- 1Key Laboratory of Model Animals and Stem Cell Biology in Hunan Province, School of Medicine, Hunan Normal University, Hunan, China
- 2Nursing Department, Changde Vocational Technical College, Changde, Hunan, China
- 3Department of Medical Consortium Work, Changsha Hospital for Maternal & Child Health Care Affiliated to Hunan Normal University, Changsha, Hunan, China
- 4Hunan Provincial People's Hospital Affiliated to Hunan Normal University, Changsha, Hunan, China
- 5Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 6Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, School of Medicine, Hunan Normal University, Changsha, Hunan, China
- 7The Engineering Research Center of Reproduction and Translational Medicine of Hunan Province, Changsha, China
- 8Department of Otolaryngology-Head and Neck Surgery, Yichang Central People's Hospital, Three Gorges University, Hubei, China
Introduction: Limited knowledge exists regarding the impact of paternal smoking and alcohol exposure on the development of allergic rhinitis in offspring. Our study aimed to investigate the potential association between preconception paternal smoking and alcohol exposure and the likelihood of children allergic rhinitis.
Methods: A retrospective case-control study of 556 prepubertal children aged 3–12 years was performed. The participants were 278 children with allergic rhinitis and 278 healthy controls matched for age and gender. Self-administered questionnaires were distributed and collected on-site, focusing on various factors related to the children's fathers, mothers, and the children themselves during the first year of life and the past 12 months, from March to October 2022.
Results: Multivariate analysis demonstrated that paternal smoking, paternal alcohol consumption prior to conception, paternal allergic diseases, children with a family history of allergies, maternal allergic diseases and pregnancy complications were identified as independent risk factors for allergic rhinitis in their offspring. Moreover, after considering confounding factors, it was observed that paternal smoking exceeding 5 cigarettes per day in the year preceding pregnancy and exceeding 11 years significantly elevated the likelihood of allergic rhinitis in children (OR = 2.009 and 2.479, respectively). Furthermore, the consumption of alcohol by the father at intervals of less than one month in the year prior to pregnancy and a duration of alcohol consumption exceeding 11 years prior to pregnancy are both associated with a significantly increased risk of allergic rhinitis in children (OR = 2.005 and 3.149, respectively).
Conclusions: Paternal smoking and alcohol consumption prior to conception contribute to an increased risk of allergic rhinitis in children, with the risk being dependent on the dosage and duration of exposure. Therefore, it is important to not only focus on personal and maternal environmental exposures when considering the occurrence risk of allergic rhinitis in children, but also to consider paternal detrimental exposures prior to conception.
1 Introduction
Allergic rhinitis, a non-infectious chronic inflammatory condition affecting the nasal mucosa, is attributed to exposure to specific allergens. Common symptoms include sneezing, runny nose, nasal irritation, and nasal congestion, with potential ocular manifestations (1). Global studies indicate an approximate worldwide incidence rate of 12.66% in children (2). Notably, the prevalence of allergic rhinitis in China has risen from 15.79% in 2016 (3) to 22.00% (4) in 2021. Allergic rhinitis (AR) is poorly controlled. Children afflicted with allergic rhinitis may experience nasal irritation, fatigue, disrupted sleep, cognitive impairment, reduced memory capacity, diminished learning efficiency, and ongoing medical costs, which can significantly lower their quality of life and that of their family (5).
The prevalence of allergic rhinitis in children is primarily influenced by genetic and environmental factors. However, these factors alone do not fully account for the high incidence of allergic rhinitis. In 1989, Barker firstly published research findings indicating that elderly individuals with a history of low birth weight or malnutrition during infancy were at a heightened risk for cardiovascular disease. In 1990, he introduced the "fetal origin of adult diseases (FOAD) hypothesis," (6). Which later evolved into the "Developmental Origin of Health and Diseases (DOHaD)" theory after extensive researches. According to this view, adverse exposures during early life stages, including sperm and oocyte development, zygote formation, fetal development, infancy, and childhood, can have long-term effects on the health and susceptibility of offspring to illness (7).
Previous research primarily focused on mothers and found that maternal factors such as age between 26 and 35 years old (8), exposure to air pollution (9) during pregnancy, passive smoking (10) and gestational hypertension (11) can elevate the likelihood of allergic rhinitis in their offspring. However, recent studies have increasingly highlighted the significant contribution of fathers in shaping the health and disease susceptibility of their children.
A multitude of detrimental factors, such as cigarette smoking and excessive alcohol consumption, have been found to have a negative impact on sperm quality (12). Pre-conception alcohol intake by fathers has been associated with an elevated likelihood of behavioral problems (13) and microcephaly in their offspring (14). Furthermore, research has indicated that paternal smoking prior to pregnancy may influence the development of asthma in their children (15). However, little is known about the effects of paternal smoking and alcohol exposure on the offspring's allergic rhinitis until now. Hence, this study will utilize the DOHaD theory as a foundation to examine the association between pre-conception factors in fathers and the prevalence of allergic rhinitis in their progeny. Special consideration will be devoted to exploring the potential influence of modifiable factors, such as paternal smoking and alcohol exposure, on the susceptibility of children to developing allergic rhinitis.
2 Methods
2.1 Study design and population
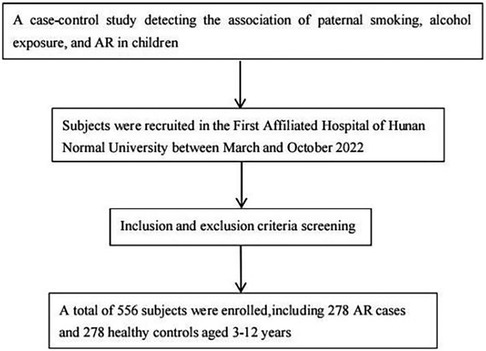
This retrospective case-control study was conducted at the First Affiliated Hospital of Hunan Normal University between March and October 2022. A total of 278 who were treated for allergic rhinitis and their parents were invited to participate in the case group, while 278 children who received physical examinations in the hospital and their parents were included in the control group. The study received approval from the Ethics Committee of the Hunan Normal University (approval number: 2022277), and informed written consent was obtained from all participants. All procedures for data collection follow-strict quality assurance and quality control guideline. Strict inclusion and exclusion criteria were applied for patient recruitment.The screening flowchart of the subjects is shown in Figure 1.
2.1.1 Inclusion criteria for case group
(1) Children who have been diagnosed with allergic rhinitis based on the diagnostic criteria of allergic rhinitis. (2) Children who are single births. (3) Children aged between 3 and 12 years old. (4) The child's father does not have any communication barriers and has no history of mental illness.
2.1.2 Exclusion criteria for case group
(1) Children who have previously suffered from immune diseases or severe organic diseases, as well as children with mental illnesses. (2) The child's father is uncooperative and does not have sufficient time to receive a questionnaire survey.
2.1.3 Inclusion criteria for control group
(1) Children have no allergic rhinitis. (2) Children who are single births. (3) Children aged between 3 and 12 years old. (4) The child's father does not have any communication barriers and has no history of mental illness.
2.1.4 Exclusion criteria for control group
(1) Children who have previously suffered from immune diseases or severe organic diseases, as well as children with mental illnesses. (2) The child's father is uncooperative and does not have sufficient time to receive a questionnaire survey.
2.2 Diagnostic criteria for allergic rhinitis in children
According to the Chinese guidelines for the Diagnosis and Treatment of Allergic Rhinitis in Children (1), these criteria require a comprehensive assessment of the allergy history, clinical symptoms, and laboratory examination results. ① Allergy history: the occurrence of allergy conditions and allergies in close relatives, including mothers and/or fathers. ② Typical clinical symptoms include sneezing, clear nasal discharge, itching, and nasal congestion. These symptoms may also be accompanied by respiratory symptoms like coughing and eye symptoms like itching and weeping. ③ Physical symptoms: The main sign of AR attack is bilateral nasal mucosal swelling pale, swollen inferior turbinate, with excessive amount of clear water like secretion in the nasal cavity. ④ Laboratory testing: a skin prick test (SPT) to identify at least one positive allergen. Detecting the presence of serum-specific IgE antibodies has high specificity and sensitivity. Additionally, the examination of nasal secretions revealed the presence of eosinophils exceeding 0.05%, and a regular blood test indicated an increase in eosinophil count.
2.3 Clinical data collection of the participants
(1) Factors related to the newborn within a year of birth: birth weight, delivery model, gravidity and parity, gestational age, mode of conception, allergic diseases, feeding methods within four months, family history of allergies, place of residence, time of adding complementary foods, types of complementary food, living environment (such as house decoration, dampness, purchase of new furniture, long-term use of blankets and carpets, frequent exposure to stuffed toys, daily ventilation time, and presence of pets), smoking habits of family members, frequency of colds, and antibiotic usage.
(2) Factors related to the child within 12 months: age, height, body weight, allergy history, place of residence, living environment (such as house decoration, dampness, purchase of new furniture, long-term use of blankets and carpets, frequent exposure to stuffed toys, daily ventilation time, presence of pets, and regular classroom ventilation), frequent utilization of air conditioning, children's lifestyle and habits [such as insufficient daily sleep duration (<8 h), daily vegetable consumption, and daily fruit consumption], as well as smoking habits of family members.
(3) Factors related to the mother: mother's age before pregnancy, height and weight before pregnancy, weight gain during pregnancy, pregnancy complications, allergic diseases, history of urticaria, and passive smoking during pregnancy.
(4) Factors related to the father: age before pregnancy, height and weight before pregnancy, birth weight, gestational age, educational level, income, engaging in high-risk occupations before pregnancy, allergic diseases, history of urticaria, pre-pregnancy hypertension, pre-pregnancy diabetes, and smoking and alcohol consumption in the year before pregnancy.
2.4 Statistical analysis
Continuous variables with normally distributed data are expressed as means and standard deviations, and a t-test was used to compare variables between two groups. The median and quartiles (Q1–Q3) were utilized for non-normally distributed continuous variables, and the Mann-Whitney non-parametric test was used to compare the variables between groups. Cases (n) and percentages (%) were employed to represent categorical variables, while the chi-square test was utilized to evaluate the qualitative data. The case–control study was matched according to gender frequency, and the baseline data of age and nationality were compared by chi-square test or nonparametric rank sum test. Include statistically significant variables from univariate analysis into multivariate analysis, and multivariate logistic regression (Forward Wald method) was used to calculate the odds ratio (OR) and 95% confidence interval (CI) to evaluate the relationship between various factors and childhood allergic rhinitis. A P value less than 0.05 was considered statistically significant. Data were analyzed using SPSS for Windows version 26.
3 Results
3.1 Participant characteristics
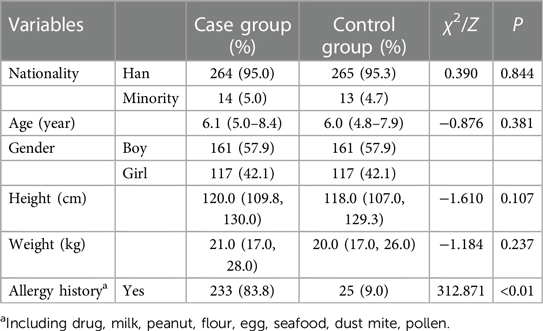
In total, 556 children with complete data were included (57.9% boys, n = 322; 42.1% girls, n = 234), with the majority of children being Han Chinese (95.1%). According to the most recent data from the previous 12 months, it was discovered that more kids in the case group have a history of allergies than in the control group (83.8% vs. 9.0%, p < 0.01). However, the study groups were relatively homogeneous in terms of nationality, age, body weight, and height. Detailed data are shown in Table 1.
3.2 Univariate analysis
3.2.1 Children indicators
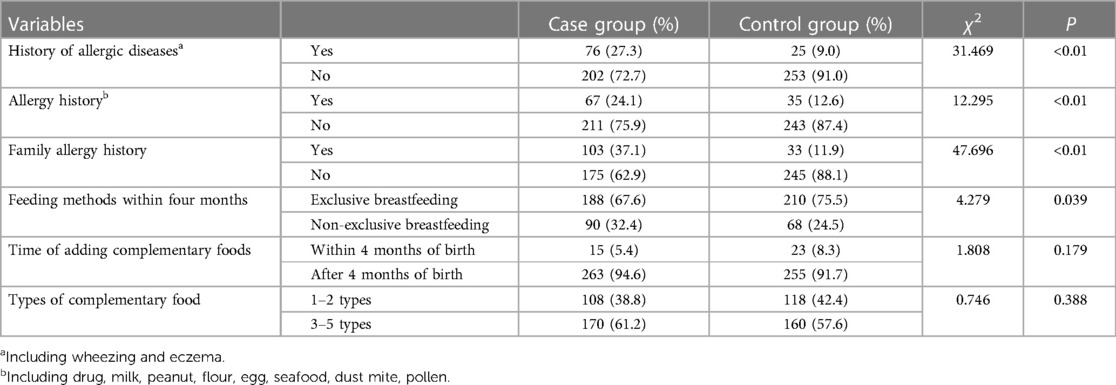
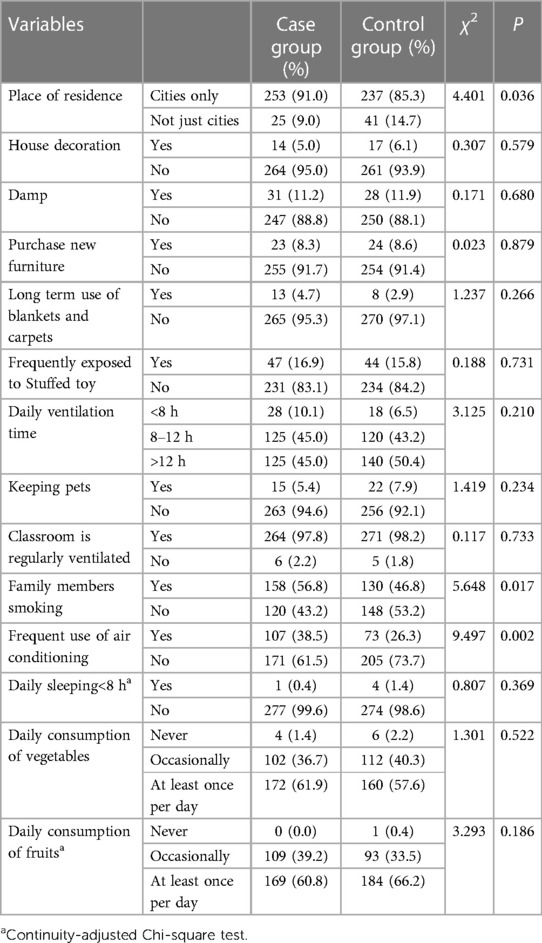
Statistically significant differences were observed between the case group and control group in various factors. These factors include family allergy history (37.1% vs. 11.9%, P < 0.01), exclusive breastfeeding during the first four months of life (67.6% vs. 75.7%, P = 0.039), urban residency (82.0% vs. 73.3%, P = 0.019), purchasing new home furniture (14.4% vs. 8.6%, P = 0.033), and experiencing frequent colds during the first year of life (22.3% vs. 14.7%, P = 0.022). Furthermore, within the past 12-month period, notable disparities were observed between the two groups concerning variables such as urban residency (91.0% vs. 85.3%, P = 0.036), familial smoking habits (56.8% vs. 46.8%, P = 0.017), and frequent use of air conditioning (38.5% vs. 26.3%, P = 0.002). These data are shown in Tables 2–5.
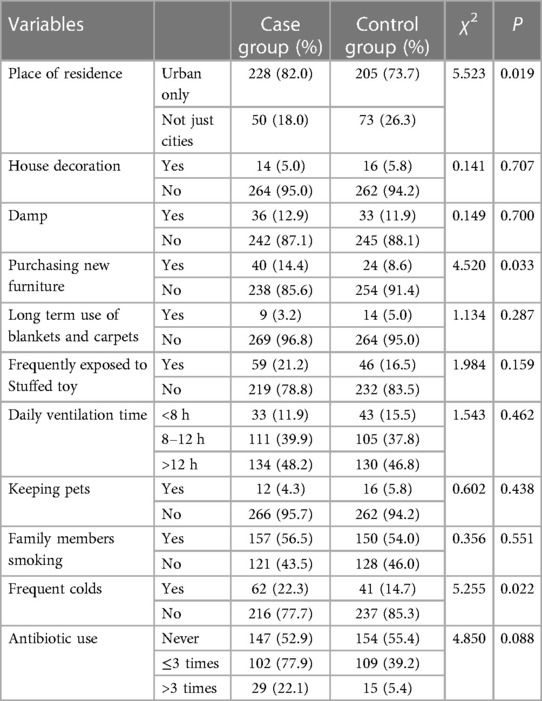
Table 4. Characteristics regarding the living environment of children within first year after birth.
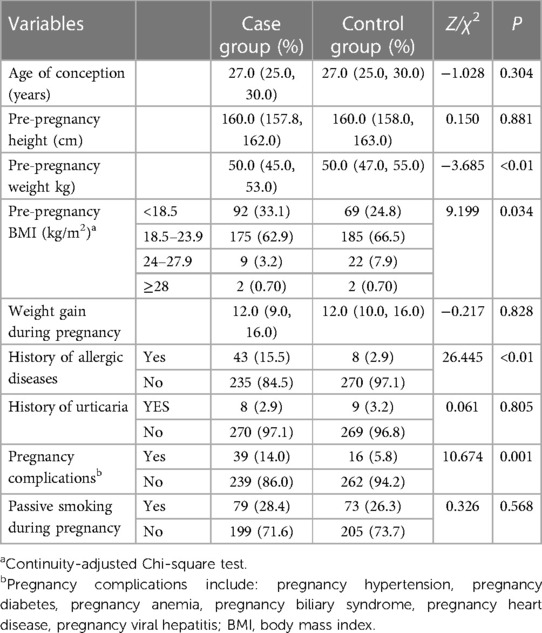
3.2.2 Maternal related indicators
Significant differences were observed between the two groups in terms of maternal allergic diseases (15.5% vs. 2.9%, P < 0.01), pre-pregnancy weight (Q1∼Q3: 45.0∼53.0 vs. 47.0∼55.0, P < 0.01), and pregnancy complications (14.0% vs. 5.8%, P = 0.001). Additional details can be found in Table 6.
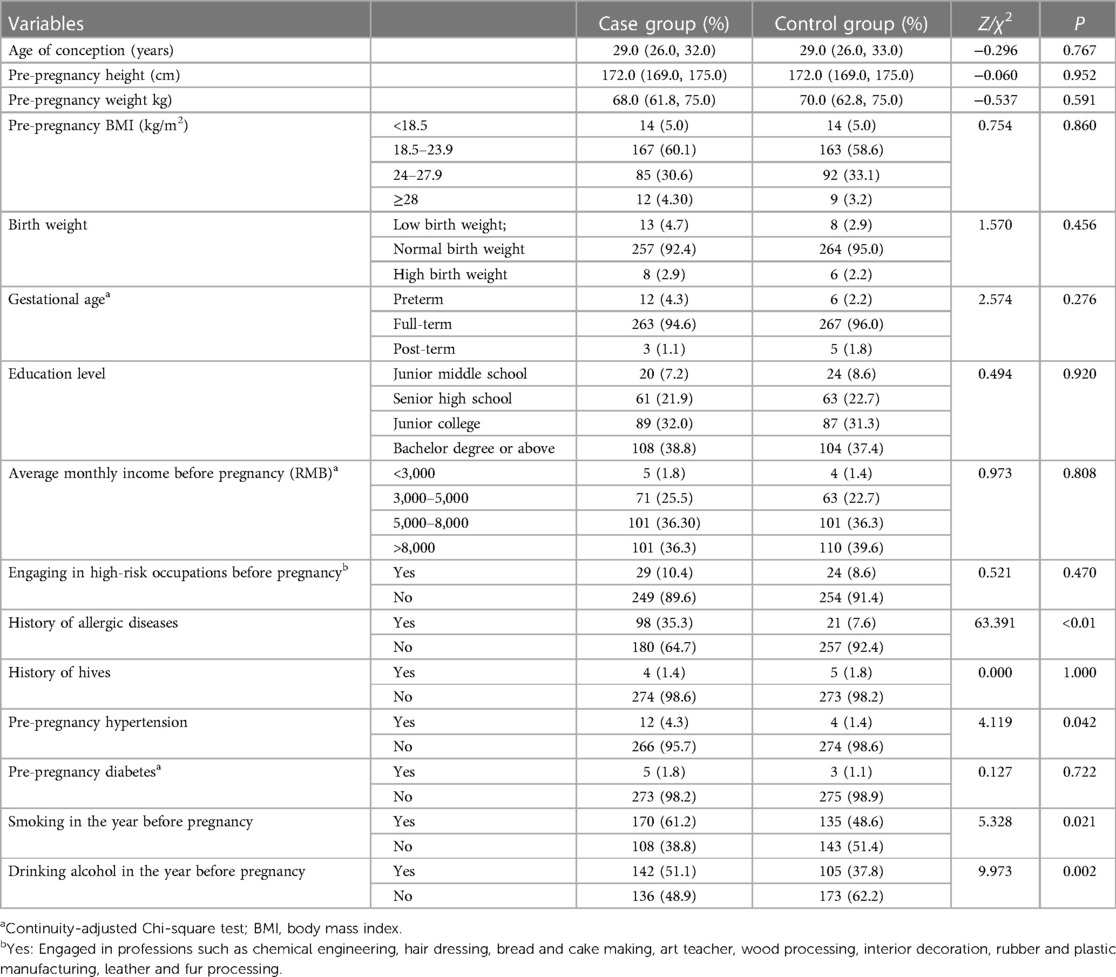
3.2.3 Paternal related indicators
The study revealed significant associations between various paternal indicators and the outcomes of interest. Specifically, there were higher rates of paternal allergic disease (35.3% vs. 7.6%, P < 0.01), paternal pre-pregnancy hypertension (4.3% vs. 1.4%, P = 0.042), paternal smoking in the year before pregnancy (61.2% vs. 48.6%, P = 0.021), and paternal alcohol consumption in the year before pregnancy (51.1% vs. 37.8%, P = 0.002) in the case group. For more comprehensive information, please refer to Table 7.
3.3 Multivariate analysis
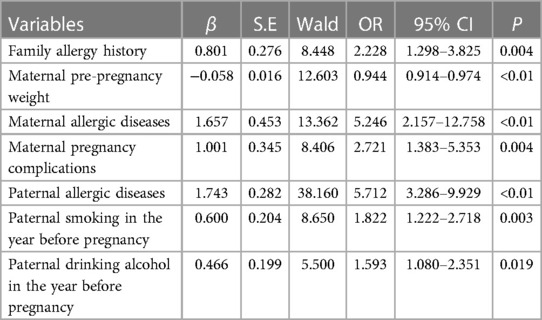
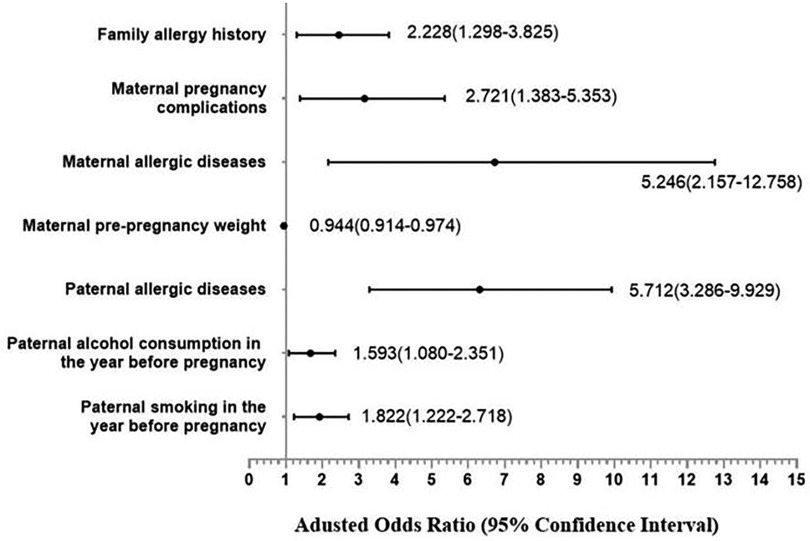
Based on the findings of the univariate analysis, it was observed that a total of 18 covariates exhibited statistically significant differences between the case and control groups. Subsequently, through the integration of professional expertise, the categories of “children with allergic diseases within one year of age” and “children with allergic history within the past 12 months” were excluded from the multivariate regression analysis. The results of the multivariate analysis indicate that several independent risk factors contribute to the development of allergic rhinitis in offspring. These factors include a family history of allergies (OR = 2.228, 95% CI: 1.298–3.825), maternal allergic diseases (OR = 5.246, 95% CI: 2.157–12.758), pregnancy complications (OR = 2.721, 95% CI: 1.383–5.353), paternal allergic diseases (OR = 5.712, 95% CI: 3.286–9.929), paternal smoking in the year before pregnancy (OR = 1.822, 95% CI: 1.222–2.718), and paternal alcohol consumption in the year before pregnancy (OR = 1.593, 95% CI: 1.080–2.351). Shown in Table 8 and Figure 2.
3.4 Correlation analysis between paternal smoking and alcohol consumption before pregnancy and children's allergic rhinitis
The following analysis aimed to examine two key modifiable factors pertaining to fathers prior to conception, with a first focus on the quantity of cigarettes smoked per day in the year before pregnancy and the duration of smoking in relation to children's allergic rhinitis. Three distinct binary logistic regression models were adjusted to examine the association between paternal smoking habits and the risk of allergic rhinitis in offspring. Our findings indicate that a heightened risk of allergic rhinitis in children is observed when the father consumes more than 5 cigarettes per day in the year before pregnancy (OR = 2.341, 95% CI: 1.458–3.758). Furthermore, a statistically significant increase in the odds ratio was observed as the number of cigarettes smoked per day escalated from exceeding 5 cigarettes. Although the relationship between the duration of smoking years and the risk of allergic rhinitis in offspring is inconclusive, nevertheless, our research indicates a noteworthy elevation in the risk of allergic rhinitis among children when the father has engaged in smoking for a minimum of 11 years prior to conception (OR = 2.479, 95% CI: 1.551–5.159), as evidenced across all three models. Detailed data can be seen in Figure 3.
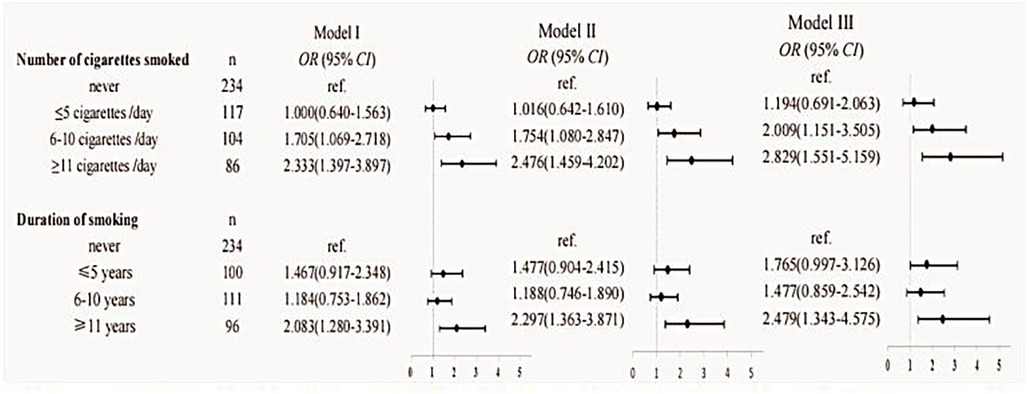
Figure 3. Results of the analyses assessing the association between paternal smoking and children's allergic rhinitis. Model I: unadjusted. Model II: adjusted for the age of conception, educational level, income, child age, child height and weight, and child's place of residence. Model III: adjusted for age of conception, educational level, income, child age, child height and weight, child's place of residence, family allergy history, paternal allergic disease, paternal alcohol consumption in the year before pregnancy, maternal weight before pregnancy, maternal allergic disease, and pregnancy complications.
And then we focus on the frequency and timing of alcohol consumption in relation to children's allergic rhinitis. The findings, supported by the upward trend observed in the overall OR value, indicate a significant correlation between the frequency or time of alcohol consumption and the occurrence of allergic rhinitis in their offspring. All three models demonstrated that paternal alcohol consumption less than one month before pregnancy and consuming alcohol for more than 11 years before conception may significantly elevate the risk of allergic rhinitis in offspring (OR = 2.668, 95% CI: 1.639–4.345 and OR = 3.149, 95% CI: 1.445–6.863, respectively). Detailed data can be seen in Figure 4.
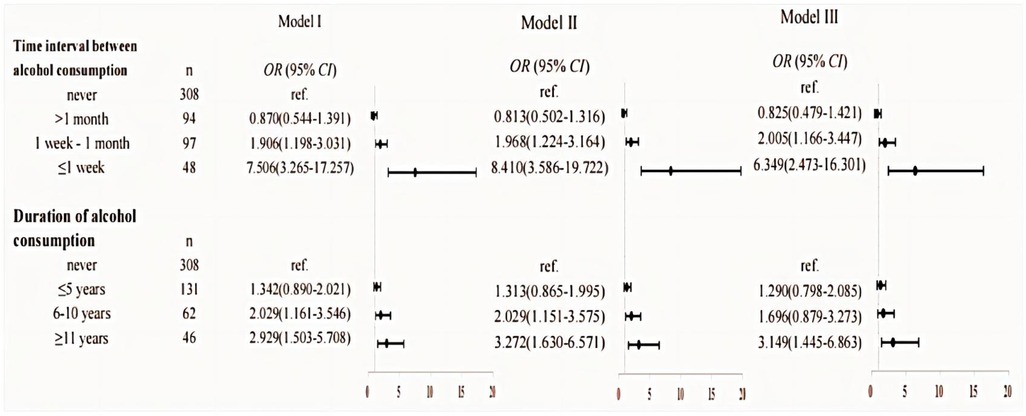
Figure 4. Results of the analyses assessing the association between paternal alcohol consumption and children's allergic rhinitis. Model I: unadjusted. Model II: adjusted for the age of conception, educational level, income, child age, child height and weight, and child's place of residence. Model III: adjusted for age of conception, educational level, income, child age, child height and weight, child's place of residence, family allergy history, paternal allergic disease, paternal smoking in the year before pregnancy, maternal weight before pregnancy, maternal allergic disease, and pregnancy complications.
In summary, the data indicates a dose-and-time correlation between the level or duration of preconception paternal smoking or alcohol consumption and the incidence of allergic rhinitis in offspring.
4 Discussion
The research reveals that fathers who engaged in smoking and alcohol consumption prior to conception significantly increased the risk of their offspring developing allergic rhinitis, with a positive correlation observed between the severity of paternal habits and the likelihood of their children being affected by the condition.
Prior studies have primarily examined the impact of maternal active smoking, maternal passive smoking during pregnancy, and child passive smoking on the development of allergy diseases in children. Many studies have indicated that maternal exposure to passive smoking during pregnancy is associated with various adverse outcomes, including gestational diabetes (16), low birth weight infants (17), small head circumference at birth (18), neural tube defects and mental health issues in offspring (19), and childhood allergic diseases, including wheezing, asthma, and allergic rhinitis, in offspring (20–22). Similarly, a recent meta-analysis has shown that children exposed to passive smoking are at an increased risk of developing allergic rhinitis (4). However, our study showed that the smoking of family members in the past 12 months were not significant risk factor in the multivariate analysis.
However, the neglect of the fact that fathers are the predominant smokers has been evident. According to the findings of the 2018 National Adult Tobacco Epidemic Survey, the prevalence of smoking among individuals aged ≥15 years in China stands at 26.6%. This rate is further disaggregated by gender, revealing that 50.5% of smokers are male, while only 2.1% are female (23). This study discovered paternal active smoking before pregnancy increased the development of allergy diseases in children. Our findings are consistent with prior research studies. Specifically, Lu et al. (24) have emphasized the association between paternal smoking and azoospermia, while Knudsen et al. (25) have identified a correlation between paternal smoking initiation before pregnancy and increased adult body mass index in their progeny. Furthermore, Kim et al. (26) have provided evidence indicating that paternal smoking both before and during pregnancy significantly heightens the risk of autism spectrum disorders in offspring. Moreover, evidence indicates that paternal prenatal smoking can result in epigenetic alterations that heighten the susceptibility of their progeny to asthma (15). A comprehensive investigation encompassing 24,168 parents of children unveiled a positive correlation between paternal smoking prior to conception and an elevated likelihood of asthma in their offspring, whereas maternal smoking prior to conception does not yield a similar impact (27).
Prior studies have shown that starting to smoke before puberty may result in a decline in lung function in offspring, especially when compared to dads who never smoke (28). Similar studies have revealed that fathers who start smoking after the age of 15 are linked to a higher BMI in their offspring (25). Intriguingly, a dose-effect relationship was found in the effect of paternal smoking on the risk of offspring allergic rhinitis. We found that paternal smoking more than 5 cigarettes per day in the year prior to pregnancy and more than 11 years increases the probability of offspring developing allergic rhinitis by 1.009 and 1.479 times, respectively. These are consistent with some previous studies. A cohort study carried out in the Netherlands found that smoking by the father for one month prior to conception was associated with a higher risk of offspring being small for gestational age, and the relative risk of smoking more than 10 cigarettes per day was higher than that of smoking less than 10 cigarettes per day (29). Conversely, other investigations have indicated that paternal smoking of at least one cigarette per day within the month prior to conception does not exhibit any significant association with either gestational age or birth weight of the progeny (30). It is presently believed that exposure to tobacco smoke may result in an immunological imbalance in Th1/Th2 cells, thereby serving as the underlying mechanism by which passive smoking in children heightens the likelihood of developing allergic diseases. Tobacco smoke can detrimentally impact children's lung development and exert cytotoxic, pro-inflammatory, and anti-inflammatory effects on nasal epithelial cells. Furthermore, a wealth of evidence suggests that oxidative stress acts as a mediator in allergy rhinitis (31, 32). According to Loffredo's research, children who are exposed to passive smoking have more Nox2 activity than the control group, which can result in oxidative stress reactions (33).
Moreover, the mechanism underlying the prenatal effects of tobacco smoke can be predominantly elucidated by alterations in epigenetic mechanisms, including histone acetylation, microRNA expression, and DNA methylation, in conjunction with individual genetic susceptibility (34). Therefore, further study is necessary to explore the exact mechanism by which allergic diseases are induced by paternal cigarette smoke.
Similarly to smoking, the majority of studies primarily examine the impact of alcohol abuse during pregnancy on fetal development and the health of subsequent generations. Prenatal alcohol abuse has been identified as a notable contributor to the increasing incidence of sudden neonatal death (35). Maternal alcohol consumption at moderate to high levels during pregnancy can result in physical and neurocognitive dysplasia in infants (36), and depression in their offspring (37). It is interesting to report a significant increase in the likelihood of atopic eczema before the ages of 3 and 5 due to maternal alcohol consumption during pregnancy (38). Similar studies have also revealed an elevated incidence of early-onset atopic dermatitis in children born to moms who consume four or more cups of alcohol per week at 30 weeks of pregnancy (39).
Just like the correlation observed with paternal smoking, scholarly investigations have also substantiated the susceptibility of offspring to emotional, physical, and socioeconomic adversities when their fathers engage in alcohol misuse. Experimental studies conducted on animals have revealed that the persistent exposure of fathers to alcohol prior to conception can impede the development of the placenta in their offspring fetuses (40). Research conducted in human clinical studies has also demonstrated that offspring of fathers who consume alcohol at least once a week during the three months preceding pregnancy face an elevated susceptibility to various adverse outcomes, including anxiety and depression, physical ailments, sleep disturbances, cognitive impairments in girls, and aberrant behaviors in boys (13). Noteworthy, this study showed that the risk of anxiety, depression, and sleep problems in 4-year-old girls increased with the father's weekly cumulative drinking dose in three months before pregnancy (13). According to Zuccolo et al.'s study (41), the likelihood of microcephaly in offspring at birth increased with the amount of alcohol the father consumed prior to becoming pregnant. While some research indicates that children born to mothers who drank alcohol at least once a month before pregnancy had greater birth weights than children born to moms who drank alcohol less than once a month, fathers were not associated in a similar way (30). Still knowledge gaps exist in the associations between paternal alcohol abuse and allergic diseases. Our findings made up for this gap by revealing that drinking alcohol one year before pregnancy was an independent risk factor for allergic rhinitis in children aged 3–12 years old. Furthermore, our findings indicate that there is a shorter interval between alcohol consumption and a higher risk of allergic rhinitis in offspring, especially when the interval between alcohol intakes is less than one week, there is a 5.349-fold increase in the risk of allergic rhinitis in offspring. Additionally, a longer duration of alcohol consumption is associated with a greater risk of allergic rhinitis in offspring. Specifically, paternal alcohol consumption exceeding 11 years prior to conception can raise the risk of allergic rhinitis in children by 2.149 times.
The consumption of alcohol by pregnant women poses a risk to the developing fetus, as it exposes them to the harmful effects of alcohol and its byproducts. Additionally, there is evidence suggesting that alcohol consumption during pregnancy may lead to an increase in IgE levels in the umbilical cord blood (42). In certain animal models investigating the association between paternal alcohol consumption and offspring, it has been observed that chronic alcohol intake negatively impacts sperm quality compared to a control group. Furthermore, RNA sequencing analysis has revealed notable differences in gene expression related to the testis between the alcohol-exposed group and the control group. These findings suggest that long-term alcohol exposure may disrupt the balance of the gut microbiota (43). Recently, the rat model, in which the paternal exposure to a combination of nicotine, ethanol, and caffeine demonstrated that such mixed adverse exposure resulted in heightened activity of the hypothalamic pituitary adrenal axis, diminished sperm quality, unfavorable outcomes in pregnancy, abnormal indicators of fetal serum metabolism, and dysfunction in multiple organs in male rats. Notably, the reduced activity of the glucocorticoid-insulin-like growth factor 1 (GC-IGF1) axis may serve as the primary mechanism underlying the developmental and multi-organ dysfunction observed in offspring due to paternal mixed exposure (44).
In conclusion, our research findings demonstrate that paternal smoking and alcohol consumption prior to pregnancy are significant risk factors for the development of allergic rhinitis in children. The potential impact of paternal smoking and alcohol consumption on sperm function may have repercussions on embryonic development, potentially heightening the susceptibility of offspring to allergic rhinitis (Figure 5). As smoking and alcohol consumption are modifiable factors, our results highlight the importance of community hospitals and healthcare providers in raising awareness about men's pre-pregnancy health state, emphasizing the necessity for men to cease or reduce their alcohol and tobacco consumption. However, it is important to note that this study is limited in its retrospective nature and single-center clinic setting. Therefore, future investigations need to consider prospective, multicentric, and mechanistic studies to further explore this topic.
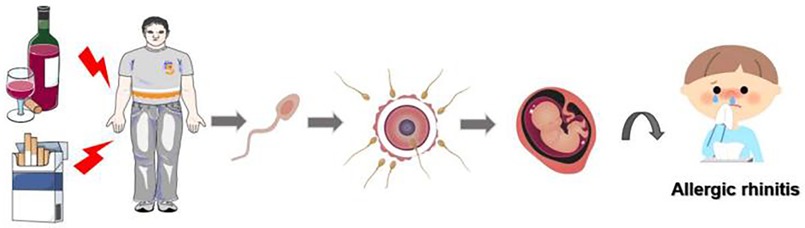
Figure 5. Schematic representation illustrating the potential impact of paternal smoking and alcohol exposure prior to conception on the likelihood of allergic rhinitis development in offspring.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Agreed by the Ethics Committee of Hunan Normal University (Ethics Number: 2022277). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JC: Data curation, Formal Analysis, Investigation, Writing – original draft. XL: Data curation, Formal Analysis, Writing – original draft. WS: Formal Analysis, Writing – review & editing. ZL: Formal Analysis, Writing – review & editing. GS: Formal Analysis, Writing – review & editing. YY: Formal Analysis, Writing – review & editing. MT: Formal Analysis, Writing – review & editing. JL: Supervision, Writing – review & editing. YD: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was partially supported by research grants from the National Natural Fund for Distinguished Young Scholars (Grant Number: 2022JJ10036) and Huxiang Young Talents project (Grant/Award Number: 2021RC3094).
Acknowledgments
All authors give final approval of the version to be published. Thanks to all authors for their contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Subspecialty Groups of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline for diagnosis and treatment of pediatric allergic rhinitis (2022, revision). Chin J Otorhinolaryngol Head Neck Surg. (2022) 57(04):392–404. doi: 10.3760/cma.j.cn115330-20220303-00092
2. Pols DH, Wartna JB, van Alphen EI, Moed H, Rasenberg N, Bindels PJ, et al. Interrelationships between atopic disorders in children: a meta-analysis based on ISAAC questionnaires. PLoS One. (2015) 10(7):e0131869. doi: 10.1371/journal.pone.0131869
3. Hu S, Wel P, Kou W, Wu X, Liu M, Chen C, et al. Prevalence and risk factors of allergic rhinitis: a meta-analysis. J Clin Otorhinolaryngol Head Neck Surg. (2017) 31(19):1485–91. doi: 10.13201/j.issn.1001-1781.2017.19.006
4. Pang K, Li G, Li M, Zhang L, Fu Q, Liu K, et al. Prevalence and risk factors for allergic rhinitis in China: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2022) 2022:7165627. doi: 10.1155/2022/7165627
5. Camelo-Nunes IC, Solé D. Allergic rhinitis: indicators of quality of life. J Bras Pneumol. (2010) 36(1):124–33. doi: 10.1590/s1806-37132010000100017
6. Barker DJ. The fetal and infant origins of adult disease. Br Med J. (1990) 301(6761):1111. doi: 10.1136/bmj.301.6761.1111
7. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. (2008) 359(1):61–73. doi: 10.1056/NEJMra0708473
8. Tong X, Tong H, Gao L, Deng Y, Xiang R, Cen R, et al. A multicenter study of prevalence and risk factors for allergic rhinitis in primary school children in 5 cities of Hubei province, China. Int Arch Allergy Immunol. (2022) 183(1):34–44. doi: 10.1159/000517948
9. Lu C, Wang F, Liu Z, Li B, Yang W, Liao H. Intrauterine and early postnatal exposure to air pollution associated with childhood allergic rhinitis. Chemosphere. (2023) 336:139296. doi: 10.1016/j.chemosphere.2023.139296
10. Rasmussen IM H, Bonefeld-Jørgensen EC, Long M. Greenlandic women's lifestyle and diet during pregnancy and child risk for asthma, eczema and allergy: an ACCEPT-substudy. Int J Circumpolar Health. (2019) 78(1):1682421. doi: 10.1080/22423982.2019.1682421
11. Henderson I, Quenby S. Gestational hypertension and childhood atopy: a millennium cohort study analysis. Eur J Pediatr. (2021) 180(8):2419–27. doi: 10.1007/s00431-021-04012-3
12. Amor H, Hammadeh ME, Mohd I, Jankowski PM. Impact of heavy alcohol consumption and cigarette smoking on sperm DNA integrity. Andrologia. (2022) 54(7):e14434. doi: 10.1111/and.14434
13. Luan M, Zhang X, Fang G, Liang H, Yang F, Song X, et al. Preconceptional paternal alcohol consumption and the risk of child behavioral problems: a prospective cohort study. Sci Rep. (2022) 12(1):1508. doi: 10.1038/s41598-022-05611-2
14. Zuccolo L, DeRoo LA, Wills AK, Davey Smith G, Suren P, Roth C, et al. Pre-conception and prenatal alcohol exposure from mothers and fathers drinking and head circumference: results from the Norwegian mother-child study (MoBa). Sci Rep. (2016) 7:39535. doi: 10.1038/srep39535
15. Wu CC, Hsu TY, Chang JC, Ou CY, Kuo HC, Liu CA, et al. Paternal tobacco smoke correlated to offspring asthma and prenatal epigenetic programming. Front Genet. (2019) 10:471. doi: 10.3389/fgene.2019.00471
16. Na J, Chen H, An H, Ren M, Jia X, Wang B, et al. Passive smoking and risk of gestational diabetes mellitus among nonsmoking women: a prospective cohort study in China. Int J Environ Res Public Health. (2022) 19(8):4712. doi: 10.3390/ijerph19084712
17. Wang R, Sun T, Yang Q, Yang Q, Wang J, Li H, et al. Low birthweight of children is positively associated with mother's prenatal tobacco smoke exposure in Shanghai: a cross-sectional study. BMC Pregnancy Childbirth. (2020) 20(1):603. doi: 10.1186/s12884-020-03307-x
18. Shiohama T, Hisada A, Yamamoto M, Sakurai K, Takatani R, Fujii K, et al. Decreased head circumference at birth associated with maternal tobacco smoke exposure during pregnancy on the Japanese prospective birth cohort study. Sci Rep. (2021) 11(1):18949. doi: 10.1038/s41598-021-98311-2
19. Zhang Y, Liu J, Zhang L, Jin L, Greene NDE, Li Z, et al. Passive smoking during the periconceptional period and risk for neural tube defects in offspring—five counties, Shanxi Province, China, 2010–2016. China CDC Wkly. (2021) 3(37):778–82. doi: 10.46234/ccdcw2021.193
20. Wada T, Adachi Y, Murakami S, Ito Y, Itazawa T, Tsuchida A, et al. Maternal exposure to smoking and infant’s wheeze and asthma: Japan environment and children’s study. Allergol Int. (2021) 70(4):445–51. doi: 10.1016/j.alit.2021.04.008
21. Toppila-Salmi S, Luukkainen AT, Xu B, Lampi J, Auvinen J, Dhaygude K, et al. Maternal smoking during pregnancy affects adult onset of asthma in offspring: a follow up from birth to age 46 years. Eur Respir J. (2020) 55(6):1901857. doi: 10.1183/13993003.01857-2019
22. Zhou Y, Chen J, Dong Y, Shen J, Tian M, Yang Y, et al. Maternal tobacco exposure during pregnancy and allergic rhinitis in offspring: a systematic review and meta-analysis. Medicine (Baltimore). (2021) 100(34):e26986. doi: 10.1097/md.0000000000026986
23. Ch W, Xiao D, Chi H. 2020 Report on health hazards of smoking in China: an updated summary. Chin Circul J. (2021) 36(10):937–52.
24. Lu YW, Li M, Wu W. Study on the relationship between smoking and the risk of nonobstructive azoospermia. J Med Postgrad. (2021) 34(12):1293–7. doi: 10.16571/j.cnki.1008-8199.2021.12.011
25. Knudsen GTM, Dharmage S, Janson C, Abramson MJ, Benediktsdóttir B, Malinovschi A, et al. Parents’ smoking onset before conception as related to body mass index and fat mass in adult offspring: findings from the RHINESSA generation study. PLoS One. (2020) 15(7):e0235632. doi: 10.1371/journal.pone.0235632
26. Kim B, Ha M, Kim YS, Koh YJ, Dong S, Kwon HJ, et al. Prenatal exposure to paternal smoking and likelihood for autism spectrum disorder. Autism. (2021) 25(7):1946–59. doi: 10.1177/13623613211007319
27. Svanes C, Koplin J, Skulstad SM, Johannessen A, Bertelsen RJ, Benediktsdottir B, et al. Father’s environment before conception and asthma risk in his children: a multi-generation analysis of the respiratory health in Northern Europe study. Int J Epidemiol. (2017) 46(1):235–45. doi: 10.1093/ije/dyw151
28. Accordini S, Calciano L, Johannessen A, Benediktsdóttir B, Bertelsen RJ, Bråbäck L, et al. Prenatal and prepubertal exposures to tobacco smoke in men may cause lower lung function in future offspring: a three-generation study using a causal modelling approach. Eur Respir J. (2021) 58(4):2002791. doi: 10.1183/13993003.02791-2020
29. Mutsaerts MA, Groen H, Buiter-Van der Meer A, Sijtsma A, Sauer PJ, Land JA, et al. Effects of paternal and maternal lifestyle factors on pregnancy complications and perinatal outcome. A population-based birth-cohort study: the GECKO drenthe cohort. Hum Reprod. (2014) 29(4):824–34. doi: 10.1093/humrep/deu006
30. Moss JL, Harris KM. Impact of maternal and paternal preconception health on birth outcomes using prospective couples’ data in add health. Arch Gynecol Obstet. (2015) 291(2):287–98. doi: 10.1007/s00404-014-3521-0
31. Koksal ZG, Uysal P, Erdogan O, Cevik O. The association between allergic rhinitis and airway dysfunction and nasal endothelial damage and oxidative stress. Rhinology. (2023) 61(3):272–82. doi: 10.4193/Rhin22.484
32. Danevska IA, Jakjovska T, Zendelovska D, Atanasovska E, Dzekova-Vidimliski P, Petrushevska M, et al. Comparison of oxidative stress levels in healthy children and children with allergic rhinitis. Pril (Makedon Akad Nauk Umet Odd Med Nauki). (2023) 44(1):17–26. doi: 10.2478/prilozi-2023-0003
33. Loffredo L, Zicari AM, Occasi F, Perri L, Carnevale R, Angelico F, et al. Role of NADPH oxidase-2 and oxidative stress in children exposed to passive smoking. Thorax. (2018) 73(10):986–8. doi: 10.1136/thoraxjnl-2017-211293
34. Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. (2018) 15(5):1033. doi: 10.3390/ijerph15051033
35. Berg V, Kuja-Halkola R, Khemiri L, Larsson H, Lichtenstein P, Latvala A. Parental alcohol and drug abuse and offspring mortality by age 10: a population-based register study. Eur J Public Health. (2022) 32(6):933–8. doi: 10.1093/eurpub/ckac142
36. Hasken JM, Adair LS, Martin SL, Thompson AL, Marais AS, de Vries MM, et al. The influence of maternal weight and alcohol exposure on infant physical characteristics and neurodevelopmental outcomes. Curr Res Toxicol. (2022) 3:100076. doi: 10.1016/j.crtox.2022.100076
37. Zhang X, Liu Y, Li J, Li B, Yang X, Sun Q, et al. Prenatal alcohol exposure and the risk of depression in offspring: a meta-analysis. Int J Clin Pract. (2022) 2022:5458611. doi: 10.1155/2022/5458611
38. Wada K, Konishi K, Tamura T, Shiraki M, Iwasa S, Nagata C. Alcohol intake during pregnancy and offspring’s atopic eczema risk. Alcoholism, clinical and experimental research. Alcohol Clin Exp Res. (2016) 40(5):1037–43. doi: 10.1111/acer.13048
39. Linneberg A, Petersen J, Grønbaek M, Benn CS. Alcohol during pregnancy and atopic dermatitis in the offspring. Clin Exp Allergy. (2004) 34(11):1678–83. doi: 10.1111/j.1365-2222.2004.02101.x
40. Thomas KN, Zimmel KN, Basel A, Roach AN, Mehta NA, Thomas KR, et al. Paternal alcohol exposures program intergenerational hormetic effects on offspring fetoplacental growth. Front Cell Dev Biol. (2022) 10:930375. doi: 10.3389/fcell.2022.930375
41. Zuccolo L, DeRoo LA, Wills AK, Smith GD, Suren P, Roth C, et al. Erratum: pre-conception and prenatal alcohol exposure from mothers and fathers drinking and head circumference: results from the Norwegian mother-child study (MoBa). Sci Rep. (2017) 7:45877. doi: 10.1038/srep45877
42. Bjerke T, Hedegaard M, Henriksen TB, Nielsen BW, Schiøtz PO. Several genetic and environmental factors influence cord blood IgE concentration. Pediatr Allergy Immunol. (1994) 5(2):88–94. doi: 10.1111/j.1399-3038.1994.tb00223.x
43. Li H, Li N, Lu Q, Yang J, Zhao J, Zhu Q, et al. Chronic alcohol-induced dysbiosis of the gut microbiota and gut metabolites impairs sperm quality in mice. Front Microbiol. (2022) 13:1042923. doi: 10.3389/fmicb.2022.1042923
Keywords: paternal smoking before pregnancy, paternal alcohol consumption before pregnancy, children allergic rhinitis, DOHaD, disease susceptibility
Citation: Chen J, Liu X, Su W, Liu Z, Sun G, Yang Y, Tian M, Li J and Dong Y (2024) Unveiling the hidden risk: paternal smoking and alcohol exposure prior to conception as independent factors for allergic rhinitis in children. Front. Pediatr. 12:1394400. doi: 10.3389/fped.2024.1394400
Received: 1 March 2024; Accepted: 2 May 2024;
Published: 30 May 2024.
Edited by:
Elena Bozzola, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Sara Manti, University of Messina, ItalyAnna Maria Zicari, Sapienza University of Rome, Italy
© 2024 Chen, Liu, Su, Liu, Sun, Yang, Tian, Li and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Li, bGlqaWFueXh5QGh1bm51LmVkdS5jbg==; Yunpeng Dong, MTEyMTE5NTg5QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Junrong Chen
Junrong Chen Xiaohua Liu
Xiaohua Liu Wenwen Su1,4
Wenwen Su1,4 Yide Yang
Yide Yang Jian Li
Jian Li