- 1Department of Pediatrics, Johns Hopkins University, Baltimore, MD, United States
- 2Department of Pediatrics, Wake Forest School of Medicine, Levine Children’s Hospital, Atrium Healthcare, Charlotte, NC, United States
- 3College of Medicine, University of Florida, Gainesville, FL, United States
- 4Department of Pediatrics, University of Florida, Gainesville, FL, United States
Introduction: Sepsis is a common cause of morbidity and mortality in the neonatal intensive care unit (NICU). The frequency and severity of sepsis-associated coagulopathy as well as its relationship to illness severity are unclear.
Methods: We performed a single-center, retrospective, observational cohort study of all infants admitted to the University of Florida Health (UF Health), level IV NICU between January 1st 2012 to March 1st 2020 to measure the frequency of sepsis-associated coagulopathy as well as its temporal relationship to critical illness in the NICU population. All clinical data in the electronic health record were extracted and deposited into an integrated data repository that was used for this work.
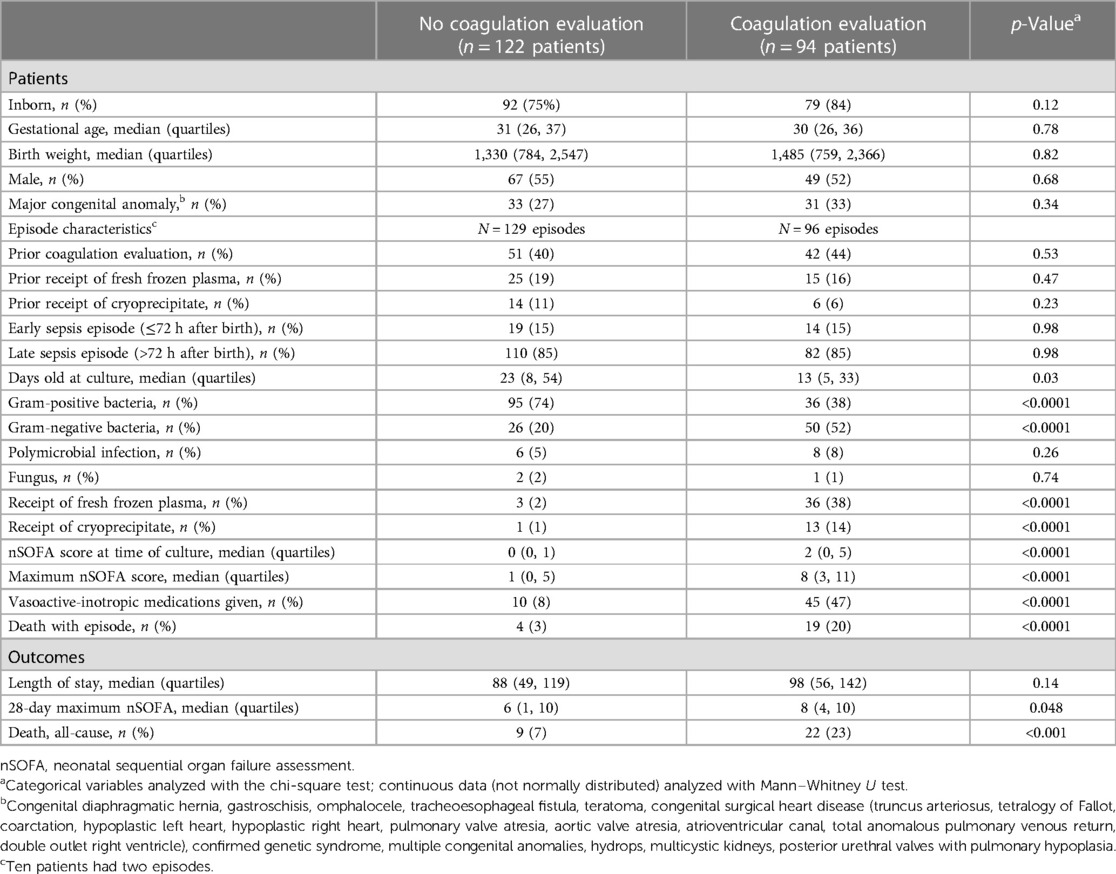
Results: We identified 225 new sepsis episodes in 216 patients. An evaluation for sepsis-associated coagulopathy was performed in 96 (43%) episodes. Gram-negative pathogen, nSOFA score at evaluation, and mortality were greater among episodes that included a coagulopathy evaluation compared with those that did not. Abnormal coagulation results were common (271/339 evaluations; 80%) and were predominantly prothrombin times. Intervention (plasma or cryoprecipitate) followed a minority (84/271; 31%) of abnormal results, occurred in 40/96 (42%) episodes that were often associated with >1 intervention (29/40; 73%), and coincided with thrombocytopenia in 37/40 (93%) and platelet transfusion in 27/40 (68%). Shapley Additive Explanations modeling demonstrated strong predictive performance for the composite outcome of death and/or treatment for coagulopathy in neonates (f1 score 0.8, area under receiver operating characteristic curve 0.83 for those with abnormal coagulation values). The three most important features influencing the composite outcome of death or treatment for coagulopathy included administration of vasoactive medications, hematologic dysfunction assessed by the maximum nSOFA platelet score, and early sepsis (≤72 h after birth).
Conclusions: A coagulopathy evaluation was performed in a minority of NICU patients with sepsis and was associated with greater illness severity and mortality. Abnormal results were common but infrequently associated with intervention, and intervention was contemporaneous with thrombocytopenia. The most important feature that influenced the composite outcome of death or treatment for coagulopathy was the administration of vasoactive-inotropic medications. These data help to identify NICU patients at risk of sepsis-associated coagulopathy.
Introduction
Sepsis is a common cause of morbidity and mortality in the neonatal population. In adults, sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (1). In children, sepsis-associated life-threatening organ dysfunction is operationalized using the Phoenix Sepsis Score (2). There is no consensus definition of sepsis in neonates. Sepsis-associated hematologic dysfunction manifesting as thrombocytopenia is well described and included in common sequential organ failure assessment (SOFA) scoring systems used in adults (3), children (pSOFA) (4), and neonates (nSOFA) (5). In addition to the consumption of platelets, clotting factor consumption commonly occurs with sepsis in children (41%) and adults (26%) (6, 7), is modified by the severity of illness, and increases the risk of poor outcomes including death (6, 8, 9). Other factors such as the host inflammatory response and pathogen modify the likelihood of coagulopathy (10). In 1976, Hutter and Colleagues described abnormalities in common coagulation assessments including fibrinogen, prothrombin time (PT), and partial thromboplastin time (PTT) among 14 infants with severe necrotizing enterocolitis (11). However, the frequency and severity of sepsis-associated coagulopathy in neonates, as well as the relationship of this entity to illness severity in this unique population, are unknown. Knowledge of the frequency of sepsis-associated coagulopathy reflected in commonly used tests in a large cohort, as well as the factors that place neonatal patients at risk for this entity, have potential to improve sepsis-related outcomes in this unique population.
Using a granular, single-center registry that included all data from the electronic health record (EHR) system for over a period of 9 years, we aimed to determine the following:(1) the frequency and timing of sepsis-associated coagulopathy evaluation, (2) the frequency, timing, and type of abnormal coagulation test results, (3) the frequency and timing of intervention with plasma or cryoprecipitate, and (4) the relation of sepsis-associated coagulopathy to illness severity in the neonatal intensive care unit (NICU) population.
Methods
Patients
This was a single-center, retrospective, observational cohort study approved by the University of Florida Health (UF Health) Institutional Review Board prior to the collection of any data. An integrated data repository (IDR) of all clinical data (structured and unstructured) in the EHR for all infants admitted to the UF Health, level IV NICU between January 1st 2012 to March 1st 2020 was created. Clinical variables that were unavailable in the IDR were collected via chart review. Demographic variables and outcomes were defined as previously reported (12–19). Maximum and hourly neonatal sequential organ failure assessment (nSOFA) scores and vasoactive-inotropic scores (VIS) were calculated as previously described (13, 19, 20).
Definition of sepsis
An episode of sepsis was defined as a new episode of bacteremia/fungemia or meningitis with bacteria or fungus that was associated with ≥5 days of antimicrobial treatment or mortality while receiving antimicrobial treatment if duration was <5 days. Episodes were excluded if there was parenteral antimicrobial exposure within 48 h of the time of the reference culture. Coagulase negative Staphylococcus (CoNS) episodes were included only if there was effective antimicrobial therapy prescribed for ≥5 days or death <5 days. Infants with positive blood cultures with organisms that are frequently considered contaminants, including Bacillus, Micrococcus, and Corynebacterium species, were excluded.
Coagulation evaluation
A coagulation evaluation was included if it included simultaneous measurement of at least two parameters of the following three tests: fibrinogen, PT, and PTT. Reference values measured at discrete chronologic ages where testing has been performed in this population vary by gestational age at birth and chronological age (21–24) (Supplementary Table S1). Because daily normative values for these tests from birth are not available for this population, we applied normative ranges for each coagulation measure by gestational age at birth and discrete postnatal age in a fill-forward manner (until the next tested interval where normative values have been described).
Analytical methods
Non-parametric continuous variables were summarized as medians with quartiles (25th and 75th percentiles). Categorical variables were presented as percentages. Demographic variables were compared using Chi-square or Mann–Whitney U test. The nSOFA scores at discrete time intervals between multiple groups were compared using a Kruskal–Wallis test with Dunn's multiple comparisons test. Shapley Additive Explanation (SHAP) values were used to determine which variables were most important in regression modeling for patients who received treatment vs. no treatment for coagulopathy. Machine learning modeling was performed for specific variables and composite outcome for death and/or treatment to prevent over-fitting. The SHAP value is the average marginal contribution of a feature value across all possible coalitions. The threshold for statistical significance was less than 0.05 for two-sided tests. Analyses were performed using GraphPad Prism (version 10) and Python (Version 3.7; SHAP).
Results
Patient characteristics
Among 7,186 patients cared for in the UF NICU during the study period, we identified 910 positive blood or cerebrospinal fluid cultures in 367 patients. After applying our sepsis definition, 225 sepsis episodes in 216 patients were studied (Table 1). Episodes associated with coagulation evaluation (n = 96) occurred at an earlier age (13 vs. 23 days old), were more often associated with gram-negative (52% vs. 20%) and less often gram-positive (38% vs. 74%) pathogens, and showed greater nSOFA scores and greater mortality as compared with episodes without coagulation evaluation.
Pathogens
Among the 225 episodes studied, 220 were associated with bacteremia and 5 were episodes of meningitis without bacteremia. Gram-positive organisms were most common (132/225; 59%), followed by gram-negative organisms (76/225; 34%), polymicrobial infections (14/225; 6%), and minimal fungus (3/225; 1%). The most common single pathogens recovered were CoNS (68/225; 30%), Escherichia coli (34/225; 15%), Staphylococcus aureus (23/225; 10%), Group B Streptococcus (20/225; 9%), and Enterococcus faecalis (15/225; 7%). Antimicrobial resistance was common and did not differ between episodes with and without coagulopathy evaluation by pathogen class (Supplementary Table S2). Among non-CoNS episodes, 61/150 (41%) were associated with pathogens resistant to one or more common antimicrobial interventions (ampicillin, penicillin + beta-lactamase inhibitor, gentamicin, oxacillin, and third generation cephalosporin). Among episodes with recovery of non-CoNS pathogens, resistance to these common antimicrobial interventions was more common among those with a coagulopathy evaluation as compared with those without an evaluation [36/76 (47%) vs. 25/74 (34%)], but this did not reach significance (p = 0.10).
Episode frequency, timing, and result of coagulation evaluation
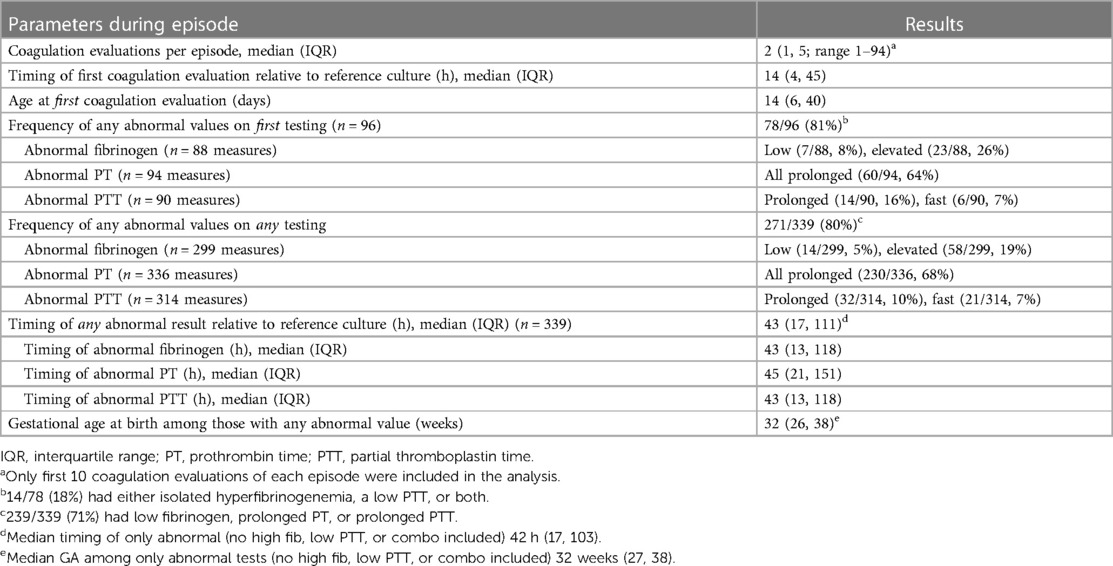
The frequency of any coagulation assessment [PT, International normalized ratio (INR), fibrinogen, PTT] at any time among all patients cared for in the UF NICU during the study period was 23% (1,654/7,186). Coagulation evaluations during sepsis events were performed at the discretion of the clinical team. Reasons for coagulation evaluation were abstracted by chart review and included concerns for or documentation of bleeding (n = 39), thrombocytopenia (n = 19), sepsis (n = 19), preoperative or unit protocol (e.g., hypoxic-ischemic encephalopathy) (n = 15), or abnormal liver function tests. An analysis of available measures for study revealed that greater than 90% of sepsis episodes were associated with ≤10 total coagulation evaluations and a median of 2 evaluations per episode (IQR 1, 5). Therefore, we restricted our analyses to the first 10 coagulation measures for each episode of sepsis, which yielded 339 episode-specific coagulation evaluations performed during 96 sepsis episodes (Table 2). The median time of the first coagulation evaluation relative to the reference culture was 14 h (IQR 4, 45) and at a median age of 14 days (IQR 6, 40). Abnormal coagulation evaluation results were similarly common in the first testing of the episode (78/96, 81%) and among all testing during the episode (271/339, 80%). The most common abnormal parameter was PT (230/336, 68%) and the median time to any abnormal result was 43 h (IQR 17, 111). An abnormal coagulation evaluation result during the episode was very common and occurred in 86/96 (90%). Episode mortality among those that had a coagulation evaluation with any abnormal result was 18/86 (21%).
Frequency and timing of intervention and repeat testing after coagulation evaluation
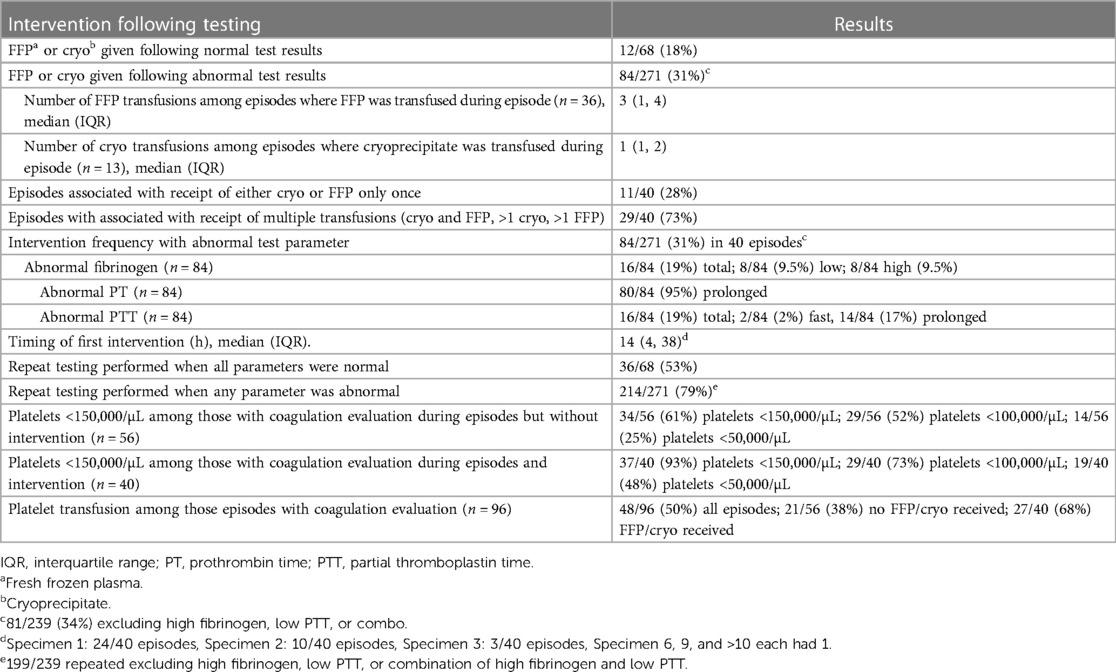
Among episodes where a coagulation evaluation was performed, 40/96 (42%) were associated with an intervention (transfusion of fresh frozen plasma or cryoprecipitate). Among all coagulation evaluations, 84/271 (31%) abnormal coagulation evaluation test results were associated with subsequent intervention and 12/68 (18%) normal test results were associated with intervention (Table 3). The median timing of the first intervention was 14 h after the reference culture (IQR 4, 38). Interventions primarily occurred following the first (24/40, 60%) or second (10/40, 25%) coagulation evaluation conducted during the episode. Most episodes (29/40, 73%) were associated with >1 intervention. Repeat testing was common. Among all normal test results, 36/68 (53%) were repeated. Among abnormal test results, 214/271 (79%) were repeated.
Frequency of concurrent abnormal platelet counts
Among the 56 episodes where a coagulation evaluation was performed without associated intervention, 34 (61%) were associated with mild thrombocytopenia (<150,000/µL), 29 (52%) with moderate thrombocytopenia (<100,000/µL), and 14 (25%) with severe thrombocytopenia (<50,000/µL) (Table 3). Among the 40 episodes where coagulation evaluation was performed with associated intervention, 37 (93%) were associated with mild thrombocytopenia, 29 (73%) with moderate thrombocytopenia, and 19 (48%) with severe thrombocytopenia. Out of the 96 episodes where a coagulation evaluation was performed (regardless of intervention), 48 episodes were associated with ≥1 platelet transfusions (27/40 (68%) episodes with intervention; 21/56 (38%) episodes without intervention).
nSOFA trajectories
We examined nSOFA scores during episodes with (lived, n = 77; died n = 19) and without (lived, n = 125) a coagulation evaluation at hourly resolution (Figure 1A) and compared scores at multiple discrete time points (Figure 1B) relative to the reference blood culture. Median nSOFA scores were 0 for survivors that did not have a coagulation evaluation at all nine discrete time points we evaluated (IQR for all nine time points: 0, 1). Survivors that had a coagulation evaluation also demonstrated a median nSOFA of 0 prior to evaluation (range of all IQR: 0, 2) but increased to 2 at evaluation (IQR 0, 4) and remained 2 at all future timepoints (range IQR 0–1, 4). Episodes with a coagulation evaluation that ended in death demonstrated a median nSOFA of 3 prior to evaluation (range IQR 0–2, 3–6) that increased to 4 (IQR 2, 8) at evaluation, 6 (IQR 3, 9) at 6 h, 8 (IQR 3, 10) at 12 h, 10 (IQR 6, 12) at 18 h, and 8 (IQR 5, 12) at 24 h.
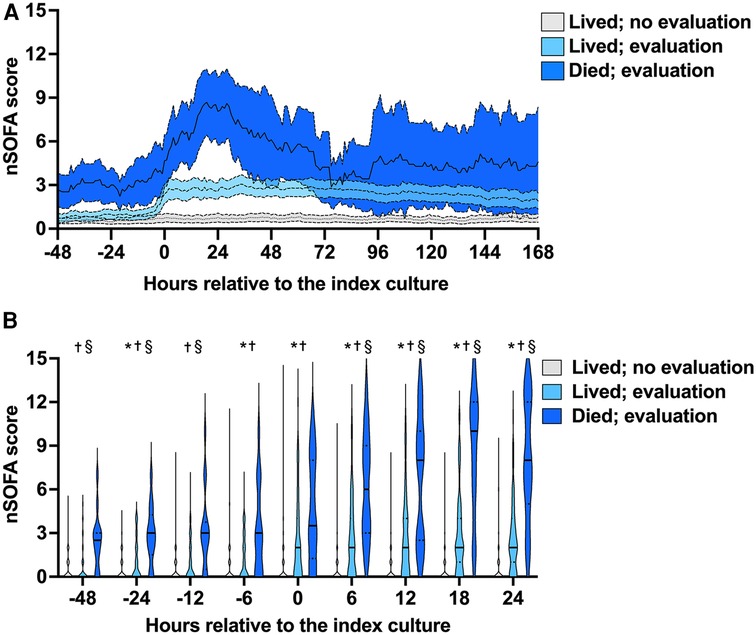
Figure 1. Neonatal sequential organ failure assessment (nSOFA) score trajectories and timed comparisons. (A) Consecutive q1-h nSOFA score mean (internal line) and 95% confidence intervals (surrounding band) during episodes with (lived, n = 77; died n = 19) and without (lived, n = 125) a coagulation evaluation (4 patients without a coagulation evaluation died and are not shown). (B) nSOFA scores at multiple discrete time points relative to the index blood culture (time 0) among the same groups. Violin plots show median (solid line) and quartiles (dotted line). Comparisons were made by Kruskal–Wallis with Dunn's multiple comparisons test. Maximum possible nSOFA score was 15 for any time point. *No evaluation vs. evaluated and lived; p ≤ 0.03; †no evaluation vs. evaluated and died; p ≤ 0.0004; §evaluated and lived vs. evaluated and died p ≤ 0.005.
Regression modeling
Logistic regression modeling and SHAP analysis were conducted to evaluate the significance of clinical variables and abnormal coagulation values in predicting outcomes (Supplementary Table S3 and Figure 2). The SHAP modeling demonstrated strong predictive performance for the composite outcome of death and/or treatment for coagulopathy in neonates (f1 score 0.8, area under receiver operating characteristic curve 0.83 for those with abnormal coagulation values). The three most important features influencing death and/or treatment for coagulopathy included administration of vasoactive medications, quantified by the VIS, hematologic dysfunction assessed by the maximum nSOFA platelet score, and early sepsis (within ≤72 h after birth).
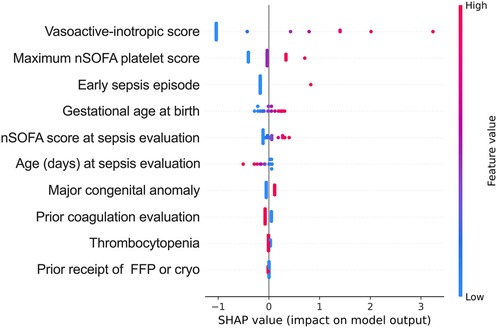
Figure 2. Shapley Additive explanation (SHAP) value plots provide a hierarchical organization of various features included in the model to predict the likelihood of death and/or treatment in the patients with abnormal coagulation values. Red and blue dots represent higher and lower values of the feature; maximum nSOFA platelet score (during episode); early sepsis episode (sepsis ≤72 h after birth); nSOFA, neonatal sequential organ failure assessment.
Discussion
This is the first comprehensive study of coagulopathy specific to common clotting parameters among patients with sepsis in the NICU population. A coagulopathy evaluation was performed in a minority of NICU patients with sepsis. Gram-negative pathogen, nSOFA score at evaluation, and mortality were greater and chronologic age at sepsis evaluation was lower among episodes that included a coagulopathy evaluation compared with those that did not. Abnormal results were common but infrequently associated with intervention, and intervention was nearly always associated with thrombocytopenia. These data may help to identify and guide treatment for future NICU patients at high risk of sepsis-associated coagulopathy.
Our findings aligned with well-described clinical risks and characteristics for coagulopathy in other populations including gram-negative pathogens, high severity of illness including shock, and coexistence of thrombocytopenia (10). A lower severity of illness and mortality frequency among those that did not have a coagulation evaluation compared with those that did in our study mirrored results in a large pediatric population evaluated for suspected sepsis in the emergency room setting (9). Among all sepsis episodes, intervention with either fresh frozen plasma or cryoprecipitate for any reason was documented in 43/225 (19%) episodes. Among patients not evaluated for coagulopathy, use of fresh frozen plasma or cryoprecipitate as well as mortality was rare and does not support that significant coagulopathy was present but missed. There are several potential reasons for neonates to exhibit reduced coagulopathy. One potential contributor to the frequency of coagulopathy in neonates is a unique host response to sepsis including a reduced inflammatory response as compared with the other age groups (25, 26). In an analysis of publicly available genome-wide expression microarrays of whole blood samples from humans of all ages with sepsis, neonates manifested a reduced pro-inflammatory response compared with other age groups (27). Furthermore, most sepsis events in neonates are associated with gram-positive bacterial recovery from blood, with CoNS being the most commonly identified gram-positive pathogen (28). The inflammatory response associated with CoNS, and thus the instigation of aberrant clotting, may be attenuated (29).
Concurrent thrombocytopenia was nearly ubiquitous among those with abnormal clotting parameters that received treatment. Coagulation dysfunction criteria in critically ill children were recently proposed following systematic review as part of the PODIUM Consensus Conference (30). Importantly, this comprehensive work was not intended for the preterm or NICU population. In addition to thrombocytopenia (<100,000 platelets/µL), the coagulation dysfunction criteria identified included INR (>1.5), fibrinogen (<150 mg/dL), and D-dimer (>10 times the upper limit of normal). The published parameters we used to guide normative coagulation values in our study reported PT rather than INR values, therefore we focused on PT. As expected, we found abnormally prolonged PT measures in most patients evaluated, which is consistent with known reduced activities of vitamin K-dependent factors in this population (31). Despite the high frequency of abnormal PT measures, most of these abnormal values were not associated with intervention. This finding suggests that the values returned were interpreted by the clinical team as acceptable, despite being considered abnormal based on the available normative data. Furthermore, perhaps because of the historical difficulty in interpretation in neonates (32), D-Dimer is neither a test routinely conducted on the NICU population nor sufficiently measured in this cohort to facilitate meaningful study (51 D-dimer measures in 22 patients among 339 coagulation tests studied). Rotational thromboelastometry, which evaluates phases of the clotting process, showed utility to identify those at the highest risk of mortality when routinely sent among a cohort of neonates with sepsis (33). As developmental normative values are being characterized, this testing may represent an additional or alternative way to measure the effectiveness of clotting in neonates with sepsis (34).
Less than half (96/225) of the patients in this near decade cohort received a coagulopathy evaluation at any time during the sepsis episode. Based on our collective clinical experiences at multiple academic institutions over several decades, we speculate the volume of blood required for coagulation testing (1.8 mL at UF) diminishes the frequency of routine coagulation evaluations in this population. Because the coagulation evaluations in this study were clinician-driven and not performed in all patients, we cannot accurately determine the frequency of coagulation abnormalities. In our cohort, 28% of infants with gram-positive bacteremia were evaluated, and 38% of those received cryoprecipitate or fresh frozen plasma. By contrast, 66% of infants with gram-negative bacteremia were evaluated for coagulopathy (2.4-fold over gram-positive) and 49% (1.3-fold over gram-positive) of those received cryoprecipitate or fresh frozen plasma. Based on our results, we suggest that coagulation evaluation be considered in all infants with presumed or confirmed sepsis and especially those with administration of vasoactive or inotropic medications. The data presented in our study does not allow for speculation regarding healthcare provider decisions to: (1) conduct assessments for coagulopathy, or (2) determine the rationale behind the treatment administered for coagulopathy.
Limitations
We acknowledge the true incidence of coagulopathy with sepsis in neonates cannot be derived from these data because the presence and timing of coagulopathy evaluation was not universal but rather was performed at the discretion of clinical providers. However, this is the largest and most comprehensive study of coagulopathy among patients with sepsis in the NICU population. Additional limitations in generalizability inherent to the single-center and retrospective nature of the study apply. Normal coagulation parameters by chronologic age, especially in the most immature infants, are unclear or in some cases entirely unavailable. Guidelines for when to measure clotting parameters and what results should guide treatment in this population are not standardized. Our study represents a first step toward addressing these many knowledge gaps and challenges.
Conclusions
In this cohort, only a minority of NICU patients with sepsis had a coagulation evaluation. Abnormal results were common but infrequently associated with intervention, and intervention was nearly always associated with thrombocytopenia. These data may help to identify future NICU patients at high risk of sepsis-associated coagulopathy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board at the University of Florida College of Medicine (approval number IRB201902780). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the retrospective study was deemed to be associated with no more than minimal risk.
Author contributions
KA: Conceptualization, Writing – original draft, Writing – review & editing. MS: Methodology, Writing – review & editing. DM: Data curation, Writing – review & editing. KW: Conceptualization, Writing – review & editing. JW: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors wish to thank the families and babies that contributed data to this work.
Conflict of interest
JW served as a one-time consultant to Sobi for neonatal immunology expertise in September of 2023. JW is a member of the Pediatric Sepsis Taskforce supported by the Society of Critical Care Medicine. JW is supported by the National Institutes of Health (GM128452; HD089939).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1364725/full#supplementary-material
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Schlapbach LJ, Watson RS, Sorce LR, Argent AC, Menon K, Hall MW, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. (2024). doi: 10.1001/jama.2024.0179
3. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
4. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. (2017) 171:e172352. doi: 10.1001/jamapediatrics.2017.2352
5. Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res. (2020) 88:85–90. doi: 10.1038/s41390-019-0517-2
6. Loberger JM, Aban IB, Prabhakaran P. Exploration of sepsis-associated coagulopathy severity and pediatric septic shock outcomes. J Pediatr Intensive Care. (2021) 10:38–44. doi: 10.1055/s-0040-1713436
7. Schmoch T, Mohnle P, Weigand MA, Briegel J, Bauer M, Bloos F, et al. The prevalence of sepsis-induced coagulopathy in patients with sepsis—a secondary analysis of two German multicenter randomized controlled trials. Ann Intensive Care. (2023) 13:3. doi: 10.1186/s13613-022-01093-7
8. Lyons PG, Micek ST, Hampton N, Kollef MH. Sepsis-associated coagulopathy severity predicts hospital mortality. Crit Care Med. (2018) 46:736–42. doi: 10.1097/CCM.0000000000002997
9. Slatnick LR, Thornhill D, Deakyne Davies SJ, Ford JB, Scott HF, Manco-Johnson MJ, et al. Disseminated intravascular coagulation is an independent predictor of adverse outcomes in children in the emergency department with suspected sepsis. J Pediatr. (2020) 225:198–206.e2. doi: 10.1016/j.jpeds.2020.06.022
10. Levi M, Van Der Poll T. Coagulation and sepsis. Thromb Res. (2017) 149:38–44. doi: 10.1016/j.thromres.2016.11.007
11. Hutter JJ Jr, Hathaway WE, Wayne ER. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr. (1976) 88:1026–31. doi: 10.1016/S0022-3476(76)81069-4
12. Alexandesr GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. (1996) 87:163–8. doi: 10.1016/0029-7844(95)00386-X
13. Aziz KB, Schles EM, Makker K, Wynn JL. Frequency of acute kidney injury and association with mortality among extremely preterm infants. JAMA Netw Open. (2022) 5:e2246327. doi: 10.1001/jamanetworkopen.2022.46327
14. Jensen EA, Dysart K, Gantz MG, Mcdonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200:751–9. doi: 10.1164/rccm.201812-2348OC
15. Lavilla OC, Aziz KB, Lure AC, Gipson D, De La Cruz D, Wynn JL. Hourly kinetics of critical organ dysfunction in extremely preterm infants. Am J Respir Crit Care Med. (2022) 205:75–87. doi: 10.1164/rccm.202106-1359OC
16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
17. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
18. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. (2015) 314:1039–51. doi: 10.1001/jama.2015.10244
19. Yeo KT, Goh GL, Park WY, Wynn JL, Aziz KB. Evaluation of the neonatal sequential organ failure assessment and mortality risk in neonates with early-onset infection. Neonatology. (2023) 120:796–800. doi: 10.1159/000533467
20. Wynn JL, Mayampurath A, Carey K, Slattery S, Andrews B, Sanchez-Pinto LN. Multicenter validation of the neonatal sequential organ failure assessment score for prognosis in the neonatal intensive care unit. J Pediatr. (2021) 236:297–300.e1. doi: 10.1016/j.jpeds.2021.05.037
21. Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, et al. Development of the human coagulation system in the healthy premature infant. Blood. (1988) 72:1651–7. doi: 10.1182/blood.V72.5.1651.1651
22. Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, et al. Development of the human coagulation system in the full-term infant. Blood. (1987) 70:165–72. doi: 10.1182/blood.V70.1.165.165
23. Neary E, Mccallion N, Kevane B, Cotter M, Egan K, Regan I, et al. Coagulation indices in very preterm infants from cord blood and postnatal samples. J Thromb Haemost. (2015) 13:2021–30. doi: 10.1111/jth.13130
24. Neary E, Okafor I, Al-Awaysheh F, Kirkham C, Sheehan K, Mooney C, et al. Laboratory coagulation parameters in extremely premature infants born earlier than 27 gestational weeks upon admission to a neonatal intensive care unit. Neonatology. (2013) 104:222–7. doi: 10.1159/000353366
25. Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. (2010) 125:1031–41. doi: 10.1542/peds.2009-3301
26. Wynn JL, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, Anas N, et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med. (2011) 17:1146–56. doi: 10.2119/molmed.2011.00169
27. Raymond SL, Lopez MC, Baker HV, Larson SD, Efron PA, Sweeney TE, et al. Unique transcriptomic response to sepsis is observed among patients of different age groups. PLoS One. (2017) 12(9):e0184159. doi: 10.1371/journal.pone.0184159
28. Flannery DD, Edwards EM, Coggins SA, Horbar JD, Puopolo KM. Late-onset sepsis among very preterm infants. Pediatrics. (2022) 150(6):e2022058813. doi: 10.1542/peds.2022-058813
29. Klingenberg C, Aarag E, Ronnestad A, Sollid JE, Abrahamsen TG, Kjeldsen G, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. (2005) 24:817–22. doi: 10.1097/01.inf.0000176735.20008.cd
30. Faustino EVS, Karam O, Parker RI, Hanson SJ, Brandao LR, Monagle P, et al. Coagulation dysfunction criteria in critically ill children: the PODIUM consensus conference. Pediatrics. (2022) 149:S79–83. doi: 10.1542/peds.2021-052888l
31. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. (2020) 12(3):780. doi: 10.3390/nu12030780
32. Hudson IR, Gibson BE, Brownlie J, Holland BM, Turner TL, Webber RG. Increased concentrations of D-dimers in newborn infants. Arch Dis Child. (1990) 65:383–4. doi: 10.1136/adc.65.4_Spec_No.383
33. Sokou R, Tsantes AG, Lampridou M, Tsante KA, Houhoula D, Piovani D, et al. Thromboelastometry and prediction of in-hospital mortality in neonates with sepsis. Int J Lab Hematol. (2024) 46(1):113–9. doi: 10.1111/ijlh.14165
Keywords: neonate, sepsis, coagulation, prothrombin time, partial thromboplastin time, NICU
Citation: Aziz KB, Saxonhouse M, Mahesh D, Wheeler KE and Wynn JL (2024) The frequency and timing of sepsis-associated coagulopathy in the neonatal intensive care unit. Front. Pediatr. 12:1364725. doi: 10.3389/fped.2024.1364725
Received: 2 January 2024; Accepted: 19 February 2024;
Published: 5 March 2024.
Edited by:
Rozeta Sokou, Nikaia General Hospital “Aghios Panteleimon,” GreeceReviewed by:
Robert Patrick Richter, University of Alabama at Birmingham, United StatesRyszard Lauterbach, University Hospital in Kraków, Poland
© 2024 Aziz, Saxonhouse, Mahesh, Wheeler and Wynn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James L. Wynn amFtZXMud3lubkBwZWRzLnVmbC5lZHU=
 Khyzer B. Aziz
Khyzer B. Aziz Matthew Saxonhouse2
Matthew Saxonhouse2 James L. Wynn
James L. Wynn