studies were added, the result was adjusted to 1.9% (95% CI: 0.49%–4.15%) (Figures 3, 4).
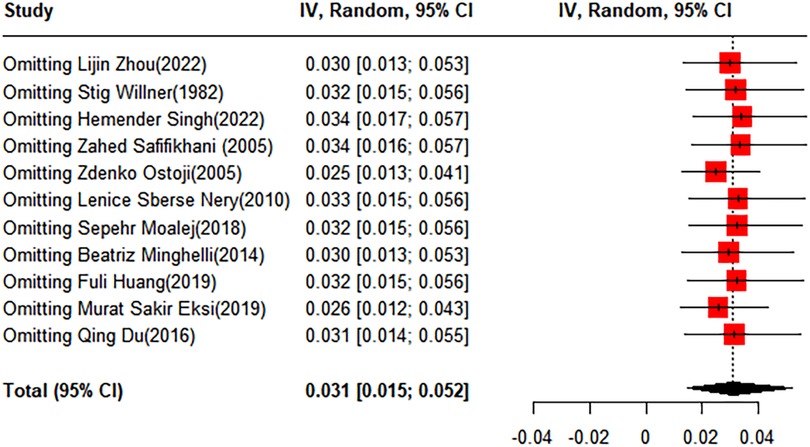
Figure 3 Forest plot for the sensitivity analysis of the prevalence of scoliosis in children and adolescents.
3.4.2 Idiopathic scoliosis
(1) Synthesized results
Out of the studies reviewed, 21 reported the prevalence of idiopathic scoliosis. A random-effects model was used, and the calculated prevalence rate for idiopathic scoliosis was found to be 1.7% (95% CI: 1.1%–2.4%) (Figure 5).
(2) Subgroup analysis
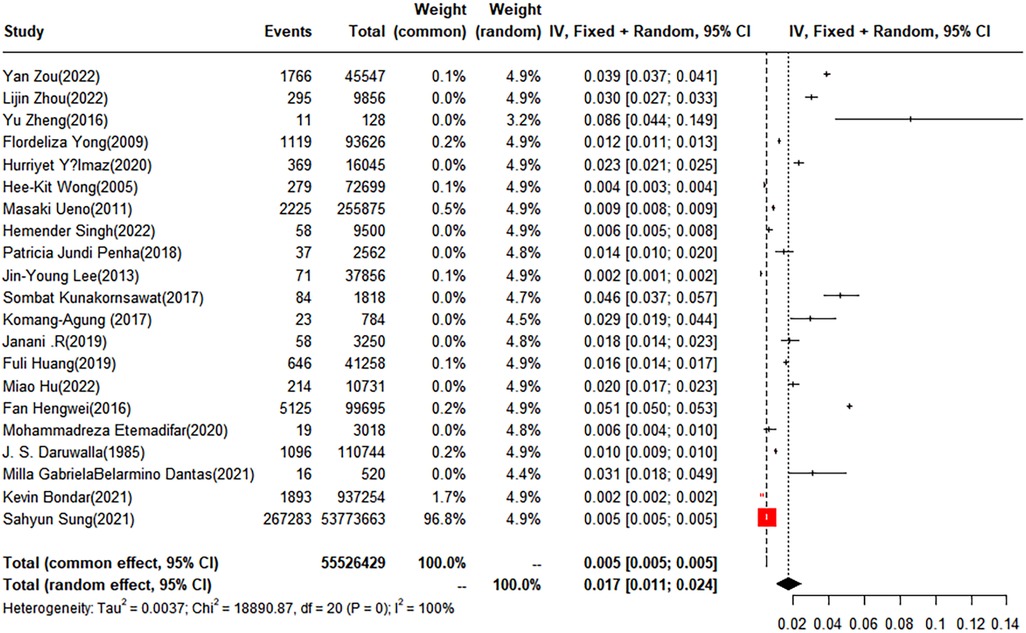
Figure 5 Forest plot for the meta-analysis of the prevalence of idiopathic scoliosis in children and adolescents.
The pooled prevalence was 1.12% (95% CI: 0.55%–1.89%) for males and 4.51% (95% CI: 0.74%–11.09%) for females. Subgroup analysis by degree unraveled that the prevalence of idiopathic scoliosis in subgroups with curves of 10–19°, 20–29°, 30–39°, and greater than 40° was 1.46% (95% CI, 0.64–2.58), 0.35% (95% CI, 0.08–0.8), 0.07% (95% CI: 0%–0.21%), and 0.19% (95% CI: 0.03%–0.48%), respectively. Geographically, the included children and adolescents were divided into four groups: Asia, Europe, South America, and North America. The prevalence of idiopathic scoliosis was 1.68% (95% CI: 0.94%–2.63%) in Asia, 1.22% (95% CI: 0.09%–3.64%) in Europe, and 2.08% (95% CI: 0.76%–4.01%) in South America. One study conducted in North America was not suitable for subgroup analysis. Five studies provided a subgroup analysis by anatomical site, categorizing the cases as thoracic, thoracolumbar, and lumbar. The prevalence rates for scoliosis within these groups were 0.44% (95% CI: 0.15%–0.88%), 0.44% (95% CI: 0.19%–0.77%), and 0.16% (95% CI: 0.02%–0.42%), respectively. Based on the developmental status of the children and adolescents, the analysis was further subdivided into three groups: normal weight, overweight, and obesity, with the prevalence rates being 1.84% (95% CI: 0%–7.44%), 1.29% (95% CI: 0%–5.80%), and 0.77% (95% CI: 0%–4.48%), respectively. In addition to these categories, we conducted a subgroup analysis based on the number of curves. The results showed that the prevalence of scoliosis stood at 0.68% (95% CI: 0.11%–1.73%) for single curve, 0.31% (95% CI: 0.03%–0.90%) for double curve, and 0.05% (95% CI: 0.03% - 0.06%) for triple curves. A subgroup analysis based on race was also performed, involving four groups: White, Black, Yellow, and Other. Two studies reported a prevalence of 0.83% (95% CI: 0%–3.20%) in the White population, and two studies reported a prevalence of 0.28% (95% CI: 0%–1.16%) in the Black population. The Yellow and Other groups each had only one study reporting prevalence; therefore, no subgroup analysis was performed (Table 3).
(3) Sensitivity analysis and reporting biases
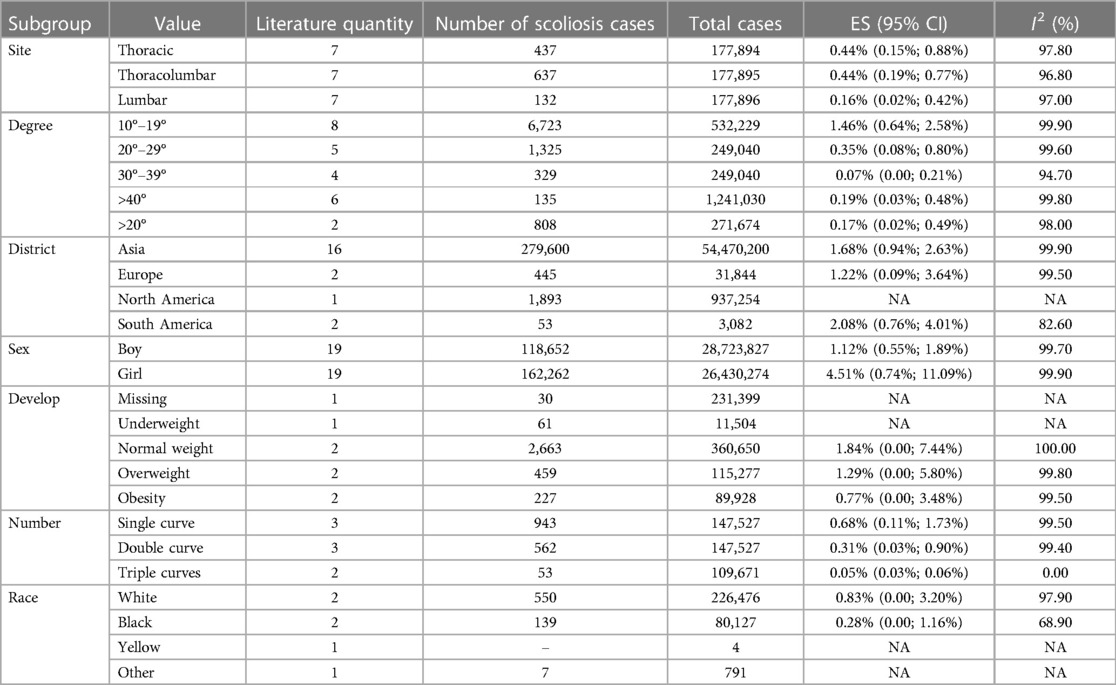
Table 3 Subgroup analysis results for prevalence of idiopathic scoliosis in children and adolescents.
To evaluate the robustness and reliability of the overall results of the meta-analysis, a sensitivity analysis was conducted. This method entailed sequentially excluding individual studies and performing a meta-analysis with the remaining data to determine whether the recalculated results were significantly different from the initial results, thereby affirming the solidity of our conclusions. The funnel plot results indicated the existence of publication bias; hence, we applied the trim-and-fill method, introducing 10 additional studies into the analysis. After this adjustment, the prevalence rate was recalculated as 0.53% (95% CI: 0.12%–1.2%) (Figures 6, 7).
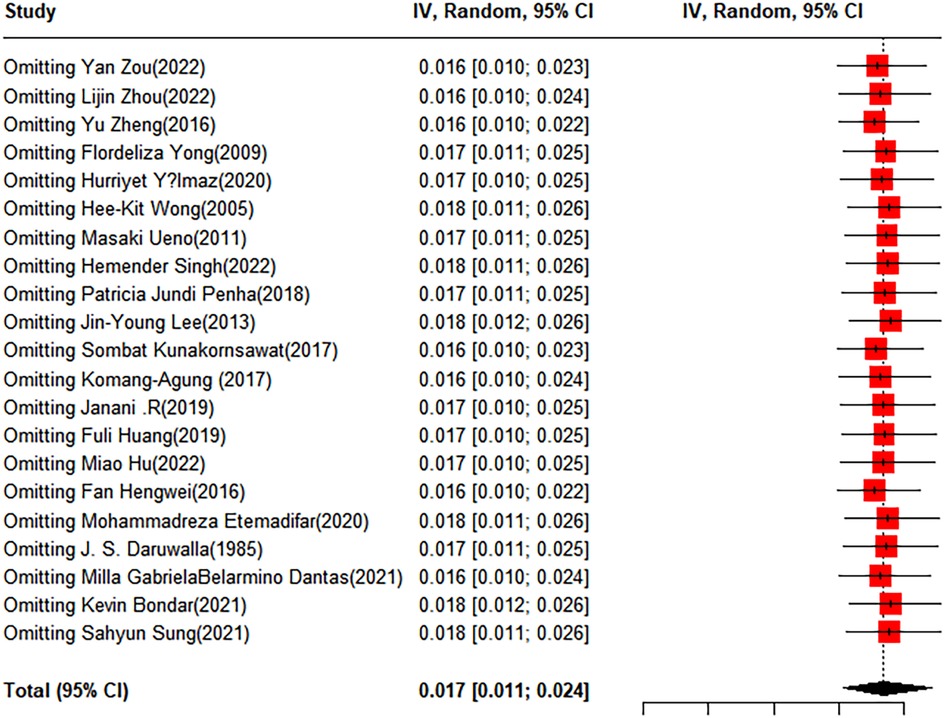
Figure 6 Forest plot for the sensitivity analysis of the prevalence of idiopathic scoliosis in children and adolescents.
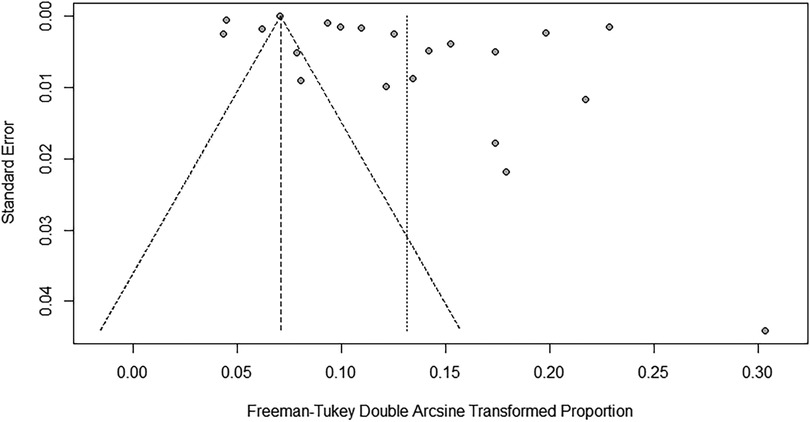
Figure 7 Funnel plot for publication bias regarding the prevalence of idiopathic scoliosis in children and adolescents.
3.4.3 Congenital scoliosis
Congenital scoliosis is a type of spinal deformity present at birth, associated with anomalies in the spine's development during the embryonic stage. This condition is distinct from AIS, which typically occurs during puberty and the cause is unknown. Congenital scoliosis can be caused by vertebral malformations, fusion of the vertebrae, or other spinal abnormalities. The incidence of congenital scoliosis is much lower than that of AIS. In this meta-analysis, four studies discussed congenital scoliosis, covering 101,548 children and adolescents, among whom 218 cases of congenital scoliosis were reported. The estimated prevalence rate of congenital scoliosi was approximately 0.215%.
3.4.4 Independent risk factors
Seven studies reported independent risk factors, with substantial heterogeneity observed among them. We identified gender, age, abnormal body mass index (BMI) (either underweight or overweight), racial differences, environmental factors, and lifestyle factors (such as prolonged sitting, lack of exercise, insufficient sleep) as independent risk factors. The use of single-strap bags and carrying overweight backpacks were also closely linked to the condition. Family income level, as an indicator of nutrition and medical conditions, was regarded as a potential risk factor. Detailed analyses can be found in Supplementary Table S3.
4 Discussion
4.1 Summary of the main findings
This study highlighted a relatively high prevalence of scoliosis among children and adolescents, with idiopathic scoliosis and congenital scoliosis warranting particular attention. The outcomes of our research indicated that the prevalence of scoliosis stood at 3.1% (95% CI: 1.5%–5.2%), idiopathic scoliosis at 1.7% (95% CI: 1.1%–2.4%), and congenital scoliosis at 0.215% (95% CI: 0.12%–1.2%).
In this study, we also discovered significant variations in the prevalence of scoliosis across different regions. The prevalence of scoliosis among children and adolescents was higher in Europe than in Asia. The incidence of AIS in South America was higher than that in Asia and Europe. The occurrence of scoliosis may be influenced by a multitude of factors including genetics, environment, and lifestyle. Typically, there are differences in these factors between Western countries and Asian nations, which could lead to varying prevalence of scoliosis across different regions. We found that the primary angle of scoliosis was 10°–19°, with a prevalence rate of 3.01%, and the number of cases decreased as the angle increased. Idiopathic scoliosis also occurred mostly at 10°–19°, and the number of affected individuals reduced as the angle became more acute. The prevalence of scoliosis in the thoracic region was 3.89%, higher than the prevalence of 1.18% in the thoracolumbar region. The prevalence rates of idiopathic scoliosis in the thoracic and thoracolumbar regions were both 0.44%, and the prevalence of lumbar scoliosis was 0.16%, meaning that in cases of idiopathic scoliosis, thoracic and thoracolumbar scoliosis tend to have higher prevalence rates.
4.2 The risk factors associated with scoliosis
Gender significantly impacts the prevalence and progression of scoliosis. Substantial research, including our study, have consistently indicated that females are disproportionally affected by scoliosis compared to males. This meta-analysis demonstrated that the prevalence of scoliosis is markedly higher in females, with a pooled prevalence rate of 4.06%, as opposed to 2.58% in males. This discrepancy is especially prominent during adolescence, a peak period for scoliosis onset. A primary factor behind this increased prevalence in females may be attributed to estrogen influences. During puberty, elevated estrogen levels in females may impact spinal column growth and development. A proposed mechanism by which estrogen contributes to AIS involves delayed menarche, leading to extended prolonged periods of rapid growth when the spine is particularly vulnerable to deformities. Further, low estrogen levels associated with delayed menarche could result in diminished bone mineralization and strength, thereby escalating the risk of spinal deformities (40). Additionally, polymorphisms in estrogen receptors (ER α and ER β) have been linked to AIS, potentially due to mutations that affect the expression of downstream genes critical for bone growth and metabolism. Evidence from several studies supports the connection between specific ER polymorphisms and the incidence of AIS, indicating a genetic predisposition moderated by estrogen signaling pathways (41–43).
Scoliosis displays variable prevalence and progression across different age brackets. Data from our research identifies adolescence as a peak period for AIS onset. According to subgroup analysis, the total scoliosis prevalence among adolescents aged 10–15 years stands at 3.01% (95% CI: 0.67%–6.94%), emphasizing the heightened risk during the rapid growth phases of adolescence. During adolescence, the body undergoes rapid skeletal growth and development, substantially increasing scoliosis incidence. The rapid growth might incite spinal instability, thereby predisposing the spine to curvature and deformation (44). While scoliosis predominantly occurs during adolescence, its presence in infancy and early childhood should not be overlooked. Infantile Idiopathic Scoliosis (IIS), for instance, involves growth suppression of the vertebral growth plates on the concavity of the deformity, leading to vertebral body wedging and spinal buckling (45). Early detection and intervention are crucial in curtailing further progression of these spinal deformities. Our study findings indicate a 0.215% overall prevalence of congenital scoliosis (95% CI: 0.12%–1.2%), underscoring the importance of routine spinal examinations during infancy.
The correlation between BMI and scoliosis is intricate, drawing considerable focus in epidemiological research. Scoliosis, a three-dimensional spinal deformity characterized by lateral curvature and vertebral rotation, may be influenced by various factors, including BMI. A Mendelian randomization analysis demonstrated a causal link between low BMI and AIS onset (46). Typically, a low BMI might reflect undernutrition or a frail physique, potentially compromising the development and support of bones and muscles, thus elevating spinal curvature risk (47). Conversely, a high BMI may increase the mechanical load on the spine, fostering structural alterations and instability. Furthermore, in overweight individuals, the distribution of body fat may influence spinal mechanics, contributing to scoliosis development (48). In a prevalence study involving 196 obese adolescents (mean BMI 36 kg/cm2), the prevalence of AIS was found to be 12.2%, which is double the rate observed in the general population (49). Another cross-sectional study revealed a higher prevalence of obesity in patients with AIS compared to healthy controls (50).
Lifestyle factors significantly influence the development and progression of scoliosis. Sedentary behaviors, marked by prolonged sitting and minimal physical activity, have been linked to an increased risk of scoliosis. Inactivity may result in insufficient engagement of spinal muscles, leading to poor posture and muscular imbalances, which could contribute to the onset of scoliosis (51). A meta-analysis indicated that exercise interventions are notably more effective than conventional therapies in reducing Cobb's angle in adolescents with AIS. Among various exercise forms, yoga demonstrated the significant impact, reducing the Cobb's angle by an average of 4.60°, followed by core strength training, Physiotherapeutic Scoliosis-Specific Exercises (PSSE), Schroth exercises, and sling exercises. Alothough all exercise forms were effective, no significant differences were observed among them. These findings underscore the potential benefits of exercise-based treatments, particularly yoga, for managing AIS (52). Further insights from a Meta-analysis of randomized controlled trials have also demonstrated that Pilates exercise training effectively reduces Cobb's Angle, decreases trunk rotation, alleviates pain, enhances trunk motion, and improves the quality of life for individuals with scoliosis (53). This study emphasizes the importance of physical activity in lowering AIS prevalence and suggests that participation in organized sports may offer a protective effect, especially for girls. Notably, a lower prevalence of AIS was noted among pupils engaged in soccer (2.8%), handball (3.4%), and martial arts (3.9%), compared to those participating in swimming (8.6%), dancing (7.7%), and volleyball (8.2%). Additionally, a positive correlation was identified between the use of handheld electronic devices and the prevalence of scoliosis, indicating that increased screen time may be associated with a higher risk of AIS (54). A case-control study conducted in China revealed that poor reading and writing posture are linked to a heightened susceptibility to AIS. Moreover, heavy school bags are significantly associated with the development of AIS. Adolescents who engage in more than two hours of screen time during weekdays are at a greater risk for developing AIS. With respect to dietary habits, individuals who avoid milk and dairy products show a higher predisposition to AIS (55). Additionally, a cross-sectional study in Japan revealed a positive association between AIS risk and factors such as increased frequency, years of experience, and duration of ballet training. It also noted that participants with mothers affected by scoliosis exhibited elevated odds of developing AIS (56).
Melatonin, a hormone secreted by the pineal gland, plays a role in regulating circadian rhythms and sleep cycles. It also possesses antioxidant and immunomodulatory properties. Emerging research suggests melatonin's involvement in the pathogenesis of scoliosis, particularly AIS (57). A study indicates that individuals with scoliosis often exhibit reduced melatonin levels. This deficiency may interfere with normal spinal growth and development, thereby potentially increasing the risk of scoliosis (58). Furthermore, animal studies, including those on salmon and chickens with induced melatonin deficits, have demonstrated a higher incidence of scoliosis. Remarkably, melatonin supplementation in these models has shown potential in partially reversing the condition (59).
4.3 The prevention strategies for scoliosis
To effectively mitigate the risks associated with scoliosis, a comprehensive and structured prevention strategy is paramount. This strategy should include regular screening, public education, lifestyle modifications, nutritional interventions, ergonomic improvements, medical interventions, and psychosocial support.
Firstly, regular screening is critical for early detection. Implementing school-based screening programs and integrating spinal examinations into routine pediatric check-ups can facilitate early identification of scoliosis, particularly during adolescence, when the risk is most pronounced. Secondly, public education plays a vital role. Informing parents, guardians, and students about the indicators and consequences of scoliosis, and emphasizing the importance of early intervention through school programs and public awareness campaigns can foster timely medical consultations and interventions. Thirdly, lifestyle modifications are necessary to mitigate risk. Promoting physical activity, especially those that enhance core strength such as yoga, Pilates, and Schroth exercises, can prevent the onset of scoliosis. Additionally, reducing sedentary behaviors, limiting screen time, and encouraging regular movement breaks can improve posture and spinal health. Fourthly, nutritional interventions are crucial. Ensuring an intake rich in calcium, vitamin D, and other vital nutrients supports bone health. Maintaining a healthy BMI through appropriate diet and regular physical activity is important, especially for children. Fifthly, ergonomic adjustments in daily activities can prevent scoliosis. Using both straps of backpacks, minimizing load, and ensuring that school furniture like desks and chairs are of appropriate sizes can promote good posture and reduce the risk of developing spinal deformities. Sixthly, For those at elevated risk, specific medical actions might be beneficial. Children with a family history or genetic predispositions to scoliosis should consider genetic counseling and monitoring of hormonal levels. Orthotic braces can manage early signs of scoliosis and prevent its progression. Lastly, psychosocial support is essential for the overall well-being of children diagnosed with scoliosis. Providing psychological support can help manage issues related to body image concerns and anxiety, ensuring a holistic approach to care for children.
By integrating these multi-faceted and logically structured prevention strategies, we can reduce the prevalence and severity of scoliosis in children and adolescents, leading to improved long-term health outcomes. Regular follow-ups and personalized care plans tailored to individual risk factors are integral to an effective scoliosis prevention program.
Aside from scoliosis, other orthopedic conditions are also prevalent in the minor population, such as fractures, osteochondritis dissecans (OCD), and Fallen Arches. OCD is a condition affecting subchondral bone and articular cartilage (60). In a study conducted by Jeffrey I Kessler in 2010 (61), the prevalence of OCD in the 6–19 age group was 9.5 per 100,000 individuals, with the highest prevalence in the 12–19 age group at 11.2 per 100,000. Multivariate logistic regression analysis indicated that the risk of developing OCD in children aged 12–19 was 3.3 times that in children aged 6–11. For children and adolescents, these orthopedic conditions can significantly impact the quality of their life later on, and should be afforded ample attention.
4.4 Advantages and limitations of the study
This systematic review and meta-analysis provide an exhaustive global perspective on the prevalence and predictors of scoliosis prevalence in children and adolescents. Rigorous methodologies coupled with detailed subgroup analyses ensure the reliablility of our findings and highlight key predictors for targeted prevention strategies. These insights provide a robust framework for improving scoliosis prevention and management.
However, there are limitations to this research. Firstly, despite its comprehensive scope, our study predominantly includes data from Asia and Europe, with scant representation from North and South America. This geographic discrepancy may rimpede the universal applicability of our conclusions. Future research should strive to encompass more studies from these under-represented regions to augment our understanding of the prevalence and predictors of scoliosis in children and adolescents globally. Additionally, conducting region-specific analyses could help identify unique risk factors and facilitate tailored prevention strategies accordingly. Secondly, the scarcity of data on congenital scoliosis hampers our capacity to thoroughly understand its epidemiology and associated risk factors. More in-depth studies focusing on congenital scoliosis are needed to refine prevalence estimates and improve preventive and therapeutic approaches.
In future research, we should incorporate literature covering wider ethnicities and diverse samples to validate the risk factors for scoliosis, aiming to develop standard or customized follow-up and screening strategies.
5 Conclusion
Our study found that the prevalence of scoliosis among children and adolescents was relatively high, with the degree mostly concentrated at 10°–19°. Female and overweight children and adolescents were more prone to developing scoliosis. The etiology of scoliosis may be related to a variety of factors, including genetic and environmental aspects. It is important to focus on prevention and timely diagnosis of scoliosis, particularly during adolescence. Preventative measures include maintaining the correct posture, engaging in physical exercise, and avoiding prolonged static positions. It should be emphasized that although scoliosis usually does not progress to a severe condition, it needs to be taken seriously, and medical evaluation and appropriate treatment should be promptly sought upon the appearance of early signs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ML: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. QN: Writing – original draft, Conceptualization, Methodology, Resources. JL: Methodology, Writing – original draft, Formal Analysis, Investigation. ZJ: Writing – original draft, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1399049/full#supplementary-material
Supplementary Table S1
Literatur search strategy.
Supplementary Table S2
Joanna Briggs Institute scale.
Supplementary Table S3
Independent risk factors.
Abbreviations
BMI, body mass index; AIS, adolescent idiopathic scoliosis; NOS, Newcastle-Ottawa scale; JBI, Joanna Briggs Institute; IIS, infantile idiopathic scoliosis; PSSE, physiotherapeutic scoliosis-specific exercises.
References
1. Lacroix M, Khalifé M, Ferrero E, Clément O, Nguyen C, Feydy A. Scoliosis. Semin Musculoskelet Radiol. (2023) 27(5):529–44. doi: 10.1055/s-0043-1772168
2. Kontodimopoulos N, Damianou K, Stamatopoulou E, Kalampokis A, Loukos I. Children’s and parents’ perspectives of health-related quality of life in newly diagnosed adolescent idiopathic scoliosis. J Orthop. (2018) 15(2):319–23. doi: 10.1016/j.jor.2018.02.003
3. de Sèze M, Cugy E. Pathogenesis of idiopathic scoliosis: a review. Ann Phys Rehabil Med. (2012) 55(2):128–38. doi: 10.1016/j.rehab.2012.01.003
4. Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J (Engl). (2020) 133(4):483–93. doi: 10.1097/cm9.0000000000000652
5. White AA 3rd, Panjabi MM. The clinical biomechanics of scoliosis. Clin Orthop Relat Res. (1976) (118):100–12954262
6. Fletcher ND, Bruce RW. Early onset scoliosis: current concepts and controversies. Curr Rev Musculoskelet Med. (2012) 5(2):102–10. doi: 10.1007/s12178-012-9116-0
7. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
8. Willner S, Uden A. A prospective prevalence study of scoliosis in Southern Sweden. Acta Orthop Scand. (1982) 53(2):233–7. doi: 10.3109/17453678208992208
9. Safikhani Z, Fakor M, Soori H, Hejazian L. Prevalence of scoliosis in female students 11–15 years of age in ahwaz, Iran. Neurosciences. (2006) 11(2):97–8.22266557
10. Ostojić Z, Krišto T, Ostojić L, Petrović P, Vasilj I, Šantić Ž, et al. Prevalence of scoliosis in school-children from Mostar, Bosnia and Herzegovina. Coll Antropol. (2006) 30(1):59–64.
11. Nery LS, Halpern R, Nery PC, Nehme KP, Stein AT. Prevalence of scoliosis among school students in a town in Southern Brazil. Sao Paulo Med J. (2010) 128(2):69–73. doi: 10.1590/s1516-31802010000200005
12. Moalej S, Asadabadi M, Hashemi R, Khedmat L, Tavacolizadeh R, Vahabi Z, et al. Screening of scoliosis in school children in Tehran: the prevalence rate of idiopathic scoliosis. J Back Musculoskelet Rehabil. (2018) 31(4):767–74. doi: 10.3233/BMR-171078
13. MŞ E, Özcan-Ekşi EE, Huet SE, Dinç T, Özmen BB, Akçal MA. Prevalence of thoracic scoliosis in adolescents in Turkey: analysis of 1,065 chest radiographs. World Neurosurg. (2020) 135:e527–40. doi: 10.1016/j.wneu.2019.12.057
14. Minghelli B, Nunes C, Oliveira R. Prevalence of scoliosis in southern Portugal adolescents. Pediatr Endocrinol Rev. (2014) 11(4):374–82.24988690
15. Du Q, Zhou X, Negrini S, Chen N, Yang X, Liang J, et al. Scoliosis epidemiology is not similar all over the world: a study from a scoliosis school screening on chongming island (China). BMC Musculoskelet Disord. (2016) 17(1):303. doi: 10.1186/s12891-016-1140-6
16. Daruwalla JS, Balasubramaniam P, Chay SO, Rajan U, Lee HP. Idiopathic scoliosis. Prevalence and ethnic distribution in Singapore schoolchildren. J Bone Joint Surg B. (1985) 67(2):182–4. doi: 10.1302/0301-620x.67b2.3980521
17. Wang F, Wang X, Medina O, Yong M, Lin G, Sun X, et al. Prevalence of congenital scoliosis in infants based on chest–abdomen x-ray films detected in the emergency department. Eur Spine J. (2021) 30(7):1848–57. doi: 10.1007/s00586-021-06779-3
18. Saifi C, Matsumoto H, Vitale MG, Roye Jr DP, Hyman JE. The incidence of congenital scoliosis in infants with tetralogy of Fallot based on chest radiographs. J Pediatr Orthop B. (2012) 21(4):313–6. doi: 10.1097/BPB.0b013e3283536872
19. Yılmaz H, Zateri C, Kusvuran Ozkan A, Kayalar G, Berk H. Prevalence of adolescent idiopathic scoliosis in Turkey: an epidemiological study. Spine J. (2020) 20(6):947–55. doi: 10.1016/j.spinee.2020.01.008
20. Penha PJ, Ramos NLJP, De Carvalho BKG, Andrade RM, Schmitt ACB, João SMA. Prevalence of adolescent idiopathic scoliosis in the state of São Paulo, Brazil. Spine. (2018) 43(24):1710–8. doi: 10.1097/BRS.0000000000002725
21. Dantas MGB, Aquino AN, Correia HJ, Ferreira KP, Do Nascimento BB, Silva LD, et al. Prevalence of back pain and idiopathic scoliosis in adolescents from the semiarid region of Brazil: a cross-sectional study. J Chiropr Med. (2021) 20(3):97–107. doi: 10.1016/j.jcm.2021.12.004
22. Bondar K, Nguyen A, Vatani J, Kessler J. The demographics and epidemiology of infantile, juvenile, and adolescent idiopathic scoliosis in a Southern California integrated health care system. Spine. (2021) 46(21):1468–77. doi: 10.1097/BRS.0000000000004046
23. Zou Y, Lin Y, Meng J, Li J, Gu F, Zhang R. The prevalence of scoliosis screening positive and its influencing factors: a school-based cross-sectional study in Zhejiang province, China. Front Public Health. (2022) 10:773594. doi: 10.3389/fpubh.2022.773594
24. Zhou LJ, Yang HH, Hai Y, Hai JJ, Cheng YZ, Yin P, et al. Scoliosis among children in Qinghai-Tibetan plateau of China: a cross-sectional epidemiological study. Front Public Health. (2022) 10. doi: 10.3389/fpubh.2022.983095
25. Zheng Y, Wu X, Dang Y, Yang Y, Reinhardt JD, Dang Y. Prevalence and determinants of idiopathic scoliosis in primary school children in Beitang district, Wuxi, China. J Rehabil Med. (2016) 48(6):547–53. doi: 10.2340/16501977-2098
26. Yong F, Wong HK, Chow KY. Prevalence of adolescent idiopathic scoliosis among female school children in Singapore. Ann Acad Med Singap. (2009) 38(12):1056–63. doi: 10.47102/annals-acadmedsg.V38N12p1056
27. Wong HK, Hui JH, Rajan U, Chia HP. Idiopathic scoliosis in Singapore schoolchildren: a prevalence study 15 years into the screening program. Spine (Phila Pa 1976). (2005) 30(10):1188–96. doi: 10.1097/01.brs.0000162280.95076.bb
28. Ueno M, Takaso M, Nakazawa T, Imura T, Saito W, Shintani R, et al. A 5-year epidemiological study on the prevalence rate of idiopathic scoliosis in Tokyo: school screening of more than 250,000 children. J Orthop Sci. (2011) 16(1):1–6. doi: 10.1007/s00776-010-0009-z
29. Sung S, Chae HW, Lee HS, Kim S, Kwon JW, Lee SB, et al. Incidence and surgery rate of idiopathic scoliosis: a nationwide database study. Int J Environ Res Public Health. (2021) 18(15):8152. doi: 10.3390/ijerph18158152
30. Stirling AJ, Howel D, Millner PA, Sadiq S, Sharples D, Dickson RA. Late-onset idiopathic scoliosis in children six to fourteen years old. A cross-sectional prevalence study. J Bone Joint Surg. (1996) 78(9):1330–6. doi: 10.2106/00004623-199609000-00006
31. Singh H, Shipra Sharma V, Sharma I, Sharma A, Modeel S, et al. The first study of epidemiology of adolescent idiopathic scoliosis shows lower prevalence in females of Jammu and Kashmir, India. Am J Transl Res. (2022) 14(2):1100–635273713
32. Lee JY, Moon SH, Kim HJ, Park MS, Suh BK, Nam JH, et al. The prevalence of idiopathic scoliosis in eleven year-old Korean adolescents: a 3 year epidemiological study. Yonsei Med J. (2014) 55(3):773–8. doi: 10.3349/ymj.2014.55.3.773
33. Kunakornsawat S, Popan N, Piyaskulkaew C, Pruttikul P, Pluemvitayaporn T, Kittithamvongs P. Prevalence of idiopathic scoliosis in Thai female students aged 11–13 years. J Med Assoc Thail. (2017) 100(5):533–8.
34. Komang-Agung IS, Dwi-Purnomo SB, Susilowati A. Prevalence rate of adolescent idiopathic scoliosis: results of school-based screening in Surabaya, Indonesia. Malays Orthop J. (2017) 11(3):17–22. doi: 10.5704/MOJ.1711.011
35. Janani R, Ramachandran A, Divya S. Analysis of prevalence and factors influencing adolescent idiopathic scoliosis among school students in Thiruvallur district. Int J Physiother. (2019) 6(3):89–94. doi: 10.15621/ijphy/2019/v6i3/183877
36. Huang F, Liu Y, Wu J, Yang J, Huang S, Zhang Z, et al. Incidence of scoliosis among junior high school students in Zhongshan City, Guangdong and the possible importance of decreased miR-30e expression. J Int Med Res. (2020) 48(6):300060519889438. doi: 10.1177/0300060519889438
37. Hu M, Zhang Z, Zhou X, Gao R, Wang C, Ma J, et al. Prevalence and determinants of adolescent idiopathic scoliosis from school screening in Huangpu district, Shanghai, China. Am J Transl Res. (2022) 14(6):4132–8.35836864
38. Hengwei F, Zifang H, Qifei W, Weiqing T, Nali D, Ping Y, et al. Prevalence of idiopathic scoliosis in Chinese schoolchildren: a large, population-based study. Spine (Phila Pa 1976). (2016) 41(3):259–64. doi: 10.1097/brs.0000000000001197
39. Etemadifar M, Hadi A, Nazem K, Esfahani M, Rabiei A, Taghvaee F, et al. Epidemiology of adolescent idiopathic scoliosis in Isfahan, Iran: a school-based study during 2014–2015. J Res Med Sci. (2020) 25(1). doi: 10.4103/jrms.JRMS_418_17
40. Grivas TB, Vasiliadis E, Mouzakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis. (2006) 1:9. doi: 10.1186/1748-7161-1-9
41. Kotwicki T, Janusz P, Andrusiewicz M, Chmielewska M, Kotwicka M. Estrogen receptor 2 gene polymorphism in idiopathic scoliosis. Spine (Phila Pa 1976). (2014) 39(26):E1599–607. doi: 10.1097/brs.0000000000000643
42. Zhao D, Qiu GX, Wang YP, Zhang JG, Shen JX, Wu ZH. Association between adolescent idiopathic scoliosis with double curve and polymorphisms of calmodulin1 gene/estrogen receptor-α gene. Orthop Surg. (2009) 1(3):222–30. doi: 10.1111/j.1757-7861.2009.00038.x
43. Zhao L, Roffey DM, Chen S. Association between the estrogen receptor beta (ESR2) Rs1256120 single nucleotide polymorphism and adolescent idiopathic scoliosis: a systematic review and meta-analysis. Spine (Phila Pa 1976). (2017) 42(11):871–8. doi: 10.1097/brs.0000000000001932
44. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. (2008) 371(9623):1527–37. doi: 10.1016/s0140-6736(08)60658-3
45. Abel MF. Infantile idiopathic scoliosis. J Neurosurg Spine. (2009) 11(1):1–2; discussion. doi: 10.3171/2009.2.Spine0982
46. Otomo N, Khanshour AM, Koido M, Takeda K, Momozawa Y, Kubo M, et al. Evidence of causality of low body mass index on risk of adolescent idiopathic scoliosis: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1089414. doi: 10.3389/fendo.2023.1089414
47. Bonjour JP, Guéguen L, Palacios C, Shearer MJ, Weaver CM. Minerals and vitamins in bone health: the potential value of dietary enhancement. Br J Nutr. (2009) 101(11):1581–96. doi: 10.1017/s0007114509311721
48. Rinonapoli G, Pace V, Ruggiero C, Ceccarini P, Bisaccia M, Meccariello L, et al. Obesity and bone: a complex relationship. Int J Mol Sci. (2021) 22(24):1581–96. doi: 10.3390/ijms222413662
49. Catanzariti JF, Rimetz A, Genevieve F, Renaud G, Mounet N. Idiopathic adolescent scoliosis and obesity: prevalence study. Eur Spine J. (2023) 32(6):2196–202. doi: 10.1007/s00586-023-07709-1
50. Escrivá D, Benet I, Burgos J, Barrios C. Adiposity-age distribution and nutritional status in girls with adolescent idiopathic scoliosis. Spine Deform. (2019) 7(4):565–70. doi: 10.1016/j.jspd.2018.10.007
51. Chopra S, Larson AN, Kaufman KR, Milbrandt TA. Accelerometer based assessment of daily physical activity and sedentary time in adolescents with idiopathic scoliosis. PLoS One. (2020) 15(9):e0238181. doi: 10.1371/journal.pone.0238181
52. Chen Y, Zhang Z, Zhu Q. The effect of an exercise intervention on adolescent idiopathic scoliosis: a network meta-analysis. J Orthop Surg Res. (2023) 18(1):655. doi: 10.1186/s13018-023-04137-1
53. Gou Y, Lei H, Zeng Y, Tao J, Kong W, Wu J. The effect of pilates exercise training for scoliosis on improving spinal deformity and quality of life: meta-analysis of randomized controlled trials. Medicine (Baltimore). (2021) 100(39):e27254. doi: 10.1097/md.0000000000027254
54. Glavaš J, Rumboldt M, Karin Ž, Matkovic R, Bilic-Kirin V, Buljan V, et al. The impact of physical activity on adolescent idiopathic scoliosis. Life (Basel). (2023) 13(5):1180. doi: 10.3390/life13051180
55. Dou Q, Zhu Z, Zhu L, Wang W, Guo L, Ru S, et al. Academic-related factors and daily lifestyle habits associated with adolescent idiopathic scoliosis: a case-control study. Environ Health Prev Med. (2023) 28:23. doi: 10.1265/ehpm.22-00243
56. Watanabe K, Michikawa T, Yonezawa I, Takaso M, Minami S, Soshi S, et al. Physical activities and lifestyle factors related to adolescent idiopathic scoliosis. J Bone Joint Surg Am. (2017) 99(4):284–94. doi: 10.2106/jbjs.16.00459
57. Gargano G, Oliva F, Migliorini F, Maffulli N. Melatonin and adolescent idiopathic scoliosis: the present evidence. Surgeon. (2022) 20(6):e315–21. doi: 10.1016/j.surge.2021.07.008
58. Sadat-Ali M, al-Habdan I, al-Othman A. Adolescent idiopathic scoliosis. Is low melatonin a cause? Joint Bone Spine. (2000) 67(1):62–4.10773970
59. Man GC, Wang WW, Yim AP, Wong JH, Ng TB, Lam TP, et al. A review of pinealectomy-induced melatonin-deficient animal models for the study of etiopathogenesis of adolescent idiopathic scoliosis. Int J Mol Sci. (2014) 15(9):16484–99. doi: 10.3390/ijms150916484
60. Accadbled F, Vial J, Sales de Gauzy J. Osteochondritis dissecans of the knee. Orthop Traumatol Surg Res. (2018) 104(1s):S97–105. doi: 10.1016/j.otsr.2017.02.016
Keywords: scoliosis, children, adolescents, prevalence, risk factors, systematic review
Citation: Li M, Nie Q, Liu J and Jiang Z (2024) Prevalence of scoliosis in children and adolescents: a systematic review and meta-analysis. Front. Pediatr. 12: 1399049. doi: 10.3389/fped.2024.1399049
Received: 11 March 2024; Accepted: 11 July 2024;
Published: 23 July 2024.
Edited by:
Rafael Lomas-Vega, University of Jaén, SpainReviewed by:
Ancuta Lupu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaZhen Liu, Nanjing Drum Tower Hospital, China
© 2024 Li, Nie, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeping Jiang, amlhbmd6ZXBpbmcyMDI0QDE2My5jb20=
 Mingyang Li1
Mingyang Li1 Qilong Nie
Qilong Nie Zeping Jiang
Zeping Jiang