
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 08 August 2024
Sec. Pediatric Endocrinology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1388437
 Domenico Corica1,†
Domenico Corica1,† Fabio Toscano1,†
Fabio Toscano1,† Mariacarla Moleti2
Mariacarla Moleti2 Giorgia Pepe1
Giorgia Pepe1 Alfredo Campenni3
Alfredo Campenni3 Guido Fadda4
Guido Fadda4 Gianlorenzo Dionigi5
Gianlorenzo Dionigi5 Carmelo Romeo6
Carmelo Romeo6 Tommaso Aversa1,‡
Tommaso Aversa1,‡ Malgorzata Wasniewska1*‡
Malgorzata Wasniewska1*‡
Thyroid nodules in children are less common than in adults but they are approximately two- to three-fold more likely to be malignant in children. Among thyroid nodular diseases, Plummer's adenoma occurs very rarely in pediatrics, and currently, there is no literature providing evidence of this diagnosis in patients with Prader–Willi syndrome (PWS). We report the case of a 9-year-old Caucasian boy affected by PWS presenting with a rapidly growing palpable mass in the thyroid lodge associated with subclinical hyperthyroidism. Laboratory and other examinations (thyroid ultrasound, fine-needle aspiration of the nodule, and scintigraphy) were strongly suggestive for Plummer's adenoma; therefore, the patient underwent left hemithyroidectomy surgery, and anatomo-pathological examination confirmed the diagnosis. Our case describes the first evidence of an isolated follicular adenoma in children with PWS. Surgery is the only therapeutic option in younger children. Further evidence is needed to assess the possible correlation between these two conditions and the existence of potential risk factors.
Thyroid nodular disease is very rare at pediatric ages. Approximately 2% of children and adolescents have palpable thyroid nodules and 0.5%–5% have ultrasonography-demonstrated nodules in the gland (1–3). Most thyroid nodules in children are benign but approximately 26% represent a differentiated thyroid cancer (DTC); therefore, although thyroid nodules in children are less common than in adults, they are approximately two- to three-fold more likely to be malignant (4). Children with thyroid nodules should be evaluated with serum thyroid-stimulating hormone (TSH) and free thyroxine (fT4), and thyroid ultrasound. Usually, the majority of children are euthyroid; in this case, a fine-needle aspiration (FNA) of the nodule should be performed if it is >1 cm or, regardless of the size, there is a suspicious clinical context or there are specific ultrasound features of malignancy (5). Furthermore, an increase in nodule size during the follow-up is considered an index of malignancy, and it is appropriate to repeat an FNA when the previous sampling was non-diagnostic (6). In 5% of cases, thyroid nodules occur with hyperthyroidism; in the presence of low/suppressed TSH levels, a Plummer's adenoma (PA) is suspected and a thyroid scintigraphy should be performed (5).
PA (or toxic adenoma or autonomous functioning thyroid adenoma) accounts for approximately 5%–10% of all solitary nodules and occurs predominantly in females (13:1), more commonly in older age groups (7). In children and adolescents, PA is an unusual finding but it seems to have a more rapidly progressive course than those in adults (8). Increased radioiodine uptake within the nodule on thyroid scintigraphy, associated with a suppression in the surrounding tissue of the gland, is consistent with PA (5). These lesions are most frequently associated with somatic activating mutations within the genes encoding the TSH receptor or the Gs-alpha subunit (9). To date, up to one-third of patients may have an incidentally discovered DTC associated with autonomous nodules. In this regard, surgical treatment (lobectomy plus isthmusectomy in most cases) is the recommended approach for most pediatric patients and patients below 10 years of age, considering that below that age, I-131 ablation is regarded as a contraindication according to the most recent European Association of Nuclear Medicine (EANM) guidelines (10), and it should be proposed at the time of the diagnosis (5).
Prader–Willi Syndrome (PWS), the most common cause of genetic obesity in the pediatric population, is a complex disorder with hypothalamus-pituitary axis abnormalities (anterior pituitary hypoplasia and an absent, small, or ectopic posterior pituitary gland) in more than 50% of patients, possibly resulting in endocrinological dysfunctions [growth hormone (GH) deficiency, hypogonadotropic hypogonadism, adrenal insufficiency, and hypothyroidism] (11–15). In this regard, the most common thyroid dysfunction in PWS seems to be central hypothyroidism (CH) (16, 17), the incidence of which varies from 2% to 32% (12, 14, 18, 19). However, some authors have reported a prevalence of CH in PWS that is similar to the general population (20, 21). Rare cases of congenital hypothyroidism (22, 23) and fetal goiter (24) have been reported, whereas descriptions of hyperthyroidism cases are sporadic (25). To date, no PA cases in either adult or pediatric subjects with PWS have been described in the scientific literature. In this report, we describe the first case of PA reported in a child with PWS presenting as a rapidly growing thyroid nodular lesion and subclinical hyperthyroidism.
A 9-year-old boy, followed at our Pediatric Endocrinology Outpatients Clinic for PWS, owing to maternal uniparental disomy for chromosome 15, presented with the appearance of a palpable non-painful mass at the left anterior cervical site in the thyroid lodge. The patient, who was receiving recombinant human growth hormone (rhGH) therapy since the age of 1 year, had regular growth in height and weight; at the time of the examination he had a stature of 130 cm (+0.05 SD), a weight of 36.7 kg (+1.48 SD), a body mass index of 21.75 kg/m2 (+1.67 SD), and a regular growth rate (7.6 cm/year). The child was prepubescent in accordance with the Tanner Stage. Familial history was negative for thyroid diseases. During outpatient follow-up, thyroid function was always within normal limits and anti-thyroid antibodies were negative, as was the case at the last check-up 6 months before the diagnosis of the palpable neck mass: TSH, 1.2 uIU/ml [normal value (n.v.) 0.27–4.2]; free triiodothyronine (fT3), 3.6 pg/ml (n.v. 2.0–4.4); and fT4, 19 pmol/L (n.v. 12.0–22.0); anti-thyroglobulin (AbTg), anti-peroxidase (AbTPO), and TSH receptor (TRAb) antibodies were negative.
Following the aforementioned finding, the child therefore underwent a thyroid ultrasound that documented the presence of a nodule in the left lobe [longitudinal diameter (LD), 17 mm] with a disomogeneous structure and microcalcifications, and without pathological findings at the color-Doppler evaluation (shown in Figure 1). Thus, considering nodule dimension, in accordance with the most recent guidelines (5), the patient underwent a fine-needle aspiration biopsy (FNAB), which suggested a non-malignant lesion (TIR2 according to the 2014 Italian consensus for the classification of thyroid cytology) (26). Over approximately 6 months, thyroid biochemical assessment shifted toward a subclinical hyperthyroidism: TSH, 0.005 uIU/ml; fT4, 20.4 pmol/L; and fT3 5.79 pg/ml. Thyroid autoantibodies were still negative. At the ultrasound reassessment, the nodule had grown in size (LD, 22 mm) and increased vascular signals were evident. A second FNAB confirmed the presence of a non-malignant lesion/adenomatous struma (TIR2) (26). Owing to the TSH suppression, a scintigraphic examination was carried out, which documented a defined focal uptake of iodine-123 in the nodule with suppressed uptake in the rest of the gland (shown in Figure 2). In light of the rapid and progressive increase in nodule size, the biochemical pattern of subclinical hyperthyroidism, and the scintigraphic finding of a hyper capturing nodule, the patient underwent left hemithyroidectomy surgery. The anatomo-pathological examination revealed a follicular adenoma, confirming the diagnosis of PA, that was hyperfunctioning on the basis of the hormonal profile. Figure 3 shows the timeline from the first clinical finding of the thyroid nodule to the diagnosis of PA. Two months after the surgery, the boy developed an acquired hypothyroidism, possibly due to a limited functionality of the residual thyroid tissue. Levothyroxine therapy was started. Five months after the hemithyroidectomy and the start of levothyroxine therapy, euthyroidism was maintained with a 1 μg/kg/day dose of levothyroxine.
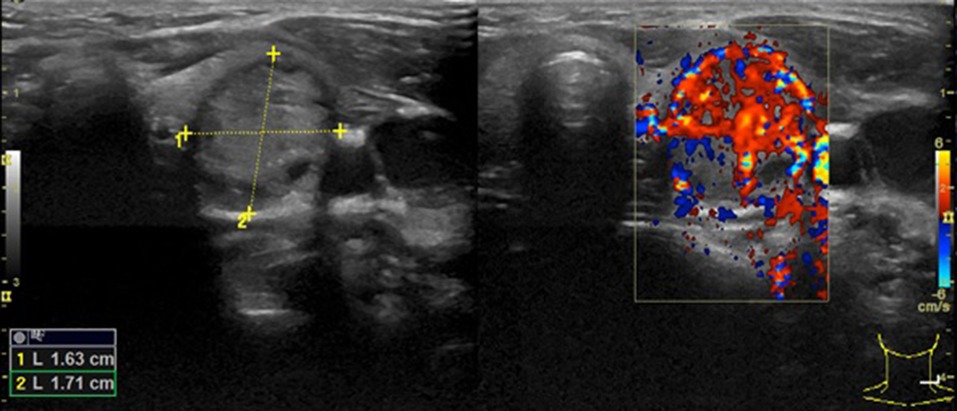
Figure 1. The first thyroid ultrasound examination showing a solitary thyroid nodule with an LD of 17 mm in the left lobe.
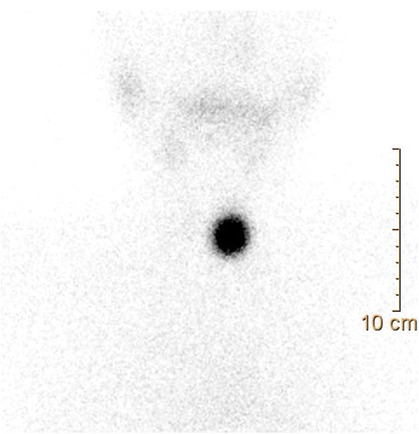
Figure 2. Thyroid scintigraphy: a scintigraphic exam documenting a hyperfunctioning nodule, as shown by the focal iodine-131 concentration on the nodule with suppression of the surrounding thyroid gland tissue.
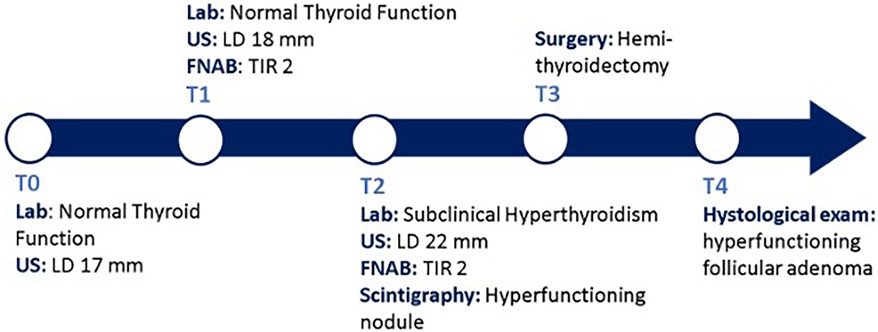
Figure 3. Diagnostic and therapeutic timeline. T0, the patient came to our attention with a palpable thyroid mass with an LD of 17 mm at the US exam, within normal thyroid function. T1, A FNAB was conducted due to the ultrasound dimensions of the nodule; malign causes were excluded. T2, after 6 months, the nodule grew up to 22 mm in LD, with evidence of some risk factors at the US exam, and TSH suppression was documented; a second FNAB confirmed a non-malignant lesion (TIR2) and the thyroid scintigraphy documented a focal iodine-132 concentration on the nodule with suppression of the surrounding gland tissue. T3, the patient underwent hemithyroidectomy surgery. T4, the histological examination confirmed the diagnosis of PA. Lab, laboratory exams; US, ultrasound; FNAB, fine-needle aspiration biopsy; PA, Plummer's adenoma; TSH, thyroid-stimulating hormone.
The clinical case described in this report is the first evidence of PA in a child with PWS. PA seems to be extremely rare in children and adolescents, even when referring to the general population. There are limited data about the incidence of PA in children. Toxic adenoma has been reported in 5%–7.5% of pediatric patients with thyroid nodules (27). Children with a hyperfunctioning autonomous nodule may indeed present with either mild or overt hyperthyroidism or sometimes euthyroidism (5). There seems to be a correlation between the size of the nodule and the biochemical picture. It has been reported that autonomous nodules less than 2.5 cm in diameter usually show incomplete suppression of the surrounding thyroid parenchyma, resulting in a subclinical hyperthyroidism. On the other hand, complete suppression is usually associated with lesions of 3 cm or more in size (7). However, the natural history of this rare condition and the correlation between the biochemical and radiological picture of the (complete or incomplete) suppression of the thyroid parenchyma surrounding the lesion have not been well defined (5). The available data on the prevalence and evolution of thyroid diseases in subjects with PWS almost exclusively concern CH and congenital hypothyroidism (14, 19), thus there are insufficient data to hypothesize a peculiar evolution of the thyroid biochemical picture of PA in PWS.
The most recent guidelines recommend surgery, lobectomy plus isthmectomy in most cases, as the optimal initial management for children with PA because of concerns about the mutagenic effect of radioiodine on the normal thyroid tissue, supported by the concern that the cancer risk in children with a thyroid nodular disease is higher than in the adult population and up to one-third of patients may have an incidentally discovered cancer associated with autonomous nodules (5, 7).
PWS is not considered a cancer predisposition syndrome: a recent retrospective study with a large international cohort of 706 patients confirmed that malignancies are rare in patients with PWS and usually due to a multifactorial etiology (28). To date, 50 published reports describe malignancies in individuals with PWS (29), including acute lymphoblastic leukemia (30), acute and chronic myeloid leukemia (31), hepatoblastoma (32), medulloblastoma (33), pulmonary carcinoid tumor (34), Wilms tumor (35), multiple endocrine neoplasia type 1 (36), a case of fetal cardiac rhabdomyoma (37), and gonadal germ cell tumors in 22.5% of all cases (11/50) (29). Maya-González et al. showed no increased risk of cancer at any age in PWS patients compared with the general population; however, among individuals developing cancer, they highlighted an increased prevalence of tumors at pediatric age in PWS patients compared with the control group (25% vs. 8.7%) (29). Moreover, they noticed an increased occurrence of gonadal (testicular or ovarian) tumors in PWS at any age in comparison with the control group (17% vs. 3%), suggesting an increased risk for these neoplasms in PWS (29). Furthermore, these authors (29) suggested a loss of imprinting (LOI) as a possible mechanism of tumorigenesis in these patients, as documented in their case report (29) and in a 13-year-old girl with PWS who also developed bilateral ovarian sex cord tumors with annular tubules (38).
The role of GH therapy in influencing the risk of cancer in PWS has been debated. GH treatment has shown significant beneficial effects on linear growth, body composition, physical strength, and mental development, with a reassuring safety profile for daily administration in children with PWS (39–41). Sjöström and Höybye documented that, over a follow-up period of approximately 20 years, GH treatment during childhood or in adulthood with PWS is not associated with an increased risk of cancer (42). Hirsch and Gross-Tsur hypothesized that increases in insulin-like factor-1 as a result of GH treatment over the course of several decades in PWS adults raise concern over the possible increase in the risk of cancer (43), although there is no evidence to date of a clear correlation between GH therapy and cancer risk in PWS subjects (14, 28). Multiple observational studies carried out in a non-PWS population did not indicate an increased risk of malignancy after treatment with GH during childhood (44, 45). However, it should be considered that the Safety and Appropriateness of Growth Hormone Treatments in Europe (SAGhE) study showed an increased incidence of cancer related to second primary malignancies in patients who received GH treatment after cancer treatment (46). Therefore, GH treatment is considered safe, although some authors suggest that the involvement of additional genetic factors and/or GH treatment in cancer development cannot be excluded (28, 30, 46).
In the few studies available, the malignancy rate of hyperfunctioning nodules at pediatric age ranges from 0% to 29% (27): Niedziela et al. reported an incidence of 29% malignancy among 31 children and adolescents with hyperfunctioning nodules detected by 99mTc scintigraphy (47). A group in Belo Horizonte, Brazil, reported an incidence of malignancy of 5.9% in a series of 13 patients with 17 hyperfunctioning nodules (48). On the other hand, Ly et al. reported no cancer in their single-center experience with 31 children diagnosed with PA, suggesting that conservative management could be offered in childhood, deferring definitive therapies until adulthood (49). Gundgurthi et al. reported the case of a 3-year-old boy with PA successfully treated with 131-iodine radio ablation (50).
Concerning our patient, no noticeable risk factors for cancer, such as obesity, a previous primary malignancy before starting GH treatment, or a suspected genetic predisposition, have been revealed according to the underlying syndrome. Nevertheless, in accordance with the most recent guidelines (5, 7), taking into account case reports of DTC found after the resection of a PA that support hemithyroidectomy as the treatment of choice (51–53), and considering that our patient with PWS and PA is a male younger than 10 years of age, with an unknown risk of malignancy in nodular disease, we decided to proceed with a hemithyroidectomy.
To the best of our knowledge, this is the first report describing PA in a patient with PWS. The management of these rare cases should refer to the most recent guidelines in the management of pediatric thyroid pathology, taking into account the patient's characteristics. Further evidence is needed to assess a possible correlation between PWS and PA, the existence of potential risk factors associated with the appearance of these lesions, and the potential risk of malignancy of hyperfunctioning nodules in PWS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies involving humans because it is not required for a Case Report. This report was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) and minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
DC: Writing – original draft, Writing – review & editing, Resources. FT: Writing – original draft, Writing – review & editing. MM: Methodology, Writing – review & editing. GP: Resources, Writing – review & editing. AC: Methodology, Writing – review & editing. GF: Methodology, Writing – review & editing. GD: Methodology, Writing – review & editing. CR: Investigation, Writing – review & editing. TA: Conceptualization, Writing – review & editing. MW: Conceptualization, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank the parents for providing consent to publish anonymized data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang H, Mehrad M, Ely KA, Liang J, Solórzano CC, Neblett WW III, et al. Incidence and malignancy rates of indeterminate pediatric thyroid nodules. Cancer Cytopathol. (2019) 127(4):231–9. doi: 10.1002/cncy.22104
2. Kaloumenou I, Alevizaki M, Ladopoulos C, Antoniou A, Duntas LH, Mastorakos G, et al. Thyroid volume and echostructure in schoolchildren living in an iodine-replete area: relation to age, pubertal stage, and body mass index. Thyroid. (2007) 17(9):875–81. doi: 10.1089/thy.2006.0327
3. Shimura H, Sobue T, Takahashi H, Yasumura S, Ohira T, Ohtsuru A, et al. Thyroid examination unit of the radiation medical center for the Fukushima Health Management Survey Group. Findings of thyroid ultrasound examination within 3 years after the Fukushima nuclear power plant accident: the Fukushima health management survey. J Clin Endocrinol Metab. (2018) 103(3):861–9. doi: 10.1210/jc.2017-01603
4. Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. (2006) 13(2):427–53. doi: 10.1677/erc.1.00882
5. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. American thyroid association guidelines task force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 25(7):716–59. doi: 10.1089/thy.2014.0460
6. Corrias A, Mussa A. Thyroid nodules in pediatrics: which ones can be left alone, which ones must be investigated, when and how. J Clin Res Pediatr Endocrinol. (2013) 5(Suppl 1):57–69. doi: 10.4274/jcrpe.853
7. Thomas CG Jr, Croom RD III. Current management of the patient with autonomously functioning nodular goiter. Surg Clin North Am. 1987;67(2):315–28. doi: 10.1016/s0039-6109(16)44186-1
8. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. (2016) 26(10):1343–421. Erratum in: Thyroid. (2017) 27(11):1462. doi: 10.1089/thy.2016.0229
9. Grob F, Deladoëy J, Legault L, Spigelblatt L, Fournier A, Vassart G, et al. Autonomous adenomas caused by somatic mutations of the thyroid-stimulating hormone receptor in children. Horm Res Paediatr. (2014) 81(2):73–9. doi: 10.1159/000357143
10. Campennì A, Avram AM, Verburg FA, Iakovou I, Hänscheid H, de Keizer B, et al. The EANM guideline on radioiodine therapy of benign thyroid disease. Eur J Nucl Med Mol Imaging. (2023) 50:3324–48. doi: 10.1007/s00259-023-06274-5
11. Butler MG, Miller JL, Forster JL. Prader-Willi syndrome—Clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. (2019) 15(4):207–44. doi: 10.2174/1573396315666190716120925
12. Alves C, Franco RR. Prader-Willi syndrome: endocrine manifestations and management. Arch Endocrinol Metab. (2020) 64(3):223–34. doi: 10.20945/2359-3997000000248
13. Diene G, Mimoun E, Feigerlova E, Caula S, Molinas C, Grandjean H, et al. French reference Centre for PWS. Endocrine disorders in children with Prader-Willi syndrome—Data from 142 children of the French database. Horm Res Paediatr. (2010) 74(2):121–8. doi: 10.1159/000313377
14. Madeo SF, Zagaroli L, Vandelli S, Calcaterra V, Crinò A, De Sanctis L, et al. Endocrine features of Prader-Willi syndrome: a narrative review focusing on genotype-phenotype correlation. Front Endocrinol. (2024) 15:1382583. doi: 10.3389/fendo.2024.1382583
15. Salvatore M, Torreri P, Grugni G, Rocchetti A, Maghnie M, Patti G, et al. The Italian registry for patients with Prader–Willi syndrome. Orphanet J Rare Dis. (2023) 18:28. doi: 10.1186/s13023-023-02633-5
16. Vaiani E, Herzovich V, Chaler E, Chertkoff L, Rivarola MA, Torrado M, et al. Thyroid axis dysfunction in patients with Prader-Willi syndrome during the first 2 years of life. Clin Endocrinol. (2010) 73(4):546–50. doi: 10.1111/j.1365-2265.2010.03840.x
17. Iughetti L, Vivi G, Balsamo A, Corrias A, Crinò A, Delvecchio M, et al. Thyroid function in patients with Prader-Willi syndrome: an Italian multicenter study of 339 patients. J Pediatr Endocrinol Metab. (2019) 32(2):159–65. doi: 10.1515/jpem-2018-0388
18. Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Speakers contributors at the second expert meeting of the comprehensive care of patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. (2008) 93(11):4183–97. Erratum in: J Clin Endocrinol Metab. (2010) 95(12):5465. doi: 10.1210/jc.2008-0649
19. Casto C, Pepe G, Li Pomi A, Corica D, Aversa T, Wasniewska M. Hashimoto’s thyroiditis and Graves’ disease in genetic syndromes in pediatric age. Genes (Basel). (2021) 12:222. doi: 10.3390/genes12020222
20. Butler MG, Theodoro M, Skouse JD. Thyroid function studies in Prader-Willi syndrome. Am J Med Genet A. (2007) 143A(5):488–92. doi: 10.1002/ajmg.a.31683
21. Sharkia M, Michaud S, Berthier MT, Giguère Y, Stewart L, Deladoëy J, et al. Thyroid function from birth to adolescence in Prader-Willi syndrome. J Pediatr. (2013) 163(3):800–5. doi: 10.1016/j.jpeds.2013.03.058
22. Bocchini S, Fintini D, Grugni G, Boiani A, Convertino A, Crinò A. Congenital hypothyroidism due to ectopic sublingual thyroid gland in Prader-Willi syndrome: a case report. Ital J Pediatr. (2017) 43(1):87. doi: 10.1186/s13052-017-0403-7
23. Sher C, Bistritzer T, Reisler G, Reish O. Congenital hypothyroidism with Prader-Willi syndrome. J Pediatr Endocrinol Metab. (2002) 15(1):105–7. doi: 10.1515/jpem.2002.15.1.105
24. Insoft RM, Hurvitz J, Estrella E, Krishnamoorthy KS. Prader-Willi syndrome associated with fetal goiter: a case report. Am J Perinatol. (1999) 16(1):29–31. doi: 10.1055/s-2007-993832
25. Pellikaan K, Snijders F, Rosenberg AGW, Davidse K, van den Berg SAA, Visser WE, et al. Thyroid function in adults with Prader-Willi syndrome: a cohort study and literature review. J Clin Med. (2021) 10(17):3804. doi: 10.3390/jcm10173804
26. Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest. (2014) 37(6):593–9. doi: 10.1007/s40618-014-0062-0
27. Hodax JK, Reinert SE, Quintos JB. Autonomously functioning thyroid nodules in patients <21 years of age: the Rhode Island hospital experience from 2003 to 2013. Endocr Pract. (2016) 22(3):328–37. doi: 10.4158/EP15905.OR
28. Pellikaan K, Nguyen NQC, Rosenberg AGW, Coupaye M, Goldstone AP, Høybye C, et al. Malignancies in Prader-Willi syndrome: results from a large international cohort and literature review. J Clin Endocrinol Metab. (2023) 108(12):e1720–30. doi: 10.1210/clinem/dgad312
29. Maya-González C, Wessman S, Lagerstedt-Robinson K, Taylan F, Tesi B, Kuchinskaya E, et al. Register-based and genetic studies of Prader-Willi syndrome show a high frequency of gonadal tumors and a possible mechanism for tumorigenesis through imprinting relaxation. Front Med. (2023) 10:1172565. doi: 10.3389/fmed.2023.1172565
30. Kato M, Mugishima H, Chin M, Urakami T, Harada K. Acute lymphoblastic leukemia in a patient with Prader-Willi syndrome under growth hormone therapy. Pediatr Int. (2005) 47(3):336–7. doi: 10.1111/j.1442-200x.2005.02068.x
31. Davies HD, Leusink GL, McConnell A, Deyell M, Cassidy SB, Fick GH, et al. Myeloid leukemia in Prader-Willi syndrome. J Pediatr. (2003) 142(2):174–8. doi: 10.1067/mpd.2003.81
32. Hashizume K, Nakajo T, Kawarasaki H, Iwanaka T, Kanamori Y, Tanaka K, et al. Prader-Willi syndrome with del(15)(q11, q13) associated with hepatoblastoma. Acta Paediatr Jpn. (1991) 33(6):718–22. doi: 10.1111/j.1442-200X.1991.tb02597.x
33. Panagopoulou P, Sattar S, Aquilina K, Jan W, Jacques T, Slater O. Challenges in the diagnosis of medulloblastoma recurrence at an unusual site in a patient with Prader-Willi syndrome. J Pediatr Hematol Oncol. (2019) 42(5):e381–4. doi: 10.1097/MPH.0000000000001555
34. Nenekidis I, Stathopoulos GT, Anagnostakou V, Kokkori A, Dedeilias P, Kokotsakis J, et al. Atypical pulmonary carcinoid tumour in a 28-year-old nonsmoker with Prader-Willi syndrome. Eur Respir J. (2011) 38(5):1230–3. doi: 10.1183/09031936.00034711
35. Coppes MJ, Sohl H, Teshima IE, Mutirangura A, Ledbetter DH, Weksberg R. Wilms tumor in a patient with Prader-Willi syndrome. J Pediatr. (1993) 122(5 Pt 1):730–3. doi: 10.1016/s0022-3476(06)80015-6
36. Nakajima K, Sakurai A, Kubota T, Katai M, Mori J, Aizawa T, et al. Multiple endocrine neoplasia type 1 concomitant with Prader-Willi syndrome: case report and genetic diagnosis. Am J Med Sci. (1999) 317(5):346–9. doi: 10.1097/00000441-199905000-00012
37. Traisrisilp K, Sirikunalai P, Sirilert S, Chareonsirisuthigul T, Tongsong T. Cardiac rhabdomyoma as a possible new prenatal sonographic feature of Prader-Willi syndrome. J Obstet Gynaecol Res. (2022) 48(1):239–43. doi: 10.1111/jog.15073
38. Eldar-Geva T, Gross-Tsur V, Hirsch HJ, Altarescu G, Segal R, Zeligson S, et al. Incomplete methylation of a germ cell tumor (seminoma) in a Prader-Willi male. Mol Genet Genomic Med. (2018) 6(5):811–8. doi: 10.1002/mgg3.448
39. Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS. Growth hormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. (2013) 98(6):E1072–87. doi: 10.1210/jc.2012-3888
40. Yang A, Choi JH, Sohn YB, Eom Y, Lee J, Yoo H, et al. Effects of recombinant human growth hormone treatment on growth, body composition, and safety in infants or toddlers with Prader-Willi syndrome: a randomized, active-controlled trial. Orphanet J Rare Dis. (2019) 14(1):216. doi: 10.1186/s13023-019-1195-1
41. Cheng RQ, Ying YQ, Qiu ZQ, Fu JF, Gong CX, Yang YL, et al. Early recombinant human growth hormone treatment improves mental development and alleviates deterioration of motor function in infants and young children with Prader-Willi syndrome. World J Pediatr. (2023) 19(5):438–49. doi: 10.1007/s12519-022-00653-y
42. Sjöström A, Höybye C. Twenty years of GH treatment in adults with Prader-Willi syndrome. J Clin Med. (2021) 10:2667. doi: 10.3390/jcm10122667
43. Hirsch HJ, Gross-Tsur V. Growth hormone treatment for adults with Prader-Willi syndrome: another point of view. Orphanet J Rare Dis. (2021) 16(1):337. doi: 10.1186/s13023-021-01952-9
44. Cianfarani S. Risk of cancer in patients treated with recombinant human growth hormone in childhood. Ann Pediatr Endocrinol Metab. (2019) 24(2):92–8. doi: 10.6065/apem.2019.24.2.92
45. Díez JJ, Sangiao-Alvarellos S, Cordido F. Treatment with growth hormone for adults with growth hormone deficiency syndrome: benefits and risks. Int J Mol Sci. (2018) 19(3):893. doi: 10.3390/ijms19030893
46. Swerdlow AJ, Cooke R, Beckers D, Borgström B, Butler G, Carel JC, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European cohort study. J Clin Endocrinol Metab. (2017) 102(5):1661–72. doi: 10.1210/jc.2016-2046
47. Niedziela M, Breborowicz D, Trejster E, Korman E. Hot nodules in children and adolescents in western Poland from 1996 to 2000: clinical analysis of 31 patients. J Pediatr Endocrinol Metab. (2002) 15(6):823–30. doi: 10.1515/jpem.2002.15.6.823
48. Rosario PW, de Castro Nicolau T. Report of one case of malignancy among 17 autonomous thyroid nodules in children and adolescents. J Paediatr Child Health. (2021) 57(6):810–2. doi: 10.1111/jpc.15322
49. Ly S, Frates MC, Benson CB, Peters HE, Grant FD, Drubach LA, et al. Features and outcome of autonomous thyroid nodules in children: 31 consecutive patients seen at a single center. J Clin Endocrinol Metab. (2016) 101(10):3856–62. doi: 10.1210/jc.2016-1779
50. Gundgurthi A, Dutta MK, Garg MK, Pandit AG. Autonomous functioning thyroid nodule successfully treated with radioiodine in a 3 and a half-year-old boy. J Pediatr Endocrinol Metab. (2012) 25(3–4):345–7. doi: 10.1515/jpem-2012-0008
51. Damle N, Gupta S, Kumar P, Mathur S, Bal C. Papillary carcinoma masquerading as clinically toxic adenoma in very young children. J Pediatr Endocrinol Metab. (2011) 24(11–12):1051–4. doi: 10.1515/jpem.2011.206
52. Ruggeri RM, Campennì A, Giovinazzo S, Saraceno G, Vicchio TM, Carlotta D, et al. Follicular variant of papillary thyroid carcinoma presenting as toxic nodule in an adolescent: coexistent polymorphism of the TSHR and Gsα genes. Thyroid. (2013) 23(2):239–42. doi: 10.1089/thy.2012.0279
Keywords: Plummer’s adenoma, subclinical hyperthyroidism, children, thyroid nodule, GH therapy
Citation: Corica D, Toscano F, Moleti M, Pepe G, Campenni A, Fadda G, Dionigi G, Romeo C, Aversa T and Wasniewska M (2024) Case Report: Plummer's adenoma in Prader–Willi syndrome. Front. Pediatr. 12:1388437. doi: 10.3389/fped.2024.1388437
Received: 28 February 2024; Accepted: 22 July 2024;
Published: 8 August 2024.
Edited by:
Mariacarolina Salerno, University of Naples Federico II, ItalyReviewed by:
Gerdi Tuli, Regina Margherita Hospital, Italy© 2024 Corica, Toscano, Moleti, Pepe, Campenni, Fadda, Dionigi, Romeo, Aversa and Wasniewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malgorzata Wasniewska, bXdhc25pZXdza2FAdW5pbWUuaXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.