- 1Rheumatology Unit, Department of Pediatrics, ASST Fatebenefratelli-Sacco, Milan, Italy
- 2Department of Pediatrics, Meyer Children’s University Hospital, Florence, Italy
Editorial on the Research Topic
Multisystem inflammatory syndrome in children
Multisystem inflammatory syndrome is a severe complication associated with COVID-19, initially recognized as a distinct clinical entity in 2020 (1). In a bulletin from the UK's National Health Service (NHS) in late April 2020, highlighting a new multi-system inflammatory condition involving a small number of children, the term “PIMS” (Pediatric Inflammatory Multisystem Syndrome associated with SARS-CoV-2) was initially used. Primarily observed in pediatric patients, it was subsequently classified by the Centers for Disease Control and Prevention as Multisystem Inflammatory Syndrome in Children (MIS-C) (2). MIS-C manifests as a rare delayed hyperinflammatory response following SARS-CoV-2 infection. Although the exact pathophysiology remains unclear, the SARS-CoV-2 coronavirus seems to trigger a dysregulated pathological immune response in the host, leading to systemic vasculitis and widespread acute organ damage (3).
Like the viral infection caused by COVID-19, also other infectious agents, such as the Epstein-Barr virus (EBV), have also provided the opportunity to analyze the complex mechanisms leading to hyperinflammatory states. EBV can influence the expression and modulation of TLR7 and TLR9 signaling pathways, and consequently the transcription factor NF-κB activation (Tan et al.) (4). Liu et al. observed how the type of interplay between EBV and TLRs defines the disease outcome. Patients with chronic active EBV (CAEBV) showed a sustained and heightened activation of TLR7 and TLR9 along with their downstream signaling mediators (Liu et al.). This could suggest how a deficit in the self-regulation of negative feedback mechanisms associated with TLRs predisposes to a prolonged, and excessive inflammatory response, potentially contributing to an unfavorable outcome.
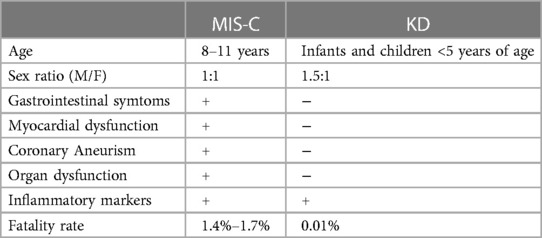
MIS-C presents with a heterogeneous clinical profile and variable severity, involving multiple organs and characterized by a state of hyperinflammation, often requiring intensive care. It shares clinical similarities with Kawasaki Disease (KD)-like shock syndrome, explaining how treatment protocols have been mostly derived from those used in KD (5).
About cardiac manifestations of these diseases, myocarditis and left ventricular systolic dysfunction are very common in MIS-C patients and rare in KD. Regarding the occurrence coronary aneurysm (CAA), a typical complication of KD, after the introduction of intravenous immunoglobulin treatment, they can be seen only in 4% of the patients, while in MIS-C, 14%–36% exhibit CAA (6).
Difference between MIS-C and KD has shown in Table 1.
This new condition has attracted significant scientific interest and has resulted in numerous publications (7–12), (Table 2). However, despite advancements, many aspects of MIS-C, including epidemiology, pathogenesis, clinical spectrum, and long-term outcomes, still remain poorly understood, providing numerous avenues for future research. Furthermore, although it has become increasingly clear that MIS-C and KD exhibit significant differences, some intriguing points of intersection seem to exist in their pathogenetic mechanisms that still need to be defined (13).
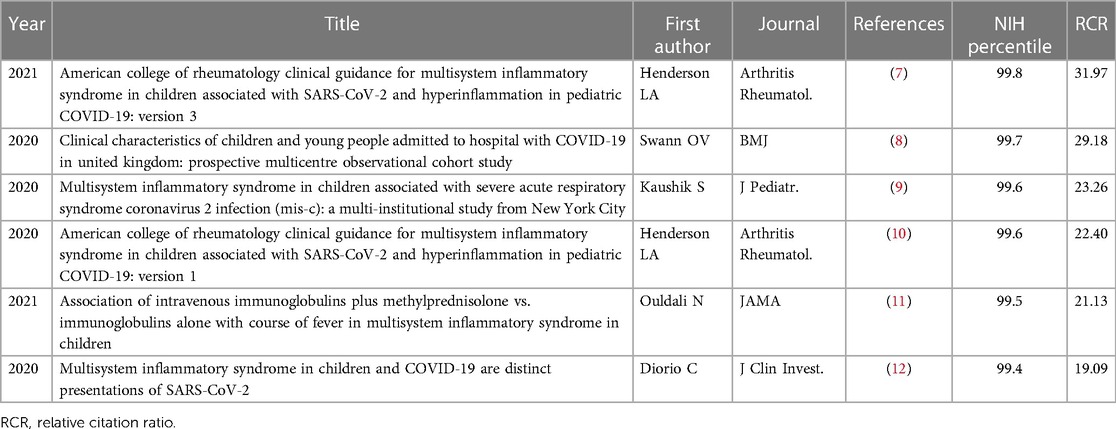
Table 2. Top 6 articles related to multisystem inflammatory syndrome, MIS-C, updated as of January 29, 2019.
At the beginning of the pandemic, differentiating children with acute severe COVID-19 infection from those who developed the post-infectious hyperinflammatory syndrome, MIS-C, proved challenging (14). In this regard, Jiju et al. in their retrospective study compared the features of 161 symptomatic acute COVID-19 and 50 MIS-C patients (≤19 years) admitted to a tertiary pediatric hospital in the North-West of England (Jiju et al.). They observed that MIS-C patients were older, with a median of 10.3 years, developed the disease later with respect to the primary infection, and often had associated comorbidities. Clinically, they typically presented with abdominal and neurological symptoms, higher inflammatory markers, and showed a more severe disease course, with a higher incidence of death.
European countries and the United States mostly contributed to the majority of scientific publications regarding MIS-C at the onset of the pandemic (15, 16). Conversely, MIS-C has rarely been reported in Chinese children. The reasons could be due to differences in prevalence rates of infection in children and differences in ethnic, genetic background, and SARS-CoV-2 subtypes (17). Wang et al. described a 4-year-old Chinese girl with severe COVID-19 infection complicated with MIS-C successfully treated according to an expert consensus statement Wang et al. (18).
MIS-C may present with a variety of clinical presentations, also in terms of severity. Efforts have been made to comprehend the role of biomarkers in predicting the disease course and outcome (19, 20). For example, older age and initial serum albumin levels have been identified as early indicators, helping to recognize children at high risk for intensive care unit admission (21).
Snipaitiene et al. in a retrospective study involving 43 patients evaluated the role of platelet (PLT) count and PLT indices (plateletcrit, mean platelet volume, and platelet distribution width) in predicting MIS-C severity in children who presented at the Hospital of Lithuanian University of Health Sciences Kauno Klinikos. They found that these markers allowed better prediction of MIS-C severity (Snipaitiene et al.). With the same purpose, Fastiggi et al. analyzed the role of the thyroid axis, Euthyroid Sick Syndrome (ESS), in a single-center observational study involving 42 patients with MIS-C, showing it to be a potential predictor of severe MIS-C course (Fastiggi et al.).
The first-line treatment for MIS-C, usually based on intravenous immunoglobulin (IVIG) and corticosteroids, aim to address the inflammatory response and symptoms associated with this condition (22).
In some cases, additional therapies may be considered based on the severity of the condition and individual patient needs. Among the treatments included in the expert consensus statement anakinra has been extensively used in this condition (23). However, data on the efficacy and safety of anakinra in patients with MIS-C are still lacking (16). Licciardi et al. in their retrospective multicenter study compared patients treated with anakinra in the ICU with those treated in the pediatric wards, observing that anakinra resulted to be efficacious and safe (Licciardi et al.).
In a MIS-C patients resistant to first line therapy, Tocilizumab, anti-IL 6, has been studied as a second line of treatment. In fact Çelikel et al. investigated the efficacy of anakinra, and/or tocilizumab in resistant patients with severe MIS-C admitted in the PICU. They enrolled at 33 patients with MIS-C with a median age of 9 years. All the patients were given first line of therapy. 23/33 (69.9) patients took Anakinra. Two patients were switched to tocilizumab because they were unresponsive to anakinra. All patients showed an increase in lymphocyte and platelet counts and a decrease in ferritin, B-type natriuretic peptide, and troponin levels after first week of treatment (24).
Moreover, Niño-Taravilla et al. describe a case of 8-year-old boy with severe MIS-C treated with tocilizumab (8 mg/kg) and corticosteroid therapy. Two days after the start of treatment, he showed an improvement in symptoms, cardiac function and laboratory tests (25).
Intravenous immunoglobulins (IVIG) is the well-established primary therapeutic option for KD and has also been extensively used in MIS-C (18). However, IVIG may entail rare, often under-recognized side effects such as headaches, hyperviscosity, and hemolysis. The passive transfer of isoagglutinins, typically anti-A or anti-B antibodies, has been recognized as the main causative factor for IVIG-associated hemolytic anemia (26).
In the context of KD, IVIG-associated hemolysis affects up to 16% of the patients. Sedlin et al. reported the first two pediatric patients, a 2 and an 8-year-old girl, diagnosed with MIS-C developing this adverse effect after IVIG therapy (27).
Evidence regarding second-line therapy in patients with resistant KD remains contradictory, a second infusion of IVIG still represents one of the most frequently employed options in such cases (Sedlin et al.). However, given the side effects associated with high IVIG doses and the availability of alternative treatments, several studies have explored their efficacy and side effects (28). Pan et al. conducted a network meta-analysis and used an aggregate Data Drug Information System software v.1.16.5 incorporating clinical trials comparing the safety and efficacy of infliximab, second IVIG infusions, and intravenous pulse methylprednisolone (IVMP) Pan et al. (29). Infliximab emerged as the optimal second-line treatment choice, although associated with an increased susceptibility to hepatomegaly. No significant differences in the risk of developing a coronary artery aneurysm among these three different options emerged.
KD and MIS-C are now recognized under the same hyperinflammatory umbrella, yet they are considered distinct diseases. However, during the early stages of the pandemic, the emergence of a new condition with a KD-like presentation posed diagnostic challenges. Initially, the boundaries between MIS-C and KD seemed blurred, sparking renewed interest among clinicians and scientists in the study of KD (30). Tan and colleagues in their bibliometric analysis have explored the current hotspots and trends in research, founding that in the period 2017–2021, more than 5,500 articles on KD have been published in the Web of Science and Scopus databases, predominantly authored by researchers from Japan, the USA, and China, with a specific focus on “COVID-19” and “multisystem inflammatory disease” (Pan et al.).
In conclusion, despite the profound impact of the COVID-19 pandemic, it has nonetheless left a significant mark on the scientific landscape. This is evident in the collaborative research endeavors that undeniably contributed to advancements in medical knowledge.
Author contributions
AM: Writing – original draft, Writing – review & editing. TG: Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395(10237):1607–8. doi: 10.1016/S0140-6736(20)31094-1
2. Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19)”. emergency.cdc.gov (May 14, 2020. Archived from the original on May 15, 2020).
3. Rowley AH, Shulman ST, Arditi M. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J Clin Invest. (2020) 130:5619–21. doi: 10.1172/JCI143840
4. Lünemann A, Rowe M, Nadal D. Innate immune recognition of EBV. Curr Top Microbiol Immunol. (2015) 391:265–87. doi: 10.1007/978-3-319-22834-1_9
5. Yeung RS, Ferguson PJ. Is multisystem inflammatory syndrome in children on the Kawasaki syndrome spectrum? J Clin Invest. (2020) 130:5681–4. doi: 10.1172/JCI141718
6. Zhang QY, Xu BW, Du JB. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment. World J Pediatr. (2021) 17:335–40. doi: 10.1007/s12519-021-00435-y
7. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus S, Bassiri H, et al. American College of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol. (2022) 74:e1–20. doi: 10.1002/art.42062
8. Swann OV, Holden KA, Turtle L, Pollok L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. Br Med J. (2020) 370:m3249. doi: 10.1136/bmj.m3249
9. Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York city. J Pediatr. (2020) 224:24–9. doi: 10.1016/j.jpeds.2020.06.045
10. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. (2020) 72:1791–805. doi: 10.1002/art.41454
11. Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. Association of intravenous immunoglobulins plus methylprednisolone vs. immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. (2021) 325:855–64. doi: 10.1001/jama.2021.0694
12. Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. (2020) 130:5967–75. doi: 10.1172/JCI140970
13. Vella LA, Rowley AH. Current insights into the pathophysiology of multisystem inflammatory syndrome in children. Curr Pediatr Rep. (2021) 9:83–92. doi: 10.1007/s40124-021-00257-6
14. Ward JL, Harwood R, Smith C, Kenny S, Clark M, Davis PJ, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med. (2022) 28:193–200. doi: 10.1038/s41591-021-01627-9
15. Giannattasio A, Orlando F, D'Anna C, Muzzica S, Angrisani F, Acierno S, et al. Distinctive phenotype of multisystem inflammatory syndrome in children associated with SARS-CoV-2 according to Patients’ age: a monocentric experience. Children (Basel). (2022) 9(4):468. doi: 10.3390/children9040468
16. Taddio A, Della Paolera S, Abbagnato L, Agrusti A, Badolato R, Biscaro F, et al. Early anakinra treatment improves cardiac outcome of multisystem inflammatory syndrome in children regardless of disease severity. Rheumatology (Oxford). (2024):63(2):366–75. doi: 10.1093/rheumatology/kead381
17. Li W, Tang Y, Shi Y, Chen Y, Liu E. Why multisystem inflammatory syndrome in children has been less commonly described in Asia? Transl Pediatr. (2020) 9:873–5. doi: 10.21037/tp-20-151
18. Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr. (2020) 16:223–31. doi: 10.1007/s12519-020-00343-7
19. Sönmez HE, Çağlayan Ş, Otar Yener G, Başar EZ, Ulu K, Çakan M, et al. The multifaceted presentation of the multisystem inflammatory syndrome in children: data from a cluster analysis. J Clin Med. (2022) 11(6):1742. doi: 10.3390/jcm11061742
20. Mauro A, Maglione M, Savoia F, Calvi M, Amoroso A, Sangerardi M, et al. Multisystem inflammatory syndrome in children: tools for a timely diagnosis in the emergency department from an Italian multicenter survey. Int J Pediatr Child Health. (2023) 11:39–49. doi: 10.12974/2311-8687.2023.11.07
21. Haslak F, Barut K, Durak C, Aliyeva A, Yildiz M, Guliyeva V, et al. Clinical features and outcomes of 76 patients with COVID-19-related multi-system inflammatory syndrome in children. Clin Rheumatol. (2021) 40:4167–78. doi: 10.1007/s10067-021-05780-x
22. Feleszko W, Okarska-Napierała M, Buddingh EP, Bloomfield M, Sediva A, Bautista-Rodriguez C, et al. Pathogenesis, immunology, and immune-targeted management of the multisystem inflammatory syndrome in children (MIS-C) or pediatric inflammatory multisystem syndrome (PIMS): EAACI position paper. Pediatr Allergy Immunol. (2023) 34:e13900. doi: 10.1111/pai.13900
23. Cattalini M, Taddio A, Bracaglia C, Cimaz R, Paolera SD, Filocamo G, et al. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): a diagnostic and treatment guidance from the rheumatology study group of the Italian society of pediatrics. Ital J Pediatr. (2021) 47:24. doi: 10.1186/s13052-021-00980-2
24. Çelikel E, Tekin ZE, Aydin F, Emeksiz S, Uyar E, Özcan S, et al. Role of biological agents in the treatment of SARS-CoV-2-associated multisystem inflammatory syndrome in children. J Clin Rheumatol. (2022) 28:e381–7. doi: 10.1097/RHU.0000000000001734
25. Niño-Taravilla C, Espinosa-Vielma YP, Otaola-Arca H, Poli-Harlowe C, Tapia LI, Ortiz-Fritz P. Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV 2 treated with tocilizumab. Pediatr Rep. (2020) 12:142–8. doi: 10.3390/pediatric12030029
26. Cattalini M, Della Paolera S, Zunica F, Bracaglia C, Giangreco M, Verdoni L, et al. Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated to SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: results from a national, multicenter survey. Pediatr Rheumatol Online J. (2021) 19(1):29. doi: 10.1186/s12969-021-00511-7
27. Bruggeman CW, Nagelkerke SQ, Lau W, Manlhiot C, de Haas M, van Bruggen R, McCrindle BW, et al. Treatment-associated hemolysis in Kawasaki disease: association with blood-group antibody titers in IVIG products. Blood Adv. (2020) 4(14):3416–26. doi: 10.1182/bloodadvances.2020002253
28. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association [published correction appears in circulation. 2019 Jul 30;140(5):e181–e184]. Circulation. (2017) 135(17):e927–99. doi: 10.1161/CIR.0000000000000484
29. Chan H, Chi H, You H, Wang M, Zhang G, Yang H, et al. Indirect-comparison meta-analysis of treatment options for patients with refractory Kawasaki disease. BMC Pediatr. (2019) 19(1):158. doi: 10.1186/s12887-019-1504-9
Keywords: multisystem inflammatory syndrome in children (MIS-C), PIMS-TS, Kawasaki disease (KD), COVID-19, children
Citation: Mauro A and Giani T (2024) Editorial: Multisystem inflammatory syndrome in children. Front. Pediatr. 12:1370467. doi: 10.3389/fped.2024.1370467
Received: 14 January 2024; Accepted: 8 March 2024;
Published: 19 March 2024.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TürkiyeReviewed by:
Aybuke Gunalp, Istanbul University-Cerrahpasa, TürkiyeHiromichi Hamada, Chiba University, Japan
© 2024 Mauro and Giani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Mauro YW5nZWxhLm1hdXJvODRAZ21haWwuY29t
 Angela Mauro
Angela Mauro Teresa Giani
Teresa Giani