- 1Division of Pediatric Nephrology, Shaare Zedek Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel
- 2Department of Paediatric Nephrology, Great Ormond Street Hospital, London, United Kingdom
- 3Pediatric Nephrology Unit, Galilee Medical Center, Nahariya, Israel
- 4Azrieli Faculty of Medicine, Bar Ilan University, Safed, Israel
- 5Division of Pediatric Nephrology and Hypertension, Mayo Clinic, Rochester, MN, United States
- 6Division of Pediatric Nephrology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, United States
- 7Hôpital Femme Mère Enfant and Centre d’Investigation Clinique Inserm, Hospices Civils de Lyon, ERKnet, Bron, France
- 8Pediatric Nephrology Department, Hôpital Robert-Debré, APHP, Paris, France
- 9Biostatistics, Alnylam Pharmaceuticals, Cambridge, MA, United States
- 10Clinical Development, Alnylam Pharmaceuticals, Cambridge, MA, United States
- 11Pediatric Nephrology Institute, Rambam Health Care Campus, and Faculty of Medicine, Technion – Israel Institute of Technology, Haifa, Israel
Background: Primary hyperoxaluria type 1 (PH1) is a genetic disorder resulting in overproduction of hepatic oxalate, potentially leading to recurrent kidney stones, nephrocalcinosis, chronic kidney disease, and kidney failure. Lumasiran, the first RNA interference therapeutic approved for infants and young children, is a liver-directed treatment that reduces hepatic oxalate production. Lumasiran demonstrated sustained efficacy with an acceptable safety profile over 12 months in infants and young children (age <6 years) with PH1 in ILLUMINATE-B (clinicaltrials.gov: NCT03905694), an ongoing, Phase 3, multinational, open-label, single-arm study.
Methods: Here, we report interim efficacy and safety findings from ILLUMINATE-B following 30 months of lumasiran treatment. Eligible patients had an estimated glomerular filtration rate (eGFR) >45 ml/min/1.73 m2 if ≥12 months old or normal serum creatinine if <12 months old, and a urinary oxalate to creatinine ratio (UOx:Cr) greater than the upper limit of normal. All 18 patients enrolled in ILLUMINATE-B completed the 6-month primary analysis period, entered an extension period of up to 54 months, and continue to participate in the study.
Results: At Month 30, mean percent change from baseline in spot UOx:Cr was −76%, and mean percent change in plasma oxalate was −42%. eGFR remained stable through Month 30. In 14 patients (86%) with nephrocalcinosis at baseline, nephrocalcinosis grade improved at Month 24 in 12; no patient worsened. In the 4 patients without baseline nephrocalcinosis, nephrocalcinosis was absent at Month 24. Kidney stone event rates were ≤0.25 per person-year through Month 30. Mild, transient injection site reactions were the most common lumasiran-related adverse events (17% of patients).
Conclusion: In infants and young children with PH1, long-term lumasiran treatment resulted in sustained reductions in urinary and plasma oxalate that were sustained for 30 months, with an acceptable safety profile. Kidney function remained stable, low kidney stone event rates were observed through Month 30, and nephrocalcinosis grade improvements were observed through Month 24.
Clinical Trial Registration: https://clinicaltrials.gov, identifier NCT03905694.
1 Introduction
Primary hyperoxaluria type 1 (PH1; OMIM #259900) is an autosomal recessive disease resulting from excess production of hepatic oxalate, potentially leading to kidney stones, nephrocalcinosis, and eventually chronic kidney disease, kidney failure, and deposition of calcium oxalate crystals in body organs, including bone, heart, and eyes (systemic oxalosis) (1–5). The phenotype is variable, with high mortality associated with infantile oxalosis (4, 6–8). Symptoms of PH1, as well as hyperhydration treatment, are associated with a substantial burden, negatively impacting quality of life (9, 10). Prompt diagnosis and treatment are critical to reduce oxalate production and mitigate the impact of excess oxalate on the kidneys and other organs (8).
Historically, treatment for PH1 has consisted mainly of supportive measures to delay or minimize oxalosis, and reactive measures to address ongoing oxalosis and associated damage (2, 11). Patients not on dialysis may be treated with hyperhydration and crystallization inhibitors, and pyridoxine (vitamin B6) may be administered. However, pyridoxine may only be effective in pyridoxine-responsive patients (e.g., those with the c.508 G>A [p.Gly170Arg] mutation (4, 12). Hemodialysis to reduce oxalate levels in the blood becomes essential as kidney function deteriorates, but it is often insufficient to prevent manifestations of systemic oxalosis (1, 13, 14). Replacement of the defective native liver carries significant risk of morbidity and mortality (1, 15).
Lumasiran, an RNA interference (RNAi) therapeutic (ie, one involving targeted inhibition of gene expression) that is directed to the liver (16), has been approved in the European Union “for the treatment of PH1 in all age groups” (17) and in the United States “for the treatment of PH1 to lower urinary oxalate (UOx) and plasma oxalate (POx) in pediatric and adult patients” (18). Lumasiran consists of a double-stranded small interfering RNA that is covalently linked to triantennary N-acetylgalactosamine (GalNAc), allowing for targeted delivery to the liver (16, 19, 20). In PH1, glyoxylate levels are increased due to pathogenic variants in the AGXT gene and deficient activity of AGT, an enzyme that metabolizes glyoxylate to glycine (21). Lumasiran causes the mRNA-encoding glycolate oxidase (GO; OMIM #605023) to be degraded, hence reducing glyoxylate, a substrate for oxalate production (19).
The lumasiran clinical development program in PH1 comprises 5 clinical trials in which a total of 98 patients were enrolled, including people of different ages and degrees of PH1 severity (19). The Phase 3, single-arm ILLUMINATE-B study (NCT03905694) is being conducted to examine lumasiran's efficacy and safety in infants and young children (age <6 years) with PH1 and estimated glomerular filtration rate (eGFR) >45 ml/min/1.73 m2 (22, 23). During the 6-month primary analysis period, lumasiran demonstrated clinically important reduction relative to baseline in spot urinary oxalate to creatinine ratio (UOx:Cr) by 72% (22). The most common treatment-related adverse events (AEs) were transient, mild injection site reactions (22). After 6 more months of treatment, during a long-term extension period, the efficacy and safety of lumasiran were maintained (23).
Here, we report efficacy and safety findings from ILLUMINATE-B following 30 months of lumasiran treatment.
2 Materials and methods
2.1 Study design and patients
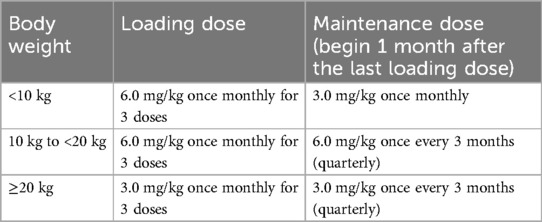
ILLUMINATE-B is an ongoing, Phase 3, multinational, open-label, single-arm study. A primary analysis was conducted at 6 months; patients are now in an extension period of up to 54 months. The study design and eligibility criteria have been described previously (22, 23). Briefly, eligible patients had a genetically confirmed diagnosis of PH1, were <6 years old at study entry, had an eGFR >45 ml/min/1.73 m2 if ≥12 months old or normal serum creatinine if <12 months old, and a UOx:Cr greater than the upper limit of normal (ULN) for age. Lumasiran was administered subcutaneously according to a dosing regimen based on body weight (Table 1). All patients received lumasiran as 3 loading doses, once monthly (at Day 1, at Month 1, and at Month 2) at a dose based on body weight category, then received lumasiran either once monthly (patients weighing <10 kg) or once every 3 months (patients weighing ≥10 kg) at the maintenance dose, beginning at Month 3.
2.2 Details of ethics approval
The study protocol and amendments and informed consent form were reviewed and approved by Independent Ethics Committees/Institutional Review Boards prior to commencement of the study. This study was conducted in accordance with Good Clinical Practice as defined by the International Council on Harmonisation, the principles defined in the Declaration of Helsinki and its amendments, and all applicable national and international laws. Legal guardians provided informed consent and patients provided assent per local regulations and institutional standards.
2.3 Endpoints
The primary endpoint was percent change in spot UOx:Cr from baseline to Month 6, as described previously (22). Spot urine samples were used as an alternative to 24-hour UOx levels due to the inability of young children to comply with 24-hour urine collections (24–26). Secondary endpoints assessed in the extension period included absolute and percent change from baseline in UOx excretion, proportion of patients with UOx excretion less than or equal to the ULN and ≤1.5 × ULN for age, absolute and percent change from baseline in POx, and change from baseline in eGFR (23). The ULN for spot UOx:Cr is age-dependent and was based on Matos et al. (1999) (27) to account for an age-related decline that occurs in infants and young children. Exploratory endpoints included changes in nephrocalcinosis grade, kidney stone event rates, and plasma glycolate (23).
2.4 Assessments
Spot UOx and plasma glycolate were measured with validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays. Spot UOx was expressed relative to creatinine (spot UOx:Cr). POx was measured with a novel, validated LC-MS/MS assay (28). eGFR was calculated for patients ≥12 months old using the Schwartz Bedside formula (29); eGFR was not calculated for patients <12 months old as the Schwartz Bedside formula is not validated for that age group (29). Drug antibodies against lumasiran were evaluated in plasma using a validated enzyme-linked immunoassay.
Kidney stone events were adjudicated by the investigator. A kidney stone event was defined as an event that included ≥1 of the following: visit to healthcare provider because of a kidney stone, medication for renal colic, stone passage, or macroscopic hematuria due to a kidney stone.
Renal ultrasounds were performed at baseline and Months 6, 12, and 24 (but not Month 30) and read by a central radiologist. Changes from baseline in nephrocalcinosis grade were categorized as follows, accounting for both kidneys: no change (stable), improving (which was further categorized into improving and improving to complete resolution), worsening, and indeterminate (defined as 1 kidney improving and 1 worsening).
2.5 Statistical analysis
This analysis was conducted using data as of a cutoff date of April 29, 2022, after all active study patients had completed their Month 30 visit.
All efficacy analyses were conducted in the efficacy analysis set, defined as all patients who received any amount of lumasiran and had ≥1 valid spot UOx:Cr value at baseline and ≥1 valid spot UOx:Cr value from assessments at Month 3 to Month 6. Percent and absolute change in POx from baseline were additionally analyzed in the POx analysis set, which included only patients in the efficacy analysis set whose baseline POx was ≥1.5 times the lower limit of quantitation (LLOQ; 5.55 µmol/L). The kidney stone event rate was calculated as the total number of kidney stone events divided by the total patient exposure time (events per person-year). The 95% CI for the kidney stone event rate was obtained using a generalized linear model for a Poisson distribution unless the rate was 0, in which case the upper bound of the 95% CI was calculated using the exact Poisson method.
A pyridoxine-responsive (PR) genotype was defined as NM_000030.3(AGXT):c.508G>A (p.Gly170Arg) or NM_000030.3(AGXT):c.454T>A (p.Phe152Ile), where N denotes nonsense and M denotes missense (30).
Cumulative safety data from the first dose of lumasiran through the data cutoff date are reported. Safety analyses were conducted in the safety analysis set, defined as all patients who received any amount of lumasiran. Duration of exposure to study drug was calculated using calendar months [duration of treatment (days)/30.44], whereas for study visits, 1 month was defined as 4 weeks (28 days).
All statistical analyses were performed using validated SAS statistical software, version 9.4.
3 Results
3.1 Patients
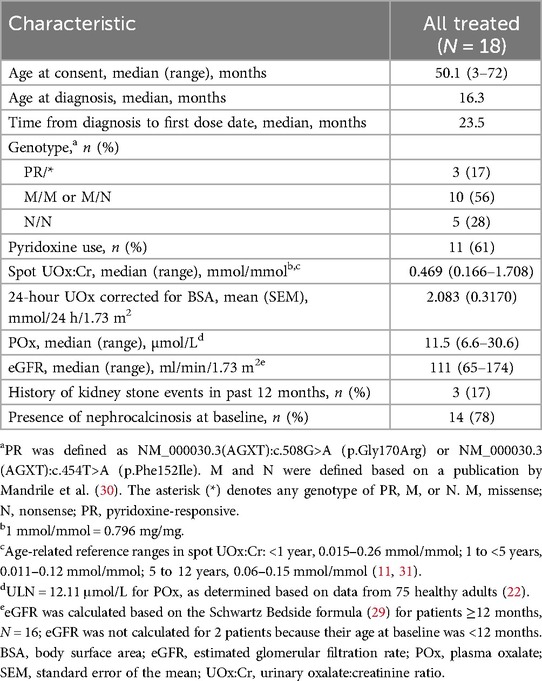
All 18 patients who enrolled in the study entered the extension and continue to participate. Baseline demographic and clinical characteristics are shown in Table 2.
3.2 Efficacy
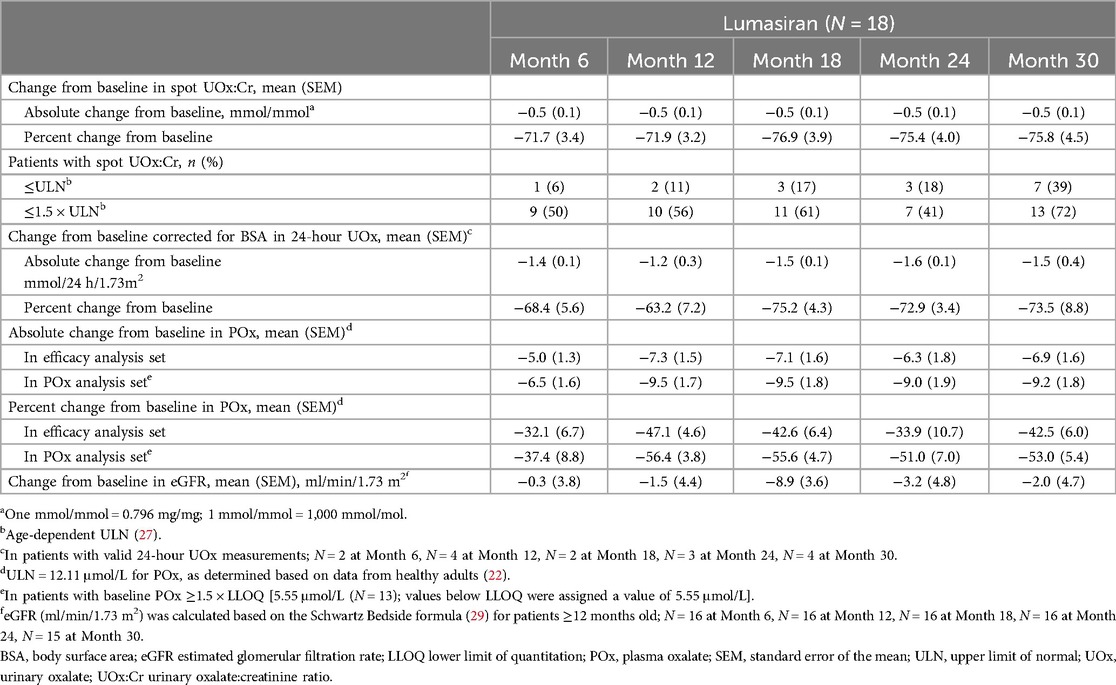
Mean spot UOx:Cr decreased from 0.63 mmol/mmol at baseline to 0.11 mmol/mmol at Month 30; mean [standard error of the mean (SEM)] percent change from baseline was −75.8% (4.5%) (Figure 1; Table 3). Thirteen of 18 patients (72%) had spot UOx:Cr values ≤1.5 × ULN at Month 30, and 7 (39%) had spot UOx:Cr values ≤ ULN (Table 3). The percent change from baseline in 24-hour UOx was similar in the 4 patients who were able to provide samples (Table 3).
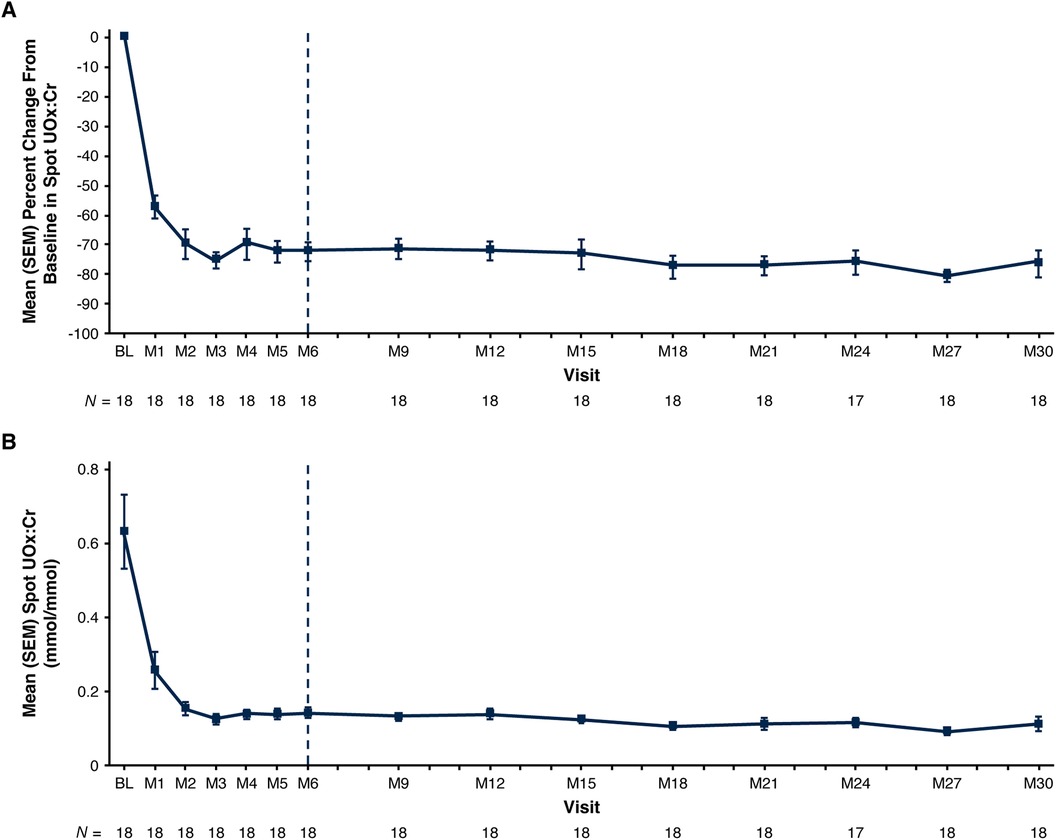
Figure 1. Mean (SEM) spot UOx:Cr. (A) Percent change from baseline at each visit and (B) actual values at each visit.a Baseline value represents the mean of all assessments collected prior to the first dose of lumasiran; 1 mmol/mmol = 0.796 mg/mg; 1 mmol/mmol = 1,000 mmol/mol. End of the primary analysis period is represented by the vertical dashed line. aThe ULN for spot UOx:Cr is age-dependent (27). Age-related reference ranges in spot UOx:Cr: < 1 year, 0.015–0.26 mmol/mmol; 1 to <5 years, 0.011–0.12 mmol/mmol; 5 to 12 years, 0.06–0.15 mmol/mmol (11, 31). BL, baseline; M, month; SEM, standard error of the mean; ULN, upper limit of normal; UOx:Cr, urinary oxalate:creatinine ratio.
Mean POx decreased from 13.2 µmol/L at baseline to 6.3 µmol/L at Month 30 (ULN: 12.11 µmol/L); mean (SEM) percent change from baseline was −42.5% (6.0%) (Figure 2; Table 3). In patients with baseline POx ≥1.5 × LLOQ (N = 13), mean (SEM) POx decreased from 15.6 µmol/L at baseline to 6.4 µmol/L at Month 30; mean percent change from baseline was −53.0% (5.4%) (Table 3).
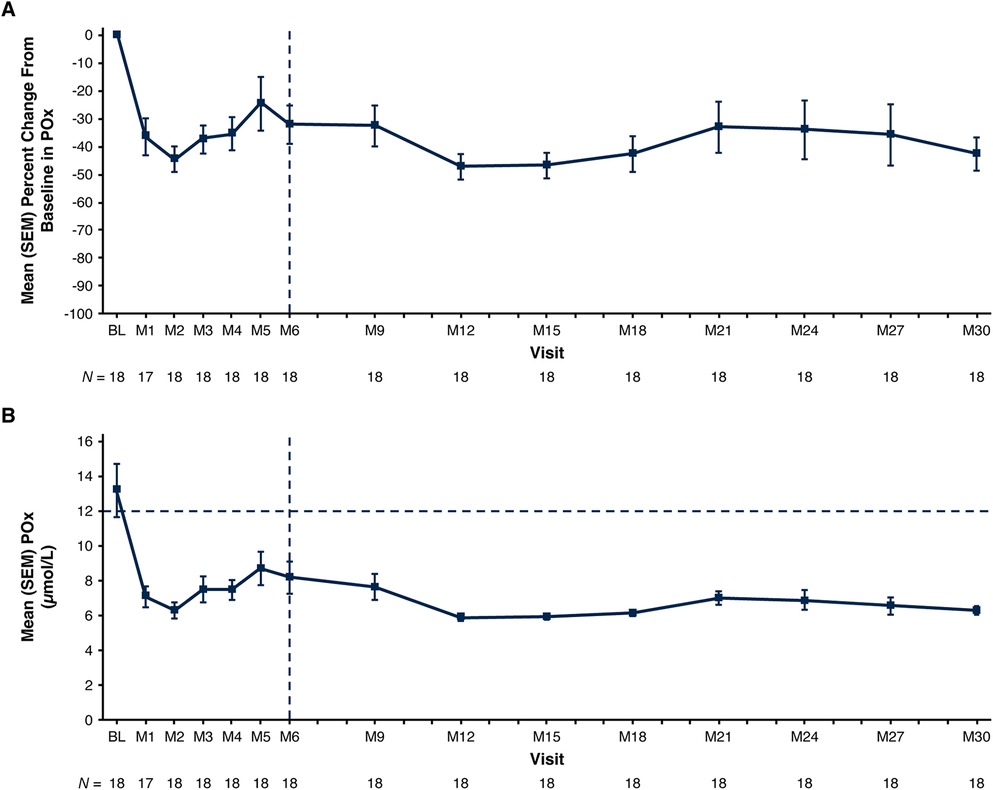
Figure 2. Mean (SEM) POx. (A) Percent change from baseline at each visit and (B) actual values at each visit. Baseline value represents the mean of all assessments collected prior to the first dose of lumasiran. The end of the primary analysis period is represented by the vertical dashed line. The ULN for POx, represented by the horizontal dashed line in panel B, is 12.11 μmol/L (determined based on data from 75 healthy adults) (22). The LLOQ is 5.55 µmol/L. Reductions in POx below the LLOQ were conservatively imputed as 5.55 µmol/L. BL, baseline; LLOQ, lower limit of quantitation; M, month; POx, plasma oxalate; SEM, standard error of the mean; ULN, upper limit of normal.
eGFR remained stable with a mean (SEM) of 112.8 (6.9) ml/min/1.73 m2 at baseline and 112.5 (6.7) ml/min/1.73 m2 at Month 30 (Figure 3; Table 3). Nephrocalcinosis was present at baseline in 14 of 18 patients. Among the 14 patients with nephrocalcinosis at baseline, nephrocalcinosis grade improved at Month 24 in 12 (86%), was indeterminate in 1 (7%), and remained stable in 1 (7%) (Figure 4). Two of the 14 patients improved to complete resolution (Figure 4). The 4 patients who had no nephrocalcinosis at baseline remained stable, with no nephrocalcinosis at Month 24. Kidney stone event rates were ≤0.25 per person-year through Month 30 (Figure 5).
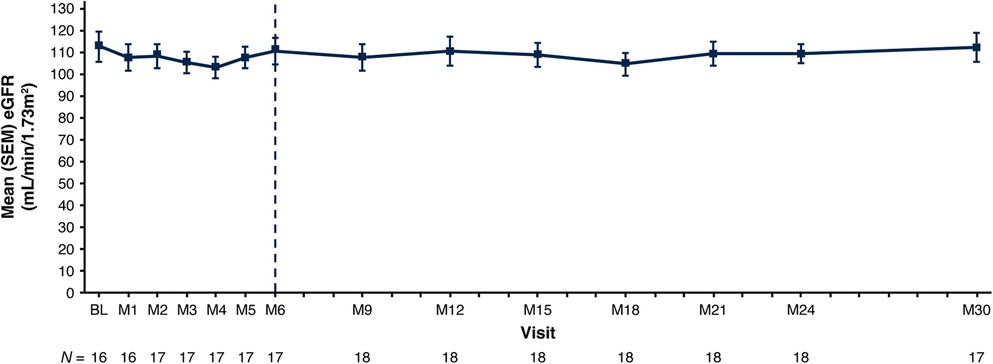
Figure 3. Mean (SEM) eGFR. Baseline is the last non-missing value collected prior to the first dose of lumasiran. The end of the primary analysis period is represented by the vertical dashed line. eGFR is calculated based on the Schwartz Bedside formula (29) in patients ≥12 months of age at the time of the assessment. BL, baseline; eGFR, estimated glomerular filtration rate; M, month; SEM, standard error of mean.
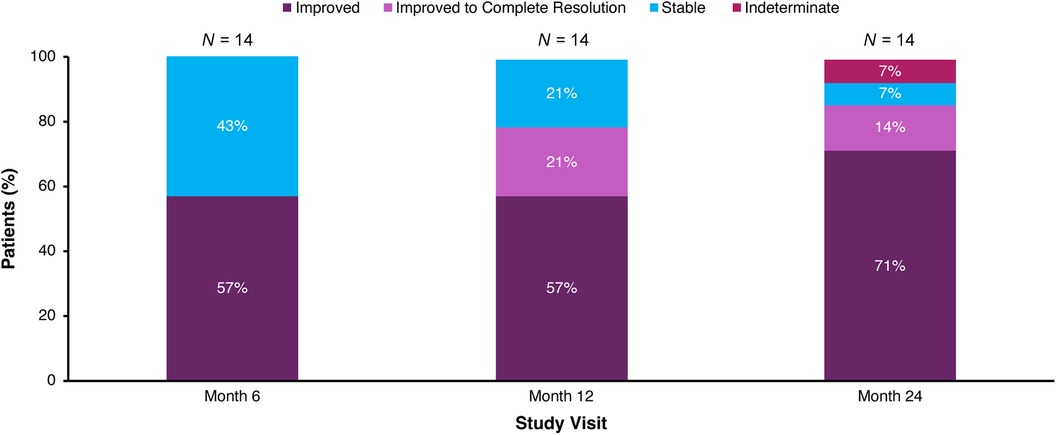
Figure 4. Change in medullary nephrocalcinosis grade in patients with nephrocalcinosis at baseline. Patients who had no nephrocalcinosis at baseline (N = 4) remained stable, with no nephrocalcinosis at Month 24; these patients are not depicted. Stable indicates grade same as baseline; improved indicates grade lower than baseline; and indeterminate indicates one side improved and the other side worsened. Renal ultrasound was not performed at Month 30.
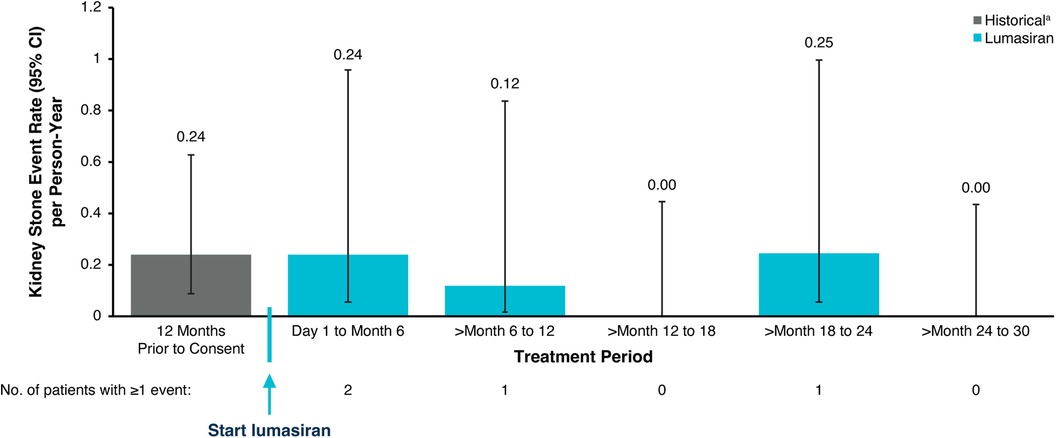
Figure 5. Kidney stone event rates. aHistorical patient-reported history of kidney stone events. An annualized rate was not calculated for patients <6 months old. CI, confidence interval.
Plasma glycolate initially increased, then plateaued, during the 6-month primary analysis period; thereafter, plasma glycolate declined slightly but remained elevated, as expected based on the mechanism of action of lumasiran (Figure 6).

Figure 6. Mean (SEM) plasma glycolate. (A) Percent change from baseline at each visit and (B) actual values at each visit. Baseline value represents the mean of all assessments collected prior to the first dose of lumasiran. The end of the primary analysis period is represented by the vertical dashed line. BL, baseline; M, month; SEM, standard error of the mean.
Decisions regarding adjustments to hyperhydration and/or vitamin B6 regimens after Month 6 were left to the discretion of study investigators. Three of 13 patients on hyperhydration at baseline decreased it during the extension period; no patients started hyperhydration during the study. After Month 6, 5 of 11 patients taking vitamin B6 at baseline stopped vitamin B6, 2 reduced their dose without stopping, and no patients started vitamin B6. There was no meaningful change in UOx:Cr ratios in patients who decreased or stopped hyperhydration or vitamin B6.
3.3 Safety
As of the cutoff date (April 29, 2022), median (range) exposure to lumasiran was 32.6 (27.5‒35.3) months. Five (28%) patients had AEs deemed by the investigator to be related to lumasiran (Table 4). The most common lumasiran-related AEs were mild, transient injection site reactions [3 patients (17%)]; symptoms included erythema, discoloration, and pain at the injection site. One patient had a serious AE of viral infection (moderate in severity and considered unrelated to lumasiran), as reported previously (23).
There were no clinically relevant changes related to lumasiran in laboratory measures, vital signs, or electrocardiograms. One patient had an AE of blood bicarbonate decreased that was deemed by the investigator to be unrelated to lumasiran. At baseline, the patient had an eGFR of 134 ml/min/1.73 m2 and a bicarbonate value of 19 mmol/L, and was on a stable dose of oral sodium bicarbonate for PH1. The AE of blood bicarbonate decreased was entered due to a bicarbonate of 18 mmol/L at Month 12; the dose of oral sodium bicarbonate was not changed. The AE was considered resolved after the bicarbonate value at Month 15 was 21 mmol/L. Plasma glycolate remained stably elevated. The patient received no treatment for the AE and remained on lumasiran.
Transient, low-titer (1:50) anti-drug antibodies were observed in 3 (17%) patients, with no observed impact on safety or efficacy. None of the patients tested positive for anti-drug antibodies at baseline.
4 Discussion
Lumasiran, the first approved treatment for PH1 (17, 18) and the first RNAi therapeutic to be studied and approved in infants and young children (22), is a disease-modifying therapy that addresses the source of hepatic oxalate overproduction in PH1 by substrate reduction leading to decreased hepatic oxalate synthesis (19). Long-term treatment with lumasiran was associated with sustained lowering of UOx excretion, stable renal function (eGFR), and improvements in medullary nephrocalcinosis in patients with PH1 who were <6 years of age and had an eGFR >45 ml/min/1.73 m2 at baseline. Kidney stone event rates remained low. Plasma glycolate levels remained elevated, consistent with reduced hepatic glycolate oxidase activity mediated by lumasiran; there are no known adverse consequences of elevated glycolate concentrations in blood (22, 23, 32).
Hyperhydration, or large daily fluid intake (proportionate to body size in children), may attenuate the effects of hyperoxaluria (9). However, hyperhydration requires a gastrostomy tube or nasogastric tube in some young children and negatively impacts quality of life; hence, adherence may be poor (9, 10, 33). In this study, hyperhydration status was recorded for all patients; 3 patients on hyperhydration decreased hyperhydration during the extension period, and none started it. Reducing the need for hyperhydration is likely to increase quality of life in patients with PH1 (10).
Vitamin B6 is recommended for patients with PH1 who have a vitamin B6–responsive genotype; it has been associated with a mean decrease in UOx of approximately 26% (34). In this study, 5 of 11 patients taking vitamin B6 at baseline stopped vitamin B6, 2 reduced their dose, and none started vitamin B6. Of the 5 patients who stopped taking vitamin B6, 3 had a pyridoxine-responsive genotype and 2 did not. This apparent reduction in the need for vitamin B6 with maintenance of UOx:Cr suppression strengthens the evidence for the efficacy of lumasiran.
Lumasiran demonstrated an acceptable safety profile; injection site reactions were the most commonly reported AE. There was only one serious AE (a viral infection) reported as of the Month 30 data cutoff, and it was not considered related to lumasiran. These findings suggest that RNAi therapy is safe for use in infants and small children. This is corroborated by recent case reports of lumasiran use in infants (35, 36) and young children (37).
Transient, low-titer (1:50) anti-drug antibodies were observed in 3 patients during the study. Similar findings of low-titer anti-drug antibodies in a minority of patients have been reported in other clinical studies of lumasiran, and, when assessed, no effect of anti-drug antibodies on lumasiran pharmacokinetics has been noted (22, 38–40). There was no observed impact of the anti-drug antibodies on efficacy or safety in this or other studies (22, 38, 40).
5 Conclusions
In infants and young children with PH1, lumasiran treatment resulted in reductions in UOx and POx that were maintained through Month 30. The safety profile of lumasiran was acceptable. Clinical assessments of kidney health were encouraging, including stable kidney function through Month 30 and improvement in nephrocalcinosis through Month 24. Kidney stone event rates were low through Month 30. The most common lumasiran-related AEs were mild, transient injection site reactions.
Data availability statement
Access to anonymized individual participant data that support these results is made available 12 months after study completion and not less than 12 months after the product and indication have been approved in the US and/or the EU. Requests for access to data can be submitted via the website http://www.vivli.org.
Ethics statement
The studies involving humans were approved by Independent Ethics Committees/Institutional Review Boards prior to commencement of the study. This study was conducted in accordance with Good Clinical Practice as defined by the International Council on Harmonisation, the principles defined in the Declaration of Helsinki and its amendments, and all applicable national and international laws. Legal guardians provided informed consent and patients provided assent per local regulations and institutional standards. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YF: Conceptualization, Investigation, Methodology, Writing – review & editing. WH: Investigation, Writing – review & editing. HS-L: Investigation, Writing – review & editing. DS: Investigation, Writing – review & editing. MM: Investigation, Writing – review & editing. A-LS-L: Investigation, Writing – review & editing. JH: Investigation, Writing – review & editing. RW: Conceptualization, Data curation, Methodology, Writing – review & editing, Formal Analysis. JG: Conceptualization, Methodology, Writing – review & editing. DM: Conceptualization, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Alnylam Pharmaceuticals.
Acknowledgments
Thank you to the patients, their families, investigators, study staff, and collaborators for their participation in the lumasiran clinical studies. Medical writing and editorial assistance was provided by Karyn Liu, PhD, Michael Morren, RPh, MBA, and Jennifer Van Winckel of Peloton Advantage, LLC, an OPEN Health company, in accordance with Good Publication Practice (GPP 2022) guidelines and funded by Alnylam Pharmaceuticals.
Conflict of interest
YF: consultancy fees from Alnylam Pharmaceuticals and membership in the safety review committee. WH: principal investigator for Alnylam Pharmaceuticals; travel and accommodation expenses from Alnylam Pharmaceuticals to attend an international investigators’ meeting. HS-L: principal investigator for Alnylam Pharmaceuticals; travel and accommodation expenses from Alnylam Pharmaceuticals to attend international investigators’ meetings. DJS: grants and other from Alnylam Pharmaceuticals and Dicerna Pharmaceuticals, and personal fees from Advicenne. MM: principal investigator for Alnylam Pharmaceuticals; served on advisory board for Novo Nordisk, Inc. ALS-L: consultancy fees from Alnylam Pharmaceuticals and Dicerna Pharmaceuticals, and principal investigator for research funded by OxThera. JH: consultancy fees from Alnylam Pharmaceuticals. RW and JMG: employees of and shareholders in Alnylam Pharmaceuticals; contributed to study design, data analysis, and (in partnership with all other authors) review and revision of the manuscript in accordance with the ethical principles of Good Publication Practice (GPP 2022) guidelines. DM: research funding, consultancy fees, and non-financial support from Alnylam Pharmaceuticals.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AEs, adverse events; BSA, body surface area; eGFR, estimated glomerular filtration rate; GalNAc, triantennary N-acetylgalactosamine; GO, glycolate oxidase; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LLOQ, lower limit of quantitation; PH1, primary hyperoxaluria type 1; POx, plasma oxalate; RNAi, RNA interference; SEM, standard error of the mean; ULN, upper limit of normal; UOx, urinary oxalate; UOx:Cr, urinary oxalate:creatinine ratio.
References
1. Cochat P, Groothoff J. Primary hyperoxaluria type 1: practical and ethical issues. Pediatr Nephrol. (2013) 28:2273–81. doi: 10.1007/s00467-013-2444-5
2. Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, et al. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant. (2012) 27:1729–36. doi: 10.1093/ndt/gfs078
3. El Hage S, Ghanem I, Baradhi A, Mourani C, Mallat S, Dagher F, et al. Skeletal features of primary hyperoxaluria type 1, revisited. J Child Orthop. (2008) 2:205–10. doi: 10.1007/s11832-008-0082-4
4. Groothoff JW, Metry E, Deesker L, Garrelfs S, Acquaviva C, Almardini R, et al. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol. (2023) 19:194–211. doi: 10.1038/s41581-022-00661-1
5. Mookadam F, Smith T, Jiamsripong P, Moustafa SE, Monico CG, Lieske JC, et al. Cardiac abnormalities in primary hyperoxaluria. Circ J. (2010) 74:2403–9. doi: 10.1253/circj.CJ-10-0107
6. Deesker LJ, Garrelfs SF, Mandrile G, Oosterveld MJS, Cochat P, Deschênes G, et al. Improved outcome of infantile oxalosis over time in Europe: data from the OxalEurope registry. Kidney Int Rep. (2022) 7:1608–18. doi: 10.1016/j.ekir.2022.04.012
7. Soliman NA, Nabhan MM, Abdelrahman SM, Abdelaziz H, Helmy R, Ghanim K, et al. Clinical spectrum of primary hyperoxaluria type 1: experience of a tertiary center. Nephrol Ther. (2017) 13:176–82. doi: 10.1016/j.nephro.2016.08.002
8. Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, et al. Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. (2010) 77:443–9. doi: 10.1038/ki.2009.435
9. Milliner DS, McGregor TL, Thompson A, Dehmel B, Knight J, Rosskamp R, et al. End points for clinical trials in primary hyperoxaluria. Clin J Am Soc Nephrol. (2020) 15:1056–65. doi: 10.2215/CJN.13821119
10. Biebuyck N, Destombes C, Prakash R, Boyer O. Is withdrawal of nocturnal hyperhydration possible in children with primary hyperoxaluria treated with RNAi? J Nephrol. (2023) 36:1473–6. doi: 10.1007/s40620-023-01611-1
11. Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. (2013) 369:649–58. doi: 10.1056/NEJMra1301564
12. Milliner DS, Harris PC, Cogal AG, Lieske JC. Primary Hyperoxaluria Type 1. Seattle, WA: University of Washington, Seattle (1993). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK1283/(update November 30, 2017).
13. Sas DJ, Enders FT, Gunderson TM, Mehta RA, Olson JB, Seide BM, et al. Natural history of clinical, laboratory, and echocardiographic parameters of a primary hyperoxaluria cohort on long term hemodialysis. Front Med (Lausanne). (2021) 8:592357. doi: 10.3389/fmed.2021.592357
14. Ben-Shalom E, Cytter-Kuint R, Rinat C, Becker-Cohen R, Tzvi-Behr S, Goichberg J, et al. Long-term complications of systemic oxalosis in children-a retrospective single-center cohort study. Pediatr Nephrol. (2021) 36:3123–32. doi: 10.1007/s00467-021-05002-1
15. Gupta A, Somers MJG, Baum MA. Treatment of primary hyperoxaluria type 1. Clin Kidney J. (2022) 15:i9–i13. doi: 10.1093/ckj/sfab232
16. Forbes TA, Brown BD, Lai C. Therapeutic RNA interference: a novel approach to the treatment of primary hyperoxaluria. Br J Clin Pharmacol. (2022) 88:2525–38. doi: 10.1111/bcp.14925
17. Oxlumo [Summary of Product Characteristics]. Amsterdam, Netherlands: Alnylam Netherlands (2022).
19. Hulton S-A. Lumasiran: expanding the treatment options for patients with primary hyperoxaluria type 1. Expert Opin Orphan Drugs. (2021) 9:189–98. doi: 10.1080/21678707.2021.2003779
20. Springer AD, Dowdy SF. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. (2018) 28:109–18. doi: 10.1089/nat.2018.0736
21. Garrelfs SF, van Harskamp D, Peters-Sengers H, van den Akker CHP, Wanders RJA, Wijburg FA, et al. Endogenous oxalate production in primary hyperoxaluria type 1 patients. J Am Soc Nephrol. (2021) 32:3175–86. doi: 10.1681/ASN.2021060729
22. Sas DJ, Magen D, Hayes W, Shasha-Lavsky H, Michael M, Schulte I, et al. Phase 3 trial of lumasiran for primary hyperoxaluria type 1: a new RNAi therapeutic in infants and young children. Genet Med. (2022) 24:654–62. doi: 10.1016/j.gim.2021.10.024
23. Hayes W, Sas DJ, Magen D, Shasha-Lavsky H, Michael M, Sellier-Leclerc AL, et al. Efficacy and safety of lumasiran for infants and young children with primary hyperoxaluria type 1: 12-month analysis of the phase 3 ILLUMINATE-B trial. Pediatr Nephrol. (2023) 38:1075–86. doi: 10.1007/s00467-022-05684-1
24. Hong YH, Dublin N, Razack AH, Mohd MA, Husain R. Twenty-four hour and spot urine metabolic evaluations: correlations versus agreements. Urology. (2010) 75:1294–8. doi: 10.1016/j.urology.2009.08.061
25. Clifford-Mobley O, Tims C, Rumsby G. The comparability of oxalate excretion and oxalate:creatinine ratio in the investigation of primary hyperoxaluria: review of data from a referral centre. Ann Clin Biochem. (2015) 52:113–21. doi: 10.1177/0004563214529937
26. Reusz GS, Dobos M, Byrd D, Sallay P, Miltényi M, Tulassay T. Urinary calcium and oxalate excretion in children. Pediatr Nephrol. (1995) 9:39–44. doi: 10.1007/BF00858966
27. Matos V, Van Melle G, Werner D, Bardy D, Guignard JP. Urinary oxalate and urate to creatinine ratios in a healthy pediatric population. Am J Kidney Dis. (1999) 34:e1. doi: 10.1016/S0272-6386(99)70380-X
28. Clausen VA, Cao KH, Gansner JM, Robbie GJ, Wu JT. Quantification of oxalate by novel LC-MS/MS: assay development, validation, and application in lumasiran clinical trials. Bioanalysis. (2023) 15:481–91. doi: 10.4155/bio-2022-0227
29. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. (2009) 20:629–37. doi: 10.1681/ASN.2008030287
30. Mandrile G, van Woerden CS, Berchialla P, Beck BB, Acquaviva Bourdain C, Hulton SA, et al. Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int. (2014) 86:1197–204. doi: 10.1038/ki.2014.222
31. Barratt TM, Kasidas GP, Murdoch I, Rose GA. Urinary oxalate and glycolate excretion and plasma oxalate concentration. Arch Dis Child. (1991) 66:501–3. doi: 10.1136/adc.66.4.501
32. Frishberg Y, Zeharia A, Lyakhovetsky R, Bargal R, Belostotsky R. Mutations in HAO1 encoding glycolate oxidase cause isolated glycolic aciduria. J Med Genet. (2014) 51:526–9. doi: 10.1136/jmedgenet-2014-102529
33. Lawrence JE, Wattenberg DJ. Primary hyperoxaluria: the patient and caregiver perspective. Clin J Am Soc Nephrol. (2020) 15:909–11. doi: 10.2215/CJN.13831119
34. Hoyer-Kuhn H, Kohbrok S, Volland R, Franklin J, Hero B, Beck BB, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol. (2014) 9:468–77. doi: 10.2215/CJN.06820613
35. Méaux MN, Sellier-Leclerc AL, Acquaviva-Bourdain C, Harambat J, Allard L, Bacchetta J. The effect of lumasiran therapy for primary hyperoxaluria type 1 in small infants. Pediatr Nephrol. (2022) 37:907–11. doi: 10.1007/s00467-021-05393-1
36. Aldabek K, Grossman OK, Al-Omar O, Fox JA, Moritz ML. Infantile primary hyperoxaluria type 1 treated with lumasiran in twin males. Cureus. (2022) 14:e21673. doi: 10.7759/cureus.21673
37. Stone HK, VandenHeuvel K, Bondoc A, Flores FX, Hooper DK, Varnell CD Jr. Primary hyperoxaluria diagnosed after kidney transplant: a review of the literature and case report of aggressive renal replacement therapy and lumasiran to prevent allograft loss. Am J Transplant. (2021) 21:4061–7. doi: 10.1111/ajt.16762
38. Frishberg Y, Deschênes G, Groothoff JW, Hulton S, Magen D, Harambat J, et al. Phase 1/2 study of lumasiran for treatment of primary hyperoxaluria type 1: a placebo-controlled randomized clinical trial. Clin J Am Soc Nephrol. (2021) 16:1025–36. doi: 10.2215/CJN.14730920
39. Michael M, Hayes W, Sas DJ, Magen D, Lavsky H, Sellier-Leclerc A, et al. Efficacy and safety of lumasiran for infants and young children with primary hyperoxaluria type 1: 12-month analysis of the phase 3 ILLUMINATE-B trial [abstract]. Annual Meeting of the Pediatric Academic Societies; 22-28 April 2022; Denver, CO, USA
Keywords: kidney, liver, lumasiran, oxalate, pediatric, rare diseases, RNA interference, primary hyperoxaluria type 1
Citation: Frishberg Y, Hayes W, Shasha-Lavsky H, Sas DJ, Michael M, Sellier-Leclerc A-L, Hogan J, Willey R, Gansner JM and Magen D (2024) Efficacy and safety of lumasiran for infants and young children with primary hyperoxaluria type 1: 30-month analysis of the phase 3 ILLUMINATE-B trial. Front. Pediatr. 12:1392644. doi: 10.3389/fped.2024.1392644
Received: 27 February 2024; Accepted: 26 August 2024;
Published: 16 September 2024.
Edited by:
Michael L. Moritz, University of Pittsburgh, United StatesReviewed by:
Daniel Turudic, University Hospital Center Zagreb, CroatiaMichiel J. S. Oosterveld, Amsterdam University Medical Center, Netherlands
Copyright: © 2024 Frishberg, Hayes, Shasha-Lavsky, Sas, Michael, Sellier-Leclerc, Hogan, Willey, Gansner and Magen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaacov Frishberg, eWFhY292ZkBla21kLmh1amkuYWMuaWw=
 Yaacov Frishberg
Yaacov Frishberg Wesley Hayes
Wesley Hayes Hadas Shasha-Lavsky
Hadas Shasha-Lavsky David J. Sas
David J. Sas Mini Michael6
Mini Michael6 Julien Hogan
Julien Hogan Richard Willey
Richard Willey Daniella Magen
Daniella Magen