- 1Universidad Europea de Madrid, Faculty of Sport Sciences, Villaviciosa de Odón, Spain
- 2Servicio Medicina Intensiva, Hospital de Mataró, Barcelona, Spain
Introduction: The objective of this review is to know the existing scientific evidence about the effects of mechanical ventilation (MV) on neurological development in low-birth-weight premature pediatric patients after 12 months of life, taking as background the direct impact that ventilation has on the central nervous system in the newborn during the first days of life.
Methods: A systematic search was carried out between 2003 and 2024 in the data bases of: PUBMED, Cochrane Library Plus, PEDro, CINAHL, and SciELO, and two investigators scored the articles according to the Newcastle-Ottawa Assessment scale.
Results: Were found 129 non-replicated articles, and 10 cohort and cross-sectional studies were selected that performed an assessment of neurodevelopment in the three spheres after 12 months of life in corrected age of premature infants exposed to ventilator support and related the two variables independently.
Conclusions: Mechanical ventilation is an independent neurodevelopmental risk factor in low-birth-weight preterm infants. The time of exposure and the type of ventilation were the variables with the most scientific evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/, Identifier CRD42023446797.
1 Introduction
Newborns born before 37 weeks of gestation are considered a premature population (1). According to the WHO, prematurity is one of the most significant risk factors for postnatal complications and development (1), because there is a greater probability of presenting surfactant deficiency due to lack of pulmonary maturation, which hinders gas exchange (2), weakness of the respiratory musculature, a more distensible rib cage as a result of the maturational deficit, and an irregular respiratory rhythm that leads to ventilatory failure (3); factors such as: hyaline membrane disease, meconium aspiration syndrome, congenital sepsis, ischemic hypoxia, and prenatal brain injuries (among others) (4), this might mean the need to initiate mechanical ventilation (MV) as life support in the first hours of the life of the premature infant. This intervention will vary depending on the infant's clinical condition.
There are two types of mechanical ventilation: invasive (IMV) and noninvasive (NIV). IMV requires endotracheal intubation through the nose or mouth (3). Three general modes of IMV are programmed according to the needs of the neonate: controlled, assisted-controlled, and spontaneous (5). NIV is the support provided without invading the airway; pressure chambers, facial/nasal masks, or high-flow cannulas are used for this type of ventilation, with two modalities: extrathoracic negative pressure or inspiratory positive pressure systems (5).
In neonates, NIV intervention has increased in delivery rooms by 47.8% with modalities such as continuous positive pressure, reducing endotracheal ventilation interventions from 6% to 4% (6).
Authors such as Van Kaam et al. (5) and López et al. (7) have published publications that explain the benefits and consequences of the choice between invasive and noninvasive ventilation; however, all three conclude that the medical team's decision to connect a patient to one support system or another should be based on the evaluation of the functioning of all systems and the patient's global condition.
MV directly impacts all the newborn's systems, mainly the heart, lungs, and brain relationship in the first days of life (2). There is an interdependence of the support and the different systems that have not matured, causing alterations in the infant's development, and it has been demonstrated that it is a high-risk factor for neurological development (8). Morbidities such as moderate sensory, motor, and severe hearing impairment are directly related to both types of ventilatory support (9, 10).
Brain lesions and developmental delay due to ventilation are due to two main causes: migration to the brain of the pulmonary inflammatory cascade, causing a focal lesion that increases markers of oxidative stress (overproduction of cytokines) and consequently injury to the white matter (11); and the second is hemodynamic instability, since the over distension of the alveoli causes compression of the capillaries, increasing pulmonary resistance and decreasing cardiac output, which leads to variable blood flow to the brain and extravasation of brain proteins (12).
Research has shown that early stimulation by the interdisciplinary therapy team benefits the development of the neonate in the critical care unit since the recognition of his environment facilitates the generation of motor action in accordance with his corrected age (13, 14).
Knowing the impact of ventilatory support on the neurological system would allow us to provide more precise information to develop personalized strategies that respond to the needs of each patient. This would reduce or prevent possible sequelae in the neurodevelopment of the premature infant, thus improving the quality of the service provided by physiotherapy professionals.
2 Materials and methods
2.1 Study selection
A systematic review of the studies published on the influence of mechanical ventilation on neurological development in preterm patients between 2003 and August 2024 was carried out, following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (15).
The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42023446797).
The literature search was conducted by two independent investigators (VV) (VB) independently, in the following databases: PUBMED, Cochrane Library Plus, PEDro, CINAHL and SciELO, following the PICO question to organize and analyze the information found, which is: How do mechanical ventilation and its variables positively or negatively influence neurological development in preterm pediatric patients with low birth weight after 12 months of life?
The search terms used for this review were “mechanical ventilation” AND (“neurological development” OR “motor development” OR “development”) in “premature”; however, when starting the search, the population filter was performed with the term “neonates” in the PUBMED and PEDro databases, since some literature uses different terminology to refer to the same population.
The inclusion criteria taken into account were:
• Analytical cohort studies (retrospective and prospective) and descriptive cross-sectional studies.
• Articles where the study population was preterm infants requiring mechanical ventilation.
• Articles in English and Spanish.
• Articles published between 2003 and August 2024, considering advancements in technology and updates to clinical practice guidelines up to this date (16, 17).
• Articles directly correlating mechanical ventilation with motor development.
And the exclusion criteria:
• Articles that within their methodology performed a direct intervention to the study population by the investigators.
• Retrospective articles of a single case.
• Articles that include in the neurodevelopmental analysis a population under 12 months of corrected age.
2.2 Classification of studies for analysis
Based on the established search criteria, studies were grouped according to the correlation between mechanical ventilation and neurodevelopment, focusing on the following variables: mechanical ventilation as a risk factor, duration of mechanical ventilation (exposure time), type of mechanical ventilation (invasive and noninvasive), and specific variables within invasive mechanical ventilation (such as ventilatory mode).
2.3 Evaluation of the methodological quality of the studies
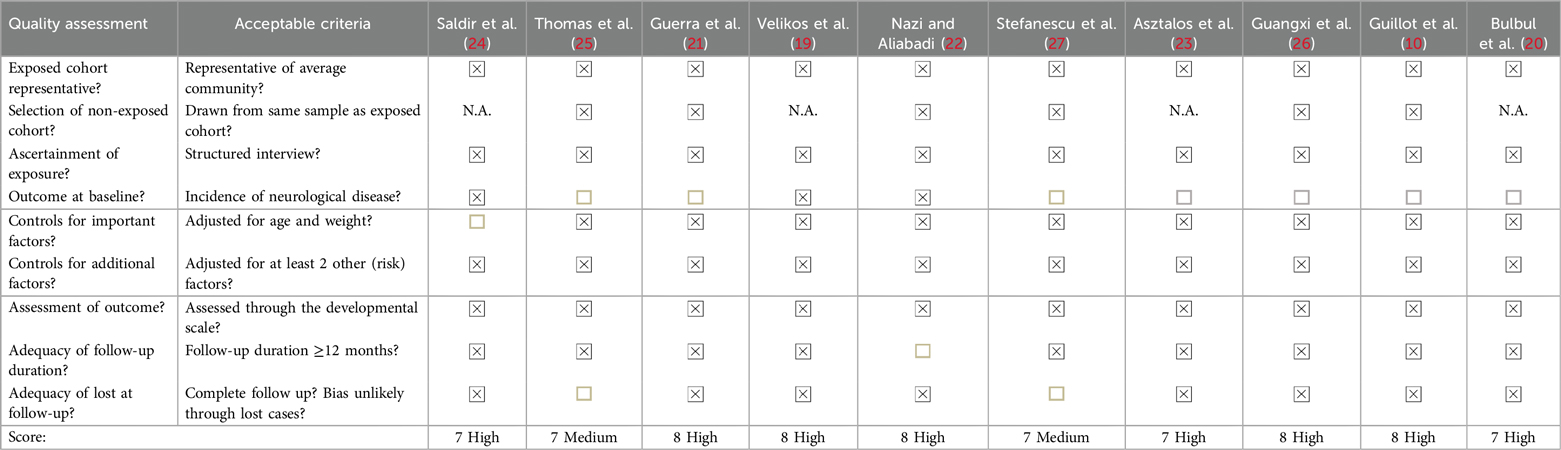
Two authors independently performed data extraction using a standardized data collection notebook, where the following data were considered: type of study, age of participants, correlation, and time elapsed between the variables studied (mechanical ventilation and neurodevelopment). The methodological quality of the articles included in this study was assessed using the Newcastle-Ottawa Assessment Scale for observational studies (18), which evaluates 8 items: patient selection (4 items), comparability (1 item), and study outcomes (3 items).
Each item allows for multiple selections, but points are only awarded to those that meet the criteria marked with an asterisk in the instrument, with a maximum possible score of 9 and a minimum of 0, as up to 2 points can be awarded for the comparability item.
To classify the quality of the studies, it is established that: a score of 80% or higher indicates a high-quality study; a score between 70% and 80% indicates medium quality; and studies scoring less than 70% are considered low quality (18).
3 Results
3.1 Selection of studies
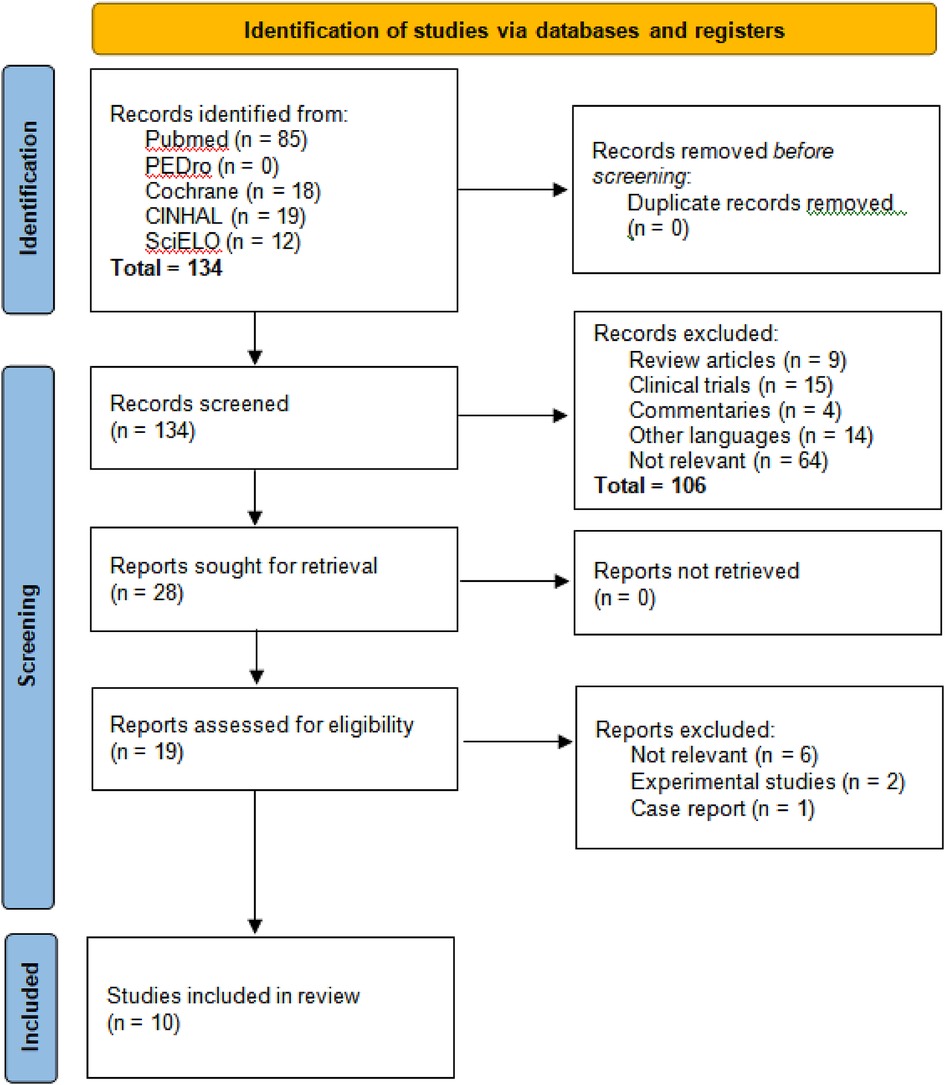
When we applied the search strategies in the five databases, we initially obtained 134 results as follows: PUBMED 85, PEDro 0, COCHRANE 18, CINAHL 19, and SciELO 12. After reviewing the titles and abstracts, 106 articles were excluded in the first stage due to the following reasons: 9 were systematic reviews of mechanical ventilation (MV) in premature neonates and its causes, 15 were clinical trials, 4 were commentaries on how the literature predictively supports the effect of MV in premature neonates, 14 were in a language other than English or Spanish, and finally, 64 were unrelated articles (Figure 1).
Subsequently, a thorough reading of the 19 preselected articles was performed, leading to a second selection phase where 9 articles were discarded based on the exclusion criteria: six (6) did not present a relationship between mechanical ventilation (MV) and neurodevelopment, two (2) involved two-part analysis studies where the first part was an experimental trial, and one (1) focused on a single case.
As a result, ten (10) observational studies were finally selected for inclusion in the present study. The second evaluator (VB) reviewed the selection and found no discrepancies in the choice of articles (Table 1).
3.2 Methodological quality of studies
The Newcastle-Ottawa Assessment Scale was used to evaluate the methodological quality of the articles (Table 2). The 10 studies scored between 7 and 8; 8 articles received a high methodological rating, and 2 were rated as medium quality. It is important to note that 4 of the articles with high ratings did not apply the cohort sample item since they are cross-sectional control studies. Out of the 90 items assessed, there was a discrepancy in only one, which was resolved by consensus between the two evaluators (VV and VB).
3.3 Study characteristics
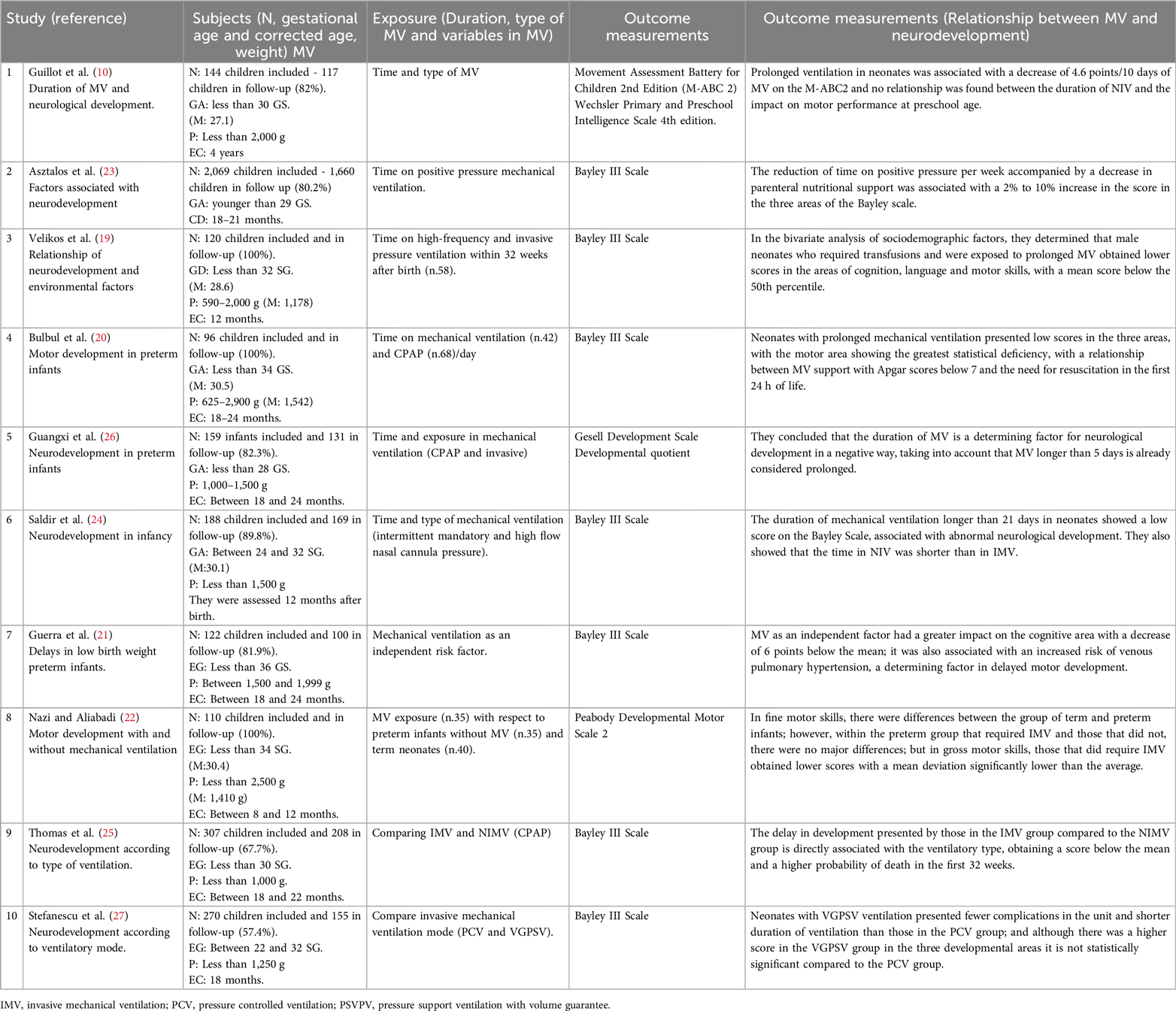
Of the studies, 4 (19–22) included preterm infants (less than 36 weeks of gestation), 4 others (10, 23–25) included very preterm infants (less than 32 weeks of gestation), and 2 studies (26, 27) included infants ranging from very preterm to extremely preterm (less than 28 weeks of gestation). All the studies are heterogeneous because the patients exposed to mechanical ventilation showed variations in the mode, type, and duration of ventilation, as there is no universal protocol for invasive methods, which varies according to the patient's clinical condition.
In the 10 articles, a total of 2,866 cases were followed up, with a mean of 286.6 ± 483.805; the range of gestational age was between 24 and 36 weeks, and birth weight ranged from 590 to 2,500 kg. Regarding data collection and analysis of the results, all the articles used neurodevelopmental assessment scales in the three domains (cognitive, language, and motor), providing a final follow-up for the patients who continued in the study. It is worth noting that only one article did not mention the average corrected age of the evaluated patients (24), and two articles had a high probability of bias due to a loss to follow-up of more than 20% of the total cases (25, 27).
3.4 Influence of mechanical ventilation on neurodevelopment
Regarding the factors of mechanical ventilation that were related to the influence on neurodevelopment in the different studies of this review, the results were analyzed and categorized as follows.
3.4.1 Duration of mechanical ventilation
Among the 10 articles reviewed, 6 investigated the duration of mechanical ventilation (MV) as a risk factor (10, 19, 20, 23, 24, 26). Five of these studies found that longer MV duration was associated with a higher likelihood of delayed motor development. The studies used different criteria to define prolonged MV: Guangxi (26) considered MV prolonged if it lasted more than 5 days, Saldir et al. (24) defined it as more than 21 days, and three other studies identified increased risk if MV extended beyond 7 days (15, 18, 21). In contrast, one study did not find a direct relationship between MV duration and delayed motor development (10), although this study involved children receiving non-invasive ventilation (NIV).
On the other hand, three articles considered factors that prolonged MV exposure, being an influential component at the moment of relating time with the consequences on development (19, 20, 23).
Velikos et al. (19) presented in their conclusions that there are greater alterations in the areas of cognition and language development, while Bulbul, et al. (20) found that the delay is greater in the motor area; and authors such as Asztalos, et al. (23) and Saldir et al. (24) determined that there is a global neurological development type, without finding significant differences between the three spheres of development.
3.4.2 Type of mechanical ventilation
Two articles studied the relationship of IMV and NIV as a risk factor for neurodevelopment (23, 25). Between these two was included an article already mentioned in the category of duration in MV (23).
Both studies agree that in NIV there is a lower probability of presenting neurodevelopmental delay. One of the two articles evaluated a population with more homogeneous characteristics and exposed to similar risk factors. Therefore, in its conclusion, they mention a direct relationship between NIV and delay in the three developmental spheres (25).
In addition, another article previously mentioned in the section on duration, in its discussion, it mentions that weaning from ventilation in neonates who were exposed to noninvasive measures was performed in less time compared to those of IMV, presenting better results (24).
3.4.3 Mechanical ventilation as a risk factor
Two articles studied MV independently as a risk factor for neurodevelopment. Both conclude that MV has a great impact on developmental delay, with the motor area showing the greatest deviation from the mean in the rating scales (21, 22); one of the articles presented more specific results, demonstrating that IMV has a greater impact on fine motor skills compared to gross motor skills in the short term in the child's motor development (22).
3.4.4 Mechanical ventilation mode
Only one article studied the IMV mode (27), where they demonstrated that, among the pressure ventilatory mode compared to a volume mode, the latter has a better impact on neurodevelopment since patients who were ventilated with this mode required less ventilation time and the results within the rating scale were not far from the mean, however due to the number of population and the little research there is about this topic the results are not statistically significant.
4 Discussion
This systematic review of observational articles aims to know the influence of mechanical ventilation and its variables on motor development in premature infants after 12 months of life (no-corrected age).
During the search we did not find in any database, reviews that related mechanical ventilation with neurodevelopment in the pediatric population (independently of gestational age). One review presented the effects of permissive hypercapnia during MV on neurodevelopment based on the alterations that can occur in the nervous system; however, they do not mention if there was a follow-up of preterm infants or if they applied neurological evaluation scales in the studies they included (28). Another review presented neurodevelopmental delay as side effects in only one ventilation modality: continuous positive airway pressure (CPAP), however, the limitations specified that only one of the comparison studies reported these results (29). In one review they studied the effects of different interfaces in NIV (30) and another one, the efficacy of different modalities in noninvasive support (31), however, they did not conclude whether they had a direct effect on neurological development, since none of the articles they included reported it. While our inclusion criteria covered studies from 2003 to August 2024, only those published between 2010 and August 2024 ultimately met these criteria, reflecting the alignment with recent advancements and evolving practices in neonatal care.
All the studies included in this review used various tools to measure neurodevelopment. Despite the variability in these tools, they are consistent in their core objective—assessing child development. Although the methodologies of the neurodevelopmental assessment tools differ, they all aim to evaluate the same fundamental concept of developmental progress. All the studies concluded that mechanical ventilation negatively impacts one or more aspects of neurodevelopment, regardless of differences in ventilation duration, type, or mode. However, in the study carried out by Saldir et al. (24) they did not find a direct relationship between alterations in development and non-invasive mechanical ventilation, possibly because this measure was taken as the main life support strategy in the first 24 h of the premature infant.
The duration of mechanical ventilation is the variable with the strongest evidence in this review. According to the included studies, prolonged ventilation is directly related to the alterations that preterm infants presented in their neurological development, because in the articles the ventilation variable was studied independently of the health conditions of the preterm infants (10, 19, 20, 23, 24, 26). The results of the scales applied in each study recorded a statistically significant deviation from typical development in premature infants who required long-term ventilatory support, so that the prognosis presented in the population under study was a delay or abnormal neurological development; even so, there is no clear consensus on how long is considered “prolonged mechanical ventilation”, with a great variety in the number of days taken as reference (from five to twenty-eight). Authors such as Pierrat et al. (32) corroborate that early weaning from ventilation decreases the probability of presenting alterations during neurological development in the first two years of life.
NIV has had a more favorable score in comparison to IMV in the neurodevelopment of premature infants and has proven to be a beneficial measure, decreasing clinical complications during exposure to ventilatory support (23, 25); likewise, the authors concluded that ventilation is an independent factor to the comorbidities of neonates, therefore, the use of less invasive measures such as continuous positive airway pressure (CPAP) and high frequency oscillatory ventilation is a factor that positively influences neonates. Onland, et al. (33) concluded that the use of less invasive measures in conjunction with other adjuvant treatments decreases the risk of developmental delay and lower probability of mortality in extremely preterm infants. However, recent studies cast doubt on this conclusion, highlighting that more research is needed to determine the failure or success of one or the other ventilatory support, as complications during pregnancy or the first 72 h of the preterm infant's life have been shown to be key factors in the medical team's decision-making process (34).
Factors such as the programming of ventilatory parameters are adjusted according to the needs and evaluation of the patients (7), therefore, the ventilatory mode is a variable dependent on the health status of each premature infant. Research has shown that volume-driven ventilatory modes minimize the risks of morbidity and mortality compared to pressure-driven modes but are not a factor influencing neurodevelopment (7, 27). Recent reviews evaluated whether permissive carbon dioxide (CO2) levels during ventilation affected different systems via respiratory rate programming, concluding that both hypercapnia (<35.3 mmHg) and hypocapnia (>30.3 mmHg) are strategies with variable and therefore contradictory results, which did not allow us to determine whether or not they influence the neurological development of the premature infant; in addition, the reference values of CO2 are subject to the weight of the neonate, so that the heterogeneity of the participants was another limitation to establish a definitive result (35).
The need for mechanical ventilation in the neonate has become a subject of study of great importance due to the implications of the use of this vital support and its relationship with neurodevelopment, which is not only altered by direct exposure, but also by associated clinical factors such as: pulmonary dysplasia, infections, pulmonary hypertension, damage to the auditory canal, damage to the white matter, among others; which implies more time in the critical care unit and longer duration of ventilation, thus increasing the probability of presenting developmental delay (36).Therefore, studies such as that of Thomas et al. (24) and Saldir et al. (25) included in their study population neonates with greater homogeneity concerning the risk factors to which they were exposed and in the studies of Velikos et al. (19) and Asztalos, et al. (23) the clinical characteristics of each of the study participants were studied independently to determine the direct impact of MV on neurodevelopment.
Knowing the role of the physical therapist in the critical care unit in premature patients with ventilatory support and the impact of early interventions in this population could help to reduce the negative effect of MV on motor development. In the study carried out by Souza et al. (37) it was shown that a therapy protocol based on physical exercise can reduce the time of exposure to mechanical ventilation and oxygen support (38), as well as obtain benefits on the autonomic system (regulation of heart rate and cardiac output), lower rate of complications, increased muscle strength and functional capacity.
Studies such as that of Zhang X, et al. (39), where it is shown that the environmental factors found in the units are a critical factor at the time of neonatal development, with a high probability of presenting different alterations, especially in the behavioral area and in sensitivity; and Mendonça et al. (40) who explain the relationship of ventilatory support with the increase of abnormal movements and atypical development, have a strong clinical implication, to take preventive measures to help reduce the complications that can occur in the neurodevelopment of premature infants. Therefore, the implementation of early care protocols could have a positive impact on preterm infants who are exposed to mechanical ventilation. Valizadeh, et al. (41) demonstrated that, although a passive physical activity program compared to restraint measures in preterm patients does not have a significant impact on neurological development, it does improve motor function of the lower extremities; on the other hand, Hae Yean, et al. (14) concluded that early physical activity programs in the units have a positive impact on the development of low-birth-weight preterm infants in mental and motor functions, achieving significant improvements.
Other research has shown that in addition to improving motor performance, the treatments implemented by physical therapists also bring benefits in bone mineralization, weight gain, better sleep quality and pain reduction (41, 42). Likewise, it has been proven that the techniques used by professionals are safe for patients in critical care units, both high and low risk; however, each case should be evaluated independently, in order to do the best possible good for our premature infants (43).
5 Limitations of the study
The primary limitation of this review is the paucity of research exploring the relationship between mechanical ventilation and neonatal neurodevelopment, as well as the complexity of neonatal outcomes and the wide variety of contributing factors, which can make it difficult to draw definitive conclusions. Some studies linked mechanical ventilation to reduced mobility but not to functional outcomes, often using non-standardized outcome measures, which further limited the sample size in the included studies. Additionally, these studies did not distinguish between low birth weight and extremely low birth weight, which would have allowed for a more detailed analysis of this variable and its relationship to mechanical ventilation.
Another limitation is that most studies did not compare outcomes with a group of preterm infants who were not exposed to ventilatory support, making it difficult to confirm the level of risk associated with mechanical ventilation in this population. Additionally, the studies did not account for differences in severity among varying degrees of prematurity, which could influence the reported outcomes.
The lack of uniformity in outcome measures, combined with the fact that the authors did not compare different MV techniques (such as nasal vs. oral intubation, or different models of invasive and non-invasive MV), prevented the possibility of conducting a meta-analysis and highlights a potential area for future research.
6 Conclusions
Studies indicate that mechanical ventilation is an independent risk factor for neurodevelopment in low-birth-weight preterm infants. Research indicates that a shorter exposure time and less invasive ventilatory measures can reduce the probability of presenting neurodevelopmental alterations; however, other factors such as ventilation modality and ventilatory parameters are not directly related to the alterations presented by premature infants in the three spheres of neurodevelopment, because they are variables that depend on the health conditions of each premature infant; in addition, the lack of research on these last two factors makes it difficult to conclude their impact on neonates. Therefore, there is a need to continue studying the relationship between ventilatory support and neurological development in a population at risk of presenting alterations during their first years of life, such as low-birth-weight preterm infants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
VV: Writing – review & editing, Conceptualization, Writing – original draft. MD: Methodology, Writing – review & editing. MS: Methodology, Writing – review & editing. MG: Writing – review & editing. FS: Writing – review & editing. CR: Supervision, Writing – review & editing. CE: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Organization WH. Preterm and low birth weight [Internet]. 2012. Available online at: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/newborn-health/preterm-and-low-birth-weight (Accessed October 09, 2024).
2. Polglase GR, Miller SL, Barton SK, Kluckow M, Gill AW, Hooper SB, et al. Respiratory support for premature neonates in the delivery room: effects on cardiovascular function and the development of brain injury. Pediatr Res. (2014) 75(6):682–8. doi: 10.1038/pr.2014.40
3. González Á, Estay A. Ventilación mecánica en el recién nacido prematuro extremo, ¿hacia dónde vamos? Rev Méd Clíni Las Condes. (2021) 32(6):682–9. doi: 10.1016/j.rmclc.2021.10.006
4. Santana YP. Ventilación mecánica en cuidados intensivos neonatales/mechanical ventilation in neonatal intensive care unit. Rev Cuba Med Intensiva Emerg. (2015) 15(1):70–7.
5. Van Kaam AH, De Luca D, Hentschel R, Hutten J, Sindelar R, Thome U, et al. Modes and strategies for providing conventional mechanical ventilation in neonates. Pediatr Res. (2021) 90(5):957–62. doi: 10.1038/s41390-019-0704-1
6. Handley SC, Salazar EG, Greenberg LT, Foglia EE, Lorch SA, Edwards EM. Variation and temporal trends in delivery room management of moderate and late preterm infants. Pediatrics. (2022) 150(2):e2021055994. doi: 10.1542/peds.2021-055994
7. López Escobar M, López Ortiz J, Bernal Sánchez JJ. Estrategia ventilatoria en neonatos que recibieron terapia de reemplazo de surfactante. Acta Colomb Cuid Intensivo. (2018) 18(2):77–83. doi: 10.1016/j.acci.2018.01.005
8. Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. (2005) 146(6):798–804. doi: 10.1016/j.jpeds.2005.01.047
9. Adams-Chapman I, Heyne RJ, DeMauro SB, Duncan AF, Hintz SR, Pappas A, et al. Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics. (2018) 141(5):e20173091. doi: 10.1542/peds.2017-3091
10. Guillot M, Guo T, Ufkes S, Schneider J, Synnes A, Chau V, et al. Mechanical ventilation duration, brainstem development, and neurodevelopment in children born preterm: a prospective cohort study. J Pediatr. (2020) 226:87–95.e3. doi: 10.1016/j.jpeds.2020.05.039
11. Cannavò L, Rulli I, Falsaperla R, Corsello G, Gitto E. Ventilation, oxidative stress and risk of brain injury in preterm newborn. Ital J Pediatr. (2020) 46:100. doi: 10.1186/s13052-020-00852-1
12. Barton SK, Tolcos M, Miller SL, Christoph-Roehr C, Schmölzer GM, Moss TJM, et al. Ventilation-Induced brain injury in preterm neonates: a review of potential therapies. Neonatology. (2016) 110(2):155–62. doi: 10.1159/000444918
13. Jeimi Lisette Rodríguez S. Fisioterapia neurológica en unidad de cuidados intensivos en población neonatal. FisioGlía Rev Divulg En Fisioter. (2017) 4(2):29–32.
14. Park HY, Maitra K, Achon J, Loyola E, Rincón M. Effects of early intervention on mental or neuromusculoskeletal and movement-related functions in children born low birthweight or preterm: a meta-analysis. Am J Occup Ther. (2014) 68(3):268–76. doi: 10.5014/ajot.2014.010371
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Rev Esp Cardiol. (2021) 74(9):790–9. doi: 10.1016/j.recesp.2021.06.016
16. Grupo Respiratorio Neonatal De La Sociedad Española De Neonatología. Neonatal conventional ventilation guidelines. An Esp Pediatr. (2001) 55(3):244–50. doi: 10.1016/S1695-4033(01)77673-4
17. Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med. (2002) 347(9):643–52. doi: 10.1056/NEJMoa012750
18. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Ottawa Hospital Research Institute. 2021. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed October 09, 2024).
19. Velikos K, Soubasi V, Michalettou I, Sarafidis K, Nakas C, Papadopoulou V, et al. Bayley-III scales at 12 months of corrected age in preterm infants: patterns of developmental performance and correlations to environmental and biological influences. Res Dev Disabil. (2015) 45-46:110–9. doi: 10.1016/j.ridd.2015.07.014
20. Bulbul L, Elitok GK, Ayyıldız E, Kabakcı D, Uslu S, Köse G, et al. Neuromotor development evaluation of preterm babies less than 34 weeks of gestation with Bayley III at 18–24 months. BioMed Res Int. (2020) 2020:5480450. doi: 10.1155/2020/5480450
21. Guerra CC, Barros MCM, Goulart AL, Fernandes LV, Kopelman BI, dos Santos AMN. Premature infants with birth weights of 1500–1999 g exhibit considerable delays in several developmental areas. Acta Paediatr. (2014) 103(1):e1–6. doi: 10.1111/apa.12430
22. Nazi S, Aliabadi F. Comparison of motor development of low birth weight (LBW) infants with and without using mechanical ventilation and normal birth weight infants. Med J Islam Repub Iran. (2015) 29:301.26913264
23. Asztalos EV, Church PT, Riley P, Fajardo C, Shah PS, Canadian Neonatal Network and Canadian Neonatal Follow-up Network Investigators. Neonatal factors associated with a good neurodevelopmental outcome in very preterm infants. Am J Perinatol. (2017) 34(4):388–96. doi: 10.1055/s-0036-1592129
24. Saldir M, Sarici SU, Bakar EE, Ozcan O. Neurodevelopmental status of preterm newborns at infancy, born at a tertiary care center in Turkey. Am J Perinatol. (2010) 27(2):121–8. doi: 10.1055/s-0029-1224863
25. Thomas CW, Meinzen-Derr J, Hoath SB, Narendran V. Neurodevelopmental outcomes of extremely low birth weight infants ventilated with continuous positive airway pressure vs. Mechanical ventilation. Indian J Pediatr. (2012) 79(2):218–23. doi: 10.1007/s12098-011-0535-5
26. Guang Xi Cooperative Research Group for Extremely Preterm Infants, Li Y, Meng DH, Wei QF, Pan XN, Liang WH, et al. Neurodevelopmental outcomes of extremely preterm infants in southern China: a multicenter study. Early Hum Dev. (2019) 133:5–10. doi: 10.1016/j.earlhumdev.2019.04.002
27. Stefanescu BM, Frewan N, Slaughter JC, O’Shea TM. Volume guarantee pressure support ventilation in extremely preterm infants and neurodevelopmental outcome at 18 months. J Perinatol. (2015) 35(6):419–23. doi: 10.1038/jp.2014.228
28. Ozawa Y, Miyake F, Isayama T. Efficacy and safety of permissive hypercapnia in preterm infants: a systematic review. Pediatr Pulmonol. (2022) 57(11):2603–13. doi: 10.1002/ppul.26108
29. Subramaniam P, Ho JJ, Davis PG. Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants. Cochrane Database Syst Rev. (2021) 2021(10):CD001243. doi: 10.1002/14651858.CD001243.pub4
30. Prakash R, De Paoli AG, Oddie SJ, Davis PG, McGuire W. Masks versus prongs as interfaces for nasal continuous positive airway pressure in preterm infants. Cochrane Database Syst Rev. (2022) 11(11):CD015129. doi: 10.1002/14651858.CD015129
31. Goel D, Oei JL, Smyth J, Schindler T. Diaphragm-triggered non-invasive respiratory support in preterm infants. Cochrane Database Syst Rev. (2020) 2020(3):CD012935. doi: 10.1002/14651858.CD012935.pub2
32. Pierrat V, Burguet A, Marchand-Martin L, Cambonie G, Coquelin A, Roze J, et al. Variations in patterns of care across neonatal units and their associations with outcomes in very preterm infants: the French EPIPAGE-2 cohort study. BMJ Open. (2020) 10(6):e035075. doi: 10.1136/bmjopen-2019-035075
33. Vliegenthart RJS, Onland W, van Wassenaer-Leemhuis AG, De Jaegere APM, Aarnoudse-Moens CSH, van Kaam AH. Restricted ventilation associated with reduced neurodevelopmental impairment in preterm infants. Neonatology. (2017) 112(2):172–9. doi: 10.1159/000471841
34. Fallahi M, Taslimi Taleghani N, Afje SA, Shamshiri AR, Esmaili F, Radfar M, et al. Predictors of success rate in different initial respiratory supports in very low birthweight infants with respiratory distress. Arch Iran Med. (2020) 23(11):724–31. doi: 10.34172/aim.2020.96
35. Wong SK, Chim M, Allen J, Butler A, Tyrrell J, Hurley T, et al. Carbon dioxide levels in neonates: what are safe parameters? Pediatr Res. (2022) 91(5):1049–56. doi: 10.1038/s41390-021-01473-y
36. Choi YB, Lee J, Park J, Jun YH. Impact of prolonged mechanical ventilation in very low birth weight infants: results from a national cohort study. J Pediatr. (2018) 194:34–9.e3. doi: 10.1016/j.jpeds.2017.10.042
37. Souza GSB, Novais MFM, Lemes GE, de Mello MLFMF, de Sales SCD, Cunha KDC, et al. Effectiveness of different physiotherapy protocols in children in the intensive care unit: a randomized clinical trial. Pediatr Phys Ther. (2022) 34(1):10–5. doi: 10.1097/PEP.0000000000000848
38. van den Adel TZ, van Dijk M, de Heer M, Hoekstra S, Steenhorst J, van Rosmalen J, et al. Quality improvement intervention to stimulate early mobilization of critically ill children. Nurs Crit Care. (2023) 28(4):545–53. doi: 10.1111/nicc.12761
39. Zhang X, Spear E, Hsu HHL, Gennings C, Stroustrup A. NICU-based stress response and preterm infant neurobehavior: exploring the critical windows for exposure. Pediatr Res. (2022) 92(5):1470–8. doi: 10.1038/s41390-022-01983-3
40. Mendonça KT, Lanza FC, de Sousa Morais RL, Camargos ACR. Clinical factors associated with abnormal general movements of preterm newborns during hospitalization in a neonatal intensive care unit. Early Hum Dev. (2022) 174:105682. doi: 10.1016/j.earlhumdev.2022.105682
41. Valizadeh L, Sanaeefar M, Hosseini MB, Asgari Jafarabadi M, Shamili A. Effect of early physical activity programs on motor performance and neuromuscular development in infants born preterm: a randomized clinical trial. J Caring Sci. (2017) 6(1):67–79. doi: 10.15171/jcs.2017.008
42. Vignochi CM, Silveira RC, Miura E, Canani LHS, Procianoy RS. Physical therapy reduces bone resorption and increases bone formation in preterm infants. Am J Perinatol. (2012) 29(8):573–8. doi: 10.1055/s-0032-1310520
Keywords: mechanical ventilation, newborn, neurodevelopment, premature birth, invasive mechanical ventilation, non-invasive ventilatory support
Citation: Vargas Caicedo V, de la Plaza San Frutos M, Sosa Reina MD, Garcia Arrabe M, Salniccia F, Reina Aguilar C and Estrada Barranco C (2024) Effects of mechanical ventilation on neurodevelopment at 12 months in preterm low birth weight pediatric patients: a systematic review. Front. Pediatr. 12:1363472. doi: 10.3389/fped.2024.1363472
Received: 30 December 2023; Accepted: 30 September 2024;
Published: 21 October 2024.
Edited by:
Mohamed E. Abdel-Latif, The Canberra Hospital, AustraliaReviewed by:
Mark Mammel, University of Minnesota Twin Cities, United StatesGiovanni Boscarino, Ospedale Universitario di Parma, Italy
Copyright: © 2024 Vargas Caicedo, de La Plaza San Frutos, Sosa Reina, Garcia Arrabe, Salniccia, Reina Aguilar and Estrada Barranco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Salniccia, ZmVkZXJpY28uc2FsbmljY2lhQHVuaXZlcnNpZGFkZXVyb3BlYS5lcw==
 Valerie Vargas Caicedo
Valerie Vargas Caicedo Marta de la Plaza San Frutos
Marta de la Plaza San Frutos Maria Dolores Sosa Reina
Maria Dolores Sosa Reina Maria Garcia Arrabe
Maria Garcia Arrabe Federico Salniccia
Federico Salniccia Clara Reina Aguilar
Clara Reina Aguilar Cecilia Estrada Barranco
Cecilia Estrada Barranco