- 1Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
- 2Children’s Minnesota, Neonatal Medicine, Minneapolis, MN, United States
- 3Stead Family Department of Pediatrics, University of Iowa, Iowa City, IA, United States
Background: Data on clinical outcomes of infants with birthweights less than 501 g (ELBW<501) are limited.
Objective: To evaluate management strategies and clinical outcomes of ELBW<501infants compared to infants weighing 501–750 g (ELBW501–750).
Methods: A retrospective study of all ELBW<501 and ELBW501–750 infants born between 2012 and 2022 at a center utilizing first intention high frequency jet ventilation was performed. Patient characteristics, clinical and outcome data were compared between the two groups.
Results: A total of 358 infants (92 ELBW<501 infants and 266 ELBW501–750) were included. The survival rate for the ELBW<501 group was 60.9% compared to 86.5% for ELBW501–750. ELBW<501 infants required more frequent use of 2.0 mm endotracheal tubes, required higher FiO2 and longer duration of mechanical ventilation. Compared to ELBW501–750 group, the ELBW<501 group were more likely to be SGA (68.2% vs. 16.5%) and more premature (23.2 vs. 24.3 weeks) with lower survival, longer length of stay, higher incidence of ROP and lower weight at discharge but comparable rates of IVH, grade 3 BPD, discharged on supplemental oxygen, and tracheostomy.
Conclusion: ELBW<501 infants are at risk for significant morbidity and mortality. However, with specialized obstetric and neonatal care, survival rates of 60% are possible with respiratory outcomes comparable to ELBW501–750 infants. However, the increased risk of severe ROP for ELBW<501 requiring either surgical or medical intervention is concerning and warrants optimal surveillance.
Introduction
The rates of survival for infants born at lower gestational ages and birthweights have been increasing over the past 2 decades. As survival of extremely low birthweight infants (ELBW, birthweight <1,000 g) has increased worldwide, the rates of infants with birthweights of <501 g who are surviving has also increased (1). Infants born with birthweights <501 g are distinctly different in terms of survival, morbidity, and long-term prognosis compared to VLBW and ELBW infants. While gestational age is often at the forefront of discussions regarding survivability and long-term outcomes, it is important to remember the role that birthweight plays in survival. Unfortunately, there is limited data on the outcomes and management strategies for this unique patient population of infants with birthweights under 501 g. The University of Iowa has had success managing infants born at the limits of viability including follow up data demonstrating a low risk of severe neurodevelopmental impairment at corrected age of 18–22 months for infants born at 22–25 weeks gestation with a standardized approach to the management of nutrition, mechanical ventilation, and hemodynamics (2–5). In the current study, we describe and compare the mortality and morbidity of inborn infants born weighing less than 501 g to infants weighing between 501 and 750 g at a single center over eleven years managed with a consistent respiratory strategy focused on first intention high frequency jet ventilation. We used our data submitted to the Vermont Oxford Network (VON) to compare mortality and morbidity between ELBW<501 and ELBW501–750 infants. From a separate database containing information from July 1, 2012 through June 30, 2017, we abstracted data comparing high frequency jet ventilator characteristics between these same two groups (6).
Methods
A retrospective cohort analysis of infants born at the University of Iowa between January 1st, 2012, and December 31st, 2022, with a birthweight ≤750 g. Infants were grouped into two birthweight categories, less than 501 g referred to as ELBW<501 and infants weighing between 501 and 750 g, referred to as ELBW501–750. Infants were excluded if they were out born, or parents declined active resuscitation at the time of birth. Neonatal characteristics, diagnoses and outcome data were compiled from Vermont Oxford Network (VON) database. All VON data were site-specific, and data were collected in accordance with the data definitions governed by VON. VON is a non-profit collaboration comprised of NICUs across the world. Racial demographic data per VON definitions.
Gestational age was determined by standard clinical guidelines using last menstrual period, prenatal ultrasonography, or physical examination. Birthweight was measured using the electronic scales on the infant beds on admission to the NICU. Antenatal steroid therapy was defined as any corticosteroid administered to the pregnant mother prior to delivery for the purpose of enhancing fetal lung maturity. Infants were classified as being small for gestational age (SGA) when the birthweight was less than the 10th percentile for gestational age and sex according to the Fenton Growth chart (7). Extreme length of stay was determined per VON definition and reflects whether an infant's total hospital stay is greater than the 95th percentile for predicted value. Bronchopulmonary dysplasia (BPD) was defined using the classification system proposed by Jensen et al. based on respiratory support required at 36 weeks postmenstrual age (PMA) (8). Grade 1 BPD is defined as oxygen by nasal cannula at ≤2 LPM flow, Grade 2 as nasal cannula flow >2 LPM flow or noninvasive positive pressure and Grade 3 is represented by invasive mechanical ventilation via endotracheal tube (8). Intraventricular hemorrhage (IVH) grade was reported based on the Papile classification system on cranial ultrasound performed in all infants during the first week of life and at 36 weeks PMA (9). Periventricular leukomalacia (PVL) was defined as radiologist identified PVL on cranial ultrasound, CT or brain MRI. Retinopathy of prematurity (ROP) presence and stage was reported based on diagnosis from ophthalmologic exam by study institution's ophthalmology team, and severe ROP was defined using VON definitions (stage 3, 4, or 5) (10).
Necrotizing enterocolitis (NEC) was defined per the VON definition as present if diagnosed at surgery, at postmortem examination, or with clinical and diagnostic imaging using the following criteria: at least one of the following clinical signs present: bilious gastric aspirate or emesis, abdominal distension or discoloration, occult or gross blood in stool (no fissure) and at least one of the following diagnostic imaging findings present: pneumatosis intestinalis, hepato-biliary gas, or pneumoperitoneum (11, 12).
From a separate time restricted (July 1, 2012 through June 30, 2017) respiratory database (University of Iowa NICU registry, electronic health record systemic, Epic, as collected in RedCap), we abstracted data comparing high frequency jet ventilator and respiratory characteristics between the <501 and the 501–750 g birthweight cohorts. Respiratory data collected included mode of ventilation and settings, days on mechanical ventilation, age at first and final successful extubation attempt, age at weaning to low flow oxygen, and days on non-invasive respiratory support capable of delivering positive pressure including non-invasive ventilation. Non-invasive ventilation included neurally adjusted ventilatory assist (NAVA), nasal pharyngeal intermittent (6) mandatory ventilation (NP-IMV), nasal pharyngeal continuous positive pressure airway pressure (NP-CPAP), RAM Cannula, and high flow nasal cannula >2 LPM.
Statistical analysis
Data were calculated using frequencies and percentages for categorical factors or with the median and interquartile range (IQR) for continuous characteristics. Demographic, survival, respiratory outcomes, ventilator characteristics and morbidity outcomes were compared between ELBW<501 and ELBW501–750 using Mann-Whitney tests for continuous variables and Chi Squared or Fisher's exact test for categorical variables. Differences were considered significant when the p-value was <0.05. No statistical adjustments were made for multiple testing. All analyses were performed using GraphPad Prism version 9.0.
Results
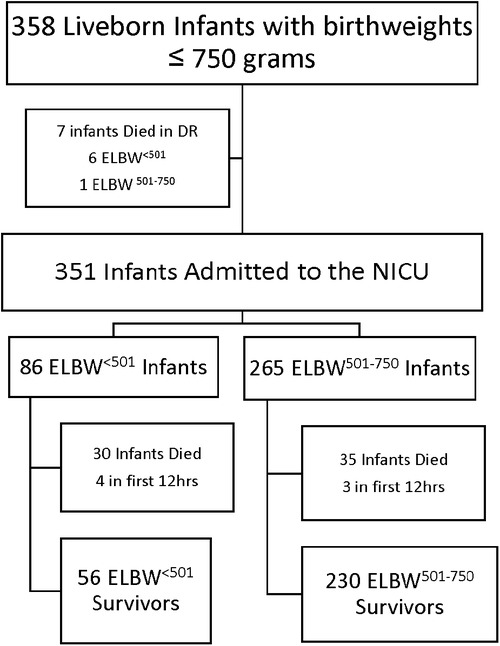
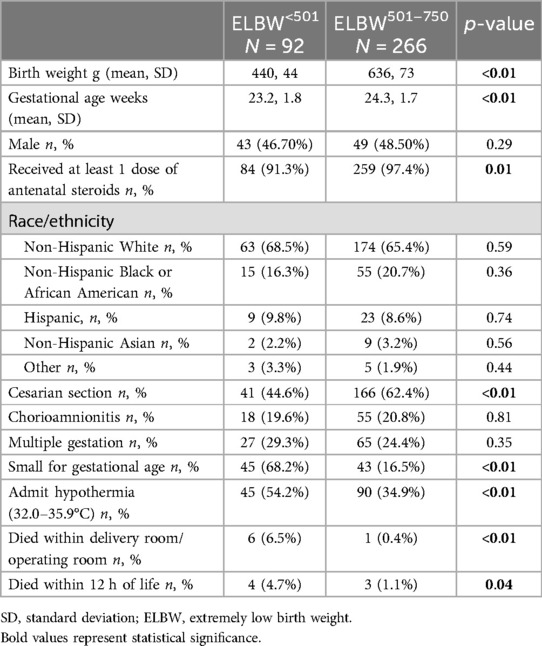
A total of 358 infants with birthweights ≤750 g were born between January 1, 2012 and December 31, 2022 (Figure 1), 92 categorized as ELBW<501 and 266 as ELBW501–750. Demographics data are presented in Table 1. There were 7 infants who died in the delivery room, 6 in the ELBW<501 group and 1 in the ELBW501–750 group (p < 0.01). There were 351 infants who were admitted to the NICU. Of these infants, 86 were born weighing less than 501 g and 265 were born weighing between 501 and 750 g. Four total infants died within the first 12 h in the ELBW<501 group and 3 infants died within the first 12 h in the ELBW501–750 group (p = 0.04). ELBW<501 infants had a mean birthweight of 440 g vs. 636 g in the ELBW501–750 group (p < 0.01). ELBW<501 infants were born at significantly lower gestational ages (median 23.2 weeks vs. 24.3 weeks, p ≤ 0.01) and had a similar percentage of male infants (46.7% vs. 48.5%, p = 0.28) compared with the ELBW501–750 group. ELBW<501 infants were less likely to have received any antenatal steroids (91.3% vs. 97.4%, p = 0.01) and were more likely to be hypothermic on admission with an initial temperature between 32.0 and 35.9 degrees Celsius when compared to the ELBW501–750 group (54.2% vs. 34.9%, p < 0.01). Caesarian sections were less common in the ELBW<501 group then in the ELBW501–750 group (44.6% vs. 63.4%, p < 0.01). Diagnosis of maternal chorioamnionitis was not different and there were similar rates of multiple gestation pregnancies between the two groups.
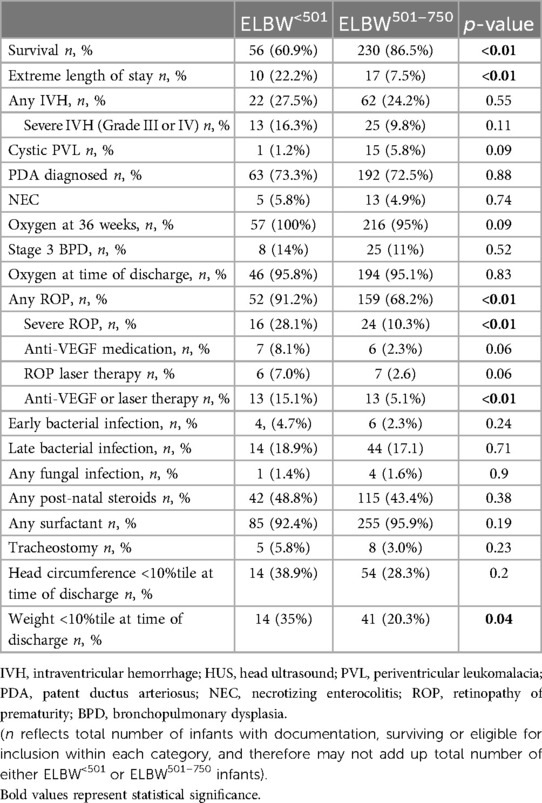
Neonatal outcomes are presented in Table 2. ELBW<501 infant survival was significantly lower compared to the ELBW501–750 infants (60.9% vs. 86.5%, p < 0.01). Extreme length of stay was significantly more common in infants born less than 501 g (22.2% vs. 7.5%). The rate of any IVH between the groups did not reach significance, neither did the presence of severe IVH or rates of cystic periventricular leukomalacia. The diagnosis of NEC did not reach statistical significance between the two groups. PDA diagnosis rates were similar between the two groups, 73.3% and 72.5% (p = 0.88). Additionally, rates of early onset bacterial sepsis, late onset bacterial sepsis, and fungal sepsis were not significantly different between the groups. ELBW<501 infants were more likely to be diagnosed with any ROP (91.2%, vs. 68.2%, p < 0.01), and to have a higher incidence of severe ROP (28.1% vs. 10.3%, p < 0.01) compared to ELBW501–750 infants. Rates of anti-VEGF medication administration or laser therapy was significantly higher in ELBW<501 (15.1% vs. 5.1%, p < 0.01). Oxygen use at 36 weeks post menstrual age for ELBW<501 infants was 100%, and 95.2% in ELBW501–750 infants (p = 0.09). There was no difference in rates of grade 3 BPD (14% vs. 11%, p = 0.52) or home going oxygen (95.8% vs. 95.1%, p = 0.83) between the groups. There was no difference seen in rates of tracheostomy placement. More infants in the ELBW<501 group had weight <10%tile at time of discharge (35% vs. 20.3%).
Respiratory and ventilator characteristics for the July 1st, 2012 through June 30th, 2017 cohort were obtained from a previous dataset and are presented in Table 3 (6). ELBW<501 infants were more likely to be initially intubated with a 2.0 mm ETT (89% vs. 32%, p < 0.01) whereas ELBW501–750 infants were intubated more often with a 2.5 mm ETT (66% vs. 11%, p < 0.01). Nearly all infants, regardless of group, were initially placed on the HFJV, consistent with unit policy (100% vs. 95%, p = 0.15). ELBW<501 infants were found to have a significantly higher oxygen need at 6 h of life (median 33% vs. 25%, p < 0.01), however no differences were seen in MAP, HFJV PIP, HFJV rate, or PCO2 at 6 h of life. At 7 days of life, ELBW<501 infants required significantly higher HFJV PIP than ELBW501–750 infants (median PIP 30 vs. 26, p < 0.01) but no difference was seen in FiO2, MAP, HFJV rate, or PCO2 at that time point. At extubation, there were no differences in MAP, HFJV PIP or rate, FiO2, or PCO2 between the two groups, however ELBW<501 infants' first trial of extubation occurred at a older age (day of life 56 vs. 44, p = 0.0007) and older PMA (31.6 vs. 30.57, p = 0.049) than ELBW501–750 infants. Additionally, successful extubation also occurred at a later day of life for ELBW<501 infants than ELBW501–750 infants (median 68.5 vs. 50.5, p < 0.01), though interestingly there was no difference in postmenstrual age at final extubation (33.36 vs. 32.12, p = 0.12). ELBW<501 infants required more days of invasive ventilation (median 64 vs. 47, p = 0.04) and more non-invasive respiratory support CPAP days (median 55 vs. 33, p < 0.01) than ELBW501–750 infants.
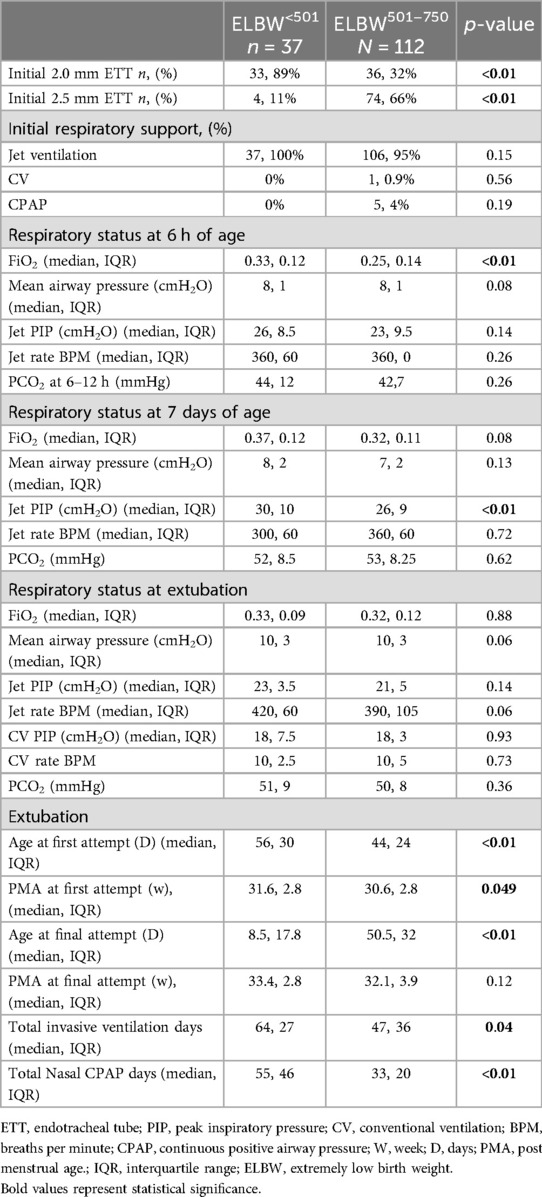
Table 3. Detailed respiratory and ventilator (first intention high frequency jet ventilation, HFJV) characteristics of ELBW<501 compared to ELBW501–750 infants from 2012 to 2017 (6).
Discussion
In our 11-year cohort study of inborn infants with birthweight ≤750 g, stratified by birthweight, survival was significantly lower for ELBW<501 compared to ELBW501–750 infants. The lower survival rate in the ELBW<501 cohort was expected due to these infants being born at significantly lower gestational ages (23.2 weeks vs. 24.3 weeks, p < 0.01). However, the survival rate of ELBW<501 infants in our cohort, at 61%, was higher than recently reported rates of survival for infants born at ≤500 g (21%–55%) (13) but not as high as survival rates reported in a small Japanese case series (n = 10 live born infants) which demonstrated 80% survival for infants born at ≤500 g with comparable birthweights, though the Japanese infants were 2 weeks more mature (median BW of 436 g, median GA of 25.2 weeks) (14).
The high rate of survival in our ELBW<501 cohort was in part due to a strong commitment to proactive prenatal and neonatal care provided as previously described by Kyser et al. (15). Provision of at least one dose of ANS was generally high in both groups, however it is notable that significantly fewer infants in the ELBW<501 group received ANS which would have impacted survival. All the infants in this study received active neonatal care, including frequent use of 2.0 mm endotracheal tube (89% of ELBW<501 infants and 32% of ELBW501–750 infants). While 2.0 mm ETTs in themselves are not advantageous compared to 2.5 mm ETT, they are a marker of the provision of active resuscitation efforts for infants <501 g as there exists in some centers, a belief that if an infant requires a 2.0 mm ETT than concurrently they must be too developmentally immature to survive (6). Previous studies have shown improved survival for infants who received antenatal corticosteroids and were delivered by cesarean section, both markers of proactive prenatal care (13, 16–19). The larger ELBW501–750 infants were found to be more likely to be delivered via cesarian section and received ANS, which may have affected survival. At the University of Iowa, antenatal steroids are given to infants starting at 21 5/7th weeks gestation, however electronic fetal monitoring and cesarean section for fetal distress is not offered until 23 0/7 weeks. There was no signifcant difference between the groups in the use of post-natal steroids for chronic lung disease or surfactant use. ELBW<501 infants' length of stay was significantly more likely to exceed predicted discharge dates in comparison to ELBW501–750 infants based on GA alone. Our data support the importance of anticipatory guidance regarding increased LOS for families with infants born less than 500 g. The significant difference in discharge weight less than the 10th percentile between the two groups (p = 0.04) indicates that growth in this population remains a challenge and highlights the relevance of comprehensive nutritional protocols.
There were significantly more SGA infants in the ELBW<501 group compared to ELBW501–750 group (68.2% vs. 16.5%, p < 0.01). Small for gestational age is often used as a surrogate marker for fetal growth restriction and has been associated with increased mortality and adverse neonatal outcomes. The risk of neonatal death has been shown to be threefold higher in premature SGA compared to appropriate for gestational age (AGA) infants (13, 20–23) with higher risk of mortality at lower gestational ages and with more severe growth restriction (24, 25). In addition, death tends to occur earlier in SGA premature infants occurring in the delivery room or within the first 12 h of life (13). Because of this, infants born extremely premature and SGA are more often offered comfort care only over aggressive medical management (22). The true effect of SGA on the rate of common neonatal morbidities (NEC, ROP, IVH, sepsis, RDS, BPD, etc.) is unknown due to conflicting results reported in the literature (20–22, 24, 25).
However, studies consistently show that SGA infants have significantly longer hospital stays often due to increased severity of illness and significant neonatal morbidities (13, 24, 25). SGA infants are more likely to have received prenatal care, antenatal steroids, and be delivered by cesarean section, especially due to non-reassuring fetal heart rate tracing (20, 22). In addition, SGA infants seem to be at higher risk for slow weight gain, postnatal growth failure, prolonged ventilator support, postnatal steroid use, and chronic lung disease (13, 20, 22).
The initial respiratory approach for extremely premature infants with birthweights of ≤750 g that need invasive ventilation at the University of Iowa is to provide first intention high frequency jet ventilation using the Bunnell Life Pulse high frequency jet ventilator (Bunnell Incorporated Salt Lake City, UT). All ELBW<501 infants and 95% of the ELBW501–750 were placed on HFJV. HFJV management focused on minimizing mechanical injury from shear force through use of low tidal volumes (below physiologic dead space to reduce volutrauma), avoiding air trapping and hyperinflation by utilizing initial rates of 300–360 BPM with a fixed inspiratory time of 0.02 s to provide adequate time for exhalation. The use of this prolonged I:E ratio allows for adequate expiratory time for ventilation to occur by passive elastic recoil. A conventional ventilator runs in tandem to provide positive end expiratory pressure (PEEP) to maintain functional residual capacity and minimize atelectasis leading to loss of oxygenation. On admission no conventional sigh breaths are used with HFJV, however they are often added in the first week of life for alveolar recruitment to treat wandering atelectasis. Once conventional sigh breaths are added they are maintained until extubation to minimize recurrence of positional (wandering) atelectasis unless air leak develops (2, 3, 26). Sigh breaths are not used to regulate PCO2 but are used for desaturation spells associated with central apnea. HFJV rate may be increased (in 60 BPM increments) to optimize alveolar ventilation when the patient is having worsening hypercarbia despite increased PIP, and/or to improve oxygenation through increased lung recruitment as mean airway pressure rises with higher rates. Conversely, HFJV rate may be decreased for persistent hypocarbia despite weaning the Jet PIP. Air trapping [pulmonary interstitial emphysema (PIE) or hyperinflation] should be screened for with frequent radiographs, especially in the first week of life. If either PIE or hyperinflation is detected, the jet rate and MAP needs to be decreased and sigh breaths discontinued (2, 3, 26). First intention HFJV was recently found to reduce Grade 3 BPD in infants ≤26 weeks gestation (27).
In our study, the ventilator settings (respiratory parameter cohort 2012–2017) at 6 h of life were not significantly different based on birthweights except for the need for higher FiO2 in ELBW<501 infants compared to ELBW501–750 infants, consistent with their younger gestational age and less mature lungs. At 7 days of life, the PIP was significantly higher in the ELBW<501 than ELBW501–750 infants again likely related to lower gestational age and the use of a 2.0 mm ETT which requires higher PIP to overcome the increased resistance through the smaller ETT to deliver a similar tidal volume. PCO2 levels are followed closely and changes in the PIP or ΔP (PIP-PEEP) are adjusted per unit protocol to keep PCO2 levels 45–55 mmHg in the first week of life and then liberalized to 50–65 mmHg following stabilization of the germinal matrix. Due to strict regulation of PCO2 targets, there were no significant differences in levels between the two weight groups.
The chronic ventilatory approach for all ventilated infants with birthweights ≤750 g is to prioritize growth with stabilization of oxygenation and ventilation and to treat any hemodynamically significant PDA in the first 2 weeks of life before evaluation for extubation (28). Extubation failure in the first 2 weeks of life for infants <26 weeks gestation should be minimized since it is associated with a 5-fold increase in mortality, higher rate of BPD, severe IVH, and sepsis (29). The criteria for extubation from HFJV include strong sustained spontaneous respiratory drive, mean air way pressure ≤10–12 cm H2O, FiO2 ≤0.45, and delta P (PIP-PEEP) <14–16 cm H2O. In our study, the median age of successful extubation for ELBW<501 was 69 days compared with 51 days of life for ELBW501–750 infants (p < 0.01). It is important to emphasis that this study looked at two specific cohorts of ELBW infants with birthweights between 501 and 750 g and birthweights less than 501 g, rather than all ELBW or VLBW infants. It is likely that larger ELBW infants, particularly those closer to 1,000 g, will not require the same duration of mechanical ventilation. As expected, PMA at time of final extubation was not significantly different with a median of 33.4 weeks for the ELBW<501 infants and 32.1 weeks for the ELBW501–750 infants. However, due to ELBW<501 infants being more premature, they spent significantly more days on invasive ventilation (64 days vs. 47 days, p = 0.04) to reach the same level of lung maturation. Both groups of infants were extubated at similar MAP, PIPs, and FiO2 (p = 0.059, p = 0.14, and p = 0.88 respectively) consistent with our standardized respiratory guidelines. Infants were primarily extubated to non-invasive Neurally Adjusted Ventilatory Assist (NIV-NAVA) through a nasal pharyngeal tube (2, 3, 26). Per unit protocol, infants generally need to be >850–900 g to attempt extubation to NIV-NAVA. If below this weight and ready for a trial of extubation, NIPPV can be used.
While the need for supplemental oxygen at 36 weeks PMA was high in both groups (100% vs. 95.2% in ELBW<501 infants and ELBW501–750 infants) this is not unexpected and is consistent with work published involving infants born at similar gestational ages (11). Importantly, the incidence of Grade 3 BPD, which is associated with a two-fold higher rate of late death, serious respiratory morbidity, and moderate-to-severe neurodevelopmental impairment, was low in both groups and not significantly different. In addition, only 5.8% of the ELBW<501 infants and 3% of the ELBW501–750 infants required tracheostomy for ongoing mechanical ventilation at home (p = 0.23).
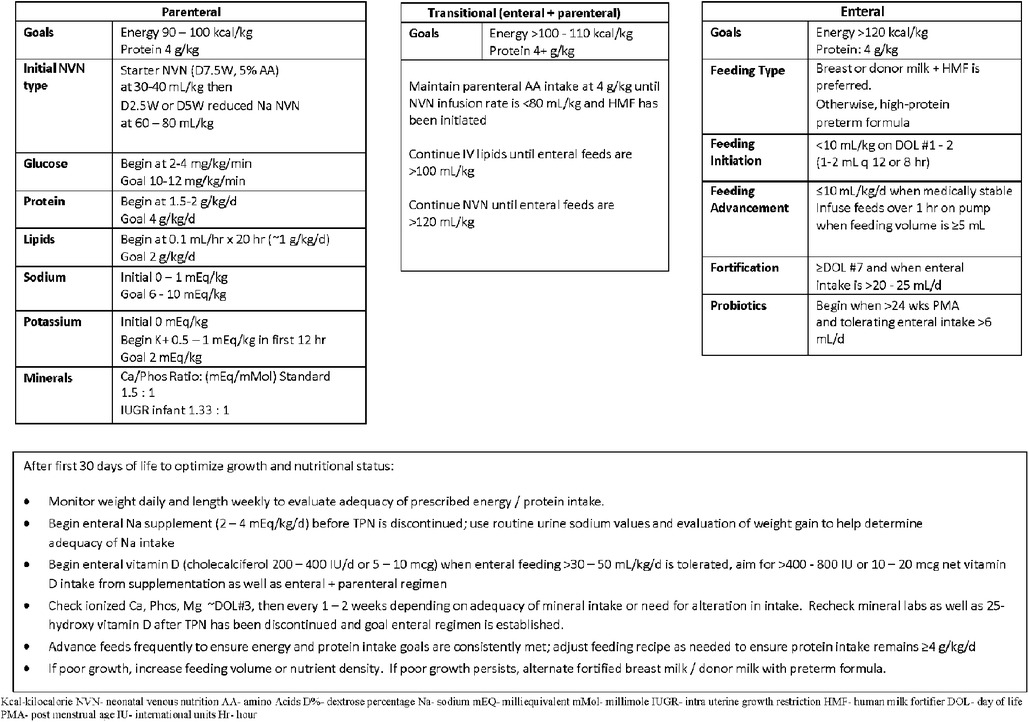
Differences and similarities were seen in non-respiratory co-morbidities between the two groups. No significant differences were seen in PVL, NEC, or infection. Infants were fed according to comprehensive feeding guidelines. The feeding protocol for infants born at 22–23 weeks involves trophic feeds at 10 ml/kg initiated within the first 24–36 h with maternal breast milk or donor milk with slow advancements of 10–12 ml/kg/day if tolerating feeds based on residuals and physical exam. Bolus feeds are switched from straight gravity to pump over 1 h when absolute volume is >4 ml, and fortification is introduced when the infants are on day of life 7 and oral intake is greater than 20–25 ml/day (Figure 2). Close attention is paid to stooling patterns, if no stooling occurs after 2–3 days the infants receive glycerin suppositories. Feeds will not advance beyond 30 ml/kg/day in the first week until transitional stools are present. By the second week of life, if no transitional stools, or not tolerating trophic feeds or exam and x-rays are consistent with suspicion for meconium obstruction of prematurity, infants receive a contrast enema performed in the NICU. The expected time of full enteral feeds is 28–30 days of life.
The rates of any ROP and severe ROP were significantly higher among ELBW<501 infant survivors compared to the ELBW501–750 infants. Additionally, ELBW<501 infants were more likely to have the composite of ROP laser surgery and/or Anti-VEGF therapy compared to ELBW501–750 infants (p < 0.01). Increased risk for ROP in ELBW<501 infants is likely related to the degree of immaturity, and greatly increased number of SGA infants. The increase in admission hypothermia in the ELBW<501 infant is consistent with the inherent physiological challenges in maintaining euthermia in the population of micro- premature infants. Maintaining neonatal euthermia is critical, and decreased admission temperature is associated with NEC, ROP, BPD, sepsis and increased mortality (30–33). Policy for infants born less than 750 g is focused on resuscitation and stabilization then admission to the NICU within the first 15 min of life. The first temperature obtained is at the time of admission, and no pre-warming or temperature probes are used prior to reaching the NICU, though these interventions have been found to be successful in thermoregulation at other centers (34). Our center engages in robust thermoregulation simulation training focused on ELBW infants, and has seen improvement in our admission temperatures, but not at the extremes of birthweight and gestation age (35). Providing effective temperature management for the smallest of infants remains an ongoing challenge.
This study has several strengths and limitations. It provides detailed information about interventions, management strategies, survival, and short-term outcomes from a subset of high-risk preterm infants born less the 501 g. Additionally, as a single center study, all infants were treated similarly based on a single institution's practices and policies. We acknowledge that our study has several limitations including a retrospective cohort design from a large database which led to the inability to obtain individualized ventilator data beyond the earlier detailed ventilator study from 2012 to 2017. Additionally, the database does not include neurodevelopmental outcomes at 18–22 months of corrected age, however we would not suspect it to be different then what was found in our previous cohort of infants born between 2006 and 2015 at 22–25 weeks gestation. It will be important to gather follow up data at 2 years corrected age in this new cohort of infants born from 2016 to 2022 to examine long term neurodevelopmental outcomes for the <501 g population compared to the 501–750 g group.
In conclusion, ELBW<501 infants are at risk for significant mortality compared to the ELBW501–750 infants. However, with proactive obstetrical care and specialized neonatal care including the use of 2.0 mm endotracheal tubes when required and first intention high frequency jet ventilation, survival rates of 61% are possible. Although 100% of these infants had BPD defined as supplemental oxygen at 36 weeks PMA, rates of grade 3 BPD (need for invasive ventilation at 36 weeks PMA) and tracheostomy were very low with the use of first intention HFJV and importantly, rates of IVH, PVL and NEC were not higher in the smaller group. ELBW<501 infants as expected have a longer hospital stay with more days of invasive ventilation and are at a much higher risk of ROP, and thus families should be counseled appropriately. With the exception of ROP (and ROP requiring intervention), this vulnerable patient population (<501 g) has comparable morbidities which may potentially impact long term neurodevelopmental outcomes. Thus, it will be important to continue to obtain and report on long term follow-up for this high-risk population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Iowa Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because retrospective analysis of the data and deidentified reporting of the data did not warrant informed consent per IRB.
Author contributions
TE: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. JB: Data curation, Writing – original draft, Writing – review & editing. DK: Investigation, Writing – original draft, Writing – review & editing. JD: Writing – original draft, Writing – review & editing. BT: Methodology, Writing – original draft, Conceptualization, Data curation, Formal Analysis. TC: Writing – original draft, Writing – review & editing, Methodology. JK: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Susan J. Carlson MMSc, RDN, CSPCC, LD, Neonatal Dietician, University of Iowa Stead Family Children's Hospital for her support in creating and sharing Figure 2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IVH, intraventricular hemorrhage; HUS, head ultrasound; PVL, periventricular leukomalacia; PIE, pulmonary interstitial emphysema; PDA, patent ductus arteriosus; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; BPD, bronchopulmonary dysplasia; ETT, endotracheal tube; PIP, peak inspiratory pressure; CV, conventional ventilation; BPM, breaths per minute; CPAP, continuous positive airway pressure; WKS, weeks; D, days; ELBW, extremely low birth weight; VLBW, very low birth weight; Kcal, kilocalorie; NVN, neonatal venous nutrition, AA, amino acids; D%, dextrose percentage; Na, sodium; mEQ, milliequivalent; mMol, millimole; IUGR, intra uterine growth restriction, HMF, human milk fortifier; DOL, day of life; PMA, post menstrual age; HFJV, high frequency jet ventilation; IU, international units; VON, Vermont Oxford Network.
References
1. Inoue H, Ochiai M, Yasuoka K, Tanaka K, Kurata H, Fujiyoshi J, et al. Early mortality and morbidity in infants with birth weight of 500 g or less in Japan. J Pediatr. (2017) 190:112–7.e3. doi: 10.1016/j.jpeds.2017.05.017
2. Elgin TG, Stanford AH, Klein JM. First intention high-frequency jet ventilation for periviable infants. Curr Opin Pediatr. (2022) 34(2):165–9. doi: 10.1097/MOP.0000000000001104
3. Dagle JM, Rysavy MA, Hunter SK, Colaizy TT, Elgin TG, Giesinger RE, et al. Cardiorespiratory management of infants born at 22 weeks’ gestation: the Iowa approach. Semin Perinatol. (2022) 46(1):151545. doi: 10.1016/j.semperi.2021.151545
4. Elgin TG, Berger JN, Thomas BA, Colaizy TT, Klein JM. Ventilator management in extremely preterm infants. Neoreviews. (2022) 23(10):e661–76. doi: 10.1542/neo.23-10-e661
5. Watkins PL, Dagle JM, Bell EF, Colaizy TT. Outcomes at 18–22 months of corrected age for infants born at 22–25 weeks of gestation in a center practicing active management. J Pediatr. (2020) 217:52–8.e1. doi: 10.1016/j.jpeds.2019.08.028
6. Berger JN, Elgin TG, Dagle JM, Klein JM, Colaizy TT. Survival and short-term respiratory outcomes of <750 g infants initially intubated with 2.0 mm vs. 2.5 mm endotracheal tubes. J Perinatol. (2022) 42(2):202–8. doi: 10.1038/s41372-021-01227-y
7. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
8. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200(6):751–9. doi: 10.1164/rccm.201812-2348OC
9. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 g. J Pediatr. (1978) 92(4):529–34. doi: 10.1016/S0022-3476(78)80282-0
10. Prakalapakorn SG, Greenberg L, Edwards EM, Ehret DEY. Trends in retinopathy of prematurity screening and treatment: 2008–2018. Pediatrics. (2021) 147(6):e2020039966. doi: 10.1542/peds.2020-039966
11. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
12. Smith LK, van Blankenstein E, Fox G, Seaton SE, Martínez-Jiménez M, Petrou S, et al. Effect of national guidance on survival for babies born at 22 weeks’ gestation in England and Wales: population based cohort study. BMJ Med. (2023) 2(1):e000579. doi: 10.1136/bmjmed-2023-000579
13. Griffin IJ, Lee HC, Profit J, Tancedi DJ. The smallest of the small: short-term outcomes of profoundly growth restricted and profoundly low birth weight preterm infants. J Perinatol. (2015) 35(7):503–10. doi: 10.1038/jp.2014.233
14. Nagara S, Kouwaki M, Togawa T, Sugiura T, Okada M, Koyama N. Neurodevelopmental outcomes at 3 years old for infants with birth weights under 500 g. Pediatr Neonatol. (2018) 59(3):274–80. doi: 10.1016/j.pedneo.2017.09.005
15. Kyser KL, Morriss FH Jr., Bell EF, Klein JM, Dagle JM. Improving survival of extremely preterm infants born between 22 and 25 weeks of gestation. Obstet Gynecol. (2012) 119(4):795–800. doi: 10.1097/AOG.0b013e31824b1a03
16. Ancel PY, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. (2015) 169(3):230–8. doi: 10.1001/jamapediatrics.2014.3351
17. Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. (2015) 372(19):1801–11. doi: 10.1056/NEJMx150023
18. Brumbaugh JE, Hansen NI, Bell EF, Sridhar A, Carlo WA, Hintz SR, et al. Outcomes of extremely preterm infants with birth weight less than 400 g. JAMA Pediatr. (2019) 173(5):434–45. doi: 10.1001/jamapediatrics.2019.0180
19. Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. (2000) 106(4):659–71. doi: 10.1542/peds.106.4.659
20. Sharma P, McKay K, Rosenkrantz TS, Hussain N. Comparisons of mortality and pre- discharge respiratory outcomes in small-for-gestational-age and appropriate-for- gestational-age premature infants. BMC Pediatr. (2004) 4:9. doi: 10.1186/1471-2431-4-9
21. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford network. Am J Obstet Gynecol. (2000) 182(1 Pt 1):198–206. doi: 10.1016/S0002-9378(00)70513-8
22. De Jesus LC, Pappas A, Shankaran S, Li L, Das A, Bell EF, et al. Outcomes of small for gestational age infants born at <27 weeks’ gestation. J Pediatr. (2013) 163(1):55–60.e1–3. doi: 10.1016/j.jpeds.2012.12.097
23. Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K, Lagercrantz H, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. (2009) 301(21):2225–33. doi: 10.1001/jama.2009.771
24. Mendez-Figueroa H, Truong VT, Pedroza C, Chauhan SP. Morbidity and mortality in small-for-gestational-age infants: a secondary analysis of nine MFMU network studies. Am J Perinatol. (2017) 34(4):323–32. doi: 10.1055/s-0036-1586502
25. Kamoji VM, Dorling JS, Manktelow BN, Draper ES, Field DJ. Extremely growth- retarded infants: is there a viability centile? Pediatrics. (2006) 118(2):758–63. doi: 10.1542/peds.2005-2399
26. Sindelar R, Nakanishi H, Stanford AH, Colaizy TT, Klein JM. Respiratory management for extremely premature infants born at 22–23 weeks of gestation in proactive centers in Sweden, Japan, and USA. Semin Perinatol. (2022) 46(1):151540. doi: 10.1016/j.semperi.2021.151540
27. Rallis D, Ben-David D, Woo K, Robinson J, Beadles D, Bernardini L, et al. Single center experience with first-intention high-frequency jet vs. volume-targeted ventilation in extremely preterm neonates. Front Pediatr. (2023) 11:1326668. doi: 10.3389/fped.2023.1326668
28. Giesinger RE, Rios DR, Chatmethakul T, Bischoff AR, Sandgren JA, Cunningham A, et al. Impact of early hemodynamic screening on extremely preterm outcomes in a high- performance center. Am J Respir Crit Care Med. (2023) 208(3):290–300. doi: 10.1164/rccm.202212-2291OC
29. Chawla S, Natarajan G, Shankaran S, Carper B, Brion LP, Keszler M, et al. Markers of successful extubation in extremely preterm infants, and morbidity after failed extubation. J Pediatr. (2017) 189:113–9.e2. doi: 10.1016/j.jpeds.2017.04.050
30. Laptook AR, Bell EF, Shankaran S, Boghossian NS, Wyckoff MH, Kandefer S, et al. Admission temperature and associated mortality and morbidity among moderately and extremely preterm infants. J Pediatr. (2018) 192:53–9.e2. doi: 10.1016/j.jpeds.2017.09.021
31. Harer MW, Vergales B, Cady T, Early A, Chisholm C, Swanson JR. Implementation of a multidisciplinary guideline improves preterm infant admission temperatures. J Perinatol. (2017) 37(11):1242–7. doi: 10.1038/jp.2017.112
32. Lyu Y, Shah PS, Ye XY, Warre R, Piedboeuf B, Deshpandey A, et al. Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks’ gestation. JAMA Pediatr. (2015) 169(4):e150277. doi: 10.1001/jamapediatrics.2015.0277
33. Chitty H, Wyllie J. Importance of maintaining the newly born temperature in the normal range from delivery to admission. Semin Fetal Neonatal Med. (2013) 18(6):362–8. doi: 10.1016/j.siny.2013.08.002
34. Bhatt DR, Reddy N, Ruiz R, Bustos DV, Peacock T, Dizon RA, et al. Perinatal quality improvement bundle to decrease hypothermia in extremely low birthweight infants with birth weight less than 1,000 g: single-center experience over 6 years. J Investig Med. (2020) 68(7):1256–60. doi: 10.1136/jim-2020-001334
35. Elgin TG, Spellman E, Schmelzel M, Colaizy TT, Rabe G, O′Connor P. The introduction of a simulated thermoregulation intervention to improve very low birth weight infant initial admission temperatures in a neonatal intensive care unit. Int J Healthcare Simul. (2022) 1(3):47–55. doi: 10.54531/DRKQ7209
Keywords: neonatalogy, HFJV - high-frequency jet ventilation, high frequency, survival, nutrition
Citation: Elgin TG, Berger JN, Kaluarachchi DC, Dagle JM, Thomas B, Colaizy TT and Klein JM (2024) Outcomes of infants with birthweights less than 501 g compared to infants weighing 501–750 g at a center utilizing first intention high frequency jet ventilation. Front. Pediatr. 12:1392079. doi: 10.3389/fped.2024.1392079
Received: 26 February 2024; Accepted: 11 June 2024;
Published: 9 September 2024.
Edited by:
Seung Han Shin, Seoul National University Children's Hospital, Republic of KoreaReviewed by:
MaryAnn Volpe, Tufts University, United StatesHasan Özkan, Dokuz Eylül University, Türkiye
Copyright: © 2024 Elgin, Berger, Kaluarachchi, Dagle, Thomas, Colaizy and Klein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy G. Elgin, dGVsZ2luQHdpc2MuZWR1
 Timothy G. Elgin
Timothy G. Elgin Jennifer N. Berger2
Jennifer N. Berger2 Dinushan C. Kaluarachchi
Dinushan C. Kaluarachchi Brady Thomas
Brady Thomas Tarah T. Colaizy
Tarah T. Colaizy Jonathan M. Klein
Jonathan M. Klein