- 1Pediatric and Adolescent Unit, Department of Internal Medicine, University of Pavia, Pavia, Italy
- 2Pediatric Department, “Vittore Buzzi” Children’s Hospital, Milan, Italy
- 3Department of Biomedical and Clinical Science, Università Degli Studi di Milano, Milan, Italy
Obesity and depression represent major health problems due to their high prevalence and morbidity rates. Numerous evidences elucidated the connections between dietary habits and the incidence or severity of depression. This overview aims to investigate the intricate relationship between dietary patterns and depression with the objective of elaborating preventive strategies for childhood obesity. Literature data recognized that there is a link between mood and food choices, with certain foods selected for their impact on the brain's reward centers. This behavior parallels the one observed in substance addiction, suggesting a specific neural mechanism for food addiction that contributes to overeating and obesity. It is important to note the significant correlation between obesity and depression, indicating a shared biological pathway influencing these conditions. Stress substantially affects also eating behaviors, often leading to increased consumption of pleasurable and rewarding foods. This can trigger a cycle of overeating, weight gain, and psychological distress, exacerbating mood disorders and obesity. In addition, consumption of certain types of foods, especially “comfort foods” high in fat and calories, may provide temporary relief from symptoms of depression, but can lead to long-term obesity and further mental health problems. Understanding these complex interactions is critical to developing preventive strategies focusing on dietary, emotional, and environmental factors, thereby reducing the risk of obesity and mood disorders.
1 Introduction
Obesity and depression represent major health problems due to their high prevalence and morbidity rates, contributors to disease burden also within the pediatric population. Adolescence, marked by significant psychosocial and physical changes, heightens the likelihood of co-occurring obesity and depression (1, 2). While depression is a common mental health disorder, its multifaceted etiological mechanisms remain not completely understood. Typical symptoms include a persistent low mood, reduced interest in usual activities, a generally negative outlook that hinders daily life, and pessimistic expectations about the future. The spectrum of effects and severity varies, ranging from mild cases characterized by a loss of interest and anhedonia to severe instances involving self-harm and suicide (1, 2).
The frequent co-occurrence of obesity and depression indicates a combined effect of various factors (Figure 1). The relationship is complex and bidirectional, but not yet fully explored. A variety of non-modifiable factors, like genetics, and modifiable factors, including psychological and environmental aspects (diet, lifestyle and social support), contribute to this intricate interplay (3). Obesity, characterized by hormonal imbalances and chronic inflammation, may play a role in precipitating depressive symptoms. Indeed, children and adolescents who are overweight or obese may often experience negative body image, stigmatization and low self-esteem increasing their vulnerability to depression. Additionally, depression may affect hormones involved in appetite-satiety circuit regulation. Sedentary lifestyle and emotional eating, intertwined with unhealthy dietary habits, represent shared aspects of both conditions (4). Several studies also highlight numerous common biological mechanisms, that are likely involved in causing both obesity and depression. These include inflammation, dysregulation of the hypothalamic-pituitary-adrenocortical axis, poor glycemic control, and impairment in neurotransmitter and neuroendocrine systems through melanocortinergic leptin-brain-derived neurotrophic factor (BNDF) signaling (5). The comprehensive exploration of these shared mechanisms and contributing factors is essential for a deeper understanding of the intricate interplay between obesity and depression, paving the way for more targeted interventions and therapeutic strategies.
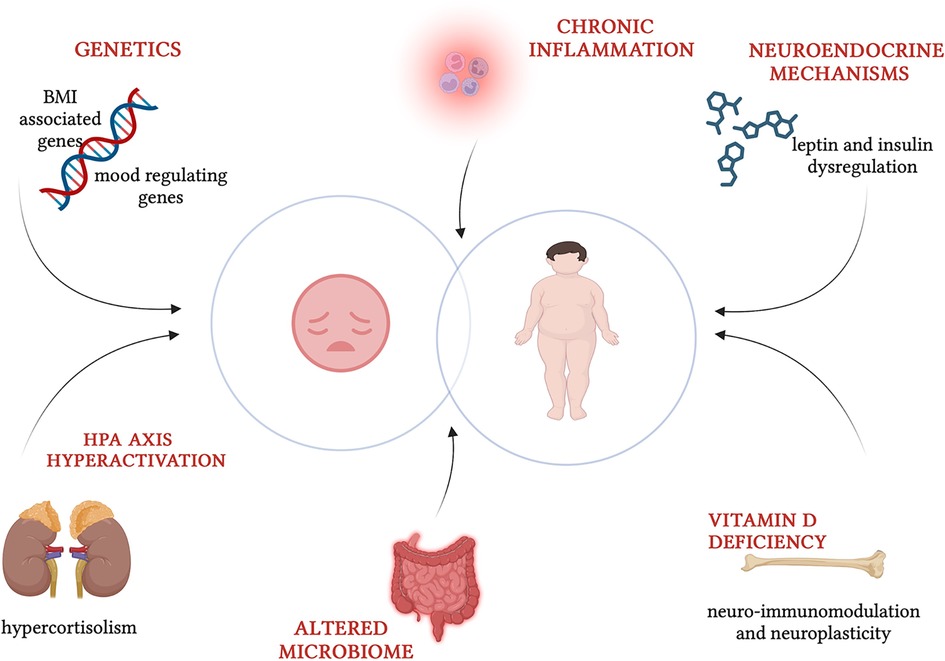
Figure 1. Common pathogenic factors in childhood obesity and depression (created with BioRender.com).
Over the past few decades, numerous systematic reviews and meta-analyses have established evidence elucidating the connections between dietary patterns, food quality and categories, macronutrients and micronutrients, and the incidence or severity of depression. Established findings highlight a negative relationship between the occurrence of depression and the intake of Mediterranean and traditional diets characterized by high levels of complex carbohydrates, B vitamins, omega-3 fatty acids and certain amino acids. Diets rich in fruits, vegetables, fish and meat have been associated with reduced symptoms of depression. Conversely, diets rich in processed or junk foods, saturated fats and sugary drinks have been linked to an increased risk of depression in adolescents (1, 3).
This overview aims to investigate the intricate association between dietary patterns and depression with the objective of elaborating preventive strategies for obesity in child and adolescent and investigating plausible impact of dietary interventions on managing depression.
2 Methods
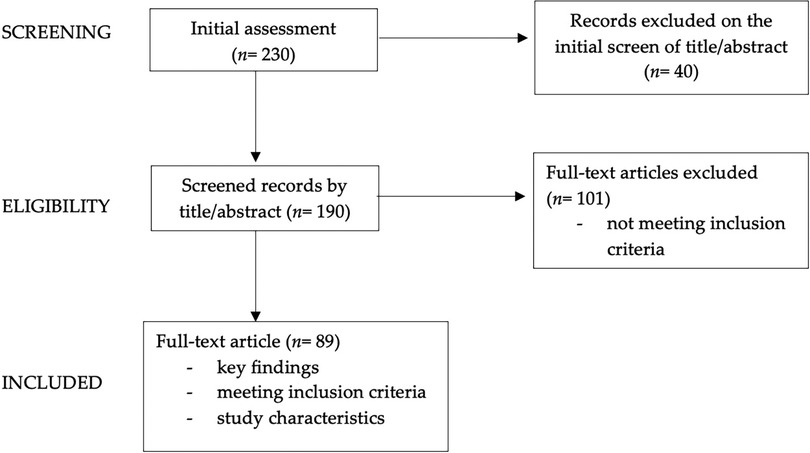
We conducted a narrative review (6, 7) to examine the relationship between dietary patterns and depression in children and adolescents with obesity. We performed a comprehensive search of literature on the PubMed database and Cochrane Library, considering English language publications from the last 25 years. Our review encompassed a diverse range of publications: original research papers, systematic reviews, meta-analyses and longitudinal studies. These encompassed studies involving both adult and pediatric populations, with a specific focus on the pediatric age group; specifically, all pediatric articles were included, whereas concerning adult data, the authors incorporated the most significant findings from adult studies into the review. The keywords used in our search and the count of articles identified and scrutinized for each section are detailed in Table 1. We started with an initial pool of 230 articles, then narrowed it down by screening abstracts (n = 190) and subsequently conducting an in-depth review of the full texts of pertinent papers (n = 89).
These articles were meticulously analyzed to facilitate an informed and critical discussion. Furthermore, we checked the references of all reviewed articles.
In Figure 2, the diagram showing graphically the process of papers selection and exclusion is reported.
3 Depression and pediatric obesity
3.1 Relationship between depression and obesity
Childhood obesity poses a pervasive global public health challenge, with far-reaching consequences and associated comorbidities that jeopardize the well-being of forthcoming generations (11). While the undeniable physical ramifications of childhood obesity demand swift intervention, it is equally imperative to acknowledge the significant impact of excess weight on mental health and quality of life.
Depression impacts over 320 million individuals globally, recognized by the World Health Organization (WHO) as an emerging global health issue and a leading contributor to the worldwide disease burden (12–14). WHO estimates that mental health conditions are responsible for more than 15% of the burden of disease and injury among teens. Depression is the fourth leading cause of illness, and 20% of teenagers manifest some level of depressive symptoms or anxiety (2). This underscores the critical imperative for timely interventions aimed at reducing its prevalence and averting detrimental long-term social consequences. This condition manifests through persistent feelings of sadness, hopelessness, disinterest, low self-esteem, and frequent thoughts of death. Moreover, it can profoundly impair one's ability to handle everyday challenges, including work, ultimately eroding the individual's life skills, leading to disability, and even premature death (12–15).
Although weight loss in the absence of intentional dieting and reduced appetite are more commonly observed symptoms of depressive disorder, the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) diagnostic criteria for a major depressive episode encompass weight gain and an increase in appetite (16). The correlation between fluctuations in weight and depression, whether occurring concurrently or at distinct times, suggests a potential shared etiology or a causal relationship where one may precipitate the other.
Sutaria et al. (17) conducted the most Comprehensive meta-analysis to date, incorporating twenty-two studies with prospective, retrospective, or cross-sectional designs. Their analysis encompassed data from 143,603 children aged 18 years or younger, drawn from general population or community settings. A noteworthy finding emerged regarding obese female children, who exhibited 44% increased odds of depression compared to their normal-weight counterparts, while no significant association was identified between overweight status in children and depression, nor among specific subgroups of obese or overweight males and depression.
Comparable findings were yielded by Moradi et al. (4), who conducted a meta-analysis comprising 28 observational studies. Their investigation not only examined the potential link between weight excess and obesity but also explored the correlation between body mass index (BMI) and the prevalence of additional psychological conditions, including low self-esteem and body dissatisfaction (4). Indeed, existing literature underscores the critical role of body dissatisfaction, marked by anxieties related to weight, body shape, and body fat. This phenomenon is of utmost importance, as it substantially contributes to the emergence of low self-esteem, thereby predisposing individuals in this age group to adverse mental health outcomes, notably depression (18).
Zhao et al. (19) conducted a cross-sectional study focusing on adults with overweight and obesity in the U.S., finding that a larger waist circumference or abdominal obesity was significantly linked to a higher probability of experiencing depressive symptoms. Hamer et al. (20) carried out a longitudinal study on adults with obesity, revealing that metabolically healthy obesity does not correlate with an increased risk of depression. Instead, the link between obesity and depression seems to hinge on the metabolic profile of the individual.
Moradi et al. (4) identified a statistically significant positive correlation between the prevalence of obesity and the likelihood of experiencing body dissatisfaction among both male and female children and adolescents. However, the association between weight excess and depression reached statistical significance solely in the case of girls (4). The authors hypothesized that the gender-specific correlation observed could be explained by psychosocial factors, particularly weight perception. Their suggestion implied that only those children who perceive themselves as overweight may develop a negative body image, subsequently contributing to the onset of depression (14). Interestingly, this relationship between body dissatisfaction and increasing BMI was less conspicuous in male children, where a higher BMI might be associated with qualities such as strength and athleticism, in contrast to their female counterparts (21). Therefore, while the association between depression and body dissatisfaction, as well as between obesity and a negative body image, appears evident in both genders, the prevalence of depression among obese females is more pronounced (4, 17, 18).
In contrast, to the aforementioned findings, Rao et al.'s (22) meta-analysis of 11 comparative studies involving 69,220 subjects revealed a significant increase in the risk of major depressive disorder (MDD) among children and adolescents with obesity when compared with healthy controls. Notably, this increased risk was observed without any gender-specific differences (17).
A key distinction between this meta-analysis and the previously mentioned studies lies in the criteria for diagnosing depressive disorder. Rao et al. (22) uniquely included studies where the diagnosis of depressive disorder was based on international diagnostic criteria, such as the International Classification of Diseases, eleventh version (ICD-11), and DSM-5 (10, 16). This selective focus on standardized diagnostic criteria limited the generalizability of the findings for clinical practice.
Another noteworthy aspect of Sutaria et al.'s (17) study is the execution of a subgroup meta-analysis specifically focusing on longitudinal studies, encompassing follow-up periods ranging from 1 to 20 years in cohort studies. This analysis unveiled an association between childhood obesity and elevated odds of developing depression in the future when compared to normal-weight children (17). This finding aligns with the understanding that the onset of depression during childhood augments the risk of experiencing subsequent depressive episodes in adult life (23).
3.2 Pathogenic mechanism of obesity and depression development
Many studies discuss multiple common pathological mechanisms involved in obesity and depression like chronic systemic inflammation (24), the dopaminergic reward system, vitamin D deficiency (25) and neuroendocrine mechanisms via leptin melanocortinergic—BDNF signaling (9).
The mechanisms behind the association between diet and -both mental and physical- health outcomes are complex, multifaceted and not restricted to any one biological pathway. Among the pathways implicated, the hyperactivation of the hypothalamus–pituitary–adrenal axis, and the consequent excessive glucocorticoids secretion, seems to have an important role both mood disorders and obesity (8, 26). Indeed, it was shown that stress and HPA-axis activation causes overconsumption of Western- style food and subsequent obesity (27). This phenomenon (known as emotional eating and comfort food) acts as attempt to mitigate stress and anxiety (27).
Another important player in this context is the neuronal reward circuitry and the neurotransmitters involved in it. Specifically, a correlation between exposure to high-fat diet (highly prevalent in subjects with obesity) and increase in serotonin and dopamine, acting on mood regulation, has been found (8, 26).
Lastly, inflammation levels seem to have a role in both mood disorders and obesity (28). Interestingly, Miller et al. reported in a cross-sectional study that depressive symptoms promote weight accumulation, that in turn activates an inflammatory response through adipose tissue release of interleukin-6 and leptin-induced upregulation of interleukin-6 release by white blood cells (29).
Indeed, obesity is characterized by chronic low-grade inflammation and depressed subjects present higher levels of inflammatory markers vs. controls according to clinical studies on cytokine-induced depression and comprehensive meta-analyses (30). In the cross-sectional study McLachlan et al. (11) demonstrated greater levels of inflammatory cytokines (C-reactive protein, tumor necrosis factor-alpha, interleukin-6) and adiposity measures such as BMI percentile, trunk/total fat ratio and total fat in depressed adolescents compared to non-depressed counterparts. Additionally, also severity depression showed a significantly positive correlation with BMI percentile and visceral adipose tissue. Thus, among therapeutic opportunities, it's crucial to focus on managing body composition reducing chronic inflammation to potentially improve outcomes (11).
Similarly, major depressive disorder is associated with expressions of genes involved in innate and adaptive immune pathways.
Neuroendocrine substances are also implicated in this intricate etiopathogenesis. Leptin influences mood acting on neuron receptors expressed in the limbic system, strengthening of neurogenesis and cortex neuroplasticity and modulation of hypothalamic-pituitary-adrenal (HPA) axis and immune system. Primary obesity is characterized by leptin resistance which is considered a possible risk phenotype for depression (31, 32). Brain regions that regulate appetite and energy (hypothalamus and pituitary gland) and mood regulation (hippocampus and limbic system) contain highly expressed genes near loci associated with BMI. These findings demonstrate its crucial role in regulating body mass and energy homeostasis, which intersect with those responsible for mood regulation (33). Neuroimmodulation and neuroplasticity vitamin D's functions could imply its role in psychiatric disorders, biologically proved also by the presence of vitamin D receptors in cerebral regions involved in depression (25). Hypothalamic-pituitary-adrenal axis dysregulation and hyperactivation is another mechanism widely reported. Long-term exposure to a high level of stress hormone like cortisol increases appetite, promotes adipogenesis and reduces energy expenditure, but also causes neurological lesions of the limbic areas (hippocampus and amygdala) affected in depression. It is plausible that the hypercortisolism of patients with obesity makes them more susceptible to metabolic complications of obesity and depression (34). These findings impact appetitive and homeostatic regulatory systems promoting obesogenic and depressogenic behaviors. In Figure 1, pathogenic factors involved in childhood obesity and depression are schematized.
4 Interaction between mood, emotional state, and feeding behaviors
4.1 Regulation of energy intake in humans
Energy balance requires that an organism match caloric intake relatively precisely with caloric expenditure (35, 36). In humans, it has been estimated that an error of only +11 kcal/day results in a one pound weight gain over the course of a year (35).
In order to face obesity and its consequences, both from the physical and from the psychological point of view, it's critical to understand the mechanisms which regulate energy homeostasis and food intake in humans.
Interesting, pioneer studies of animal behavior highlighted the existence of two mechanisms involved in the regulation of food intake: satiation and satiety (37). Satiation is defined as a set of complex processes that inhibit the motivation to eat during an eating event. Generally, the beginning of a meal is due to different stimulations, involving physiological factors (hunger), sensory cues (olfactory, gustatory, visual), and other factors linked to the environment (time of day, mood, the social situation); progressively, food is eaten internal inhibitory influences (sensory, cognitive, gastric, hormonal, neural) develop and bring ingestion to an end (37, 38).
Satiety instead is the inhibitory mechanism occurring at the end of eating and prevents the return of hunger for variable time. In efficient appetite and energy control, hunger, satiation, and satiety occur in succession and balance energy intake to energy needs. Instead, the current epidemic of obesity shows that different things can alter appetite control and energy intake, because of various environmental and psychological influences, as emotional eating (EE), tend to override physiological mechanisms (37). In order to better understand this issue, it's fundamental to briefly review the physical mechanisms involved in energy intake regulation.
Food intake is regulated by activation of peripheral signals, the so called “afferent system” (e.g., in the gastrointestinal tract and adipose tissue), that directly monitor incoming nutrient and nutrient stores, and central systems (e.g., the hypothalamus, the hippocampus, the hindbrain), which receive these signals and modify behavioral and metabolic output to balance energy intake through the “efferent system”. These signals interact with one another, making other signals more or less effective (35, 37).
In Table 2, the main key peptides and neurotransmitters involved are resumed (39).
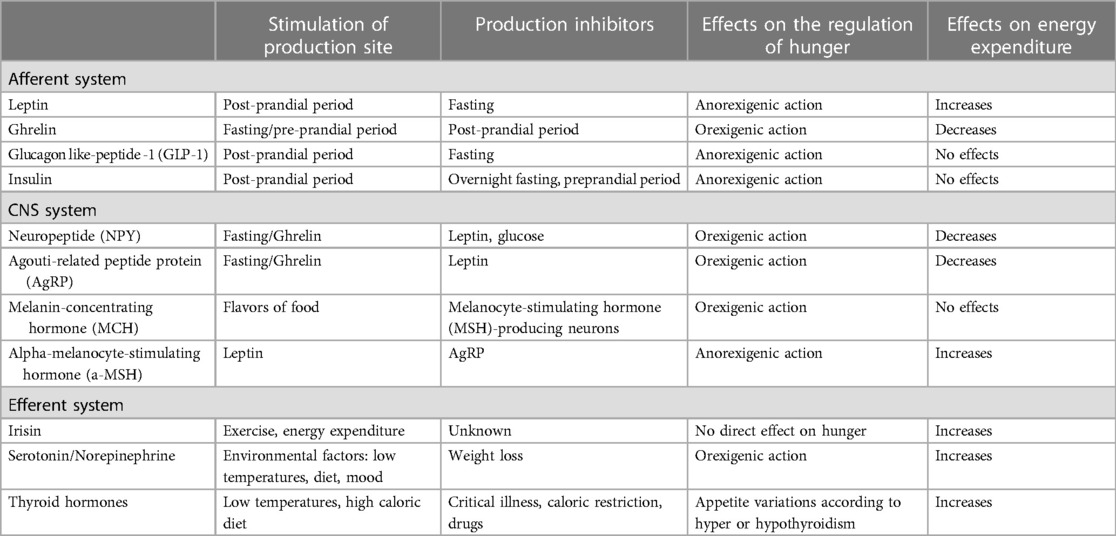
Table 2. Key peptides and neurotransmitters involved in the regulation of energy intake (39).
Interestingly, overweight, or chronic consumption of a high-fat, calorie-rich diet, has been correlated to a change in the sensitivity to these signals and a decreased ability to regulate energy balance and satiety (35, 40). Moreover, obesity seems to affect also cognitive function and sensitivity to the rewarding aspects of food (35). This may further contribute to the persistence of weight gain and overweight status (35, 40).
4.2 Emotional eating
Individuals engage in diverse methods to manage their mood, with food consumption being a prevailing approach (41). The interaction between mood and food intake is reciprocal, involving both the emotional well-being of the individual and the selection and amount of food consumed (42).
Emotional eating, characterized by eating in reaction to emotions, especially negative ones like sadness, anger, or boredom, and notably when not physically hungry, is a recognized feeding behavior (43–56). This dietary pattern often involves eating foods that provide pleasure, serving as a means able to alleviate negative emotional states through hedonic satisfaction (47, 48). Notably, EE can begin as early as the age of two (46, 49). While the exact origins of EE remain unclear, heritability estimates suggest a minimal role for genetic factors, suggesting that EE is predominantly a learned behavior, driven mainly by environmental factors (50).
Therefore, mood effect on reward mechanisms influenced by food intake is well-established (26). Notably, in specific emotional states, certain foods are chosen for their impact on the reward-processing areas of the brain (42, 51–53).
Intriguingly, highly appealing foods have been shown to stimulate brain regions tied to reward and pleasure, in a manner akin to the neural activations seen in drug addiction (54). This suggests the existence of a specialized neural mechanism for food addiction, which plays a significant role in prompting overeating and contributing to the development of obesity (42, 55–59).
Two parallel signaling systems, interoceptive and exteroceptive, ensure normal eating behavior by finely regulating energy intake and the emotional-motivational processes underlying feeding (53, 60). Endocrine and metabolic signals regulate energy consumption and expenditure in response to the body's requirements, through their interaction with the hypothalamic and brainstem nuclei (53). This system is interconnected with a different neural system that oversees the hedonic pleasure derived from food consumption, eliciting emotional responses via the brain's reward system (53, 60, 61). This includes regions like the substantia nigra and ventral tegmental area, along with several cerebral areas such as the amygdala and the orbitofrontal cortex (53, 60, 61). Together, these systems assimilate signals pertaining to the nutritional and sensory state of food, consequently affecting its rewarding qualities, the appetite, and eating behaviors (62, 63). Under typical conditions, the homeostatic and hedonic pathways interact and mutually influence each other. However, in scenarios involving stress or disorders like obesity or depression, this delicate equilibrium can be disrupted (53, 64, 65).
Alterations in dietary habits often serve as a key indicator of mood disorders (42). As stated before, there is a notable and significant link between obesity and depression, a relationship well-documented through extensive research in both human and animal models (42, 66–69).
Preferring palatable foods seems to alleviate stress and anxiety symptoms (70–72). Such dietary patterns are observed in both humans and animals (73–76), suggesting the involvement of a common neurobiological pathway in the decision-making process regarding food choices and eating habits under stress.
Consuming highly palatable, calorie-dense “comfort foods” appears to offer a transient amelioration of depressive symptoms (42). Though, chronic adherence to a high-fat diet is associated with the induction of negative affective states, increased stress sensitivity, and alterations in the basal levels of corticosterone (42). This scenario culminates in a recurrent pattern of excessive food intake, progressive weight gain, and deteriorating mood states, thereby perpetuating a cycle of overeating and psychological distress.
Numerous systematic reviews and meta-analyses have highlighted substantial evidence linking specific dietary patterns, overall diet quality, and various food groups, including macro- and micronutrients, with the onset of depression and the severity of its symptoms (3, 77, 78).
Furthermore, food, mood, and stress are intricately linked (42, 68, 79). Stressors, whether acute or chronic, significantly affect eating behaviors, leading to either an increase or decrease in both the quantity and quality of food consumed (68, 79, 80).
5 Dietary habits and the risk of depression
The nutritional quality of children's diet has markedly declined in recent decades. This low-quality diet may adversely affect children's health, increasing the risk of dental issues, childhood obesity and related complications, subpar academic performance, and diminished self-esteem. This deterioration, coupled with a seemingly parallel rise in the prevalence of adolescent depression, has spurred increased interest in evaluating the potential dietary role in the development or exacerbation of depressive symptoms (81–85). Considering the developmental processes of brain during childhood, the influence of diet on mental health may arguably be more significant during this period than in subsequent stages of life (86). It is crucial to consider as confounding variables various influencing factors conditioning socio-economic status as these can impact diet. On the whole, researches focusing on adults and their diet's relationship with mental health have suggested a multifaceted and potentially bidirectional connection (86).
In epidemiological studies examining correlations among adults, a Westernized diet has been associated with a heightened likelihood of mental disorders and psychiatric unease. Conversely, it has been demonstrated that a healthy diet is related to enhanced mental well-being (81, 87, 88). Similarly, a study conducted by Jacka et al. (89) has revealed that adolescents adhering to a healthy diet exhibit a lower likelihood of reporting symptomatic depression. On the contrary, adolescents who consumed a higher amount of processed foods have a higher risk of experiencing depression. Furthermore, a year later, Jacka et al. has demonstrated that betterment of eating habits is correlated with improvement of mental health (89). Converging results have been found out by a recent meta-analysis conducted by Orlando et al. this research revealed a noteworthy association between adopting a healthy eating pattern and fewer depressive symptoms among children and adolescents (90).
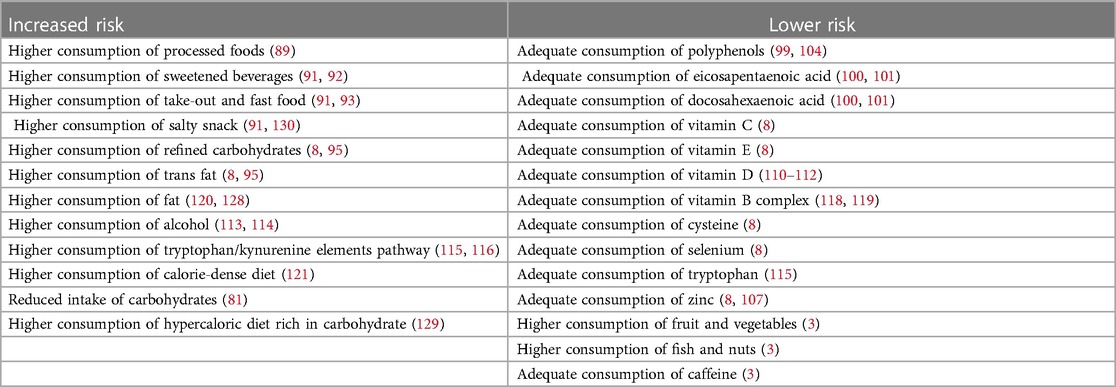
Over the years, various studies have examined the influence of diet on mood disorders, and a correlation has been observed between depression and sweetened beverages by Zahedi (91) and Hoare et al. (92), take-out and fast food consumption by Castillo (93) and Zahedi et al. (91), daily salty snack intake by Zahedi et al. (91). Therefore, findings from these studies indicate that foods with elevated levels of starch, fat, and salt exhibit a more pronounced association with depressive symptoms compared to sweet foods.
Recent observational research has underscored that individuals grappling with severe mental illness display markedly heightened levels of dietary inflammation when compared to the broader population. More specifically, children and adolescents grappling with major depressive disorders exhibit heightened levels of pro-inflammatory cytokines and this escalation in pro-inflammatory markers served as a predictive factor for depression symptoms in this age group (30, 94). Furthermore, unhealthy eating behaviors contribute to the worsening of depression symptoms by fostering increased inflammation. The latter derives from an increased intake of pro-inflammatory foods, including refined carbohydrates and trans fats, coupled with diminished consumption of anti-inflammatory nutrients sourced from whole foods and plants. Additionally, meta-analyses of longitudinal studies has demonstrated that individuals adhering to a more inflammatory dietary pattern face a heightened risk of developing depression over an extended period (8, 95).
In Dehghan et al.'s research (96), a substantial negative association has been observed between dietary antioxidant index and depression in adolescent girls. This emphasizes the significance of maintaining a healthy and anti-inflammatory nutrition for adolescents' mental well-being (96). As demonstrated by a recent study conducted by Sureda et al. (97), adolescents who more strictly adhere to the Mediterranean diet exhibit lower levels of C reactive protein. All these pieces of evidence support how enhanced compliance with the Mediterranean diet has the potential to mitigate the impact of inflammation induced by stress and lower the likelihood of future mental health issues (98). A healthy diet encompasses various nutritional components, with certain elements standing out for their anti-inflammatory properties.
5.1 Foods and nutrients associated with depression
Phytochemicals like polyphenols found in blueberries, and cocoa exhibit strong anti-inflammatory effects (99). Eicosapentaenoic acid and docosahexaenoic acid, omega-3 fatty acids abundant in marine products like salmon, have anti-inflammatory properties. These fatty acids also demonstrate a capacity to delay cytokine-induced depression (100, 101). Moreover, results from studies on animals have indicated that omega-3 fatty acids may mitigate inflammation-induced decreases in neurogenesis to a degree similar to that of antidepressants (8). Oxidative stress is implicated in cellular damage associated with depression. The meta-analysis of 115 articles conducted by Liu et al. (102) has shown elevated oxidative stress markers and reduced antioxidant markers in individuals with depression compared to healthy controls (102). Antidepressant treatment is linked to a reduction in oxidative stress markers, suggesting a causal relationship. Moreover, diet plays a pivotal role, influencing oxidative stress by either depleting or enhancing dietary compounds with antioxidant properties (102). Studies on animals, such as the research conducted on aged mice by Morrison et al. (103), have suggested that high-fat Western-style diets can raise indicators of oxidative stress both in brain and periphery. Given the reported elevated levels of oxidative stress in individuals with mental disorders, improving dietary quality emerged as a potential strategy to restore oxidative balance (8). Vitamins C and E as direct free radical scavengers and selenium, zinc and cysteine as cofactors for antioxidant system combat oxidative stress (8). Furthermore, according to Zhang et al. (104), also polyphenols enhance antioxidant defenses nuclear factor erythroid, nuclear factor k B and mitogen-activated protein (MAP) kinase pathway signaling (104). Polyphenols have demonstrated the ability to influence the gut microbiota and function as anti-inflammatory agents (105).
Some of above-mentioned micronutrients have a potential antidepressant action, independent from the antioxidant effect. Vitamin C, beyond antioxidative properties and consequent neuroprotective action, serves as a neuromodulator within the brain, influencing neurotransmission mediated by both dopamine and glutamate and exert an impact on 5- Serotonin 1A (HT1A) receptor (106–109). Zinc regulates N-methyl-D-aspartate (NMDA) receptor function and boosts neuroplasticity and neurogenesis. This micronutrient impacts serotonin receptors and regulates immune responses (107). Vitamin D exhibits various potential mechanisms with antidepressant effects: it modulates innate and adaptive immune response through regulation of inflammation and production of antimicrobial substances, it influences hypothalamic-pituitary-adrenal axis, and it regulates serotonin and dopamine synthesis (110–112). A recent umbrella review published by Xu et al. (3), has affirmed an inverse link between depression risk and intake of fruits and vegetables, particularly noticeable in high vs. low consumption meta-analysis, albeit with evidence of modest quality. A heightened presence of saturated fats in red and processed meat has been associated with reduced brain-derived neurotrophic factor, irregular neuroplasticity, and compromised cognitive function—all implicated in depression's pathogenesis.
Conversely, fish and nuts, abundant in n-3 polyunsaturated fatty acids, exhibited potential benefits in preventing depression through anti-inflammatory processes, neuro-endocrine modulation, and neurotransmitter activation (3). The ANIVA (Antropometria y Nutricion Infantil de Valencia) study, a descriptive cross-sectional study conducted by Rubio-Lopez et al. (81), has shown a reduced intake of carbohydrates in children displaying symptoms of depression than those without such symptoms.
Carbohydrates exert a profound influence on nervous system, mood and behavior: their ingestion induces to supply glucose and energy, and conditions neurotransmitter synthesis. The consumption of a high amount of carbohydrates induces the release of insulin, facilitating the entry of blood sugar into cells for energy while simultaneously promoting the entry of tryptophan into the brain (81).
As shown by Teesson et al. (113) and Chou et al. (114), alcohol consumption by adults is linked to depression. The association between alcohol and depression has been reported, with past cross-sectional studies suggesting a higher susceptibility to depression in individuals facing alcohol-related issues. However, it is crucial to note that those with depression may drink alcohol as a means for alleviating distress, potentially leading to an overestimation of alcohol's impact on depression risk.
In psychiatry, the exploration of tryptophan availability and metabolism has predominantly focused on its conversion into serotonin, a pivotal target for most antidepressants. Nevertheless, the primary physiological route for tryptophan entails the kynurenine pathway, giving rise to both neurotoxic quinolinic acid and neuroprotective kynurenic acid (115). Exploring tryptophan supplementation as a strategy in managing depression, studies have yielded diverse results. Within the brain, tryptophan influences neurotransmitter levels as the precursor to brain serotonin. Individuals with diminished brain serotonin levels are deemed susceptible to depression (8, 81). However, in instances of heightened tryptophan metabolism through the kynurenine pathway (e.g., induced by stress or immune activation), there could be an augmented generation of the neurotoxic quinolinic acid. As shown by O' Connor et al. (116) giving L-kynurenine to mice with no prior exposure results in dose-dependent induction of behavior resembling depression. An essential cofactor in the metabolism of tryptophan is vitamin B6 that acts facilitating its conversion into the neurotransmitter serotonin.
Vitamins from the B-complex play a central role in energy metabolism, mitochondrial function, and the production of neurotransmitters (107, 117). The cross-sectional analysis conducted by Murakami et al. has demonstrated a negative correlation between riboflavin level and depression in females aged 12–15 years, but no association with B12 levels in both sexes (118). In another cross-sectional study conducted by Herbison et al. (119) females and males aged 17 years characterized by a low intake of vitamin B6 and folate had higher internalizing behavior scores measured by Youth Self Report. Although mechanisms behind are unknown, also caffeine consumption seems associated with a reduced risk of depression. Several theoretical biological rationales exist, according a plausible theory coffee, with its abundant caffeine content, can stimulate the central nervous system and augment dopaminergic neurotransmission (3). As demonstrated by studies conducted with animal models, high fat or high calories diet can modify gut microbiome increasing Firmicutes/Bacteroidetes ratio (120) and Clostridiales, Ruminococcaceae, and Bacteroidales (121) contributing to behavioral changes similar to symptoms of depression and anxiety. As shown by Hiel et al. (122), a diet rich in inulin-type fructans, a type of fermentable dietary fiber, increases Bifidobacterium spp., enhancing satiety and intrapersonal competence without affecting mood or perceived stress (122). Liu et al. (123) through a random-effects meta-analysis of 34 controlled clinical trials evaluating the effects of probiotics and prebiotic on depression and anxiety has shown that addition of live microorganisms, either Lactobacillus spp. alone or in conjunction with Bifidobacterium spp., has the potential to positively impact both depression and anxiety. Contrariwise the use of prebiotic has shown no significant difference in symptoms compared to control, but the sample was limited and composed especially by non-clinical participants. Nevertheless, according to the major literature, also prebiotic supplementation reverses stress-induced microbiota changes preserving beneficial Bifidobacterium spp. or Lactobacillus spp. and mitigates depressive behaviors in mice. Fermented foods, incorporating functional microorganisms, prebiotics, and biogenics, constitute a food category with the potential to influence gut-brain communication (123, 124). Exact pathways which connect the gut microbiota to diet-brain are still under study, but various mechanisms have been suggested, such as through immune-immunomodulatory action of microbial metabolites as short-chain fatty acids which derives from dietary fiber, neuronal and endocrine pathways through nervus vagus and microbioma direct-indirect control of tryptophan metabolism pathway or of other neurotransmitters such as γ-Aminobutyric acid (GABA) and the hypothalamus–pituitary–adrenal axis (8). Among studies suggesting involvement of hypothalamic-pituitary-adrenal axis, the one conducted by Barbadoro et al. (125) has highlighted improvement in cortisol levels in both healthy and depressed adult through administration of omega-3 fatty acid. Moreover, the study conducted by Tsang et al. (126) has demonstrated similar result through the administration of polyphenol-rich foods in adults recruited from a health and social care setting. The involvement of hypothalamic-pituitary-adrenal axis seems implicated in the pathophysiology of neuropsychiatric disorders: more than 60% of individuals with depression display heightened cortisol production or disruptions in this axis (8).
Results from animal studies have indicated that an elevated sugar diet during the puberal transition fosters increased depressive tendencies in adulthood by activating the hypothalamic-pituitary-adrenal axis and elevating glucocorticoids. Considering that teenagers constitute the major consumers of sugar-sweetened beverages, an overabundance of added sugar during this developmental phase could contribute to the enduring dysregulation of stress response and predispose to depressive symptoms (3, 127). Also, mitochondrial dysfunction is linked to depression and extensive preclinical data indicated that an inadequate diet might play a role in causing mitochondrial dysfunction. As illustrated by Kuipers et al. (128), Sihali-Beloui et al. (129) and Woodman et al. (130), respectively a high fat diet a hypercaloric diet rich in carbohydrate and a diet high in salt are linked to irregular mitochondrial biogenesis, a phenomenon also correlated with heightened free radical generation and inflammation, conditions associated with depressive symptoms.
In Table 3, a list of foods and nutrients associated with higher or lower risk to develop depression are resumed.
5.2 Gut microbiota and the risk of depression
As demonstrated by studies conducted with animal models, high fat or high calories diet can modify gut microbiome increasing Firmicutes/Bacteroidetes ratio (120) and Clostridiales, Ruminococcaceae, and Bacteroidales (121) contributing to behavioral changes similar to symptoms of depression and anxiety. As shown by Hiel et al. (122), a diet rich in inulin-type fructans, a type of fermentable dietary fiber, increases Bifidobacterium spp., enhancing satiety and intrapersonal competence without affecting mood or perceived stress (122). Liu et al. (123) through a random-effects meta-analysis of 34 controlled clinical trials evaluating the effects of probiotics and prebiotic on depression and anxiety has shown that addition of live microorganisms, either Lactobacillus spp. alone or in conjunction with Bifidobacterium spp., has the potential to positively impact both depression and anxiety. Contrariwise the use of prebiotic has shown no significant difference in symptoms compared to control, but the sample was limited and composed especially by non-clinical participants. Nevertheless, according to the major literature, also prebiotic supplementation reverses stress-induced microbiota changes preserving beneficial Bifidobacterium spp. or Lactobacillus spp. and mitigates depressive behaviors in mice. Fermented foods, incorporating functional microorganisms, prebiotics, and biogenics, constitute a food category with the potential to influence gut-brain communication (123, 124). Exact pathways which connect the gut microbiota to diet-brain are still under study, but various mechanisms have been suggested, such as through immune-immunomodulatory action of microbial metabolites as short-chain fatty acids which derives from dietary fiber, neuronal and endocrine pathways through nervus vagus and microbioma direct-indirect control of tryptophan metabolism pathway mentioned above or of other neurotransmitters such as GABA and the hypothalamus–pituitary–adrenal axis (8).
Exploring the immune modulation along the brain-gut-microbiota axis, extensive research has delved into variations in inflammatory cytokines to probe the immune dynamics in depression. Nonetheless, the intricate immune signaling network underlying these inflammatory fluctuations, encompassing innate and adaptive immune modulation within the gut, brain, and systemic circulation, has surfaced as an integral component of the communication framework among the microbiota, gut, and brain in depression (131, 132).
In 2015 Erny et al. demonstrated the importance of the host microbiota in microglial maintenance, with germ-free mice showing widespread microglial abnormalities characterized by an immature phenotype and defects in innate immunity. Reintroducing a diverse microbiota partially restored normal microglial characteristics. Glial cells engage with neurons, impacting brain well-being and conditions like depression (132, 133).
The gut microbiota can influence glial functions via neural and chemical signaling pathways, modulating microglial activation and playing a role in triggering neuroinflammatory processes in depression (131).
Moreover, research indicates that Th17 and Treg cells, influenced by the gut microbiota, play a crucial role in depression, impacting neuroinflammation and immune balance in the brain and gut (134).
Imbalances in (Thelper) Th17/T regulator (Treg) ratios are linked to depression, affecting the brain-gut-microbiota axis. Specific gut microbiota (e.g., Clostridia, Bacteroides fragilis, Lactobacillus reuteri, and Bifidobacterium) and their metabolites also shape Th17/Treg activity, influencing susceptibility to stress and antidepressant effects (135). Further studies are needed to understand this interplay fully.
Research suggests that commensal microbes in the mammalian gastrointestinal (GI) tract significantly influence intestinal tryptophan availability, impacting tryptophan metabolism. While most ingested tryptophan is absorbed in the small intestine, some reaches the large intestine, where commensal microbes degrade it into tryptamine, a serotonin-related compound (136). Germ-free mice lacking microorganisms show reduced tryptamine levels but increased tryptophan in blood, suggesting microbiota involvement in tryptophan metabolism modulation (137). Additionally, gut microbiota metabolizes tryptophan into indole derivatives and affect serotonin levels in various ways, including promoting serotonin biosynthesis in colonic cells and directly producing serotonin. Microbial modulation of the kynurenine pathway, influenced by inflammatory mediators and microbial metabolites like short-chain fatty acids, further highlights the intricate relationship between the gut microbiota and tryptophan metabolism, with implications for immune function and neuroactive compound production (56, 136).
Particular strains of Lactobacillus and Bifidobacterium produce gamma-aminobutyric acid, a primary inhibitory neurotransmitter in the central nervous system. According to literature, disrupted or altered gamma-aminobutyric acid signaling pathways are associated with anxiety disorders and depression (138, 139). In individuals with unipolar depression, GABA levels in cerebrospinal fluid and plasma are reduced compared to those in control subjects (139). In 2002 Sanacora and co-authors (140) have utilized in vivo proton magnetic resonance spectroscopy to demonstrate decreased GABA levels in the occipital cortex of depressed patients, with a elevation observed in patients undergoing treatment with selective serotonine reuptake inhibitors (139, 140).
Depression correlates with the signaling of the vagus nerve, which plays a role in regulating inflammation and is influenced by neuroactive substances. The vagus nerve establishes a vital connection between the brain, the microbiota in the gut, and the immune system (132). In 2011 Bravo et al. discovered that oral administration of Lactobacillus rhamnosus mitigated stress-induced depressive behaviors in mice when compared to the control group. Furthermore, they found that vagotomy hindered the effects of Lactobacillus rhamnosus on neurochemical processes and on behavioral effects (132, 141). As shown by Wang et al. in 2020 (142), consumption of microbes related to depression such as Lactobacillus intestinalis and Lactobacillus reuteri resulted in depression-like characteristics, elevated levels of interleukin (IL)-6 in the bloodstream and decreased synaptic protein expression in the prefrontal cortex of antibiotic-treated mice. Remarkably, selective dorsal vagotomy prevented the onset of depression-like behaviors, rise in plasmatic IL-6 levels, and decline in synaptic proteins in the prefrontal cortex following oral introduction of Lactobacillus intestinalis and Lactobacillus reuteri (132, 142). These studies acknowledge the vagus as a crucial regulatory communication pathway linking gut-exposed bacteria to the brain.
Among studies suggesting involvement of hypothalamic-pituitary-adrenal axis, the one conducted by Barbadoro et al. (125) has highlighted improvement in cortisol levels in both healthy and depressed adult through administration of omega-3 fatty acid. Moreover, the study conducted by Tsang et al. (126) has demonstrated similar result through the administration of polyphenol-rich foods in adults recruited from a health and social care setting. The involvement of hypothalamic-pituitary-adrenal axis seems implicated in the pathophysiology of neuropsychiatric disorders: more than 60% of individuals with depression display heightened cortisol production or disruptions in this axis (8).
6 Benefit of diet interventions on obesity and depression
It's widely known that diet is the core intervention in the treatment of obesity, both in adulthood and in the pediatric field (143). Interestingly, growing evidence supports the potential use of dietary interventions also as an adjunctive treatment for depression and mood disorders, either associated or not with obese phenotype (144). Indeed, accumulating evidence indicates that obesity and mood disorders are intrinsically linked (26) and the two conditions share clinical, neurobiological, genetic and environmental factors (26).
The emerging area of research working on the potential use of nutritional intervention as adjunctive treatment for depression and mood disorders is known as “Nutritional Psychiatry” (144–146). The dietary interventions studied in this context include nutrient interventions, food interventions and/or whole diet interventions (e.g., Mediterranean or Ketogenic diet) (8).
Unfortunately, there is still insufficient evidence on nutrient-intervention in the treatment of mood disorders as the results of the meta-analyses are still conflicting (144, 145, 147) and evidences lack in the pediatric field. Table 4, reassumes the nutrients studied and the mechanisms of action proposed (144). However, among the supplements studied, omega-3 polyunsaturated fatty acids (n-3 PUFAs) have the greatest evidence for use in the treatment of mood disorder, especially eicosapentaenoic acid (EPA) (148). The beneficial role of n-3 PUFAs has also been shown in subjects with obesity, with and without mental disorders (158, 159), but studies on children are still lacking.
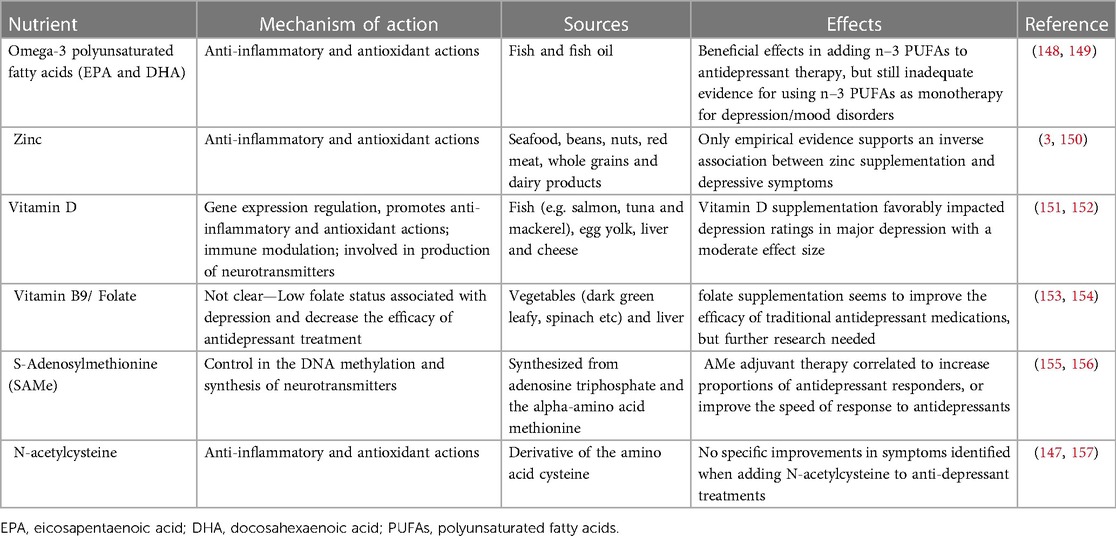
Table 4. Nutritional supplements tested in the treatment of depression/mood disorders—adapted from (144).
The Mediterranean diet (MD), detected in the 1950s and 1960s among Mediterranean populations, emphasizes consumption of plant-based foods, moderate fish, and olive oil as the main fat source. The diet is based on moderate intake of white meat, low-fat dairy, eggs, and low red wine for adults, whereas the consumption of red and processed meats, and sweets is limited. (MD) values seasonality, biodiversity, and consumption of traditional local foods (77, 160, 161). On the contrary, the prevalent Western diet in developed countries leans towards convenience and highly processed foods. It involves high intake of processed and red meat, fried foods, sweets, high-fat dairy, and refined grains, with low consumption of plant-based foods such as whole grains, legumes, vegetables, and fruits.
Among the whole diet interventions studies, different meta-analyses of observational studies evidenced the association between the (MD) and decreased risk of psychiatric disorders (144, 162–164). Specifically, the protective effect of MD against depression was recently confirmed by Lasse et al. and, previously, by Psaltopoulou et al. (144, 163). Recently, Salari-Moghaddam et al. performed a cross-sectional study on 3,176 subjects and demonstrated that high adherence to a regime called Mediterranean-Dietary Approach to Stop Hypertension (DASH) Diet Intervention for Neurodegenerative Delay (MIND), also decreases the risk of developing depression (165). This dietary intervention was created mixing the MD and the DASH diet (165). Interestingly, Jacka et al. performed a randomized controlled trial, aiming to investigate the efficacy of a dietary improvement program for the treatment of major depressive episodes (166). The randomized controlled trial was called “Supporting the Modification of Lifestyle In Lowered Emotional States” (SMILES) and evaluated the effect of a dietary improvement program based on a modified MD (“ModiMedDiet”) in reducing the severity of depressive symptoms in patients with major depression (166). The ModiMedDiet is similar to the MD, but encourages a moderate consumption of red meat and dairy (166). At the end of the 12 weeks of intervention, the subjects that followed the ModiMedDiet had a lower score for depressive symptoms and a higher frequency of remission (166). However, further studies are needed to confirm the benefits of these dietary regimens for depression and other mood disorders, both in adults and in adolescents and children.
Another dietary plan analyzed in the treatment of neuropsychiatric diseases is the Ketogenic diet (KD) (144, 167, 168). KD, used to treat drug-refractory pediatric epilepsy for over 100 years (169) and known to work also on obesity (170), is becoming increasingly popular for the treatment of other neurological conditions, including mental illnesses (167, 168). This diet consists in a reduction of carbohydrate intake (usually less than 20 g a day), in order to stimulate the production of ketone bodies, used as source of energy by the brain (144). Different promising studies were performed evaluating KD in subjects affected by bipolar disorder (171–173), but works in the children or adolescents affected by depression or other mood disorders are missing.
In sum, the results of the studies cited show the need for more clinical trials to assess the effect of nutritional interventions in the treatment of patients (both adults and children) with mood disorders, either normal weight or obese.
7 Conclusions
It is widely recognized that there is a link between mood and food choices, with certain foods selected for their impact on the brain's reward centers. This behavior parallels the one observed in substance addiction, suggesting a specific neural mechanism for food addiction that contributes to overeating and obesity. It is important to note the significant correlation between obesity and depression, indicating a shared biological pathway influencing these conditions. In addition, stress substantially affects eating behaviors, often leading to increased consumption of pleasurable and rewarding foods. This can trigger a cycle of overeating, weight gain, and psychological distress, exacerbating mood disorders and obesity. In addition, consumption of certain types of foods, especially “comfort foods” high in fat and calories, may provide temporary relief from symptoms of depression, but can lead to long-term obesity and further mental health problems.
Understanding these complex interactions is critical to developing preventive strategies focusing on dietary, emotional, and environmental factors, thereby reducing the risk of obesity and mood disorders.
This review suggests that, due to the current paucity of studies in pediatric obesity and depression, for the moment there is not sufficient evidence to drive clear conclusions about the possibility to carry out some therapeutical or preventive strategies in children and adolescence, but only in adults. Moreover, it's worth to underline that in children a specific and rigid dietary plan, as the KD, may need constant clinical evaluations of its possible side effects, with a precise evaluation of growth and nutritional status, in order to avoid possible malnutrition and ensure a linear growth (174). In addition, adherence to this plan may be difficult in the long term, but short nutritional intervention may be anyway sufficient to obtain health improvements (175).
Despite these challenges, dietary interventions including KD represents a possible preventive and therapeutic option for pediatric patients, thus, further research is needed to better understand the potential side effects of this nutritional approach and to find strategies to increase the adherence to the plan.
In conclusion, this analysis highlights the complex relationship between dietary habits, emotional states, and dietary patterns. Therefore, in order to manage both depression and obesity, a holistic and personalized approach, is often necessary and the potential benefits of nutritional interventions should also be considered. Healthcare professionals, including mental health specialists, nutritionists and primary care providers, can provide support for a combination of psychotherapy, medication, lifestyle changes, and other healthcare issues. Integrated care that addresses both mental and physical health is crucial for effective management and long-term well-being.
Author contributions
VC: Conceptualization, Methodology, Supervision, Writing – original draft. VR: Investigation, Methodology, Writing – original draft. VCM: Investigation, Methodology, Writing – original draft. PB: Investigation, Methodology, Writing – original draft. RG: Investigation, Methodology, Writing – original draft. ML: Investigation, Methodology, Writing – original draft. VF: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. GZ: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Project performed in the context of (1) National Recovery and resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3-Call for proposal No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union-NextGenerationEU. Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods-Research and innovation network on food and nutrition Sustainability, Safety and Security-Working ON Foods”; (2) PODiaCar project Call: EU4H-2022-PJ-3 — EU4H-PJG; (3) Together grant, Regione Lombardia (Italy) (CUP: E82C22000570002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chopra C, Mandalika S, Kinger N. Does diet play a role in the prevention and management of depression among adolescents? A narrative review. Nutr Health. (2021) 27(2):243–63. doi: 10.1177/0260106020980532
2. Kokka I, Mourikis I, Bacopoulou F. Psychiatric disorders and obesity in childhood and adolescence—a systematic review of cross-sectional studies. Children. (2023) 10(2):285. doi: 10.3390/children10020285
3. Xu Y, Zeng L, Zou K, Shan S, Wang X, Xiong J, et al. Role of dietary factors in the prevention and treatment for depression: an umbrella review of meta-analyses of prospective studies. Transl Psychiatry. (2021) 11(1):478. doi: 10.1038/s41398-021-01590-6
4. Moradi M, Mozaffari H, Askari M, Azadbakht L. Association between overweight/obesity with depression, anxiety, low self-esteem, and body dissatisfaction in children and adolescents: a systematic review and meta-analysis of observational studies. Crit Rev Food Sci Nutr. (2022) 62(2):555–70. doi: 10.1080/10408398.2020.1823813
5. Piao J, Wang Y, Zhang T, Zhao J, Lv Q, Ruan M, et al. Antidepressant-like effects of representative types of food and their possible mechanisms. Molecules. (2023) 28(19):6992. doi: 10.3390/molecules28196992
6. Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. (2019) 4(1):5. doi: 10.1186/s41073-019-0064-8
7. Gregory AT, Denniss AR. An introduction to writing narrative and systematic reviews — tasks, tips and traps for aspiring authors. Heart Lung Circ. (2018) 27(7):893–8. doi: 10.1016/j.hlc.2018.03.027
8. Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. (2021) 26(1):134–50. doi: 10.1038/s41380-020-00925-x
9. Su SC, Sun MT, Wen MJ, Lin CJ, Chen YC, Hung YJ. Brain-derived neurotrophic factor, adiponectin, and proinflammatory markers in various subtypes of depression in young men. Int J Psychiatry Med. (2011) 42(3):211–26. doi: 10.2190/PM.42.3.a
10. World Heath Organization (WHO). International Statistical Classification of Diseases and Related Health Problems (ICD) (2023). Available online at: https://www.who.int/standards/classifications/classification-of-diseases (accessed February 02, 2024).
11. McLachlan C, Shelton R, Li L. Obesity, inflammation, and depression in adolescents. Front Psychiatry. (2023) 14:1221709. doi: 10.3389/fpsyt.2023.1221709
12. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7
13. Sinyor M, Rezmovitz J, Zaretsky A. Screen all for depression. Br Med J. (2016) 352:i1617. doi: 10.1136/bmj.i1617
14. World Heath Organization (WHO). Depression (2023). Available online at: https://www.who.int/health-topics/depression#tab=tab_1 (accessed February 01, 2024).
15. Lohoff FW. Overview of the genetics of Major depressive disorder. Curr Psychiatry Rep. (2010) 12(6):539–46. doi: 10.1007/s11920-010-0150-6
16. Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM. 5th ed Washington, D.C: American Psychiatric Association (2013). p. 947.
17. Sutaria S, Devakumar D, Yasuda SS, Das S, Saxena S. Is obesity associated with depression in children? Systematic review and meta-analysis. Arch Dis Child. (2019) 104(1):64–74. doi: 10.1136/archdischild-2017-314608
18. Paxton SJ, Neumark-Sztainer D, Hannan PJ, Eisenberg ME. Body dissatisfaction prospectively predicts depressive mood and low self-esteem in adolescent girls and boys. J Clin Child Adolesc Psychol. (2006) 35(4):539–49. doi: 10.1207/s15374424jccp3504_5
19. Zhao G, Ford ES, Li C, Tsai J, Dhingra S, Balluz LS. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: national health and nutrition examination survey 2005–2006. BMC Psychiatry. (2011) 11(1):130. doi: 10.1186/1471-244X-11-130
20. Hamer M, Batty GD, Kivimaki M. Risk of future depression in people who are obese but metabolically healthy: the english longitudinal study of ageing. Mol Psychiatry. (2012) 17(9):940–5. doi: 10.1038/mp.2012.30
21. Austin SB, Haines J, Veugelers PJ. Body satisfaction and body weight: gender differences and sociodemographic determinants. BMC Public Health. (2009) 9(1):313. doi: 10.1186/1471-2458-9-313
22. Rao WW, Zong QQ, Zhang JW, An FR, Jackson T, Ungvari GS, et al. Obesity increases the risk of depression in children and adolescents: results from a systematic review and meta-analysis. J Affect Disord. (2020) 267:78–85. doi: 10.1016/j.jad.2020.01.154
23. Johnson D, Dupuis G, Piche J, Clayborne Z, Colman I. Adult mental health outcomes of adolescent depression: a systematic review. Depress Anxiety. (2018) 35(8):700–16. doi: 10.1002/da.22777
24. Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. (2013) 11(1):200. doi: 10.1186/1741-7015-11-200
25. Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202(2):100–7. doi: 10.1192/bjp.bp.111.106666
26. Mansur RB, Brietzke E, McIntyre RS. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci Biobehav Rev. (2015) 52:89–104. doi: 10.1016/j.neubiorev.2014.12.017
27. Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food.”. Proc Natl Acad Sci. (2003) 100(20):11696–701. doi: 10.1073/pnas.1934666100
28. Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, et al. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun. (2018) 69:1–8. doi: 10.1016/j.bbi.2017.08.026
29. Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. (2003) 17(4):276–85. doi: 10.1016/S0889-1591(03)00057-6
30. Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. (2020) 277:940–8. doi: 10.1016/j.jad.2020.09.025
31. Milaneschi Y, Simmons WK, Van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24(1):18–33. doi: 10.1038/s41380-018-0017-5
32. Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BWJH. Leptin dysregulation is specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biol Psychiatry. (2017) 81(9):807–14. doi: 10.1016/j.biopsych.2015.10.023
33. Milaneschi Y, Lamers F, Peyrot WJ, Baune BT, Breen G, Dehghan A, et al. Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry. (2017) 74(12):1214. doi: 10.1001/jamapsychiatry.2017.3016
34. Noppe G, Van Den Akker ELT, De Rijke YB, Koper JW, Jaddoe VW, Van Rossum EFC. Long-term glucocorticoid concentrations as a risk factor for childhood obesity and adverse body-fat distribution. Int J Obes. (2016) 40(10):1503–9. doi: 10.1038/ijo.2016.113
35. Tracy AL, Hazeltine G, Wee CJM, Benoit SC. Regulation of energy intake in humans. In: Feingold KR, Anawalt B, Blackman MR, et al. editors. Endotext [Internet]. South Dartmouth: MDText.com, Inc. (2013). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK279112/
36. Fernández-Verdejo R, Mey JT, Ravussin E. Effects of ketone bodies on energy expenditure, substrate utilization, and energy intake in humans. J Lipid Res. (2023). 64(10):100442. doi: 10.1016/j.jlr.2023.100442
37. Tremblay A, Bellisle F. Nutrients, satiety, and control of energy intake. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. (2015) 40(10):971–9. doi: 10.1139/apnm-2014-0549
38. de Castro JM. The control of food intake of free-living humans: putting the pieces back together. Physiol Behav. (2010) 100(5):446–53. doi: 10.1016/j.physbeh.2010.04.028
39. Moehlecke M, Canani LH, Silva LOJE, Trindade MRM, Friedman R, Leitão CB. Determinants of body weight regulation in humans. Arch Endocrinol Metab. (2016) 60(2):152–62. doi: 10.1590/2359-3997000000129
40. Webber ES, Bonci A, Krashes MJ. The elegance of energy balance: insight from circuit-level manipulations. Synap N Y N. (2015) 69(9):461–74. doi: 10.1002/syn.21837
41. Morris WN, Reilly NP. Toward the self-regulation of mood: theory and research. Motiv Emot. (1987) 11(3):215–49. doi: 10.1007/BF01001412
43. Macht M. Characteristics of eating in anger, fear, sadness and joy. Appetite. (1999) 33(1):129–39. doi: 10.1006/appe.1999.0236
44. Macht M. How emotions affect eating: a five-way model. Appetite. (2008) 50(1):1–11. doi: 10.1016/j.appet.2007.07.002
45. Arnow B, Kenardy J, Agras WS. The emotional eating scale: the development of a measure to assess coping with negative affect by eating. Int J Eat Disord. (1995) 18(1):79–90. doi: 10.1002/1098-108X(199507)18:1%3C79::AID-EAT2260180109%3E3.0.CO;2-V
46. Stone RA, Blissett J, Haycraft E, Farrow C. Emotional eating following a laboratory mood induction: the interaction between parental feeding practices and child temperament. Food Qual Prefer. (2023) 112:105008. doi: 10.1016/j.foodqual.2023.105008
47. Nguyen-Michel ST, Unger JB, Spruijt-Metz D. Dietary correlates of emotional eating in adolescence. Appetite. (2007) 49(2):494–9. doi: 10.1016/j.appet.2007.03.005
48. van Strien T, Gibson EL, Baños R, Cebolla A, Winkens LHH. Is comfort food actually comforting for emotional eaters? A (moderated) mediation analysis. Physiol Behav. (2019) 211:112671. doi: 10.1016/j.physbeh.2019.112671
49. Haycraft E, Blissett J. Predictors of paternal and maternal controlling feeding practices with 2- to 5-year-old children. J Nutr Educ Behav. (2012) 44(5):390–7. doi: 10.1016/j.jneb.2010.03.001
50. Herle M, Fildes A, Llewellyn CH. Emotional eating is learned not inherited in children, regardless of obesity risk. Pediatr Obes. (2018) 13(10):628–31. doi: 10.1111/ijpo.12428
51. Rangel A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci. (2013) 16(12):1717–24. doi: 10.1038/nn.3561
52. Jauch-Chara K, Oltmanns KM. Obesity—a neuropsychological disease? Systematic review and neuropsychological model. Prog Neurobiol. (2014) 114:84–101. doi: 10.1016/j.pneurobio.2013.12.001
53. Weltens N, Zhao D, Van Oudenhove L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol Motil. (2014) 26(3):303–15. doi: 10.1111/nmo.12309
54. Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. In: Carter CS, Dalley JW, editors. Brain Imaging in Behavioral Neuroscience. Berlin, Heidelberg: Springer Berlin Heidelberg (2011). p. 1–24. (Current Topics in Behavioral Neurosciences; vol. 11). Available online at: https://link.springer.com/10.1007/7854_2011_169
55. Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that “food addiction” is a valid phenotype of obesity. Appetite. (2011) 57(3):711–7. doi: 10.1016/j.appet.2011.08.017
56. Davis C, Levitan RD, Kaplan AS, Kennedy JL, Carter JC. Food cravings, appetite, and snack-food consumption in response to a psychomotor stimulant drug: the moderating effect of "food-addiction". Front Psychol. (2014) 5:403. doi: 10.3389/fpsyg.2014.00403
57. DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. (2012) 15(10):1330–5. doi: 10.1038/nn.3202
58. Potenza MN. Obesity, food, and addiction: emerging neuroscience and clinical and public health implications. Neuropsychopharmacology. (2014) 39(1):249–50. doi: 10.1038/npp.2013.198
59. Pai N, Vella SL, Richardson K. Is food addiction a valid phenomenon through the lens of the DSM-5? Aust N Z J Psychiatry. (2014) 48(3):216–8. doi: 10.1177/0004867413512384
60. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. (2006) 443(7109):289–95. doi: 10.1038/nature05026
61. Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. (2008) 59:55–92. doi: 10.1146/annurev.psych.59.103006.093551
62. Rolls ET. Understanding the mechanisms of food intake and obesity. Obes Rev Off J Int Assoc Study Obes. (2007) 8(Suppl 1):67–72. doi: 10.1111/j.1467-789X.2007.00321.x
63. Rolls ET. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. (2006) 361(1471):1123–36. doi: 10.1098/rstb.2006.1852
64. Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes. (2009) 33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65
65. Tryon MS, Carter CS, Decant R, Laugero KD. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav. (2013) 120:233–42. doi: 10.1016/j.physbeh.2013.08.010
66. Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem. (2007) 282(31):22448–59. doi: 10.1074/jbc.M700265200
67. Singh M, Zimmerman MB, Beltz TG, Johnson AK. Affect-related behaviors in mice misexpressing the RNA editing enzyme ADAR2. Physiol Behav. (2009) 97(3–4):446–54. doi: 10.1016/j.physbeh.2009.03.029
68. Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. (2010) 21(3):159–65. doi: 10.1016/j.tem.2009.10.004
69. Akubuiro A, Bridget Zimmerman M, Boles Ponto LL, Walsh SA, Sunderland J, McCormick L, et al. Hyperactive hypothalamus, motivated and non-distractible chronic overeating in ADAR2 transgenic mice. Genes Brain Behav. (2013) 12(3):311–22. doi: 10.1111/gbb.12020
70. Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. (2012) 63(1):97–110. doi: 10.1016/j.neuropharm.2012.04.017
71. Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci. (2010) 107(47):20529–34. doi: 10.1073/pnas.1007740107
72. Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. (2011) 192:351–60. doi: 10.1016/j.neuroscience.2011.06.072
73. Christiansen AM, Herman JP, Ulrich-Lai YM. Regulatory interactions of stress and reward on rat forebrain opioidergic and GABAergic circuitry. Stress. (2011) 14(2):205–15. doi: 10.3109/10253890.2010.531331
74. Park E, Kim JY, Lee JH, Jahng JW. Increased depression-like behaviors with dysfunctions in the stress axis and the reward center by free access to highly palatable food. Neuroscience. (2014) 262:31–9. doi: 10.1016/j.neuroscience.2013.12.054
75. Rho SG. Sweet food improves chronic stress-induced irritable bowel syndrome-like symptoms in rats. World J Gastroenterol. (2014) 20(9):2365. doi: 10.3748/wjg.v20.i9.2365
76. Gibson EL. The psychobiology of comfort eating: implications for neuropharmacological interventions. Behav Pharmacol. (2012) 23(5 and 6):442–60. doi: 10.1097/FBP.0b013e328357bd4e
77. Opie RS, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, et al. Dietary recommendations for the prevention of depression. Nutr Neurosci. (2017) 20(3):161–71. doi: 10.1179/1476830515Y.0000000043
78. Kris-Etherton PM, Petersen KS, Hibbeln JR, Hurley D, Kolick V, Peoples S, et al. Nutrition and behavioral health disorders: depression and anxiety. Nutr Rev. (2021) 79(3):247–60. doi: 10.1093/nutrit/nuaa025
79. Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. (2006) 89(1):53–61. doi: 10.1016/j.physbeh.2006.01.024
80. Yau YHC, Potenza MN. Stress and eating behaviors. Minerva Endocrinol. (2013) 38(3):255–67.24126546
81. Rubio-López N, Morales-Suárez-Varela M, Pico Y, Livianos-Aldana L, Llopis-González A. Nutrient intake and depression symptoms in Spanish children: the ANIVA study. Int J Environ Res Public Health. (2016) 13(3):352. doi: 10.3390/ijerph13030352
82. Kulkarni AA, Swinburn BA, Utter J. Associations between diet quality and mental health in socially disadvantaged New Zealand adolescents. Eur J Clin Nutr. (2015) 69(1):79–83. doi: 10.1038/ejcn.2014.130
83. Adair LS, Popkin BM. Are child eating patterns being transformed globally? Obes Res. (2005) 13(7):1281–99. doi: 10.1038/oby.2005.153
84. Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev Off J Int Assoc Study Obes. (2004) 5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x
85. Maunder EMW, Nel JH, Steyn NP, Kruger HS, Labadarios D. Added sugar, macro- and micronutrient intakes and anthropometry of children in a developing world context. PloS One. (2015) 10(11):e0142059. doi: 10.3389/fpsyg.2014.00403
86. Khalid S, Williams CM, Reynolds SA. Is there an association between diet and depression in children and adolescents? A systematic review. Br J Nutr. (2016) 116(12):2097–108. doi: 10.1017/S0007114516004359
87. Murphy SP, Barr SI. Practice paper of the American dietetic association: using the dietary reference intakes. J Am Diet Assoc. (2011) 111(5):762–70. doi: 10.1016/j.jada.2011.03.022
88. Barrett-Connor E. Nutrition epidemiology: how do we know what they ate? Maturitas. (1992) 14(3):245. doi: 10.1016/0378-5122(92)90139-U
89. Jacka FN, Kremer PJ, Berk M, de Silva-Sanigorski AM, Moodie M, Leslie ER, et al. A prospective study of diet quality and mental health in adolescents. PloS One. (2011) 6(9):e24805. doi: 10.1371/journal.pone.0024805
90. Orlando L, Savel KA, Madigan S, Colasanto M, Korczak DJ. Dietary patterns and internalizing symptoms in children and adolescents: a meta-analysis. Aust N Z J Psychiatry. (2022) 56(6):617–41. doi: 10.1177/00048674211031486
91. Zahedi H, Kelishadi R, Heshmat R, Motlagh ME, Ranjbar SH, Ardalan G, et al. Association between junk food consumption and mental health in a national sample of Iranian children and adolescents: the CASPIAN-IV study. Nutr Burbank Los Angel Cty Calif. (2014) 30(11–12):1391–7. doi: 10.1016/j.nut.2014.04.014
92. Hoare E, Millar L, Fuller-Tyszkiewicz M, Skouteris H, Nichols M, Malakellis M, et al. Depressive symptomatology, weight status and obesogenic risk among Australian adolescents: a prospective cohort study. BMJ Open. (2016) 6(3):e010072. doi: 10.1136/bmjopen-2015-010072
93. Castillo F, Francis L, Wylie-Rosett J, Isasi CR. Depressive symptoms are associated with excess weight and unhealthier lifestyle behaviors in urban adolescents. Child Obes Print. (2014) 10(5):400–7. doi: 10.1089/chi.2014.0042
94. Arouca A, Michels N, Moreno LA, González-Gil EM, Marcos A, Gómez S, et al. Associations between a Mediterranean diet pattern and inflammatory biomarkers in European adolescents. Eur J Nutr. (2018) 57(5):1747–60. doi: 10.1007/s00394-017-1457-4
95. Firth J, Stubbs B, Teasdale SB, Ward PB, Veronese N, Shivappa N, et al. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry Off J World Psychiatr Assoc WPA. (2018) 17(3):365–7. doi: 10.1002/wps.20571
96. Dehghan P, Nejati M, Vahid F, Almasi-Hashiani A, Saleh-Ghadimi S, Parsi R, et al. The association between dietary inflammatory index, dietary antioxidant index, and mental health in adolescent girls: an analytical study. BMC Public Health. (2022) 22(1):1513. doi: 10.1186/s12889-022-13879-2
97. Sureda A, Bibiloni MDM, Julibert A, Bouzas C, Argelich E, Llompart I, et al. Adherence to the Mediterranean diet and inflammatory markers. Nutrients. (2018) 10(1):62. doi: 10.3390/nu10010062
98. Carvalho KMB, Ronca DB, Michels N, Huybrechts I, Cuenca-Garcia M, Marcos A, et al. Does the Mediterranean diet protect against stress-induced inflammatory activation in European adolescents? The HELENA study. Nutrients. (2018) 10(11):1770. doi: 10.3390/nu10111770
99. Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. (2018) 10(11):1618. doi: 10.3390/nu10111618
100. Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. (2019) 9(1):190. doi: 10.1038/s41398-019-0515-5
101. Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JPC, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry. (2014) 76(7):559–66. doi: 10.1016/j.biopsych.2014.01.008
102. Liu T, Zhong S, Liao X, Chen J, He T, Lai S, et al. A meta-analysis of oxidative stress markers in depression. PloS One. (2015) 10(10):e0138904. doi: 10.1371/journal.pone.0138904
103. Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. (2010) 114(6):1581–9. doi: 10.1111/j.1471-4159.2010.06865.x
104. Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. (2016) 8:33–42. doi: 10.1016/j.cofs.2016.02.002
105. Pacheco-Ordaz R, Wall-Medrano A, Goñi MG, Ramos-Clamont-Montfort G, Ayala-Zavala JF, González-Aguilar GA. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett Appl Microbiol. (2018) 66(1):25–31. doi: 10.1111/lam.12814
106. Moretti M, Colla A, de Oliveira Balen G, dos Santos DB, Budni J, de Freitas AE, et al. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J Psychiatr Res. (2012) 46(3):331–40. doi: 10.1016/j.jpsychires.2011.11.009
107. Lopresti AL. A review of nutrient treatments for paediatric depression. J Affect Disord. (2015) 181:24–32. doi: 10.1016/j.jad.2015.04.014
108. Rebec GV, Christopher Pierce R. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. (1994) 43(6):537–65. doi: 10.1016/0301-0082(94)90052-3
109. Binfaré RW, Rosa AO, Lobato KR, Santos ARS, Rodrigues ALS. Ascorbic acid administration produces an antidepressant-like effect: evidence for the involvement of monoaminergic neurotransmission. Prog Neuropsychopharmacol Biol Psychiatry. (2009) 33(3):530–40. doi: 10.1016/j.pnpbp.2009.02.003
110. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. (2014) 28(6):2398–413. doi: 10.1096/fj.13-246546
111. Kesby JP, Cui X, Ko P, McGrath JJ, Burne THJ, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. (2009) 461(2):155–8. doi: 10.1016/j.neulet.2009.05.070
112. Zhang Y, Leung DYM, Goleva E. Vitamin D enhances glucocorticoid action in human monocytes: involvement of granulocyte-macrophage colony-stimulating factor and mediator complex subunit 14*. J Biol Chem. (2013) 288(20):14544–53. doi: 10.1074/jbc.M112.427054
113. Teesson M, Hall W, Slade T, Mills K, Grove R, Mewton L, et al. Prevalence and correlates of DSM-IV alcohol abuse and dependence in Australia: findings of the 2007 national survey of mental health and wellbeing. Addict Abingdon Engl. (2010) 105(12):2085–94. doi: 10.1111/j.1360-0443.2010.03096.x
114. Chou SP, Lee HK, Cho MJ, Park JI, Dawson DA, Grant BF. Alcohol use disorders, nicotine dependence, and co-occurring mood and anxiety disorders in the United States and South Korea—a cross-national comparison. Alcohol Clin Exp Res. (2012) 36(4):654–62. doi: 10.1111/j.1530-0277.2011.01639.x
115. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. (2017) 357(6349):eaaf9794. doi: 10.1126/science.aaf9794
116. O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. (2009) 14(5):511–22. doi: 10.1038/sj.mp.4002148
117. Woolf K, Manore MM. B-vitamins and exercise: does exercise alter requirements? Int J Sport Nutr Exerc Metab. (2006) 16(5):453–84. doi: 10.1123/ijsnem.16.5.453
118. Murakami K, Miyake Y, Sasaki S, Tanaka K, Arakawa M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: ryukyus child health study. Pediatrics. (2010) 126(3):e623–30. doi: 10.1542/peds.2009-3277
119. Herbison CE, Hickling S, Allen KL, O’Sullivan TA, Robinson M, Bremner AP, et al. Low intake of B-vitamins is associated with poor adolescent mental health and behaviour. Prev Med. (2012) 55(6):634–8. doi: 10.1016/j.ypmed.2012.09.014
120. Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, et al. Effects of lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. (2013) 38(9):1738–47. doi: 10.1016/j.psyneuen.2013.02.008
121. Magnusson KR, Hauck L, Jeffrey BM, Elias V, Humphrey A, Nath R, et al. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. (2015) 300:128–40. doi: 10.1016/j.neuroscience.2015.05.016
122. Hiel S, Bindels LB, Pachikian BD, Kalala G, Broers V, Zamariola G, et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr. (2019) 109(6):1683–95. doi: 10.1093/ajcn/nqz001
123. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. (2019) 102:13–23. doi: 10.1016/j.neubiorev.2019.03.023
124. Aslam H, Green J, Jacka FN, Collier F, Berk M, Pasco J, et al. Fermented foods, the gut and mental health: a mechanistic overview with implications for depression and anxiety. Nutr Neurosci. (2020) 23(9):659–71. doi: 10.1080/1028415X.2018.1544332
125. Barbadoro P, Annino I, Ponzio E, Romanelli RML, D’Errico MM, Prospero E, et al. Fish oil supplementation reduces cortisol basal levels and perceived stress: a randomized, placebo-controlled trial in abstinent alcoholics. Mol Nutr Food Res. (2013) 57(6):1110–4. doi: 10.1002/mnfr.201200676
126. Tsang C, Hodgson L, Bussu A, Farhat G, Al-Dujaili E. Effect of polyphenol-rich dark chocolate on salivary cortisol and mood in adults. Antioxid Basel Switz. (2019) 8(6):149. doi: 10.3390/antiox8060149
127. Thoma MV, Gianferante D, Hanlin L, Fiksdal A, Chen X, Rohleder N. Stronger hypothalamus-pituitary-adrenal axis habituation predicts lesser sensitization of inflammatory response to repeated acute stress exposures in healthy young adults. Brain Behav Immun. (2017) 61:228–35. doi: 10.1016/j.bbi.2016.11.030
128. Kuipers EN, Held NM, In Het Panhuis W, Modder M, Ruppert PMM, Kersten S, et al. A single day of high-fat diet feeding induces lipid accumulation and insulin resistance in brown adipose tissue in mice. Am J Physiol Endocrinol Metab. (2019) 317(5):E820–30. doi: 10.1152/ajpendo.00123.2019
129. Sihali-Beloui O, Aroune D, Benazouz F, Hadji A, El-Aoufi S, Marco S. A hypercaloric diet induces hepatic oxidative stress, infiltration of lymphocytes, and mitochondrial reshuffle in Psammomys obesus, a murine model of insulin resistance. C R Biol. (2019) 342(5–6):209–19. doi: 10.1016/j.crvi.2019.04.003
130. Woodman AG, Mah R, Keddie DL, Noble RMN, Holody CD, Panahi S, et al. Perinatal iron deficiency and a high salt diet cause long-term kidney mitochondrial dysfunction and oxidative stress. Cardiovasc Res. (2020) 116(1):183–92. doi: 10.1093/cvr/cvz029
131. Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. (2020) 136:104714. doi: 10.1016/j.nbd.2019.104714
132. Chang L, Wei Y, Hashimoto K. Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res Bull. (2022) 182:44–56. doi: 10.1016/j.brainresbull.2022.02.004
133. Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18(7):965–77. doi: 10.1038/nn.4030
134. Beurel E, Lowell JA. Th17 cells in depression. Brain Behav Immun. (2018) 69:28–34. doi: 10.1016/j.bbi.2017.08.001
135. Cheng H, Guan X, Chen D, Ma W. The Th17/treg cell balance: a gut Microbiota-modulated story. Microorganisms. (2019) 7(12):583. doi: 10.3390/microorganisms7120583
136. Gao K, long MC, Farzi A, yun ZW. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. (2020) 11(3):709–23. doi: 10.1093/advances/nmz127
137. Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, et al. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. (2013) 7(10):1933–43. doi: 10.1038/ismej.2013.89
138. Cryan JF, Kaupmann K. Don’t worry “B” happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci. (2005) 26(1):36–43. doi: 10.1016/j.tips.2004.11.004
139. University Psychiatric Clinic Ljubljana, Ljubljana S, Zalar B, Haslberger A. University of Vienna, department of nutritional sciences, Vienna, Austria, peterlin B, clinical institute of medical genetics, university medical centre Ljubljana, Ljubljana, Slovenia. The role of microbiota in depression—a brief review. Psychiatr Danub. (2018) 30(2):136–41. doi: 10.24869/spsih.2018.136
140. Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. (2002) 159(4):663–5. doi: 10.1176/appi.ajp.159.4.663
141. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. (2011) 108(38):16050–5. doi: 10.1073/pnas.1102999108
142. Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, et al. Ingestion of lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflammation. (2020) 17(1):241. doi: 10.1186/s12974-020-01916-z
143. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity—assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102(3):709–57. doi: 10.1210/jc.2016-2573
144. Martins LB, Braga Tibães JR, Sanches M, Jacka F, Berk M, Teixeira AL. Nutrition-based interventions for mood disorders. Expert Rev Neurother. (2021) 21(3):303–15. doi: 10.1080/14737175.2021.1881482
145. Adan RAH, Van Der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, et al. Nutritional psychiatry: towards improving mental health by what you eat. Eur Neuropsychopharmacol. (2019) 29(12):1321–32. doi: 10.1016/j.euroneuro.2019.10.011
146. Bremner J, Moazzami K, Wittbrodt M, Nye J, Lima B, Gillespie C, et al. Diet, stress and mental health. Nutrients. (2020) 12(8):2428. doi: 10.3390/nu12082428
147. Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. (2016) 173(6):575–87. doi: 10.1176/appi.ajp.2016.15091228
148. Guu TW, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Hamazaki K, et al. International society for nutritional psychiatry research practice guidelines for Omega-3 fatty acids in the treatment of Major depressive disorder. Psychother Psychosom. (2019) 88(5):263–73. doi: 10.1159/000502652
149. Mocking RJT, Harmsen I, Assies J, Koeter MWJ, Ruhé HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. (2016) 6(3):e756–e756. doi: 10.1038/tp.2016.29
150. Wang J, Um P, Dickerman B, Liu J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. (2018) 10(5):584. doi: 10.3390/nu10050584
151. Vellekkatt F, Menon V. Efficacy of vitamin D supplementation in major depression: a meta-analysis of randomized controlled trials. J Postgrad Med. (2019) 65(2):74–80. doi: 10.4103/jpgm.JPGM_571_17
152. Jamilian H, Amirani E, Milajerdi A, Kolahdooz F, Mirzaei H, Zaroudi M, et al. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: a systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 94:109651. doi: 10.1016/j.pnpbp.2019.109651
153. Bender A, Hagan KE, Kingston N. The association of folate and depression: a meta-analysis. J Psychiatr Res. (2017) 95:9–18. doi: 10.1016/j.jpsychires.2017.07.019
154. Hsieh YC, Chou LS, Lin CH, Wu HC, Li DJ, Tseng PT. Serum folate levels in bipolar disorder: a systematic review and meta-analysis. BMC Psychiatry. (2019) 19(1):305. doi: 10.1186/s12888-019-2269-2
155. Sarris J, Schoendorfer N, Kavanagh DJ. Major depressive disorder and nutritional medicine: a review of monotherapies and adjuvant treatments. Nutr Rev. (2009) 67(3):125–31. doi: 10.1111/j.1753-4887.2009.00180.x
156. Hancock CR, Han D, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. (2011) 25(2):785–91. doi: 10.1096/fj.10-170415
157. Kishi T, Miyake N, Okuya M, Sakuma K, Iwata N. N-acetylcysteine as an adjunctive treatment for bipolar depression and major depressive disorder: a systematic review and meta-analysis of double-blind, randomized placebo-controlled trials. Psychopharmacology (Berl). (2020) 237(11):3481–7. doi: 10.1007/s00213-020-05629-2
158. Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, et al. Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J Nutr Biochem. (2018) 58:1–16. doi: 10.1016/j.jnutbio.2018.02.012
159. Salman HB, Salman MA, Yildiz EA. The effect of omega-3 fatty acid supplementation on weight loss and cognitive function in overweight or obese individuals on weight-loss diet. Nutr Hosp. 39(4):803–13. doi: 10.20960/nh.03992
160. Zielińska M, Łuszczki E, Michońska I, Dereń K. The Mediterranean diet and the western diet in adolescent depression-current reports. Nutrients. (2022) 14(20):4390. doi: 10.3390/nu14204390
161. Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients. (2015) 7(11):9139–53. doi: 10.3390/nu7115459
162. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99(1):181–97. doi: 10.3945/ajcn.113.069880
163. Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. (2013) 74(4):580–91. doi: 10.1002/ana.23944
164. Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. (2019) 24(7):965–86. doi: 10.1038/s41380-018-0237-8
165. Salari-Moghaddam A, Keshteli AH, Mousavi SM, Afshar H, Esmaillzadeh A, Adibi P. Adherence to the MIND diet and prevalence of psychological disorders in adults. J Affect Disord. (2019) 256:96–102. doi: 10.1016/j.jad.2019.05.056
166. Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial). BMC Med. (2017) 15(1):23. doi: 10.1186/s12916-017-0791-y
167. Norwitz NG, Sethi S, Palmer CM. Ketogenic diet as a metabolic treatment for mental illness. Curr Opin Endocrinol Diabetes Obes. (2020) 27(5):269–74. doi: 10.1097/MED.0000000000000564
168. Operto FF, Matricardi S, Pastorino GMG, Verrotti A, Coppola G. The ketogenic diet for the treatment of mood disorders in comorbidity with epilepsy in children and adolescents. Front Pharmacol. (2020) 11:578396. doi: 10.3389/fphar.2020.578396
169. Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy—a review. Nutrients. (2020) 12(6):1809. doi: 10.3390/nu12061809
170. Partsalaki I, Karvela A, Spiliotis BE. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocrinol Metab. (2012) 25(7–8):697–704. doi: 10.1515/jpem-2012-0131
171. Campbell IH, Campbell H. Ketosis and bipolar disorder: controlled analytic study of online reports. BJPsych Open. (2019) 5(4):e58. doi: 10.1192/bjo.2019.49
172. Phelps JR, Siemers SV, El-Mallakh RS. The ketogenic diet for type II bipolar disorder. Neurocase. (2013) 19(5):423–6. doi: 10.1080/13554794.2012.690421
173. Chmiel I. Ketogenic diet in therapy of bipolar affective disorder—case report and literature review. Psychiatr Pol. (2022) 56(6):1345–63. doi: 10.12740/PP/OnlineFirst/136356
174. Corsello A, Scatigno L, Pascuzzi MC, Calcaterra V, Dilillo D, Vizzuso S, et al. Nutritional, gastrointestinal and endo-metabolic challenges in the management of children with spinal muscular atrophy type 1. Nutrients. (2021) 13(7):2400. doi: 10.3390/nu13072400
Keywords: dietary habits, diet, depression, obesity, children, adolescents, prevention, mental health
Citation: Calcaterra V, Rossi V, Magenes VC, Baldassarre P, Grazi R, Loiodice M, Fabiano V and Zuccotti G (2024) Dietary habits, depression and obesity: an intricate relationship to explore in pediatric preventive strategies. Front. Pediatr. 12:1368283. doi: 10.3389/fped.2024.1368283
Received: 10 January 2024; Accepted: 28 February 2024;
Published: 8 March 2024.
Edited by:
Anna Di Sessa, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Szymon Skoczen, Jagiellonian University Medical College, PolandHugo Martinez-Rojano, Escuela Superior de Medicina (IPN), Mexico
© 2024 Calcaterra, Rossi, Magenes, Baldassarre, Grazi, Loiodice, Fabiano and Zuccotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Calcaterra dmFsZXJpYS5jYWxjYXRlcnJhQHVuaXB2Lml0 Virginia Rossi dmlyZ2luaWEucm9zc2lAdW5pbWkuaXQ=
 Valeria Calcaterra
Valeria Calcaterra Virginia Rossi
Virginia Rossi Vittoria Carlotta Magenes
Vittoria Carlotta Magenes Paola Baldassarre
Paola Baldassarre Roberta Grazi2
Roberta Grazi2 Valentina Fabiano
Valentina Fabiano Gianvincenzo Zuccotti
Gianvincenzo Zuccotti