- 1Faculty of Health, School of Nursing, Dalhousie University, Halifax, NS, Canada
- 2Department of Pediatrics, IWK Health, Halifax, NS, Canada
- 3Canadian Premature Babies Foundation, Toronto, ON, Canada
- 4Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
- 5Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada
- 6Department of Pediatrics, St. Michael’s Hospital, Toronto, ON, Canada
- 7Niagara Region Public Health, Niagara, ON, Canada
- 8Faculty of Medicine, Dalhousie University, Halifax, NS, Canada
- 9Maritime SPOR SUPPORT Unit, Nova Scotia Health, Halifax, NS, Canada
- 10Department of Laboratory Medicine & Pathobiology, University of Toronto, Toronto, ON, Canada
- 11Department of Nursing, University of Prince Edward Island, Charlottetown, PE, Canada
- 12Loyalist College, Belleville, ON, Canada
- 13Humber River Health, Toronto, ON, Canada
- 14Département de Pédiatrie, Centre Hospitalier Universitaire Sainte-Justine, Montréal, QC, Canada
- 15Pickle Planet, Moncton, NB, Canada
- 16Department of Pediatrics, Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada
- 17Department of Pediatrics, Mount Sinai Hospital, Toronto, ON, Canada
- 18Department of Department of Paediatrics, University of Toronto, Toronto, ON, Canada
- 19Children’s Healthcare Canada, Ottawa, ON, Canada
- 20Department of Paediatrics, SickKids Hospital, Toronto, ON, Canada
Aim: To co-create parental presence practice recommendations across Canadian NICUs during pandemics caused by respiratory pathogens such as COVID-19.
Methods: Recommendations were developed through evidence, context, Delphi and Values and Preferences methods. For Delphi 1 and 2, participants rated 50 items and 20 items respectively on a scale from 1 (very low importance) to 5 (very high). To determine consensus, evidence and context of benefits and harms were presented and discussed within the Values and Preference framework for the top-ranked items. An agreement of 80% or more was deemed consensus.
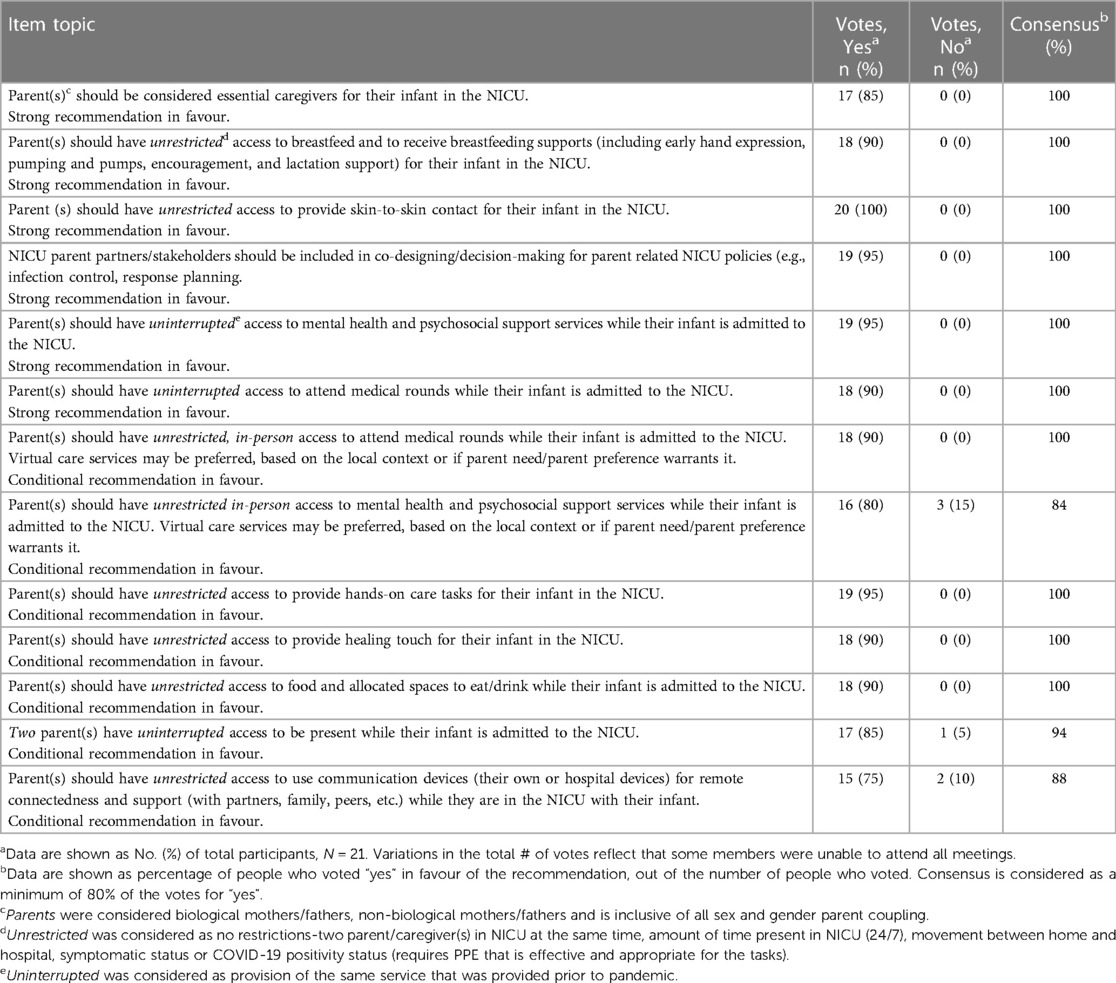
Results: After two Delphi rounds (n = 59 participants), 13 recommendations with the highest rated importance were identified. Consensus recommendations included 6 strong recommendations (parents as essential caregivers, providing skin-to-skin contact, direct or mothers' own expressed milk feeding, attending medical rounds, mental health and psychosocial services access, and inclusion of parent partners in pandemic response planning) and 7 conditional recommendations (providing hands-on care tasks, providing touch, two parents present at the same time, food and drink access, use of communication devices, and in-person access to medical rounds and mental health and psychosocial services).
Conclusion: These recommendations can guide institutions in developing strategies for parental presence during pandemics caused by respiratory pathogens like COVID-19
1 Introduction
Nearly 400,000 babies are born in Canada each year. Of these, approximately 15,000 babies are admitted to tertiary neonatal intensive care units (NICUs) annually (1). About 8% will be born preterm (<37 weeks gestational age), with many requiring neonatal intensive care (1). Infants born extremely preterm are the most vulnerable, but even those delivered one to two weeks early can face immediate and long-term challenges, including developmental delays, and social and behavioural problems (2).
Despite significant improvement in survival rates of preterm infants over the past decade, there has been less improvement in associated morbidity and need for additional health care or neurodevelopmental supports (3). Emphasis on greater parental presence, with parents being designated as essential caregivers in the NICU, has shown to be a beneficial component of care (2, 4). Greater parental presence has also been associated with improved infant growth and developmental outcomes, faster time to reach full enteral feeding, greater provision of mother's own breastmilk (MOM), and reduced sepsis, medical procedures, pain response, physiologic stress, duration of hospital stay, and major morbidities (5).
The pandemic has heightened social inequities and mental health concerns (6). While there was some variation on parent presence policies, primarily regarding siblings, extended family or support people, before the COVID-19 pandemic, 24/7 parent presence was the accepted practice in most Canadian NICU's. Parents of infants requiring neonatal care report higher levels of immediate and posttraumatic stress, anxiety, depression, and adverse parenting outcomes than parents of healthy newborns, all of which have been worsened during the COVID-19 pandemic (7, 8).
With the aim to reduce viral spread, policymakers had to rapidly institute public health policies, often with limited information and engagement with families, patients or other relevant partners. Most hospitals restricted access, many leaving patients, including infants, without a support person or parent, even during extreme illness or death (8). At the pandemic's onset, most Canadian NICUs imposed varying degrees of parental presence restrictions, even within similar viral spread communities (9). Presently, there is continued variation in parental presence policies across Canadian NICUs, with some reverting to pre-COVID policies and others maintaining restrictions on parent and family access.
These restrictive COVID-19 policies have been associated with adverse infant health and parent mental health outcomes, reduced family-centered care delivery, and parent access to education, support, and physical needs (7, 10). Globally, NICUs' pandemic response led to significant policy changes, negatively affecting breastfeeding, parental bonding, caregiving involvement, parental mental health, and staff stress (4).
The aim of this study was to co-create Canadian consensus practice recommendations regarding physical parental presence in NICUs during pandemics caused by respiratory pathogens such as COVID-19.
2 Methods
2.1 Study design and population
The recommendation development process was guided using the AGREE II tool (11). Eligible participants included parents of NICU infants, neonatal healthcare providers and managers, and infectious disease, public or child health care providers or policy makers. The study was approved by the IWK Health Research Ethics Board #1025748, and all participants provided informed consent.
The study was conducted between April 2022 and July 2023 and was carried out in two phases. For phase 1, the Delphi (12) method was used to determine priority recommendations of importance to participants, using two rounds of surveys. The aim was to identify the top 10–12 recommendations. This number, while arbitrary is in keeping with many practice recommendations, and reflects a broad but manageable number to aid in practice uptake and implementation (13). For phase 2, the consensus team was guided by best evidence synthesis, national context, and values and preferences to reach a consensus for the recommendations (14).
2.2 Recruitment
A purposive sampling method was used to help facilitate a diverse and cross sectoral representation (e.g., geographic location, parents and multidisciplinary care providers, leaders and policymakers) for all phases of the study.
For phase 1, we aimed to include a minimum of 20–40 participants across relevant groups: parents of NICU infants, multi-disciplinary healthcare providers with expertise in neonatal care, infectious disease, and public health, health system leaders, and policy makers. To recruit participants, the Delphi surveys were disseminated electronically via email or social media channels across the study teams networks including the Canadian Premature Babies Foundation (CPBF), Canadian Neonatal Network (CNN), and Children's Healthcare Canada. Surveys were accessed on the Research Electronic Data Capture (REDCap) platform hosted at IWK Health, located in Eastern Canada (15). Surveys were open until our goal sample was reached.
For phase 2, we aimed to include a purposive sample of a minimum of 20 participants.
2.3 Data collection
The Delphi surveys were co-created by the research team based on previously circulated national neonatal survey data (parents, health care providers and leaders) and available peer-reviewed published data on the most reported practice gaps, consequences, or impact of the COVID-19 pandemic on families of infants requiring care in a NICU (9, 16–18).
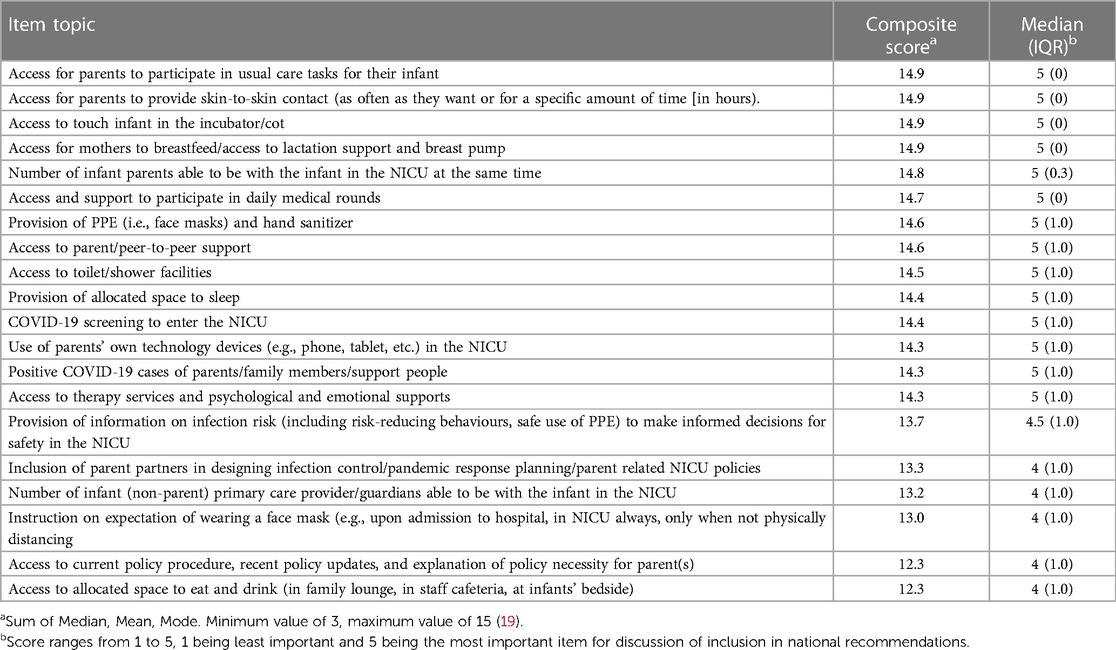
The first Delphi included 50 possible recommendation items to be included as national recommendations (Supplementary Materials). Participants were asked to rate each item on their perceived importance on a Likert scale from 1 (very low importance) to 5 (very high importance). A composite score for each item was calculated based on the sum of the mode, median, and mean. Any item with a mode, median, or mean of less than “4” on the 5-point Likert scale (i.e., rated less than “high” or “very high” importance), along with any interquartile range of more than 1-point difference, was removed from the next round because they were not of high importance.
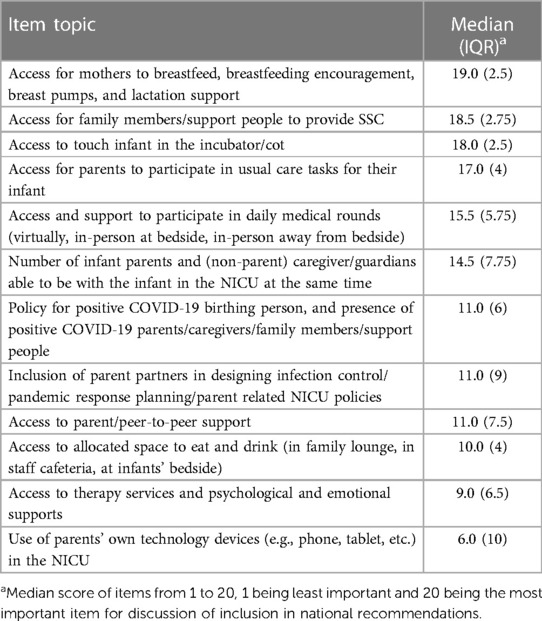
The top 20 items with the highest composite score proceeded to the second round of the Delphi (Table 1). During the second Delphi, participants were asked to rank each item in order of importance from greatest (1) to least (20) impact on safety, parental and healthcare provider mental health, and infant health outcomes. Items were reverse scored (i.e., a rank of 1 was scored as a 20) and scores were summed for each question across respondents to calculate priority scores. The top-rated items with the highest priority scores were items moved forward to the next stage of the study to be considered for the national consensus recommendations.
Survey data were collected through REDCap, a secure web application for building and managing online research surveys and databases, which can be accessed through a shareable public web address (15). Data were collected up to the point that participants completed the survey. If participants chose to withdraw before the survey ended, REDCap recorded the data that the participant completed up until the point of withdrawal.
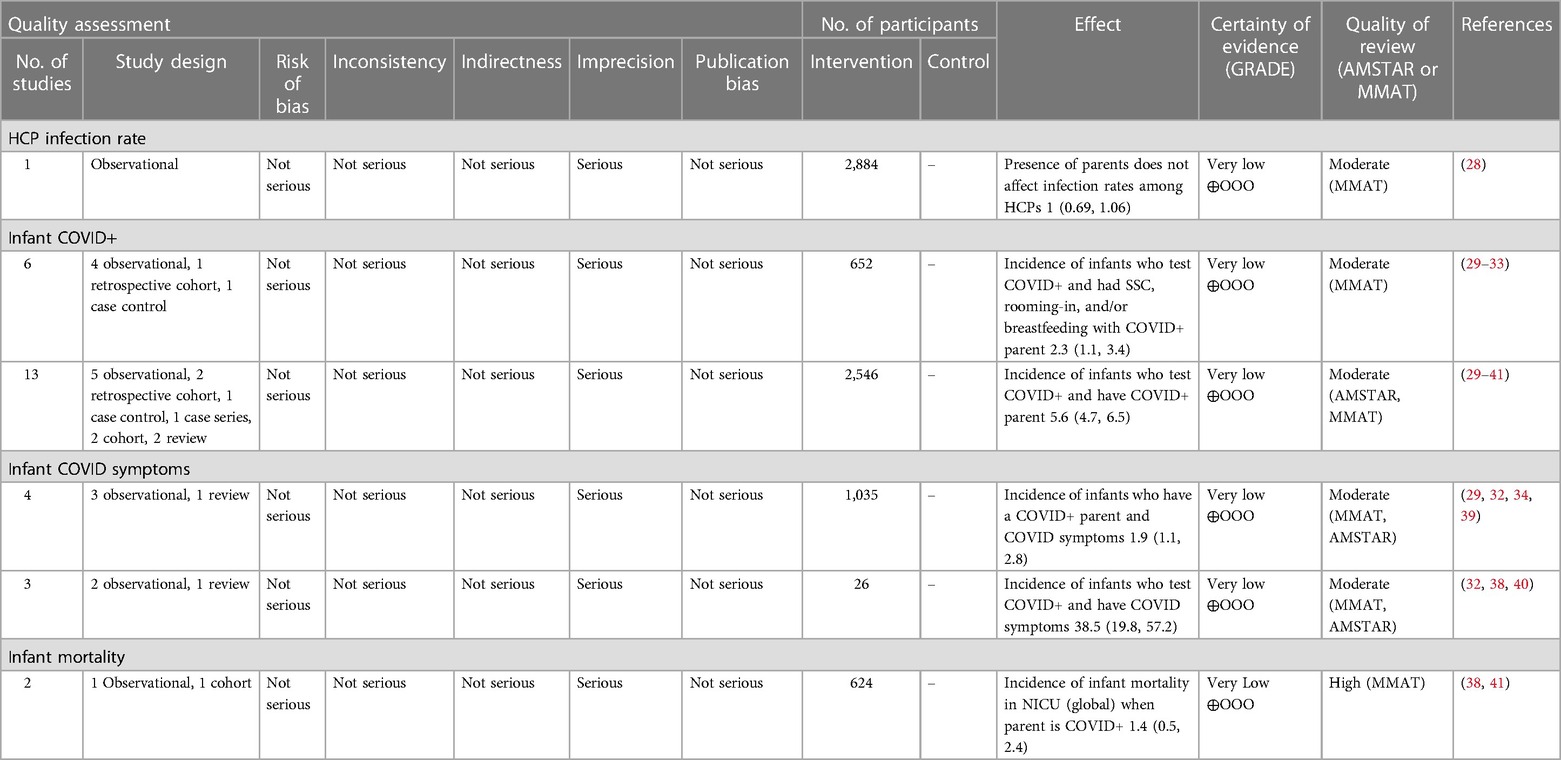
For phase 2, a rapid synthesis of the evidence related to the benefits and harms associated with each of the top ranked items was presented to the consensus panel. See Supplementary Material 1 for search strategy. The rapid review was conducted to accelerate the synthesis process by streamlining the systematic review methods via limiting the search to published literature, including English articles only and having one person screen and abstract the data and another verify (20). Consensus was established when threshold for agreement was met, if voting was equal to or exceeding 80% agreement (21).
A decision aid summarizing the evidence was presented for each item, which included the certainty of evidence for each outcome. Items were discussed, considering context, values and preferences. Panel participants were then asked to vote, yes or no, via an anonymous online portal regarding the recommendation of each of the top ranked items.
Recommendations for each item were either “strong” or “conditional” based upon the certainty of evidence for the outcomes. A recommendation was deemed “strong” if the consensus panel was confident that the desirable effects of adherence to a recommendation outweighed the undesirable effects and that given the certainty of the evidence and consistency of values and preference, it would be unlikely that the recommendation would change with new evidence (21). A recommendation was deemed “conditional” if there was a small margin between favorable and unfavorable outcomes, the consensus panel concluded that the desirable effects of adherence to the recommendation probably outweighed the undesirable effects or if, the evidence was of lower quality, there was greater variability in individual values and preferences and there was a possibility that the recommendation may change with new evidence (21). Recommendations were based on current COVID data regarding virulence of covid-19 and associated low risk for newborns.
2.4 Analysis
Categorical data are expressed as frequencies and percentages. Continuous data are expressed as means and standard deviations (SD) for parametric data, and median and interquartile range (IQR) for nonparametric data. The median was used as it is the recommended measure for Likert scales (19). The IQR was applied, as it considered the spread of responses around the median to gauge the level of agreement when establishing consensus. To discern the hierarchy of items, a composite score, incorporating the mean, median, and mode, was computed (19).
Following a rapid synthesis of evidence, certainty of evidence was determined using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework (21). In keeping with this framework, certainty of evidence was determined from, risk of bias, inconsistency, indirectness, imprecision, and publication bias (50).
The methodological quality of systematic reviews including clinical trials was determined using AMSTAR (Assessment of Multiple Systematic Reviews) (22). For instances where little to no quantitative data existed, narrative summaries of qualitative data were provided, and the quality of the data was determined using the Mixed Methods Appraisal Tool (MMAT) (23).
3 Results
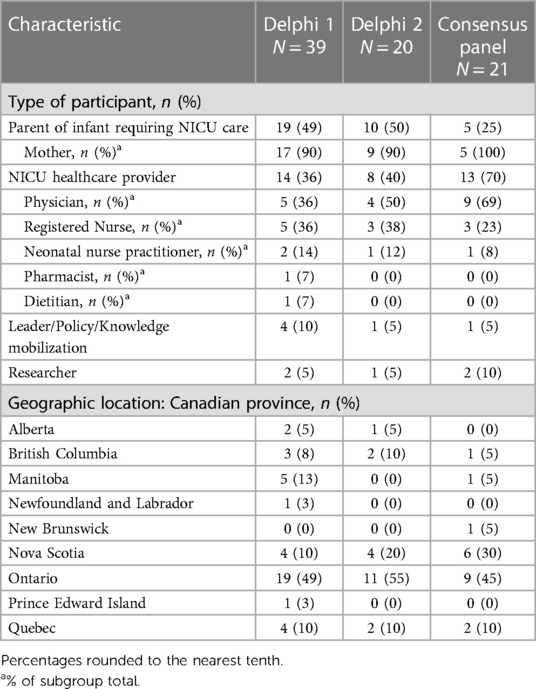
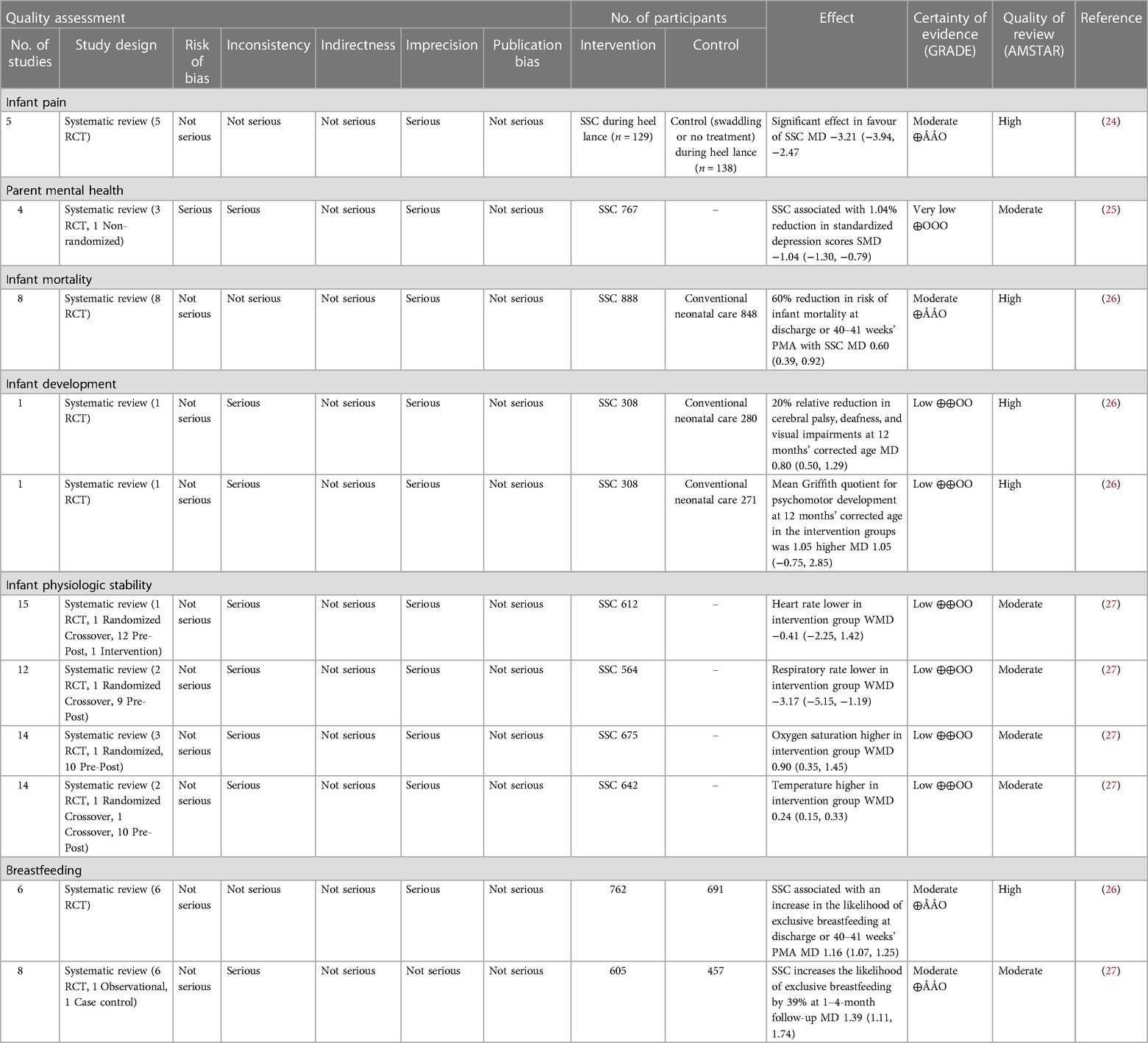
The demographic information of the Delphi participants (n = 59) and consensus panel members can be found in Table 2. The national consensus panel consisted of 21 participants including: parents of NICU babies; health care providers (neonatology, pediatrics, infectious disease, public health), health system leaders, and policy makers (Table 2). Results from the first and second Delphi surveys can be found in Tables 1, 3 respectively. National recommendations were established through discussion at 13 consensus panel meetings held between October 2022 and July 2023. An example of evidence presented to the consensus panel to support the benefits and harms associated with each recommendation can be found in Tables 4, 5. Certain items of importance from the second Delphi (Table 3) were combined, divided, added, or removed after discussions with the panel. Given the current evidence available in the literature as well as the values and preferences of members of our consensus panel, our final number of recommendations totaled 13. We aimed to establish a maximum of 10–12 national recommendations, to facilitate practice uptake (42). Of the 13 recommendations, 6 were strong recommendations in favour with 100% consensus, and 7 were conditional recommendations in favour with consensus ranging from 84%–100% (Table 6 and for a visual summary, Supplementary Material 2).
During consensus panel meetings, there was considerable discussion surrounding the language used for each recommendation. The term “unrestricted” is used for recommendations that would have no restrictions on (a) two parents in the NICU at the same time, (b) amount of time present in the NICU, (c) movement between home and hospital, (d) COVID-19 symptom status, or (e) COVID-19 positivity status (requires personal protective equipment that is effective and appropriate for the tasks being carried out). The term “uninterrupted” was used to describe provision of services that had been in place before the pandemic (i.e., attendance at medical rounds, mental health support).
4 Discussion
The purpose of this study was to co-create national Canadian consensus recommendations to address the unattended negative consequences specific to parental NICU presence and support policies, through engaging stakeholders such as parents, families, healthcare providers, researchers, leaders, and policymakers. These recommendations incorporate best available evidence, context, values, preferences, and priorities for post-COVID-19 recovery, and future pandemics caused by respiratory pathogens that are adaptive and responsive across communities.
To our knowledge, we are the first to propose a set of consensus recommendations that can be applied to the post COVID-19 pandemic recovery and future pandemics caused by respiratory pathogens.
Overall, the panel collectively agreed on the paramount importance of parental access to their babies and the imperative to prevent parent restrictions on parental access in future pandemics. Supported by the evidence, the presence of parents in the NICU demonstrated a substantial positive impact on infant and parent outcomes, outweighing the potential harms or risks associated with being present in the NICU. The first strong recommendation, highlighting that parents are essential caregivers, was added by the consensus panel. This recommendation is imperative for addressing concerns regarding organizational and health system policy formation and the inclusion of families in care practices for post-COVID-19 and future pandemic and other planning (4). Ultimately, this recommendation recognizes parents as essential caregivers, as opposed to visitors in the NICU, and supports that policies be changed to reflect this distinction.
Breastfeeding, provision of MOM and SSC recommendations both had strong, high-quality evidence to support their inclusion as unrestricted in the national recommendations. The COVID-19 pandemic caused widespread decreased in breastfeeding, provision of MOM and SSC, leading to a high risk of negative outcomes for both mothers and their infants (43). Evidence to support breastfeeding and provision of MOM and SSC and their associated immediate and long-term positive outcomes in neonates far outweighed the potential negative effects from COVID-19 (43, 44).
Parent access to psychological support services and to attend medical rounds were each split into two recommendations of “uninterrupted” (strong) and “unrestricted” (conditional). The decision to split these recommendations was made as there was not sufficient evidence in the literature to support that in-person access to these services was significantly more beneficial than virtual access in the context of COVID-19. Parent access to personal support systems using personal communication devices were combined to form one “unrestricted” (conditional) recommendation. This recommendation aligns with previous data reporting wide variation in policies allowing communication devices in NICUs (18).
While the recommendations were co-created using rigorous methods, certain limitations must be acknowledged in this study. Firstly, the phase 1 sample size comprised of only 59 participants which, while adhering to minimum Delphi methods, may limit the generalization across top-ranked items. However, the included items align with findings from larger parent and healthcare provider surveys (9). Secondly, while our findings relate to the Canadian context, the decisions made by the panelists were based on available global evidence on COVID-19 up until the consensus panel meeting. Although re-evaluation may be needed with emerging knowledge in future pandemics, this work provides a robust foundation for guiding current system change and steering future decision-making to reduce future unintended harms during future pandemics across Canada and worldwide. Lastly, the impact of parent, family and care provider restrictions during the pandemic extended beyond the NICU, affecting various care areas, including intensive care, pediatric care, general medical care, hospital care, palliative care, and nursing home care (45). Although our recommendations are tailored to the NICU setting, the framework can be applied to formulate presence recommendations and support policies in these other care contexts.
5 Conclusion
Preventing parental presence in the NICU has significant negative effects on parent mental health and well-being, as well as on infant health outcomes. Nationwide consensus in parental presence policies must be achieved to optimize parent and infant health outcomes and ensure equitable provision of neonatal care. Immediate implementation of the recommendations established from this study will reduce unintended harms associated with parent restriction and are useful for current post-recovery and future pandemics caused by respiratory pathogens.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by IWK Health Research Ethics Board #1025748. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MC-Y: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FB: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – review & editing. LA: Data curation, Formal Analysis, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. SM: Funding acquisition, Methodology, Validation, Visualization, Writing – review & editing, Investigation. MM: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. MB: Funding acquisition, Investigation, Writing – review & editing. TB: Writing – review & editing, Investigation. DC: Investigation, Writing – review & editing. AC: Funding acquisition, Writing – review & editing, Investigation. JC: Funding acquisition, Investigation, Writing – review & editing. JD: Funding acquisition, Writing – review & editing. AG: Funding acquisition, Investigation, Methodology, Writing – review & editing. JG: Funding acquisition, Investigation, Writing – review & editing. BH: Funding acquisition, Writing – review & editing. AH: Funding acquisition, Writing – review & editing. DI: Funding acquisition, Investigation, Writing – review & editing. AL: Investigation, Writing – review & editing. YL: Funding acquisition, Investigation, Writing – review & editing. TL: Funding acquisition, Investigation, Writing – review & editing. JM: Investigation, Writing – review & editing. MN: Funding acquisition, Investigation, Writing – review & editing. KO: Writing – review & editing. PR: Funding acquisition, Investigation, Writing – review & editing. MS: Funding acquisition, Investigation, Writing – review & editing. PS: Funding acquisition, Investigation, Writing – review & editing. LW: Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Funding for this study was provided through a grant from the Canadian Institutes of Health Research (CIHR). Award number: 464929
Acknowledgments
This paper is submitted on behalf of the Canadian Neonatal Network Investigators.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1390209/full#supplementary-material
References
2. Woythaler M. Neurodevelopmental outcomes of the late preterm infant. Semin Fetal Neonatal Med. (2019) 24(1):54–9. doi: 10.1016/j.siny.2018.10.002
3. Lui K, Lee SK, Kusuda S, Adams M, Vento M, Reichman B, et al. Trends in outcomes for neonates born very preterm and very low birth weight in 11 high-income countries. J Pediatr. (2019) 215:32–40.e14. doi: 10.1016/j.jpeds.2019.08.020
4. van Veenendaal NR, Deierl A, Bacchini F, O’Brien K, Franck LS. Supporting parents as essential care partners in neonatal units during the SARS-CoV-2 pandemic. Acta Paediatr. (2021) 110(7):2008–22. doi: 10.1111/apa.15857
5. O’Brien K, Robson K, Bracht M, Cruz M, Lui K, Alvaro R, et al. Effectiveness of family integrated care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health. (2018) 2(4):245–54. doi: 10.1016/S2352-4642(18)30039-7
6. Paremoer L, Nandi S, Serag H, Baum F. COVID-19 pandemic and the social determinants of health. BMJ. (2021) 372:n129. doi: 10.1136/bmj.n129
7. Cena L, Biban P, Janos J, Lavelli M, Langfus J, Tsai A, et al. The collateral impact of COVID-19 emergency on neonatal intensive care units and family-centered care: challenges and opportunities. Front Psychol. (2021) 12:630594. doi: 10.3389/fpsyg.2021.630594
8. Bembich S, Tripani A, Mastromarino S, Di Risio G, Castelpietra E, Risso FM. Parents experiencing NICU visit restrictions due to COVID-19 pandemic. Acta Paediatr. (2020) 110(3):940–1. doi: 10.1111/apa.15620
9. Campbell-Yeo M, Dol J, McCulloch H, Hughes B, Hundert A, Bacchini F, et al. The impact of parental presence restrictions on Canadian parents in the NICU during COVID-19: a national survey. J Fam Nurs. (2023) 29(1):18–27. doi: 10.1177/10748407221114326
10. Campbell-Yeo M, Dol J, Richardson B, McCulloch H, Hundert A, Foye S, et al. A co-design of clinical virtual care pathways to engage and support families requiring neonatal intensive care in response to the COVID-19 pandemic (COVES study). J Neonatal Nurs.. (2021) 27(6):463–70. doi: 10.1016/j.jnn.2021.06.010
11. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J. (2010) 182(18):E839–42. doi: 10.1503/cmaj.090449
12. Trevelyan EG, Robinson N. Delphi methodology in health research: how to do it? Eur J Integr Med. (2015) 7(4):423–28. doi: 10.1016/j.eujim.2015.07.002
13. Van Den Broek-Altenburg E, Atherly A. Using discrete choice experiments to measure preferences for hard to observe choice attributes to inform health policy decisions. Health Econ Rev. (2020) 10(1):18. doi: 10.1186/s13561-020-00276-x
14. Zhang Y, Coello PA, Brożek J, Wiercioch W, Etxeandia-Ikobaltzeta I, Akl EA, et al. Using patient values and preferences to inform the importance of health outcomes in practice guideline development following the GRADE approach. Health Qual Life Outcomes. (2017) 15(1):52. doi: 10.1186/s12955-017-0621-0
15. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
16. MacNeil M, Campbell-Yeo M, McCulloch H, Hughes B, Dol J, Marriott N, et al. Parental perspectives on impact of parental presence restrictions in the neonatal intensive care unit during the COVID-19 pandemic: a cross-sectional study. J Perinat Neonatal Nurs. (2023) 37(4):E17–23. doi: 10.1097/JPN.0000000000000714
17. McCulloch H, Campbell-Yeo M, Richardson B, Dol J, Hundert A, Dorling J, et al. The impact of restrictive family presence policies in response to COVID-19 on family integrated care in the NICU: a qualitative study. HERD. (2022) 15(2):49–62. doi: 10.1177/19375867211065178
18. Campbell-Yeo M, McCulloch H, Hughes B, Hundert A, Dol J, Smit M, et al. Parental perspectives on technology use to enhance communication and closeness during the COVID-19 parental presence restrictions. J Neonatal Nurs. (2023) 29(1):169–73. doi: 10.1016/j.jnn.2022.05.002
19. Sullivan GM, Artino AR. Analyzing and interpreting data from Likert-type scales. J Grad Med Educ. (2013) 5(4):541–42. doi: 10.4300/JGME-5-4-18
20. Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. (2021) 130:13–22. doi: 10.1016/j.jclinepi.2020.10.007
21. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336(7650):924–26. doi: 10.1136/bmj.39489.470347.AD
22. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. (2007) 7(1):10. doi: 10.1186/1471-2288-7-10
23. Hong QN, Gonzalez-Reyes A, Pluye P. Improving the usefulness of a tool for appraising the quality of qualitative, quantitative and mixed methods studies, the mixed methods appraisal tool (MMAT). J Eval Clin Pract. (2018) 24(3):459–67. doi: 10.1111/jep.12884
24. Johnston C, Campbell-Yeo M, Disher T, Benoit B, Fernandes A, Streiner D, et al. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst Rev. (2017) 2:CD008435. doi: 10.1002/14651858.CD008435.pub3
25. Scime NV, Gavarkovs AG, Chaput KH. The effect of skin-to-skin care on postpartum depression among mothers of preterm or low birthweight infants: a systematic review and meta-analysis. J Affect Disord. (2019) 253:376–84. doi: 10.1016/j.jad.2019.04.101
26. Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. (2016) 8:CD002771. doi: 10.1002/14651858.CD002771.pub4
27. Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics. (2016) 137(1):e20152238. doi: 10.1542/peds.2015-2238
28. De La Rosa-Zamboni D, Ortega-Riosvelasco F, González-García N, Gamiño-Arroyo AE, Espinosa-González GA, Valladares-Wagner JM, et al. Tracing COVID-19 source of infection among health personnel in a pediatric hospital. Front Pediatr. (2022) 10:897113. doi: 10.3389/fped.2022.897113
29. Ferreira M, Garcia C, Barroso R. Characteristics of newborns from mothers with SARS-CoV-2 infection in a Portuguese hospital. Acta Médica Portuguesa. (2021) 34(10):650–56. doi: 10.20344/amp.16180
30. Dumitriu D, Gyamfi-Bannerman C. Understanding risk for newborns born to SARS-CoV-2-positive mothers. JAMA. (2021) 325(20):2051. doi: 10.1001/jama.2021.6210
31. Elenga N, Wandji M-J, Siban J, Nacher M, Demar M. Neonatal outcomes related to maternal SARS-CoV-2 infection in French guiana: a case-control study. J Infect Public Health. (2022) 15(7):746–51. doi: 10.1016/j.jiph.2022.06.003
32. Ibrahim CPH, Lobko FO, Alchamat GA, Swilam WG, Wani SR, Said ST, et al. Management of infants born to mothers with SARS-CoV2 infection: a prospective observational study. BMJ Paediatr Open. (2020) 4(1):e000824. doi: 10.1136/bmjpo-2020-000824
33. Vila-Candel R, González-Chordá VM, Soriano-Vidal FJ, Castro-Sánchez E, Rodríguez-Blanco N, Gómez-Seguí A, et al. Obstetric–neonatal care during birth and postpartum in symptomatic and asymptomatic women infected with SARS-CoV-2: a retrospective multicenter study. Int J Environ Res Public Health. (2022) 19(9):5482. doi: 10.3390/ijerph19095482
34. AlQurashi MA, Alattas A, Shirah B, Mustafa A, Al-Hindi MY, Alrefai A, et al. Clinical characteristics of newborn infants delivered to pregnant women with laboratory-confirmed COVID-19: a single-center experience from Saudi Arabia. Cureus. (2021) 13(10):e18573. doi: 10.7759/cureus.18573
35. Baquedano-Lobera I, Lalaguna-Mallada P, Barberá-Pérez P. Pediatric hospitalization due to COVID-19: experience in a regional hospital. Bol Med Hosp Infant Mex. (2022) 79(2):7457. doi: 10.24875/BMHIM.21000147
36. Congdon JL, Kair LR, Flaherman VJ, Wood KE, LoFrumento MA, Nwaobasi-Iwuh E, et al. Management and early outcomes of neonates born to women with SARS-CoV-2 in 16 U.S. hospitals. Am J Perinatol. (2021) 38(06):622–31. doi: 10.1055/s-0041-1726036
37. Giuliani F, Oros D, Gunier RB, Deantoni S, Rauch S, Casale R, et al. Effects of prenatal exposure to maternal COVID-19 and perinatal care on neonatal outcome: results from the INTERCOVID multinational cohort study. Am J Obstet Gynecol. (2022) 227(3):488.e1–17. doi: 10.1016/j.ajog.2022.04.019
38. Gupta V, Yadav Y, Sharma R, Mishra M, Ambedkar D, Gupta V. Maternal and perinatal outcomes of hospitalized COVID-19 positive pregnant women. Cureus. (2022) 14(2):e21817. doi: 10.7759/cureus.21817
39. Kyle MH, Glassman ME, Khan A, Fernández CR, Hanft E, Emeruwa UN, et al. A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. (2020) 44(7):151286. doi: 10.1016/j.semperi.2020.151286
40. Shalish W, Lakshminrusimha S, Manzoni P, Keszler M, Sant’Anna GM. COVID-19 and neonatal respiratory care: current evidence and practical approach. Am J Perinatol. (2020) 37(08):780–91. doi: 10.1055/s-0040-1710522
41. Verulava T, Galogre N. Epidemiological characteristics of neonates born to mothers infected with COVID-19: a single-centre observational study. J Neonatal Perinatal Med. (2022) 15(2):291–95. doi: 10.3233/NPM-210883
42. Gupta S, Rai N, Bhattacharrya O, Cheng AYY, Connelly KA, Boulet L-P, et al. Optimizing the language and format of guidelines to improve guideline uptake. Can Med Assoc J. (2016) 188(14):E362–8. doi: 10.1503/cmaj.151102
43. Spatz DL, Davanzo R, Müller JA, Powell R, Rigourd V, Yates A, et al. Promoting and protecting human milk and breastfeeding in a COVID-19 world. Front Pediatr. (2021) 8:633700. doi: 10.3389/fped.2020.633700
44. Vassilopoulou E, Feketea G, Koumbi L, Mesiari C, Berghea EC, Konstantinou GN. Breastfeeding and COVID-19: from nutrition to immunity. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.661806
Keywords: COVID-19, neonatal care, parental presence, practice recommendations, participatory research (PR), pandemic planning, respiratory pathogens
Citation: Campbell-Yeo M, Bacchini F, Alcock L, Mitra S, MacNeil M, Mireault A, Beltempo M, Bishop T, Campbell DM, Chilcott A, Comeau JL, Dol J, Grant A, Gubbay J, Hughes B, Hundert A, Inglis D, Lakoff A, Lalani Y, Luu TM, Morton J, Narvey M, O’Brien K, Robeson P, Science M, Shah P and Whitehead L (2024) Practice recommendations regarding parental presence in NICUs during pandemics caused by respiratory pathogens like COVID-19. Front. Pediatr. 12:1390209. doi: 10.3389/fped.2024.1390209
Received: 22 February 2024; Accepted: 17 May 2024;
Published: 13 June 2024.
Edited by:
Kee Thai Yeo, KK Women's and Children's Hospital, SingaporeReviewed by:
Nai Ming Lai, Taylor's University, MalaysiaAlvin S. M. Chang, KK Women's and Children's Hospital, Singapore
© 2024 Campbell-Yeo, Bacchini, Alcock, Mitra, MacNeil, Mireault, Beltempo, Bishop, Campbell, Chilcott, Comeau, Dol, Grant, Gubbay, Hughes, Hundert, Inglis, Lakoff, Lalani, Luu, Morton, Narvey, O'Brien, Robeson, Science, Shah and Whitehead. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marsha Campbell-Yeo, bWFyc2hhLmNhbXBiZWxsLXllb0BkYWwuY2E=
 Marsha Campbell-Yeo
Marsha Campbell-Yeo Fabiana Bacchini
Fabiana Bacchini Lynsey Alcock
Lynsey Alcock Souvik Mitra4
Souvik Mitra4 Amy Mireault
Amy Mireault Marc Beltempo
Marc Beltempo Douglas M. Campbell
Douglas M. Campbell Thuy Mai Luu
Thuy Mai Luu Michael Narvey
Michael Narvey Prakesh Shah
Prakesh Shah