
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 30 April 2024
Sec. General Pediatrics and Pediatric Emergency Care
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1366953
Objectives: Chest pain is a common chief complaint in pediatric emergency departments (EDs). Coronavirus disease-2019 (COVID-19) has been shown to increase the risk of cardiac disease. It remains unclear how COVID-19 changed how pediatric emergency clinicians approach patients presenting with chest pain. The goal of this study was to characterize the diagnostic testing for chest pain in a pediatric ED before and during the COVID-19 pandemic.
Methods: This was a retrospective study of children between the ages of 2–17 years presenting to a pediatric ED from 1/1/2018–2/29/2020 (Pre-COVID-19) and 3/1/2020–4/30/2022 (COVID-19) with chest pain. We excluded patients with a previous history of cardiac disease.
Results: Of the 10,721 encounters during the study period, 5,692 occurred before and 5,029 during COVID-19. Patient demographics showed minor differences by age, weight, race and ethnicity. ED encounters for chest pain consisted of an average of 18% more imaging studies during COVID-19, including 14% more EKGs and 11% more chest x-rays, with no difference in the number of echocardiograms. Compared to Pre-COVID-19, 100% more diagnostic tests were ordered during COVID-19, including cardiac markers Troponin I (p < 0.001) and BNP (p < 0.001). During COVID-19, 1.1% of patients had a cardiac etiology of chest pain compared with 0.7% before COVID-19 (p = 0.03).
Conclusions: During COVID-19, pediatric patients with chest pain underwent more diagnostic testing compared to Pre-COVID-19. This may be due to higher patient acuity, emergence of multisystem inflammatory syndrome in children (MIS-C) that necessitated more extensive testing and possible changes in ED clinician behavior during COVID-19.
Chest pain is a common chief complaint in pediatric emergency departments (EDs), accounting for 0.3%–2% of all ED visits (1, 2). In contrast to the adult population where cardiac etiologies are the most common cause of chest pain (3), pediatric chest pain is often due to benign, noncardiac etiologies (4). To differentiate benign from more serious etiologies, pediatric chest pain can be broadly categorized into noncardiac vs. cardiac. The reported incidence of chest pain of cardiac etiology presenting to pediatric EDs is highly variable, ranging from 0.6% to 12.6% of all ED visits for chest pain (1, 2, 5, 6).
Although pediatric chest pain typically has benign causes and prognoses, ED diagnostic testing for chest pain can lead to significant utilization of resources and healthcare costs, including consults and referrals to cardiology, and unnecessary testing (7, 8). The lack of consistency in the diagnostic approach is thought to be a contributing factor to this phenomenon (7, 9). As a result, there have been concerted efforts to implement standardized clinical evaluation pathways to reduce unnecessary resource utilization, thereby decreasing practice variation and healthcare costs (2, 7–10). The goal of standardized diagnostic approaches is to rule out serious cardiac etiologies of chest pain based on the history, physical exam and an electrocardiogram (EKG) so as to reserve more extensive testing for patients more likely to have a cardiac etiology.
With the outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a global pandemic due to coronavirus disease-2019 (COVID-19) was declared in March 2020. As of May 2023, more than 15 million children in the U.S. have tested positive for COVID-19 (11). Acute infection with COVID-19 can cause myocarditis and arrhythmia and has been shown to confer a 15.7-fold increase in the risk for myocarditis compared to individuals who do not have COVID-19 (12). Although relatively rare, a post-infectious inflammatory syndrome known as multisystem inflammatory syndrome in children (MIS-C) develops in 0.5%–3.1% of children typically 3–6 weeks after infection with SARS-CoV-2 (13). MIS-C can cause myocarditis, coronary artery aneurysms and cardiovascular collapse (14). Similarly, myopericarditis post-mRNA COVID-19 vaccination is a recognized complication and tends to affect the pediatric population in the 14–18 years range, with the highest incidence among 12–40 year olds (15). Furthermore, it is estimated that up to 30% of patients with COVID-19 may continue to complain of chest pain months after acute infection (“long COVID”) (16). Taken together, the COVID-19 pandemic has added another layer of complexity to the differential diagnoses of chest pain in children.
It is unclear if and to what extent the COVID-19 pandemic has affected how pediatric emergency clinicians approach patients presenting with chest pain. We speculate that pediatric emergency clinicians have taken a more cautious approach in evaluating patients with chest pain during the COVID-19 pandemic with increased diagnostic testing. This study aims to characterize the extent of diagnostic workup for chest pain in a pediatric ED before and during the COVID-19 pandemic.
This was a retrospective study of children presenting to the pediatric EDs on the Children's Medical Center (CMC) campuses in Dallas, TX and Plano, TX with the chief complaint of chest pain. The CMC EDs have an annual volume of approximately 120,000 patient visits a year. Clinical pathways for pediatric chest pain did not exist at our institution in either of the two emergency departments during the study period. Data was abstracted from electronic medical records (Epic Systems Corporation, Verone, WI). The University of Texas Southwestern Medical Center Institutional Review Board exempted this work as non-human subject research.
We included pediatric patients aged 2–17 years who presented with the chief complaint of chest pain and excluded patients with a history of cardiac disease using International Classification of Diseases, Tenth Revision (ICD-10) codes (Supplementary Table S1). We abstracted data from ED encounters between 1 January 2018 and 30 April 2022. We defined the Pre-COVID-19 period as 1 January 2018–29 February 2020 and the COVID-19 period as 1 March 2020–30 April 2022. Data obtained were sex, age, weight, race, ethnicity, ED length of visit, disposition, chief complaints, imaging studies [EKG, chest x-ray (CXR), echocardiogram, point-of-care ultrasound (POCUS) echocardiogram, computed tomography (CT) of the chest, and others], laboratory tests, cardiology consults, cardiology outpatient referrals and ICD-10 codes of final diagnoses.
Cardiac diagnoses were identified using ICD-10 codes (Supplementary Table S1). The timing of cardiac diagnoses during COVID-19 was visually represented in relationship to the weekly number of COVID-19 cases in the U.S. Data on weekly COVID-19 cases were downloaded from the Centers for Disease Control (CDC): https://covid.cdc.gov/covid-data-tracker/#trends_weeklycases_select_00.
Continuous variables are reported as mean ± standard deviation and were tested for normality using Kolmogorov–Smirnov test. Normally distributed data were analyzed using the Student's t-test and data not following normal distribution were analyzed using Mann–Whitney test. Categorical variables are presented as numbers and percentages and were compared using χ2 test to compute odds ratios (ORs) with confidence intervals (CIs) and p-values. ORs are presented with the Pre-COVID-19 group as the reference. All tests were two-tailed. Data analysis was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA; Version 2301) and Graphpad Prism software (Dotmatics, Boston, MA; Version 8.4.3). Statistical significance was defined as p < 0.05.
There were a total of 473,321 ED visits during the study period of which 11,169 visits presented with a chief complaint of chest pain. Of those, 10,721 visits met the inclusion criteria. There were 5,692 ED visits during Pre-COVID-19 and 5,029 ED visits during COVID-19 for chest pain (Figure 1). During COVID-19, the absolute number of ED visits for chest pain significantly decreased at the onset of the pandemic, while the relative number of ED visits for chest pain remained stable, then steadily increased throughout the year (Figure 2). ED visit trends for chest pain in 2021 were overall similar to pre-pandemic years. While the absolute number of ED visits for chest pain was similar between Pre-COVID-19 and COVID-19 (218 vs. 193 ED visits for chest pain per month, p = 0.10), there was a significant increase in the relative number of ED visits for chest pain during COVID-19 (2.1% vs. 2.5%, p = 0.001).
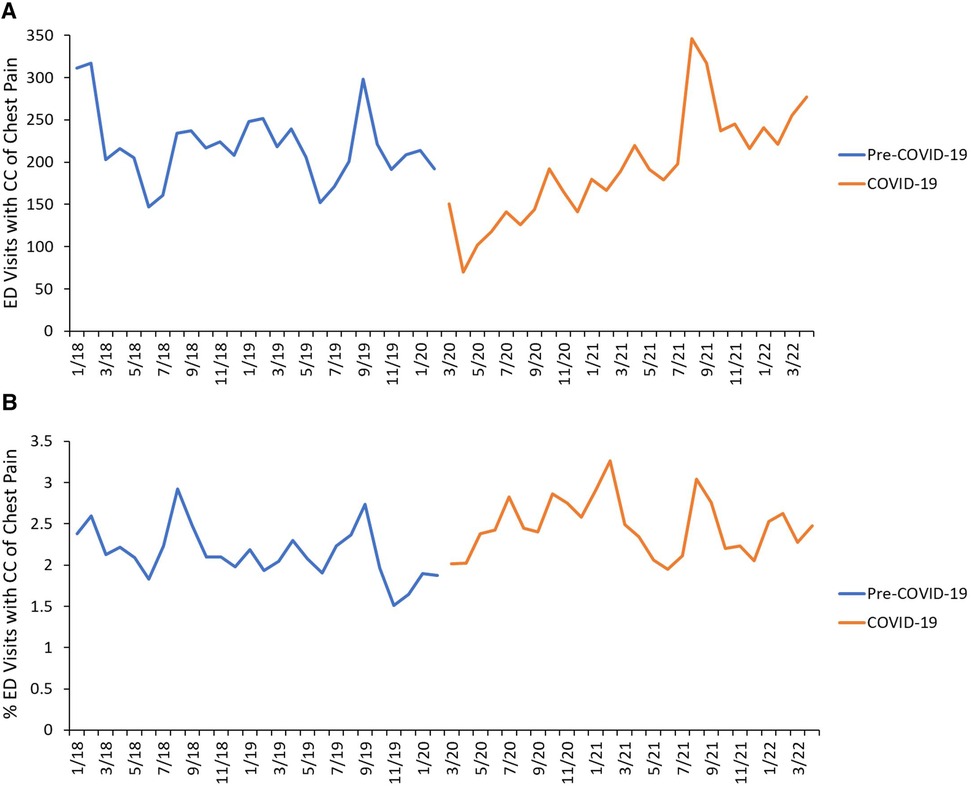
Figure 2. Timeline of ED visits. (A) Absolute numbers of ED visits for CC of chest pain during the study period, color-coded by study period. (B) Relative frequencies of ED visits for CC of chest pain during the study period, color-coded by study period. The Pre-COVID-19 period was defined as 1 January 2018–29 February 2020 and the COVID-19 period was defined as 1 March 2020–30 April 2022.
To evaluate for differences in the characteristics of patients who visited the ED before and during the COVID-19 pandemic, we analyzed patient demographics, and found small differences by age, weight and visit length (Table 1). There were no significant differences by race, ethnicity and disposition of patients.
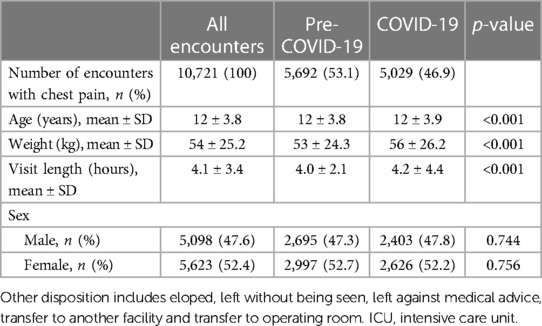
Table 1. Patient demographics of ED visits for CC of chest pain before and during the COVID-19 pandemic.
We then evaluated for differences in the diagnostic testing during ED visits for chest pain before and during the pandemic. During the pandemic, 18.2% more imaging studies were obtained per ED visit compared to pre-pandemic (1.3 vs. 1.1 per ED visit, p < 0.001; Table 2). The odds of undergoing no imaging study were lower during the pandemic (OR, 0.7, 95% CI, 0.7–0.8, p < 0.001), while the odds of receiving two imaging studies was higher during the pandemic (OR, 1.4, 95% CI, 1.3–1.5, p < 0.001). The majority of patients before and during the pandemic received an EKG (57.7% vs. 65.7%) and/or CXR (53.6% vs. 59.6%), both of which were done more frequently during the pandemic (EKG, OR, 1.4, 95% CI, 1.3–3.0, p < 0.001 and CXR, OR, 1.3, 95% CI, 1.2–2.8, p < 0.001). While there was no significant difference in the number of echocardiograms ordered during the pandemic compared to pre-pandemic (OR, 1.6, 95% CI, 0.9–2.9, p = 0.18), 34 encounters during COVID-19 underwent POCUS echocardiography that was newly introduced in our ED during the pandemic.
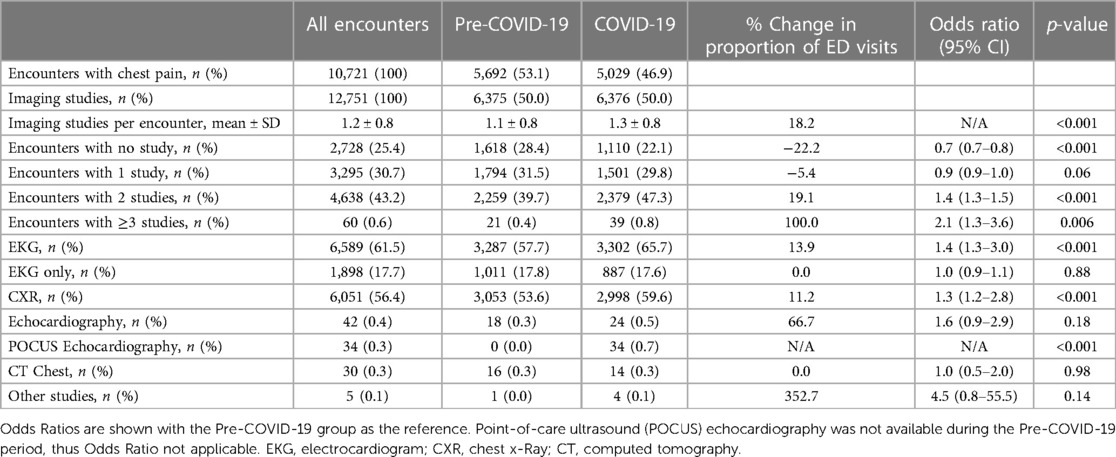
Table 2. Rates of imaging studies for ED visits for chest pain before and during the COVID-19 pandemic.
We next analyzed the rates of diagnostic laboratory tests and found that during COVID-19 twice as many tests were obtained per ED visit compared to Pre-COVID-19 (1.8 vs. 0.9 tests per ED visit, p < 0.001). Rates of selected laboratory tests are shown in Supplementary Table S2. The number of encounters with at least one laboratory test was 42.4% during the pandemic and 26.2% pre-pandemic (OR, 2.1, 95% CI, 1.5–2.3, p < 0.001). During COVID-19, 11.2% and 3.9% of encounters underwent testing for cardiac markers Troponin I and brain natriuretic peptide (BNP), respectively, while the rates were 3.5% and 0.8%, respectively, before the pandemic (Troponin I, OR, 3.4, 95% CI, 2.9–4.1, p < 0.001; BNP, OR, 4.2, 95% CI, 3.7–7.2, p < 0.001). At least 16 (0.3%) encounters during COVID-19 received diagnostic testing for MIS-C as shown by the rates of tests for interleukin-6 and SARS-COV-2 IgG antibody. Lastly, during COVID-19, cardiology was consulted twice as often (1.8% vs. 0.9%, OR, 1.9, 95% CI, 1.4–2.7, p < 0.001) and referrals for outpatient cardiology follow-up five times more frequently (0.5% vs. 0.1%, OR, 4.1, 95% CI, 1.8–9.7, p < 0.001) compared to Pre-COVID-19. Taken together, our data demonstrate that during COVID-19 the diagnostic evaluation for pediatric chest pain included higher rates of diagnostic testing and cardiology involvement compared to Pre-COVID-19.
We then analyzed for differences in cardiac diagnoses before and during the pandemic. The incidence of cardiac diagnoses was 0.9% (98 cases) during the study period; 0.7% Pre-COVID-19 (41 cases) and 1.1% during COVID-19 (57 cases; OR, 1.6, 95% CI, 1.1–2.4, p = 0.02; Table 3). The most common cardiac diagnosis was arrhythmia and accounted for 41.8% of all cardiac diagnoses; 35.1% during COVID-19 and 51.2% during Pre-COVID-19 (OR, 0.5, 95% CI, 0.2–1.2, p = 0.11). Although myocarditis was more frequent during COVID-19, the difference was not statistically significant (15.8% vs. 4.9%, OR, 3.7, 95% CI, 0.9–17.5, p = 0.09). There were 5 encounters with a diagnosis of MIS-C accounting for 8.8% of cardiac diagnoses during COVID-19.
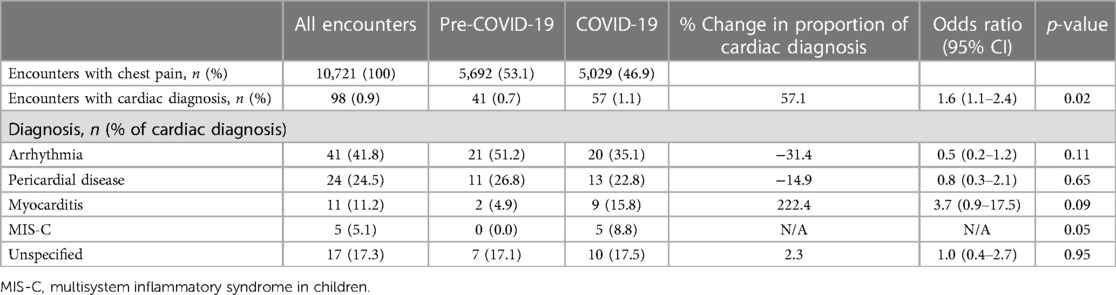
Table 3. Rates of cardiac diagnoses of ED visits for chest pain before and during the COVID-19 pandemic.
Infection with SARS-COV-2 has been shown to increase the risk of cardiac disease (12, 13, 16). We analyzed the timing of cardiac diagnoses during COVID-19 in our study in correlation with the rates of weekly COVID-19 cases in the U.S. based on publicly available data from the CDC (Supplementary Figure S1). While we did not identify clear patterns of cardiac diagnoses, there was qualitatively a cluster of diagnoses of pericardial disease following the surge of COVID-19 cases in December 2020 and December 2021. Two of the five MIS-C diagnoses followed the surge of COVID-19 cases in December 2020 and another two followed the surge of cases in August 2021.
We next analyzed the extent of diagnostic testing of encounters with a cardiac vs. non-cardiac diagnosis during the entire study period and found that encounters with a cardiac diagnosis received higher rates of imaging studies compared to non-cardiac diagnosis (1.9 vs. 1.2 studies per ED visit, p < 0.001, Supplementary Table S3). All encounters with a cardiac diagnosis underwent at least one imaging study. This is in contrast to non-cardiac diagnosis where only 74.3% of encounters underwent imaging studies. Encounters with a cardiac diagnosis received more laboratory tests compared to non-cardiac diagnosis (6.8 vs. 1.4 tests per ED visit, p < 0.001), including cardiac markers Troponin I (52.0% vs. 6.7%, OR, 15.1, 95% CI, 10.1–45.1, p < 0.001) and BNP (27.6% vs. 2.0%, OR, 18.7, 95% CI, 11.7–29.7, p < 0.001), and were more likely to receive at least one laboratory test (OR, 7.8, 95% CI, 4.8–12.6, p < 0.001). These data demonstrate that encounters with a cardiac diagnosis underwent more extensive diagnostic testing.
We next analyzed the rates of diagnostic testing in encounters with a cardiac diagnosis before and during the pandemic. Although the rates of imaging studies were higher during COVID-19 compared to Pre-COVID-19, the differences were not statistically significant (1.9 vs. 1.7 studies per ED visit, p = 0.08, Supplementary Table S4). Encounters during COVID-19 were less likely to receive an isolated EKG study without other tests (OR, 0.3, 95% CI, 0.1–0.8, p = 0.009). Rates of diagnostic laboratory tests were higher during COVID-19 than Pre-COVID-19 (8.7 vs. 4.1 tests per ED visit, p < 0.001). The number of encounters with at least one laboratory test was 87.7% during the pandemic and 68.3% pre-pandemic (OR, 3.3, 95% CI, 1.2–8.5, p < 0.001). During COVID-19, 59.6% and 36.8% of encounters underwent Troponin I and BNP studies, respectively, while the rates were 41.5% and 14.6%, respectively, before the pandemic (Troponin I, OR, 2.1, 95% CI, 0.9–4.7, p = 0.08; BNP, OR, 3.4, 95% CI, 1.2–9.4, p = 0.02). Taken together, our data suggest that encounters with a cardiac diagnosis underwent higher rates of laboratory testing, but similar rates of imaging studies during COVID-19 compared to Pre-COVID-19.
We found that during COVID-19, compared to pre-pandemic (1) there was an increase in the percentage of ED visits for chest pain, (2) the diagnostic evaluation for chest pain included higher rates of testing and cardiology involvement (3) the incidence of cardiac diagnoses was greater and (4) encounters with a cardiac diagnosis received more diagnostic testing compared to encounters with a non-cardiac diagnosis.
We found that early during the pandemic the number of ED visits for chest pain declined sharply. This is in line with the decreased number of pediatric ED visits across the U.S. during the pandemic (17). Despite overall declines in ED visits, we found that the percentage of ED visits for chest pain increased during the pandemic, similar to findings that ED visits for certain types of injuries (e.g., drug poisonings, self-harm, and firearm injuries) and mental health complaints increased during the pandemic (17, 18). A large retrospective study did not find differences in the relative proportion of pediatric ED visits for chest pain during the pandemic, however, the study was limited to the first year of the pandemic (19). Non-cardiac chest pain in children has been shown to be associated with psychosocial distress, sleep problems, restriction of activities and anxiety symptoms (20, 21). It is possible that the increased mental health issues children experienced during the pandemic contributed to increased incidence of chest pain, especially since the majority of encounters included in our study had a non-cardiac diagnosis.Infection with SARS-CoV-2 has been shown to place children at risk for long term sequelae (“long COVID”), including persistent chest pain symptoms in up to 4.6% of patients with COVID-19 (22) lasting at least two months after acute infection (23), possibly contributing to ED visits for chest pain during the pandemic. However, it is also possible that a decreased number of ED visits during COVID-19, especially in the first year of the pandemic from March 2020 to March 2021, may have led to an increased percentage of ED visits for chest pain. We recognize that there various potential causes of the difference in the percentage of ED visits for chest pain between both study periods, which is beyond the scope of this discussion.
The number of imaging studies obtained during ED visits for pediatric chest pain varies widely in the literature, ranging from 16% (24) to 94% (6) for CXR, 20% (24) to 100% (25) for EKG and 1% (2, 5) to 8% (24) for echocardiograms. While the number of CXR and EKG obtained in our study was within the range reported in the literature, echocardiograms were less frequently obtained in our study. The frequency with which laboratory tests are obtained for pediatric chest pain ED visits also varies widely in the literature, from 5% (2) to 62% (6). The number of laboratory tests obtained in our study was within this range. Our study showed that patients with chest pain underwent more diagnostic testing during the pandemic. The reasons for these observations are manifold. First, it is possible that patients presented with higher acuity during the pandemic, warranting more extensive testing. Second, during times of high COVID-19 incidence, the index of suspicion for severe illness was likely higher, leading to more extensive testing. Third, the morbidity and mortality associated with COVID-19 likely changed the behavior of ED clinicians, such as their comfort level with making disposition decisions without diagnostic testing. It is well known that infectious disease outbreaks have the potential to cause significant psychological stress to physicians (26), which in turn may affect their clinical decision making. The increased diagnostic testing, however, was arguably appropriate and clinically indicated, given that they resulted in a higher number of cardiac diagnoses during COVID-19. Fourth, the diagnosis of MIS-C involves a plethora of laboratory tests, including cardiac biomarkers and potentially an echocardiogram (27), therefore the increased number of tests during COVID-19 was likely skewed by the diagnostic criteria for MIS-C. Fifth, certain tests such as procalcitonin, were not available prior to the pandemic. Technological advancement therefore represents an important aspect of how clinicians approach patients presenting to the ED. Lastly, a respiratory virus panel became an admission criterion during the pandemic, whereby the test had to be obtained even if it did not contribute to the diagnostic testing for chest pain.
We found that cardiac etiologies were more likely to be the underlying cause of chest pain in children during the pandemic than pre-pandemic. The incidence of cardiac-related chest pain in our study during both study periods was within previously reported range of 0.6%–12.6% (1, 2, 5, 6). Although statistically not significant, we found a three-fold higher incidence of myocarditis during COVID-19, suggesting that the pandemic may have been a contributing factor to the increased incidence. The incidence of pericardial disease was not different pre-pandemic compared to during the pandemic. Both myocarditis and pericarditis are predominantly caused by viruses via airborne transmission (28, 29). However, the prevalence of community respiratory viruses, including those that have been shown to cause both myocarditis and pericarditis, such as influenza viruses, adenovirus and enteroviruses, significantly declined at the onset of pandemic and increased to 50% of pre-pandemic prevalence only in Fall 2021 (30). The majority of myocarditis and pericarditis diagnoses in our study occurred prior to Fall 2021, suggesting that a viral etiology other than SARS-CoV-2 may have been unlikely, although this does not rule out other etiologies, such as bacterial infections and non-infectious causes. In our analysis, we found a cluster of pericardial disease diagnoses following the surge of COVID-19 cases in December 2020 and December 2021, suggesting a link to acute infection with SARS-CoV-2. However, we did not demonstrate a causative relationship between infection or vaccine and subsequent myocarditis or pericardial disease.
Taken together, our study showed that during COVID-19 pediatric patients visiting the ED for the chief complaint of chest pain received more diagnostic testing than pre-pandemic, which was followed by a proportional increase of the incidence of cardiac diagnoses. It is possible that the higher patient acuity seen in pediatric EDs during the COVID-19 pandemic (31, 32) resulted in higher testing rates and cardiac diagnoses. The higher acuity could be partly attributed to the effects of COVID-19 on the cardiovascular system through direct infection, post-infectious sequelae and vaccination (12–16). This was likely exacerbated by the pandemic causing delays medical care due to widespread outpatient clinic closures and fear of exposure to SARS-CoV-2 in healthcare settings (33).
Our study has several limitations. First, despite being one of the largest pediatric EDs in the country, our single-center results may not be generalizable to other patient populations across the U.S. or in other countries. Second, our large sample size may have contributed to statistical significance for differences that are clinically insignificant. Lastly, we cannot exclude the possibility that the clinicians' decision to order more testing during the COVID-19 pandemic was due to their prior expectation of cardiac disease given the relationship between COVID-19 and cardiac disease.
In conclusion, during COVID-19 the proportion of ED visits for chest pain was higher, more diagnostic testing was done, and the incidence of cardiac diagnoses was higher compared to the pre-pandemic period. This may be due to higher patient acuity during the pandemic and thus higher pre-test probability for cardiac disease, technological advancement leading to novel imaging tools and laboratory tests, and the emergence of MIS-C which necessitated more extensive testing when suspected. Alternatively, our data suggest a possible change in ED clinician behavior during the pandemic. Given the higher rate of cardiac diagnoses during COVID-19, the increased diagnostic testing may have been justified. Our data suggest that when the local prevalence of infectious diseases known to increase the risk of cardiac disease in children, such as COVID-19, is high, a reasonably intensified diagnostic assessment may be warranted to rule out life-threatening cardiac disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The University of Texas Southwestern Medical Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. KY: Conceptualization, Supervision, Writing – review & editing. AZ: Conceptualization, Formal Analysis, Investigation, Project administration, Supervision, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Halim Hennes, MD, MS from the UTSW Division of Pediatric Emergency Medicine for his support. We thank Sergey Nayman from the Children's Medical Center Department of Analytics and Informatics for his assistance with data abstraction from electronic medical records.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1366953/full#supplementary-material
Supplementary Table S1
ICD-10 codes for cardiac disease.
Supplementary Table S2
Rates of laboratory tests for ED visits for chest pain before and during the COVID-19 pandemic.
Supplementary Table S3
Rates of imaging studies for ED visits for chest pain with non-cardiac vs cardiac diagnosis during the entire study period.
Supplementary Table S4
Rates of imaging studies for ED visits for chest pain with cardiac diagnosis before and during the COVID-19 pandemic.
Supplementary Figure S1
Temporal plot of the incidence of cardiac diagnoses during COVID-19 and weekly COVID-19 cases in the U.S. Weekly numbers of COVID-19 cases in the U.S. were obtained from the website of the CDC (see Methods). Cardiac diagnoses made during the COVID-19 pandemic as shown in Table 3 were plotted according to the date of the respective ED encounter. Each dot represents one ED encounter and is color-coded by cardiac diagnosis.
1. Massin MM, Bourguignont A, Coremans C, Comté L, Lepage P, Gérard P. Chest pain in pediatric patients presenting to an emergency department or to a cardiac clinic. Clin Pediatr. (2004) 43:231–8. doi: 10.1177/000992280404300304
2. Mohan S, Nandi D, Stephens P, M'Farrej M, Vogel RL, Bonafide CP. Implementation of a clinical pathway for chest pain in a pediatric emergency department. Pediatr Emerg Care. (2018) 34:778–82. doi: 10.1097/PEC.0000000000000861
3. Knockaert DC, Buntinx F, Stoens N, Bruyninckx R, Delooz H. Chest pain in the emergency department: the broad spectrum of causes. Eur J Emerg Med. (2002) 9:25–30. doi: 10.1097/00063110-200203000-00007
4. Barbut G, Needleman JP. Pediatric chest pain. Pediatr Rev. (2020) 41:469–80. doi: 10.1542/pir.2019-0058
5. Drossner DM, Hirsh DA, Sturm JJ, Mahle WT, Goo DJ, Massey R, et al. Cardiac disease in pediatric patients presenting to a pediatric ED with chest pain. Am J Emerg Med. (2011) 29:632–8. doi: 10.1016/j.ajem.2010.01.011
6. Kim J, Park E, Park M, Cho J, Son MH. Clinical features of adolescents who visited the emergency department with chest discomfort: the importance of recognizing underlying medical conditions. Pediatr Emerg Med J. (2020) 7:70–6. doi: 10.22470/pemj.2020.00101
7. Verghese GR, Friedman KG, Rathod RH, Meiri A, Saleeb SF, Graham DA, et al. Resource utilization reduction for evaluation of chest pain in pediatrics using a novel standardized clinical assessment and management plan (SCAMP). J Am Heart Assoc. (2012) 1:e000349. doi: 10.1161/JAHA.111.000349
8. Harahsheh AS, O'Byrne ML, Pastor B, Graham DA, Fulton DR. Pediatric chest pain-low-probability referral: a multi-institutional analysis from standardized clinical assessment and management plans (SCAMPs®), the pediatric health information systems database, and the national ambulatory medical care survey. Clin Pediatr. (2017) 56:1201–8. doi: 10.1177/0009922816684605
9. Friedman KG, Kane DA, Rathod RH, Renaud A, Farias M, Geggel R, et al. Management of pediatric chest pain using a standardized assessment and management plan. Pediatrics. (2011) 128:239–45. doi: 10.1542/peds.2011-0141
10. Angoff GH, Kane DA, Giddins N, Paris YM, Moran AM, Tantengco V, et al. Regional implementation of a pediatric cardiology chest pain guideline using SCAMPs methodology. Pediatrics. (2013) 132:e1010–e7. doi: 10.1542/peds.2013-0086
11. American Academy of Pediatrics. Children and COVID-19: State-Level Data Report; Available online at: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ American Academy of Pediatrics (accessed August 5, 2022).
12. Chin SE, Bhavsar SM, Corson A, Ghersin ZJ, Kim HS. Cardiac complications associated with COVID-19, MIS-C, and mRNA COVID-19 vaccination. Pediatr Cardiol. (2022) 43:483–8. doi: 10.1007/s00246-022-02851-x
13. Duarte-Salles T, Vizcaya D, Pistillo A, Casajust P, Sena AG, Lai LYH, et al. Thirty-day outcomes of children and adolescents with COVID-19: an international experience. Pediatrics. (2021) 148(3):e2020042929. doi: 10.1542/peds.2020-042929
14. Wu EY, Campbell MJ. Cardiac manifestations of multisystem inflammatory syndrome in children (MIS-C) following COVID-19. Curr Cardiol Rep. (2021) 23:168. doi: 10.1007/s11886-021-01602-3
15. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. (2022) 327:331–40. doi: 10.1001/jama.2021.24110
16. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. (2021) 40(12):e482–7. doi: 10.1097/INF.0000000000003328
17. Radhakrishnan L, Carey K, Hartnett KP, Kite-Powell A, Zwald M, Anderson KN, et al. Pediatric emergency department visits before and during the COVID-19 pandemic—United States, January 2019–January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:313–8. doi: 10.15585/mmwr.mm7108e1
18. Bolt J, Patel F, Stone L, Pandian D, Manuel MM, Gaines N. Impact of COVID-19 on pediatric mental and behavioral health visits to the emergency department. Pediatr Emerg Care. (2022) 38:409–15. doi: 10.1097/PEC.0000000000002794
19. Barbiellini Amidei C, Buja A, Bardin A, Bonaldi F, Paganini M, Manfredi M, et al. Pediatric emergency department visits during the COVID-19 pandemic: a large retrospective population-based study. Ital J Pediatr. (2021) 47:218. doi: 10.1186/s13052-021-01168-4
20. Lee JL, Gilleland J, Campbell RM, Johnson GL, Simpson P, Dooley KJ, et al. Internalizing symptoms and functional disability in children with noncardiac chest pain and innocent heart murmurs. J Pediatr Psychol. (2012) 38:255–64. doi: 10.1093/jpepsy/jss111
21. Lipsitz JD, Masia-Warner C, Apfel H, Marans Z, Hellstern B, Forand N, et al. Anxiety and depressive symptoms and anxiety sensitivity in youngsters with noncardiac chest pain and benign heart murmurs. J Pediatr Psychol. (2004) 29:607–12. doi: 10.1093/jpepsy/jsh062
22. Lopez-Leon S, Wegman-Ostrosky T, Ayuzo del, Valle NC, Perelman C, Sepulveda R, Rebolledo PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. (2022) 12:9950. doi: 10.1038/s41598-022-13495-5
23. Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Petersen MNS, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. (2022) 6:614–23. doi: 10.1016/S2352-4642(22)00154-7
24. Gesuete V, Fregolent D, Contorno S, Tamaro G, Barbi E, Cozzi G. Follow-up study of patients admitted to the pediatric emergency department for chest pain. Eur J Pediatr. (2020) 179:303–8. doi: 10.1007/s00431-019-03495-5
25. Brancato F, De Rosa G, Gambacorta A, Nunziata A, Ferrara P, Buonsenso D, et al. Role of troponin determination to diagnose chest pain in the pediatric emergency department. Pediatr Emerg Care. (2021) 37(12):e1589–92. doi: 10.1097/PEC.0000000000002123
26. Fiest KM, Parsons Leigh J, Krewulak KD, Plotnikoff KM, Kemp LG, Ng-Kamstra J, et al. Experiences and management of physician psychological symptoms during infectious disease outbreaks: a rapid review. BMC Psychiatry. (2021) 21:91. doi: 10.1186/s12888-021-03090-9
27. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. (2020) 72:1791–805. doi: 10.1002/art.41454
28. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. (2021) 18:169–93. doi: 10.1038/s41569-020-00435-x
29. Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA. (2015) 314:1498–506. doi: 10.1001/jama.2015.12763
30. Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. (2023) 21:195–210. doi: 10.1038/s41579-022-00807-9
31. Finkelstein Y, Maguire B, Zemek R, Osmanlliu E, Kam AJ, Dixon A, et al. Effect of the COVID-19 pandemic on patient volumes, acuity, and outcomes in pediatric emergency departments: a nationwide study. Pediatr Emerg Care. (2021) 37:427–34. doi: 10.1097/PEC.0000000000002484
32. Stowell JR, Henry MB, Pugsley P, Edwards J, Burton H, Norquist C, et al. Impact of the COVID-19 pandemic on emergency department encounters in a major metropolitan area. J Emerg Med. (2024) 66:e383–e90. doi: 10.1016/j.jemermed.2023.10.007
Keywords: chest pain, diagnostic testing, COVID-19, MIS-C, cardiac disease
Citation: Atmanli A, Yen K and Zhou AZ (2024) Diagnostic testing for chest pain in a pediatric emergency department and rates of cardiac disease before and during the COVID-19 pandemic: a retrospective study. Front. Pediatr. 12:1366953. doi: 10.3389/fped.2024.1366953
Received: 7 January 2024; Accepted: 19 April 2024;
Published: 30 April 2024.
Edited by:
Stephen Aronoff, Temple University, United StatesReviewed by:
Duraisamy Balaguru, Harvard Medical School, United States© 2024 Atmanli, Yen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy Z. Zhou YW15Lnpob3VAdXRzb3V0aHdlc3Rlcm4uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.