- 1Department of Pediatrics and Neonatal Nursing, Debre Tabor University, Debre Tabor, Ethiopia
- 2Department of Nursing, Debre Tabor University, Debre Tabor, Ethiopia
- 3Department of Pediatrics and Child Health, Debre Tabor Comprehensive Specialized Hospital, Debre Tabor, Ethiopia
Background: Unlike in developed countries, neonatal morbidity and mortality are the leading challenges associated with easily preventable and treatable disorders during the neonatal period in low- and middle-income countries. However, evidence-based data about prolonged transitional hypoglycemia and associated factors are highly limited in Ethiopia and resource-limited countries.
Methods: An institution-based prospective cross-sectional study was conducted at public hospitals in South Gondar in neonatal intensive care units (NICUs). The data were entered and analyzed using SPSS version 23. Descriptive statistics were used to summarize maternal characteristics. Multivariate binary logistic regression at a p value <0.05 was used.
Results: A total of 400 neonates, admitted to NICUs in public hospitals within 48–72 h of birth between October 2, 2021, and June 30, 2022, were included in the study. The incidence of prolonged transitional neonatal hypoglycemia (PTHG) was 23.5% (19.3%–28%). The factors associated with PTHG were hypothermia (AOR = 4.41; 95% CI = 2.72–10.92), preterm birth (AOR = 3.5; 95% CI = 1.69–11.97), perinatal asphyxia (AOR = 2.5; 95% CI = 1.34–9.67), and pathological jaundice (AOR = 2.3; 95% CI = 1.21–10.34). In contrast, spontaneous vaginal delivery (SVD) was a protective factor (AOR = 0.72; 95% CI = 0.35–0.88).
Conclusions: The incidence of (PTHG) was nearly one-fifth. Factors increasing the risk of PTHG were hypothermia, preterm birth, perinatal asphyxia (PNA), early onset of sepsis (EONS), and pathological jaundice. Spontaneous vaginal delivery (SVD) was also a protective factor. Preventing neonatal hypothermia was the main measure used to reduce PTHG in the study area. Special attention could be given to neonates with prematurity, early onset neonatal sepsis (EONS), birth asphyxia, and pathological jaundice, as monitoring their RBS could lead to a significant change in reducing PTHG.
Background
Deviations from the normal transition can occur as a result of a wide range of factors predisposing individuals to neonatal hypoglycemia. Thus, the main mechanisms by which these factors result in hypoglycemia include disruption of gluconeogenesis decreased alternate fuel production, increased glucose demand, and failure to receive or absorb nutrients (1–4).
However, in instances of prolonged hypoglycemia, the liver produces ketone bodies, which are partly able to produce fuel for the brain's metabolism; lactate may also be used as fuel, which is associated with metabolic acidosis, which in turn affects central nervous system symptoms (5–7).
The variability of neonatal blood glucose levels and responses and lack of outcome data are the challenges of defining neonatal hypoglycemia consistently across various populations. Ideally, clinically significant neonatal hypoglycemia is defined as the blood glucose concentration at which an intervention should be initiated to avoid significant morbidity, especially neurologic defect (1–3, 8, 9).
Neonatal hypoglycemia is still a severe disease associated with an important risk of rapidly developing severe mental visual deficit, intellectual disability, and epilepsy. According to the Women and Child Health section of the WHO, preterm and small for gestational age (SGA) neonates exhibit a constrained blood glucose regulatory mechanism for hypoglycemia. Thus, detection and treatment of these groups of neonates are targeted to reduce neonatal death, mainly in low-resource countries (10–13).
A random blood glucose level (RBS) less than 47 mg/dl (2.6 mmol/L), if present after 48 h during the first two months of life, was significantly correlated with acute seizure activity, abnormal motor manifestations, and impaired intellectual performance at 18 months of age (1, 3).
Despite progress over the past two decades, the age distribution of the mortality of children and young adolescents shows that the highest risk of death occurs during the neonatal period (the first 28 days of life), during which preventable causes accounted for 2.5 million out of 6.2 million deaths of children and young adolescents in 2018 alone. The neonatal mortality rate was estimated to be 18 deaths/1,000 live births globally. The probability of dying after the first month and before reaching age 1 was 11 per 1,000 live births (3, 14).
Prolonged neonatal hypoglycemia (PTHG) is an under-recognized cause of morbidity and mortality. In some studies, the incidence of PTHG varies due to different factors; as a result, it cannot be estimated easily by findings from other countries (1, 15–18).
The increased prevalence of prematurity and low birth weight make such studies vital to the formulation of recommendations in the most significant population of neonates to reduce neonatal mortality in specific and under-five children in general. According to the Ethiopian Demographic Health Survey (EDHS), Ethiopia has one of the highest neonatal mortality rates in the world (29 per 1,000 live births), which represents poor progress in reducing the rate of neonatal mortality from 2000 to 2016 according to the 2016 EDHS. Decreasing preventable neonatal death is an essential public health concern for resource-limited countries, particularly Ethiopia (15, 19–22).
Although a preprint of this manuscript has previously been published in Research Square (19), there is no known documented evidence of the incidence of PTHG in Ethiopia specifically or in Africa at large. The incidence of PTHG in Ethiopia is not clearly understood or documented. Hence, this study identified this important public health problem in the neonatal period. The authors also identified the factors associated with PTHG, which in turn contributes to neonatal mortality.
Methods
Study design and setting
This was an institutional-based prospective cross-sectional study of admitted neonates at South Gondar public hospitals in neonatal intensive care units (NICUs) from 02 October 2021 to 30 June 2022. In the study area, South Gondar Zone, there are eight public hospitals, namely, Debre Tabor Specialized and Comprehensive Hospital, Addis Zemen District Hospital, Ebnat District Hospital, Mekane-Eyesus District Hospital, Andabet District Hospital, Wogeda District Hospital, Nefas Mewucha District Hospital, and Tach Gayint District Hospital, through which neonatal care services were delivered. There are maternal and child health (MCH), inpatient, outpatient (OPD), emergency, and referral services in all the woredas in which the data were collected.
In addition to the zonal-level health care delivery system, the woreda level provides services in its catchment area. There is at least one health center and one district hospital in each woreda. At each district hospital, there is at least one tertiary neonatal nurse (a Bsc neonatal nurse professional) providing neonatal care to general practitioners (GPs) in the neonatal intensive care unit (NICU).
Debre Tabor Comprehensive and Specialized Hospital at the zonal level and the remaining seven public district hospitals were clustered in the zone and each of the woreda, respectively, within their catchment area. The data collection procedure was conducted at each cluster proportional to size (PPS) based on the annual neonatal case flow data. All neonates aged 48–72 h were selected from among all admitted neonates in this study.
Sample size measurements and data collection procedures
The single population proportion was calculated with the following assumptions: 95% confidence interval (CI), 5% margin of error, and 50% incidence of PHG.
Where
N = minimum sample size required for the study
Z = standard normal distribution (Z = 1.96) with a confidence interval of 95%
P = proportion of the PTHG (0.5)
d = is a tolerable margin of error (d = 0.05). The sample size calculation to determine the incidence of PHG by using the above formula was 384.
By adding a 10% nonresponse rate, the final sample size (N) was 422.
Study participants and sampling method
A cluster sampling technique proportional to size (PPS) was employed among admitted neonates in the intensive care unit (NICU) at public hospitals in South Gondar. Before a maternal interview, the data collectors ascertained the case in the neonatal intensive care unit (NICU) and extracted information from the checklist for a neonatal biophysical profile, such as cause of admission, gestational age, mode of delivery, presentation, rupture of membranes, duration of labor and other information from the labor delivery room referral report form.
The data were extracted (collected) by BSc neonatal nurses and health information technicians or delivery ward coordinators for verification of the data at each health facility. The data collectors and supervisors had one day of training from a principal investigator about the tool for data collection and abstraction.
Data collection tool and procedures
The random glucose level of the neonates (RBS) was measured by using a glucometer device with blood glucose test strips, and the selected study participants were tested for hypoglycemia by piercing the outer part of their heel as per routine measurement procedures in each hospital. The measurements were taken twice a day at 6 AM in the morning and at 6 PM in the afternoon within 48–72 h of neonatal age. Two RBS measurements, morning and afternoon, were subsequently recorded on the data collection form.
Clinical information such as gestational age (GA), mode of delivery, duration of labor, and causes of admission, such as jaundice, prenatal asphyxia (PNA), and early-onset neonatal sepsis (EONS), was collected from individual neonates (Neonatal Chart). The maternal interview was conducted after taking her neonatal RBS record in the mother's waiting room at her convenient time and with her verbal consent.
The tool was formulated after referring to various pieces of literature. The interview questionnaire consisted of information on socio-demographic characteristics; pregnancy and obstetric factors; medical and other factors; and neonatal profiles from labor delivery referral forms and admission history (4, 12, 16, 17, 21–23).
Data quality control plan
Data accuracy, completeness, and timeliness were checked by the following activities. The training of the data collectors and supervisors for one day regarding the objective of the study, the data collection tool, and the methods of data collection, checking the completeness of the data collection tool, and maintaining confidentiality by coding and limiting access to the collected pieces of information was completed.
The data collection tool was pretested by Debre Tabor Comprehensive Specialized Hospital. A pretest was administered, questions that were not clear were corrected, and a discussion was held with the data collectors and supervisors on the problems they encountered in completing the checklist. The data were checked for completeness before data entry into epi version 4.2. Proper coding of the data and categorization of outcome variables into persistent hypoglycemia and not were maintained for the quality of the data to be analyzed. Similarly, temperature data were categorized as hypothermic or normothermic according to the operationalized definition used in this study. Double data entry was performed for validity, and the data were compared to the original data. Simple frequencies and cross-tabulations were used to check missing and outlier variables, which were cross-checked with hard copies of the collected data.
Outcome variable measurement
Prolonged transitional hypoglycemia (PTHG) was defined as a random blood glucose level (RBS) < 47 mg/dl measured by a tool labeled as Gmate-Voice at 6 AM on the morning and at 6 PM in the afternoon for any gestational age within 48–72 h of life after delivery (1, 10, 24, 25).
Hyperglycemia: The latest record of a random blood sugar level (RBS) > 125 mg/dl was obtained at 6 AM in the morning and at 6 PM in the afternoon within 48–72 h after delivery (25).
Normglycemic status: The latest record of a random blood glucose level (RBS) of 47 mg/dl–125 mg/dl was obtained at 6 AM in the morning and at 6 PM in the afternoon within 48–72 h after delivery (24, 25).
Hypothermia was defined as the most recent measurement of an auxiliary body temperature within 48–72 h of age, which was less than 36.5°C (23).
Neonate: Newborns within the first 28 days of age after live birth with a viable gestational age (GA > 30 weeks) were included in this study.
Data processing and analysis
The data were entered and analyzed using EpiData 4.2 and SPSS version 23. Descriptive analysis was performed by computing proportions and summary statistics. The data are presented as simple frequencies, summary measures, tables, and figures. Missing values were analyzed by using multiple imputation techniques.
Binary logistic regression was used to analyze the outcome variables. Bivariate and multivariate analyses were performed to determine the associations between the outcome variables and each independent variable. The assumptions for binary logistic regression were checked. The goodness of fit was tested by the Hosmer–Lemeshow test and Omnibus test. All variables with P < 0.25 in the bivariate analysis were included in the final model of multivariate analysis to control for all possible confounders, and the variables were selected by the method.
The direction and strength of the associations were measured by odds ratios (ORs) with 95% confidence intervals (CIs). The adjusted odds ratio (OR) and 95% confidence interval (CI) were estimated to identify factors associated with persistent neonatal hypoglycemia via multivariate analysis via logistic regression. In this study, a P value < 0.05 was considered to indicate statistical significance.
Results
Maternal socio-demographic characteristics
Out of 422 mothers whose neonates were admitted to neonatal intensive care units in the study areas fulfilled the criteria for enrollment in the study. Approximately 284 (67.30%) of the participants were aged 20–34 years, whereas the majority of the mothers were married [392 (92.90%)] (Table 1).
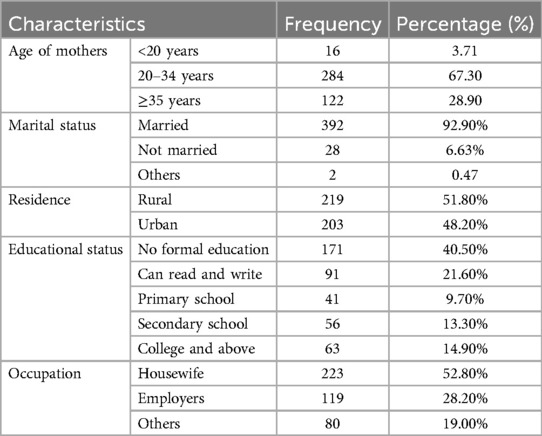
Table 1. Maternal socio-demographic characteristics of prolonged transitional hypoglycemia and associated factors among admitted neonates at South Gondar public hospitals, 2022.
Incidence of prolonged transitional hypoglycemia
The median and range of random blood glucose levels at 48–72 h of age were 62 gm/dl, and the RBS ranged from 35 gm/dl to a high level, which was not recorded by a glucometer. For instance, high RBS levels can reach more than 250 mg/dl.
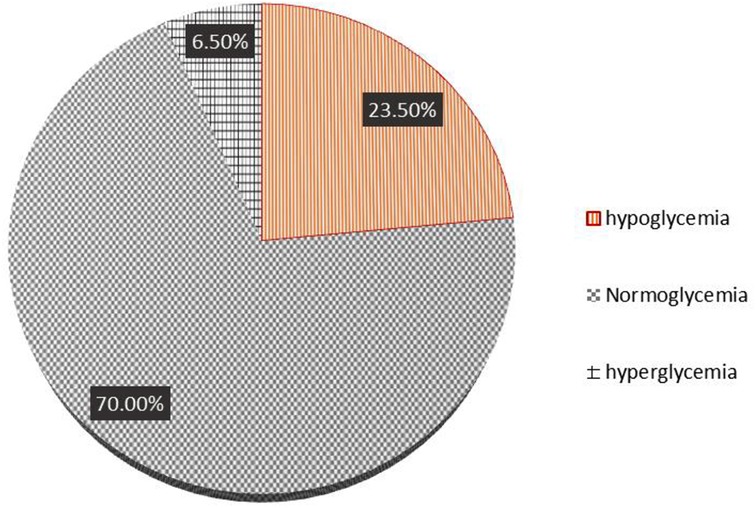
The incidence of persistent hypoglycemia (PTHG) was 94 (23.5%) (19.3%–28%), 280 (70%) neonates were in a normoglycemic state, and approximately 26 (6.50%) were hyperglycemic. Figure 1 shows the incidence of persistent neonatal hypoglycemia.
Pregnancy and obstetric characteristics
Approximately 218 (56.41%) mothers had a duration of labor between 1 and 12 h, and the majority of them delivered their current pregnancy through SVD, accounting for 96 (70.26%) of the mothers. The majority of the patients had a rupture of membrane (ROM) 12 h or less [365 (93.84%) of the patients] (Table 2).
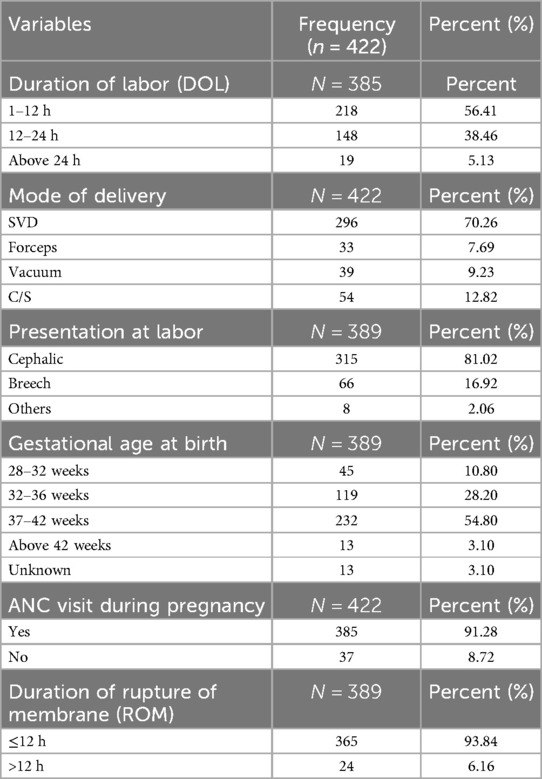
Table 2. Pregnancy and obstetrics characteristics of prolonged transitional hypoglycemia and associated factors among admitted neonates in South Gondar public hospitals, 2022.
Maternal health problems during pregnancy
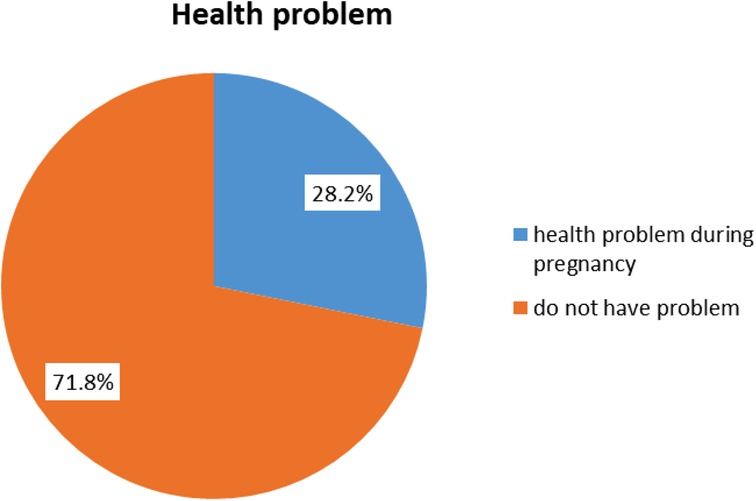
The majority of the 303 (71.8%) women did not have health problems during pregnancy, while the remaining 119 (28.2%) had different health problems during their latest pregnancy. Figure 2 shows maternal health problems during pregnancy.
Types of health problems during pregnancy
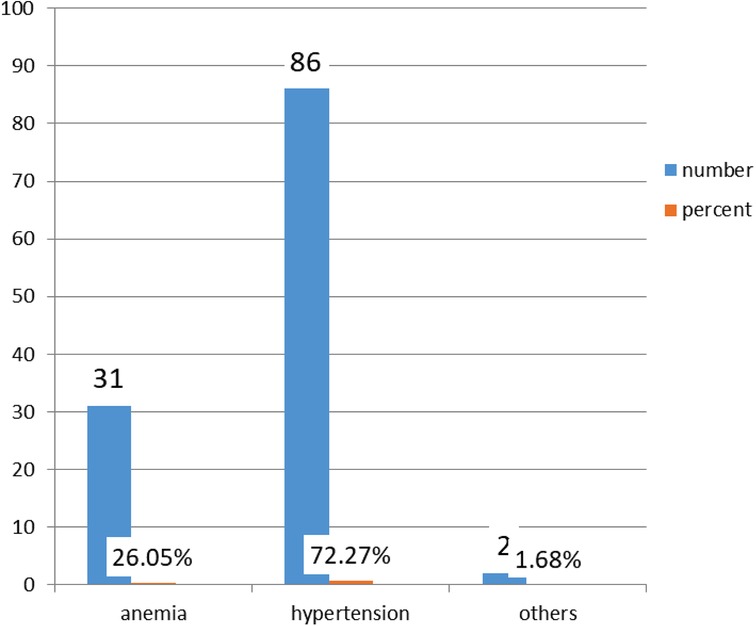
Among the 119 women who had a health problem, 86 (72.27%) had hypertensive disorders during pregnancy and 2 had other disorders (1.68%). Figure 3 shows the types of maternal pregnancy health problems.
Neonatal profiles at admission
The most common cause of admission was hypothermia (100, 25%), the least common cause was other causes (20, 5%), and the majority of neonates were born at term (n = 232, 54.80%). The number of very-low-birth-weight neonates was the lowest among the groups, accounting for 10 (2.56%) (Table 3).
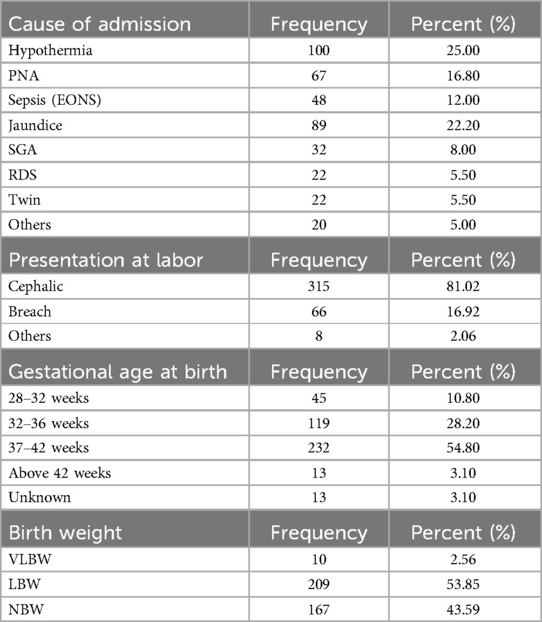
Table 3. Neonatal profile for incidence and associated factors of prolonged transitional neonatal hypoglycemia among admitted neonates in the NICU at South Gondar public hospitals, 2022.
Factors associated with prolonged transitional hypoglycemia
Binary logistic regression was used to identify factors associated with PTHG. There was no marked variation according to the number of deliveries, intentions to current birth, pregnancy disorders, educational status, or maternal age group, even though these factors were associated with each other according to bivariate analysis.
Preterm birth, hypothermia, perinatal asphyxia (PNA), early onset of sepsis (EONS), and clinical pathological jaundice were risk factors for PTHG, while spontaneous vaginal delivery (SVD) was protective.
Neonates with hypothermia were 4 times more likely to develop PTHG than were those with normothermic hypothermia (AOR = 4.41; 95% CI = 2.72–10.92); preterm birth was 3.5 times more likely to be associated with PTHG than was term birth (AOR = 3.5; 95% CI = 1.69–11.97); perinatal asphyxia was 2.5 times more likely to lead to PTHG than was no asphyxia (AOR = 2.5; 95% CI = 1.34–9.67); and neonates with pathological jaundice were almost 2 times more likely to have PTHG than were those with no jaundice problems (AOR = 2.3; 95% CI = 1.21–10.34).
In contrast, spontaneous vaginal delivery (SVD) was protective and was 72% less likely to develop vaginal delivery than was cesarean section delivery (AOR = 0.72; 95% CI = 0.35–0.88) (Table 4).
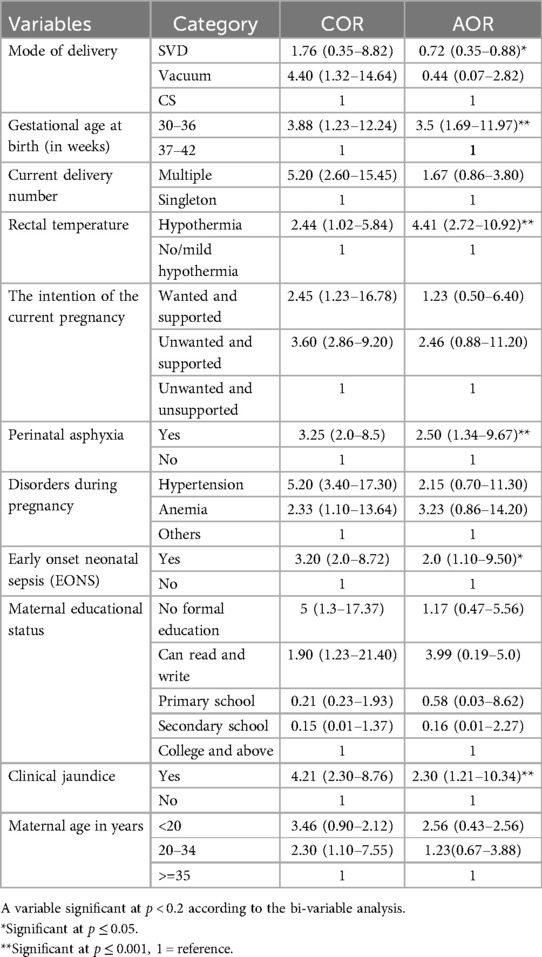
Table 4. Factors associated with the incidence of PTHG among admitted neonates in the NICU at South Gondar public hospitals, 2022.
Discussion
Incidence of prolonged transitional neonatal hypoglycemia (PTHG)
The incidence of PTHG was 94 (23.5%; 19.3%–28%) in this study. According to the literature, under diagnosing or misdiagnosing neonatal hypoglycemia can lead to neonatal morbidity and mortality, which can be attributed to the aggravating factors of hypoglycemia. There have been underreporting or nearly absent studies about neonatal hypoglycemia globally and in Africa about persistent hypoglycemia.
The incidence estimates of hypoglycemia in the newborn depend both on the definition of the condition and the methods by which blood glucose concentrations are measured as well as the cutoff point of time for taking the measurement.
Studies Globally, conducted in Royal Alexandra Hospital, Canada, Denver Colorado, Waikato District Health Board, Hamilton, New Zealand, according to the American Association of Pediatrics in the entire population estimate, Boston, USA, showed that the incidence of neonatal hypoglycemia within 48 h of age ranged from 30%–63%, which was higher than that in this study (14, 16–19).
Unpublished findings indicated that 25% of neonatal hypoglycemic agents were diagnosed at St. Paul Millennium Hospital Medical College in Ethiopia (2).
The variation might be because the studies used transient hypoglycemia within 48 h after delivery, which in turn consisted of physiological hypoglycemia that subsequently returned to the normal glycemic state after the physiological process (48–72 h). This discrepancy might arise from the sample size variation and some clinical setup variation found among studies.
According to other reports, for early neonatal hypoglycemia, which was screened in Turkey, Denver Colorado, and Israel, the incidence of hypoglycemia was 12%–17%, and 12.1%, which was lower than that in this study (20, 26, 27).
This variation might arise from the definition of the condition and the methods by which blood glucose concentrations are measured as well as the cutoff point of time for taking the measurement.
Factors associated with PTHG
Preterm birth, hypothermia, perinatal asphyxia (PNA), early onset of sepsis (EONS), and pathological jaundice were factors associated with PTHG, while spontaneous vaginal delivery (SVD) was protective.
Compared with normothermic neonates, neonates with hypothermia were 4 times more likely to develop PTHG; preterm birth was 3.5 times more likely to be associated with; and perinatal asphyxia was 2.5 times more likely to develop than it was in noasphyxia. Neonates with pathological jaundice were almost 2 times more likely to be PTHG agents than were those with no jaundice.
There are various risk factors for neonatal hypoglycemia, as reported in the literature, depending on the population and area of study, making it difficult to use these data consistently worldwide.
In line with these findings, in Israel and West Virginia University Hospital, hypothermia, low birth weight (<2,500 g), prematurity, small for gestational age (SGA), and perinatal asphyxia were found to be independent predictors of neonatal hypoglycemia (2, 13, 27).
This might be because hypothermia causes an increased demand for glucose metabolism for thermoregulation in addition to the demand for an extra uterine adaptation process. When the neonate is cold, he or she uses more glycogen to stay warm. Then, he utilized his glucose stores to stay warm, his blood sugar concentration decreased, and he became hypoglycemic and hypothermic.
Similarly, premature individuals are predisposed to developing hypoglycemia and associated complications due to their limited glycogen and fat stores, inability to generate new glucose via gluconeogenesis pathways, increased metabolic demand due to relatively greater brain size, and inability to mount a counter regulatory response to hypoglycemia.
In Ethiopia, the risk factors associated with neonatal hypoglycemia were birth weight, duration of labor, maternal age, duration of feeding initiation, hypothermia, and respiratory distress syndrome, and some of the factors leading to hypoglycemia were identified as consistent with the findings of this study (2).
These factors associated with hypoglycemia increase the risk of developing hypoglycemia because hypothermia, low birth weight, and respiratory distress syndrome affect the normal life of neonates from intrauterine to extra uterine life.
Conversely, this study revealed that spontaneous vaginal delivery (SVD) was protective and was 72% less likely to develop than cesarean section delivery was.
Preventing and treating PTHG is required because it leads to neurological damage, intellectual disability, epilepsy, personality disorders, impaired cardiac performance, and muscle weakness (23–29).
Strength and limitation
Preventing and treating PTHG prevents neurological damage, intellectual disability, epilepsy, personality disorders, impaired cardiac performance, and muscle weakness attributed to PTHG during the neonatal period. It highlights the consequences of adequate postnatal care in preventing persistent neonatal hypoglycemia. However, because of the cross-sectional nature of the study, we could not determine the causal relationships.
Conclusions
The PTHG was lower than that reported in previous studies, while factors increasing the risk of PTHG were preterm birth, hypothermia, perinatal asphyxia (PNA), early onset of sepsis (EONS), and clinical pathological jaundice. Spontaneous vaginal delivery (SVD) was found to be a protective factor. Preventing neonatal hypothermia is the main measure used to reduce the PTHG in the study area as well as in resource-limited areas in Ethiopia. Preventing neonatal hypothermia was the main measure used to reduce PTHG in the study area. Close follow-up of neonates with prematurity, early onset neonatal sepsis (EONS), birth asphyxia, and pathological jaundice due to hypoglycemia could lead to a significant change in reducing PTHG.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Debre Tabor University, research ethics review committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the data collection procedure was part of a routine neonatal care in included hospitals.
Author contributions
SK: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. TA: Investigation, Methodology, Writing – original draft, Writing – review & editing. KA: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. WA: Methodology, Software, Writing – original draft, Writing – review & editing. NM: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful for the cooperation of the hospital NICU staff, data collectors, and mothers whose neonates were admitted for their cooperation during the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AOR, adjusted odd ratio; COR, crude odd ratio; CS, cesarean section; EONS, early onset of neonatal sepsis; GA, gestational age; NICU, neonatal intensive care unit; PNA, perinatal asphyxia; PTHG, persistent hypoglycemia; RBS, random blood sugar; SGA, small for gestational age; SVD, spontaneous vaginal delivery; WHO, World Health Organization.
References
1. Cornblath M, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, Schwartz R, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. (2000) 105(5):1141–5. doi: 10.1542/peds.105.5.114110790476
2. Fantahun BY, Nurussen I. Prevalence and Risk Factors of Hypoglycaemia in Neonates at St. Paul’s Hospital Millennium Medical College Neonatal Intensive Care Unit, Ethiopia: A Cross-Sectional Study. Unpublished manuscript. (2020).
3. Taranushenko TE, Antsiferova EV, Kiseleva NG. Hypoglycaemia in the early neonatal period in newborns. Am J Pediatr. (2022) 8(1):17–22. doi: 10.11648/j.ajp.20220801.15
4. Thompson-Branch A, Havranek T. Neonatal hypoglycemia. Pediatr Rev. (2017) 38(4):147–57. doi: 10.1542/pir.2016-006328364046
5. Bhand SA, Sheikh F, Siyal AR, Nizamani MA, Saeed M. Neonatal hypoglycemia: presenting pattern and risk factors of neonatal hypoglycemia. Professional Med J. (2014) 21(04):745–9. doi: 10.29309/TPMJ/2014.21.04.5568
6. McKinlay CJ, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. (2015) 373(16):1507–18. doi: 10.1056/NEJMoa150490926465984
7. Tin W. Defining neonatal hypoglycaemia: a continuing debate. In: Seminars in Fetal and Neonatal Medicine (Vol. 19, No. 1). WB Saunders (2014). p. 27–32.
8. Gustafsson J. Neonatal energy substrate production. Indian J Med Res. (2009) 130(5):618–23.20090117
9. Menni F, de Lonlay P, Sevin C, Touati G, Peigné C, Barbier V, et al. Neurologic outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycemia. Pediatrics. (2001) 107(3):476–9. doi: 10.1542/peds.107.3.47611230585
10. Tam EW, Widjaja E, Blaser SI, Macgregor DL, Satodia P, Moore AM. Occipital lobe injury and cortical visual outcomes after neonatal hypoglycemia. Pediatrics. (2008) 122(3):507–12. doi: 10.1542/peds.2007-200218762519
11. McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. (2017) 171(10):972–83. doi: 10.1001/jamapediatrics.2017.157928783802
12. Sweet CB, Grayson S, Polak M. Management strategies for neonatal hypoglycemia. J Pediatr Pharmacol Ther. (2013) 18(3):199. doi: 10.5863/1551-6776-18.3.19924052783
13. You D, Hug L, Ejdemyr S, Idele P, Hogan D, Mathers C, et al. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN inter-agency group for child mortality estimation. Lancet. (2015) 386(10010):2275–86. doi: 10.1016/S0140-6736(15)00120-826361942
14. Gebremichael SG, Fenta SM. Under-five mortality and associated risk factors in rural settings of Ethiopia: evidences from 2016 Ethiopian demographic and health survey. Adv Public Health. (2020) 2020(1):8430246. doi: 10.1155/2020/8430246
15. Mitchell NA, Grimbly C, Rosolowsky ET, O'Reilly M, Yaskina M, Cheung PY, et al. Incidence and risk factors for hypoglycemia during fetal-to-neonatal transition in premature infants. Front Pediatr. (2020) 8:34. doi: 10.3389/fped.2020.0003432117839
16. Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. (2012) 161(5):787–91. doi: 10.1016/j.jpeds.2012.05.02222727868
18. Kebede S, Munye T, Agmas K, Necho W, Moges N. Incidence of Persistent Neonatal Hypoglycemia and Associated Factors among Neonatal Intensive Care Unit Admissions in South Gondar Public Hospitals, Northcentral Ethiopia: a prospective cross sectional study, 04 January 2024, PREPRINT (Version 1) available at Research Square. doi: 10.21203/rs.3.rs-3823404/v1
19. James-Todd T, March MI, Seiglie J, Gupta M, Brown FM, Majzoub JA. Racial differences in neonatal hypoglycemia among very early preterm births. J Perinatol. (2018) 38(3):258–63. doi: 10.1038/s41372-017-0003-929209031
20. Hosagasi NH, Aydin M, Zenciroglu A, Ustun N, Beken S. Incidence of hypoglycemia in newborns at risk and an audit of the 2011 American academy of pediatrics guideline for hypoglycemia. Pediatr Neonatol. (2018) 59(4):368–74. doi: 10.1016/j.pedneo.2017.11.00929198616
21. Mukunya D, Odongkara B, Piloya T, Nankabirwa V, Achora V, Batte C, et al. Prevalence and factors associated with neonatal hypoglycemia in northern Uganda: a community-based cross-sectional study. Trop Med Health. (2020) 48:1–8. doi: 10.1186/s41182-020-00275-y31920458
22. Ababa A. Neonatal intensive care unit (NICU) training. Federal Ministry of Health of Ethiopia. (2014).
23. Bhand SA, Sheikh F, Siyal AR, Nizamani MA, Saeed M. Neonatal hypoglycemia: presenting pattern and risk factors of neonatal hypoglycemia. Prof Med J. (2014) 21(04):745–9. doi: 10.17957/PMJ.21.04.2014.217
24. Frank-Briggs AI, Ojule AC, Nkanginieme KE. Neonatal hypoglycaemia: prevalence and clinical manifestations in Port Harcourt, Nigeria. Port Harcourt Med J. (2008) 2(2):166–70. doi: 10.1016/j.phmj.2008.06.002
25. Vannucci RC, Vannucci SJ. Glucose metabolism in the developing brain. In Seminars in Perinatology (Vol. 24, No. 2). WB Saunders (2000). p. 107–15.
26. Lubchenco LO, Bard H. Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics. (1971) 47(5):831–8. doi: 10.1542/peds.47.5.8315573868
27. Bromiker R, Perry A, Kasirer Y, Einav S, Klinger G, Levy-Khademi F. Early neonatal hypoglycemia: incidence of and risk factors. A cohort study using universal point of care screening. J Matern Fetal Neonatal Med. (2019) 32(5):786–92. doi: 10.1080/14767058.2017.139178129020813
28. Stomnaroska O, Petkovska E, Jancevska S, Danilovski D. Neonatal hypoglycemia: risk factors and outcomes. Prilozi. (2017) 38(1):97–101. doi: 10.1515/prilozi-2017-001328593892
Keywords: prolonged hypoglycemia, neonatal hypoglycemia, newborn hypoglycemia, abnormal transition, persistent hypoglycemia of infant, South Gondar public hospitals
Citation: Kebede SD, Aytenew TM, Agmas K, Asferie WN and Moges N (2024) Incidence of prolonged transitional neonatal hypoglycemia and associated factors among neonatal admissions in South Gondar public hospitals, North-Central Ethiopia: a prospective cross-sectional study. Front. Pediatr. 12:1381867. doi: 10.3389/fped.2024.1381867
Received: 4 February 2024; Accepted: 30 September 2024;
Published: 24 October 2024.
Edited by:
Nicola Improda, AORN Santobono-Pausilipon, ItalyReviewed by:
Francesco Dituri, ASL Roma, ItalyAntonella Poloniato, San Raffaele Hospital (IRCCS), Italy
Gisella Garbetta, Vita-Salute San Raffaele University, Italy
Copyright: © 2024 Kebede, Aytenew, Agmas, Asferie and Moges. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Solomon Demis Kebede, c29sb21vbmRlbWlzQGdtYWlsLmNvbQ==
 Solomon Demis Kebede
Solomon Demis Kebede Tigabu Munye Aytenew
Tigabu Munye Aytenew Kindu Agmas3
Kindu Agmas3 Worku Necho Asferie
Worku Necho Asferie Natnael Moges
Natnael Moges