- 1Department of Woman and Child Health and Public Health, Catholic University of Sacred Heart, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy
- 2Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy
Background: There are no guidelines regarding enteral feeding (EF) of infants with hypoxic-ischemic encephalopathy (HIE) during and shortly after therapeutic hypothermia; consequently, clinical practice is, to date, still variable. The objective of this study is to assess whether a minimal EF strategy during therapeutic hypothermia may be associated with a shorter time to full EF of infants with HIE and to identify the clinical variables that independently affect the time to full EF.
Methods: A retrospective study, covering the period from 1 January 2015 to 30 June 2022 was performed at the Neonatal Intensive Care Unit of the Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS, Rome, which compared infants with HIE who received minimal EF during therapeutic hypothermia with those who did not.
Results: Seventy-eight infants received minimal EF during therapeutic hypothermia, while 75 did not. Infants who were fed reached full EF significantly faster than those who were not. Moreover, they received parenteral nutrition and maintained central venous lines for a shorter time. A multivariate analysis, taking into account the variable of clinical severity, confirmed that minimal EF is an independent beneficial factor for reaching full EF in a shorter time and mechanical ventilation and seizures are independent factors for a longer time to full EF.
Conclusions: Minimal EF during therapeutic hypothermia is associated with a shorter time to full EF in stable infants with HIE. Further prospective studies are needed to better define the enteral nutrition strategy for infants during therapeutic hypothermia, regardless of the severity of clinical conditions.
1 Introduction
Hypoxic-ischemic encephalopathy (HIE) represents a substantial cause of death and disability during the neonatal age (1). Currently, therapeutic hypothermia is the only validated strategy for reducing the long-term sequelae of HIE (2).
The optimal enteral feeding (EF) strategy for infants with HIE during therapeutic hypothermia is still debated (3). The studies available until now show the beneficial effect of EF without adverse effects, even if we remain uncertain about how to administer enteral nutrition to infants with HIE, and, above all, whether EF can be administered regardless of clinical severity (4–8).
A survey conducted in 2017 on the nutritional practices in neonatal intensive care units (NICUs) in the United Kingdom for infants treated with therapeutic hypothermia revealed that, of the 49 NICUs that answered, only 59% started EF during therapeutic hypothermia, with most initiating as a minimal EF (9).
The key reason for withholding EF during therapeutic hypothermia is the fear of necrotizing enterocolitis (NEC), which, although typically associated with prematurity, is described in full-term neonates with HIE (10).
Hypoxia is an established risk factor for NEC; hypoxia affects the gastrointestinal (GI) tract, decreasing perfusion and motility (11, 12). Given these assumptions, withholding EF might seem the most logical choice. However, from studies on very low birth weight (BW) infants, who are at greatest risk of NEC, it is known that the early provision of minimal EF is beneficial, as it stimulates GI motility, promotes GI hormone secretions, and favors beneficial microflora, thus improving GI tract maturation and feeding tolerance without increasing the risk of developing NEC (13). Moreover, therapeutic hypothermia seems to have a beneficial effect on GI function and feeding tolerance in moderate-to-severe HIE, as shown in studies comparing cooled infants with non-cooled ones (14–16).
Birth asphyxia typically affects visceral hemodynamics, with an increase in the superior mesenteric and celiac artery velocities in non-cooled infants with mild-to-moderate HIE during the first day of life and very low velocities in severe HIE. Therapeutic hypothermia plays a beneficial role by modulating the reperfusion phase and keeping blood flow stable during treatment, thus reducing the risk of reperfusion injury (17).
In this study, we aimed to assess whether a minimal EF strategy during therapeutic hypothermia may be associated with a shorter time to full EF in infants with HIE. In addition, we intended to identify the clinical variables that independently influence the achievement of full EF.
2 Methods
2.1 Settings and patients
We performed a monocentric retrospective cohort study at the NICU of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome between 1 January 2015 and 30 June 2022.
We included all infants with HIE undergoing therapeutic hypothermia. Therapeutic hypothermia was initiated within 6 h of life in infants with a gestational age (GA) of ≥35 weeks and a BW of ≥1,800 g if the following two criteria were met: (1) intrapartum hypoxia with at least one of the following: (A) Apgar score ≤5 at 10 min, (B) persistent need for resuscitation at 10 min, and (C) blood gas acidosis characterized by either a pH of ≤7.0 or negative base excess of ≥12 mmol/L in the first hour of life; and (2) moderate to severe encephalopathy evaluated by standardized neurologic examination (18). Neurological involvement was confirmed by an amplitude-integrated electroencephalogram (aEEG), which was defined as moderately abnormal with an upper margin of >10 μV and a lower margin of <5 μV, severely abnormal with an upper margin of <10 μV and a lower margin of <5 μV, or burst suppression. Infants missing relevant nutritional and clinical data for the study were excluded.
Institutional review boards approved the study. Parents gave written informed consent to the processing of personal data.
2.2 Nutritional protocol during therapeutic hypothermia
In our NICU, the nutritional practice for infants with HIE undergoing therapeutic hypothermia involved the initiation of minimal EF if they did not have systemic hypotension requiring vasoactive therapy, disseminated intravascular coagulation, acidosis defined by a pH of <7.15 for more than 2 h, hypoxia defined by a PaO2 of <45 mmHg for more than 2 h, and abnormal abdominal clinical findings. Minimal EF was intended as trophic feeding with administration of 12–24 ml/kg/day of human or formula milk, delivered as an intermittent bolus by a nasogastric or an orogastric tube every 3 h. The total intravenous fluid volume was maintained between 50 and 60 ml/kg/day during therapeutic hypothermia.
Feeding was defined as well tolerated in the absence of pathological abdominal signs (distension, color changes, visible intestinal loops, or pain), pathological gastric residue (>100% of previous feeding, biliary, blood, or fecal content), vomiting, bloody stools, and associated cardiorespiratory events.
2.3 Primary and secondary outcomes
The primary outcome was the day of life at which a full EF of 150 ml/kg/day of milk was achieved and well tolerated for at least three consecutive days. Secondary outcomes included the duration of parenteral nutrition (PN) and central venous (CV) lines, breastfeeding rates at discharge, and length of hospital stay.
2.4 Data collection and definitions
For every newborn, we extracted data from the clinical records on sex, GA (determined by the best obstetric estimate based on the first day of the last menstrual period, prenatal ultrasound, and postnatal physical examination), BW, and small for GA (SGA) infants, defined with the Italian Neonatal Study (INeS) reference values as those with a BW z-score below −1.28 standard deviations (SDs) (19). Obstetrical diseases, the occurrence of sentinel events, outborn status, and mode of delivery were also recorded.
As for the severity of HIE, we detailed the Apgar score at 1, 5, and 10 min of life, the acid–base status at birth, the neurological examination before starting therapeutic hypothermia, the temperature on NICU admission, the aEEG pattern, the timing of the commencement of therapeutic hypothermia, and its target temperature.
We recorded data on the following neonatal morbidities: seizures; respiratory distress syndrome (RDS); pneumothorax; persistent pulmonary hypertension of the newborn (PPHN); transient tachypnea of the newborn; hypotension requiring inotropic support; acute kidney injury; anemia; thrombocytopenia and coagulation issues; pneumonia, defined by a positive bronchoalveolar lavage culture; late-onset culture-proven sepsis, defined by a positive blood culture occurring at or after 72 h of life; NEC according to Bell staging criteria (20); hypoglycemia, defined as a blood glucose concentration <47 mg/dl (21); cholestasis defined as the presence of serum-conjugated bilirubin with a level of >1 mg/dl when the total bilirubin was <5 mg/dl or a conjugated component of >20% of the total when the total bilirubin was >5 mg/dl (22).
We recorded data on neonatal treatments such as surfactant delivery, the percentage of infants on mechanical ventilation (MV) during therapeutic hypothermia, and the duration of MV, oxygen therapy, steroid treatment, inotropic drugs, diuretics, blood product transfusions, and vitamin K supplementation.
As for EF during therapeutic hypothermia, we registered the feeding volumes (ml/kg/day) for each day of therapeutic hypothermia, the type of milk (human milk or formula), feeding technique, and time to full EF.
Data on the duration of PN and CV lines, breastfeeding rates at discharge, and the length of neonatal unit stay were also registered.
2.5 Sample size and statistical analysis
We based the sample size calculation on our observational data, which showed that among the unfed infants undergoing therapeutic hypothermia, the mean ± SD time to reach the full EF was 10.0 ± 5.5 days. Sample size calculation demonstrated that at least 53 patients for each group were needed to detect a 3-day advantage in achieving full EF in fed infants with a power of 80% and an α error of 0.05 (23).
Data are reported as numbers (percentages) and mean ± SD for normally distributed variables and as median (interquartile range, IQR) for non-normally distributed variables.
We tested normal distribution using the Shapiro–Wilk test; we compared categorical variables with the chi-squared test or Fisher's exact test and continuous variables with the Student t-test for the independent sample or the Mann–Whitney U test.
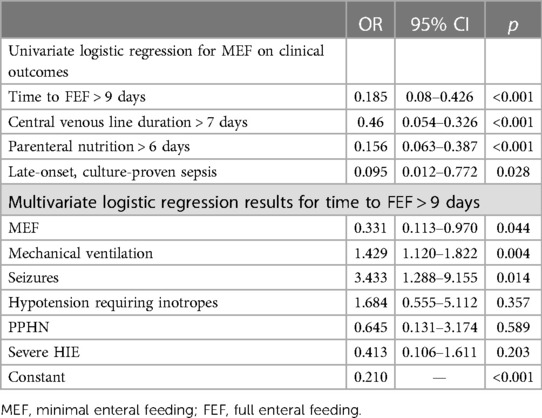
We performed a univariate logistic regression analysis with minimal EF (stated as ml/kg/day on the third day of therapeutic hypothermia) as the independent variable and with time to full EF (stated as time to full EF of >9 days), CV line duration of >7 days, PN duration of >6 days, and sepsis as the dependent variables. We then performed a multivariate logistic regression analysis with minimal EF, MV, hypotension requiring inotropes, PPHN, severe HIE, and seizures as the independent variables and with time to full EF (stated as time to full EF of >9 days), CV line duration of >7 days, PN duration of >6 days, and sepsis as the dependent variables. A p-value <0.05 was considered statistically significant. We used software STATA 16 (StataCorp 2017).
3 Results
In total, 153 newborns met the inclusion criteria during the study period; among them, 78 infants received minimal EF during therapeutic hypothermia (fed infants), and 75 were not fed during therapeutic hypothermia (unfed infants).
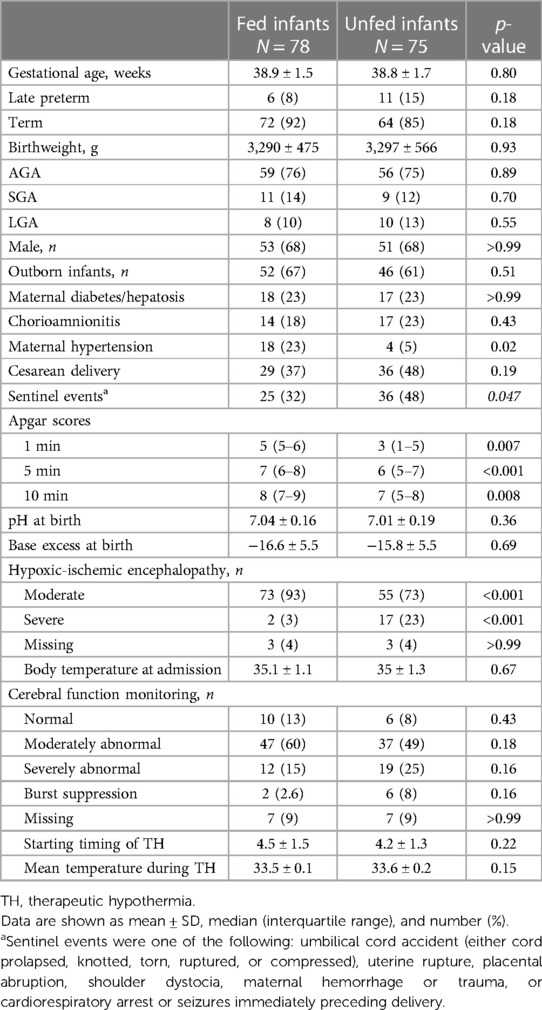
Infants’ characteristics at baseline and obstetrical conditions are reported in Table 1. There were no significant differences in baseline characteristics and obstetrical conditions, except for a higher occurrence of sentinel events in the unfed infants and a higher occurrence of maternal hypertension among the fed infants. Unfed infants exhibited more severe initial clinical conditions, as evidenced by the lower Apgar scores at 1, 5, and 10 min and by the higher frequency of severe HIE according to the modified Sarnat staging system compared to the fed infants.
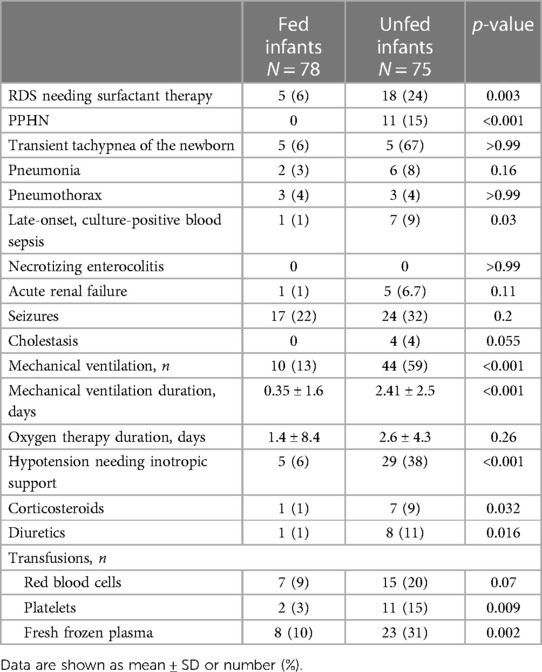
Unfed infants were also sicker than fed infants, as evidenced by the higher rates of RDS requiring surfactant administration, PPHN, and hypotension requiring inotropic support (Table 2). The unfed cohort also showed significantly higher rates of MV during therapeutic hypothermia, late-onset culture-proven sepsis, and a longer duration of MV; they required corticosteroid and diuretic treatment more frequently, as well as platelet and fresh frozen plasma transfusions (Table 2).
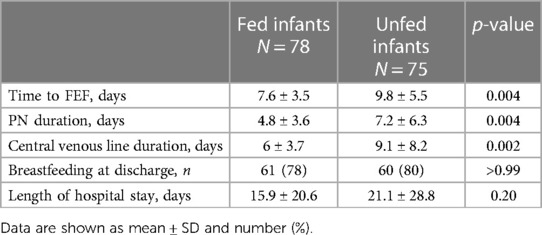
Fed infants achieved full EF sooner, received fewer days of PN, and spent fewer days with a CV line compared to unfed infants. No statistically significant differences were found in the breastfeeding rates at the time of discharge or in the mean length of hospital stay between the groups (Table 3).
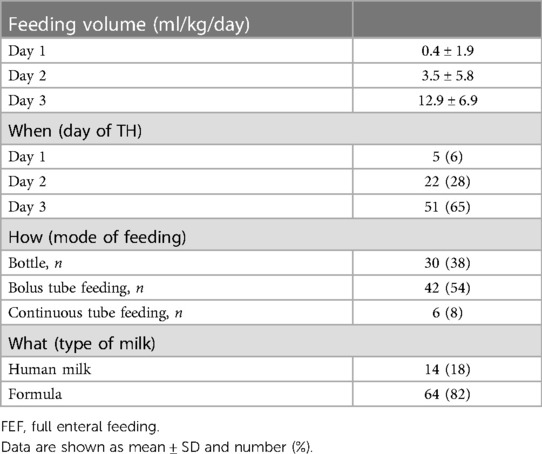
Regarding the fed infants, Table 4 presents the day of therapeutic hypothermia at which minimal EF started, the volume of feedings, the type of milk, and the feeding technique. Most patients started minimal EF on the third day of therapeutic hypothermia. Only a minority of patients received minimal EF throughout the entire therapeutic hypothermia course. All patients received a true minimal EF on day 3 of therapeutic hypothermia. The most common feeding technique was bolus tube feeding, but a large number of infants were also bottle-fed. Only a minority of infants received their mother's milk.
We then performed a logistic regression analysis to examine whether minimal EF was associated with a shorter time to full EF and with other clinical outcomes. In the univariate regression analysis, minimal EF emerged as a beneficial factor for achieving time to full EF of less than 9 days, as well as for CV line duration of less than 7 days, PN duration of less than 6 days, and for preventing late-onset sepsis (Table 5). In the multivariate regression analysis, we included the variable of clinical severity (the need for mechanical ventilation seizures, hypotension requiring inotropes, PPHN, and severe HIE). Minimal EF was confirmed as an independent beneficial factor for a time to full EF of less than 9 days, whereas MV and the presence of clinical seizures were independent variables associated with a longer time to full EF (Table 5).
4 Discussion
In this monocentric retrospective study, we found that feeding infants with minimal EF during therapeutic hypothermia was associated with a significant reduction of the time to full EF and a reduction of the PN and CV line duration.
Moreover, we identified minimal EF as a beneficial independent factor for a time to full EF of less than 9 days, whereas MV and the presence of clinical seizures during therapeutic hypothermia were identified as independent variables associated with a longer time to full EF.
The available reports on the effects of minimal EF on the time to full EF are conflicting. A retrospective study comparing 34 cooled infants cared for in the United Kingdom, where feeds are commonly withheld, with 51 infants cared for in Sweden, where feeding was commonly administered during therapeutic hypothermia, failed to find a difference in the time to full EF between the two groups (4).
Our findings are instead in agreement with those of another small, retrospective, matched case–control study, which compared 17 cooled infants who received minimal EF with 17 infants who did not; in that study, minimal EF during hypothermia was associated with a reduced length of stay and a shorter time to full EF (5).
Our results are also in agreement with a recent randomized controlled trial aiming to explore the effect of early (during therapeutic hypothermia) vs. delayed (after therapeutic hypothermia) enteral nutrition on the incidence of feeding intolerance among cooled infants (8). Although the study found a similar incidence of feeding intolerance between the two study groups, the time to full EF was shorter in cooled infants who received EF during therapeutic hypothermia compared with those who received enteral nutrition after rewarming. In that study, clinical variables that could have influenced the time to full EF, such as the percentage of infants on MV during therapeutic hypothermia, the duration of MV, or the presence of seizures, were not declared.
At last, a large retrospective cohort study involving 6,030 newborns with HIE assessed the effect of EF during therapeutic hypothermia on the rates of NEC, finding that the incidence of NEC was lower in cooled infants who were fed (6). There was no mention of the time to full EF, although the duration of PN, which can be considered a surrogate for the earlier achievement of full EF, was shorter in the fed infants (6). In the same study, some clinical variables suggestive of sickness that are discernible to clinicians and could influence decision making regarding enteral nutrition, such as MV during therapeutic hypothermia or the presence of seizures, were not reported; however, above all, despite the great methodological soundness of the study, the criteria for identifying the most appropriate infants with HIE to start feeding early were not established.
In our cohort of cooled infants, 65% of infants started minimal EF on the third day of therapeutic hypothermia and only 6% of infants received minimal EF throughout the entire therapeutic hypothermia course. The starting time of enteral nutrition was not reported in all of the previous papers, but it appears that enteral nutrition was started on average on the second day of therapeutic hypothermia (5, 8). In our study, all infants received a true minimal EF (an amount of milk ranging from 12 to 24 ml/kg/day) on the third day of therapeutic hypothermia. These data appear to be comparable to that reported by other studies (4, 5, 8).
Therefore, the debate about enteral nutrition strategy during therapeutic hypothermia is still ongoing, and several questions still need to be addressed: Should all asphyxiated infants receive EF during the course of therapeutic hypothermia, regardless of the severity of the illness? What is the optimal day for initiating EF? What should be the optimal feeding volume?
Randomized controlled trials are warranted to develop an efficient feeding protocol for asphyxiated infants undergoing therapeutic hypothermia.
The major limitation of our study is its retrospective nature, which did not allow us to have two cohorts of infants with comparable clinical features of severity and to exclude other variables, in addition to those considered, that could have had an effect on the time to full EF. Another limitation of the study is that only 6% of the fed infants received minimal EF throughout the entire therapeutic hypothermia course, while 65% began minimal EF on the third day of therapeutic hypothermia, leading to enteral nutritional disparity within the group. This may reflect clinicians’ fear of feeding cooled infants even when clinical conditions do not contraindicate starting minimal EF.
Our study also has many strengths: the study was well equipped to detect differences in the primary outcome; furthermore, many variables indicative of illness severity were measured, and, most importantly, a statistical analysis was conducted to identify the clinical variables potentially associated with time to full EF.
5 Conclusions
The introduction of minimal EF during therapeutic hypothermia is associated with a shorter time to full EF, a shorter duration of PN and CV lines, and a lower incidence of late-onset sepsis. Randomized controlled trials are still necessary to identify the optimal enteral nutrition strategy for cooled infants to understand whether they could be fed regardless of illness severity, to determine when enteral nutrition should be started during therapeutic hypothermia, and to know how cooled infants should be fed, including considerations such as the quantity and quality of milk.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy (approval number 12692/22 ID 4867). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SC: Conceptualization, Writing – original draft, Validation. IR: Writing – original draft, Data curation, Validation. SF: Formal analysis, Methodology, Validation, Writing – review & editing. FS: Data curation, Validation, Writing – review & editing. FP: Data curation, Validation, Writing – review & editing. MC: Data curation, Validation, Writing – review & editing. ET: Investigation, Validation, Writing – review & editing. MT: Investigation, Validation, Writing – review & editing. PC: Data curation, Writing – review & editing. GV: Conceptualization, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Ministero della Salute – Ricerca Corrente 2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin Perinatol. (2016) 40(8):549–55. doi: 10.1053/j.semperi.2016.09.007
2. Overview. Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury | Guidance | NICE. Available online at: https://www.nice.org.uk/guidance/ipg347 (accessed August 7, 2023).
3. Ojha S, Dorling J, Battersby C, Longford N, Gale C. Optimising nutrition during therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. (2019) 104(3):F230–1. doi: 10.1136/archdischild-2018-315393
4. Thyagarajan B, Tillqvist E, Baral V, Hallberg B, Vollmer B, Blennow M. Minimal enteral nutrition during neonatal hypothermia treatment for perinatal hypoxic-ischaemic encephalopathy is safe and feasible. Acta Paediatr. (2015) 104(2):146–51. doi: 10.1111/apa.12838
5. Chang LL, Wynn JL, Pacella MJ, Rossignol CC, Banadera F, Alviedo N, et al. Enteral feeding as an adjunct to hypothermia in neonates with hypoxic-ischemic encephalopathy. Neonatology. (2018) 113(4):347–52. doi: 10.1159/000487848
6. Gale C, Longford NT, Jeyakumaran D, Ougham K, Battersby C, Ojha S, et al. Feeding during neonatal therapeutic hypothermia, assessed using routinely collected National Neonatal Research Database data: a retrospective, UK population-based cohort study. Lancet Child Adolesc Health. (2021) 5(6):408–16. doi: 10.1016/s2352-4642(21)00026-2
7. Alburaki W, Scringer-Wilkes M, Dawoud F, Oliver N, Lind J, Zein H, et al. Feeding during therapeutic hypothermia is safe and may improve outcomes in newborns with perinatal asphyxia. J Matern Fetal Neonatal Med. (2022) 35(25):9440–4. doi: 10.1080/14767058.2022.2041594
8. Hu Y, Chen F, Xiang X, Wang F, Hua Z, Wei H. Early versus delayed enteral nutrition for neonatal hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia: a randomized controlled trial. Ital J Pediatr. (2022) 48(1):146. doi: 10.1186/s13052-022-01342-2
9. Hazeldine B, Thyagarajan B, Grant M, Chakkarapani E. Survey of nutritional practices during therapeutic hypothermia for hypoxic-ischaemic encephalopathy. BMJ Paediatr Open. (2017) 1(1):e000022. doi: 10.1136/bmjpo-2017-000022
10. Goldberg R, Thomas D, Sinatra F. Necrotizing enterocolitis in the asphyxiated full-term infant. Am J Perinatol. (2008) 1(01):40–2. doi: 10.1055/s-2007-1000050
11. Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. (2007) 27(7):437–43. doi: 10.1038/sj.jp.7211738
12. Lu Q, Cheng S, Zhou M, Yu J. Risk factors for necrotizing enterocolitis in neonates: a retrospective case-control study. Pediatr Neonatol. (2017) 58(2):165–70. doi: 10.1016/j.pedneo.2016.04.002
13. Kwok TC, Dorling J, Gale C. Early enteral feeding in preterm infants. Semin Perinatol. (2019) 43(7):151159. doi: 10.1053/j.semperi.2019.06.007
14. Azzopardi DV, Strohm B, Edwards AD, Med F, Leigh Dyet MB, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. (2009) 361(14):1349–58. doi: 10.1056/NEJMoa0900854
15. Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial: a randomized controlled trial. Arch Pediatr Adolesc Med. (2011) 165(8):692–700. doi: 10.1001/archpediatrics.2011.43
16. Thornton KM, Dai H, Septer S, Petrikin JE. Effects of whole body therapeutic hypothermia on gastrointestinal morbidity and feeding tolerance in infants with hypoxic ischemic encephalopathy. Int J Pediatr. (2014) 2014:643689. doi: 10.1155/2014/643689
17. Sakhuja P, More K, Ting JY, Sheth J, Lapointe A, Jain A, et al. Gastrointestinal hemodynamic changes during therapeutic hypothermia and after rewarming in neonatal hypoxic-Ischemic encephalopathy. Pediatr Neonatol. (2019) 60(6):669–75. doi: 10.1016/j.pedneo.2019.04.003
18. Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, Mcdonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. (2005) 353(15):1574–84. doi: 10.1056/NEJMcps050929
19. Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. (2010) 51(3):353–61. doi: 10.1097/MPG.0b013e3181da21
20. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. (1987) 17(4):213–88. doi: 10.1016/0045-9380(87)90031-4
21. Adamkin DH. Neonatal hypoglycemia. Semin Fetal Neonatal Med. (2017) 22(1):36–41. doi: 10.1016/j.siny.2016.08.007
22. Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. (2017) 64(1):154–68. doi: 10.1097/mpg.0000000000001334
23. Brooks S. Comparing Two Means—Sample Size. Exeter, UK: Select Statistical Consultants (2016). Available online at: https://select-statistics.co.uk/calculators/sample-size-calculator-two-means/ (accessed August 7, 2023).
Keywords: birth asphyxia, enteral feeding, minimal enteral feeding, nutritional strategy, therapeutic hypothermia
Citation: Costa S, Rizzo ID, Fattore S, Serrao F, Priolo F, Corsello M, Tiberi E, Tana M, Catalano P and Vento G (2024) Enteral nutritional strategy during therapeutic hypothermia: who? when? what?. Front. Pediatr. 12:1357831. doi: 10.3389/fped.2024.1357831
Received: 18 December 2023; Accepted: 22 May 2024;
Published: 25 June 2024.
Edited by:
Simone Pratesi, Careggi University Hospital, Italy© 2024 Costa, Rizzo, Fattore, Serrao, Priolo, Corsello, Tiberi, Tana, Catalano and Vento. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simona Fattore, c2ltb25hLmZhdHRvcmVAZ3Vlc3QucG9saWNsaW5pY29nZW1lbGxpLml0
 Simonetta Costa
Simonetta Costa Irene Del Rizzo2
Irene Del Rizzo2 Simona Fattore
Simona Fattore Francesca Serrao
Francesca Serrao Eloisa Tiberi
Eloisa Tiberi Milena Tana
Milena Tana Giovanni Vento
Giovanni Vento