- 1Department of Neonatology, The Canberra Hospital, Canberra, ACT, Australia
- 2Australian National University Medical School, Australian National University, Canberra, ACT, Australia
- 3Department of Neonatology, Westmead Hospital, Sydney, NSW, Australia
- 4The Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
Congenital diaphragmatic hernia (CDH) is a developmental defect of the diaphragm resulting in herniation of viscera into the chest. This condition is characterized by pulmonary hypoplasia, pulmonary hypertension (PH) and cardiac ventricular dysfunction. PH is a key component of the pathophysiology of CDH in neonates and contributes to morbidity and mortality. Traditionally, PH associated with CDH (CDH-PH) is thought to be secondary to increased pulmonary arterial resistance and vasoreactivity resulting from pulmonary hypoplasia. Additionally, there is increasing recognition of associated left ventricular hypoplasia, dysfunction and elevated end diastolic pressure resulting in pulmonary venous hypertension in infants with CDH. Thus, hemodynamic management of these infants is complex and cautious use of pulmonary vasodilators such as inhaled nitric oxide (iNO) is warranted. We aim to provide an overview of different phenotypic profiles of CDH associated PH and potential management options based on current evidence and pathophysiology.
1 Introduction
Congenital diaphragmatic hernia (CDH) is a rare congenital anomaly secondary to a developmental defect of the diaphragm that produces the herniation of abdominal organs into the thorax of the fetus. This pathology affects approximately 1 in 3,500 liveborn infants (1). The anatomical defect of the diaphragm is more frequent on the left side (75%–90% of cases) (2). The herniation of abdominal contents into the chest during important stages of the embryonic and fetal development produces a complex condition characterized classically by pulmonary hypoplasia, pulmonary hypertension (PH), and more recently identified left ventricular dysfunction (3, 4). Despite considerable advances in antenatal and perinatal diagnosis and management, CDH-PH continues to be an important cause of neonatal morbidity and mortality and most challenging aspect of CDH care (3, 5). This article aims to describe different phenotypic profiles of CDH-PH and provide recommendations on diagnosis and management of these infants.
2 Definition of PH in CDH
Traditionally in children, PH is defined as a mean pulmonary arterial pressure (PAP) of ≥25 mmHg on cardiac catheterization (6, 7). Neonates have physiological PH during normal postnatal transition. Hence a simple pressure-based definition is not possible. Echocardiography or point of care ultrasound (POCUS) is the mainstay for diagnosis of CDH-PH as invasive testing is not practical or feasible. CDH-PH can be defined as raised PAP relative to systemic blood pressure resulting in dysfunctional pulmonary blood flow, poor oxygenation, cardiac dysfunction and/or systemic hypotension (3, 8).
3 Pathophysiology of PH and circulatory dysfunction in CDH
The pathophysiology of CDH-PH is multifactorial in nature contributed by abnormal pulmonary parenchymal and vascular development, pulmonary vasoreactivity and left ventricular hypoplasia and/or dysfunction as described below.
3.1 Pulmonary vascular remodeling
The anatomical abnormality of the diaphragm and subsequent herniation of abdominal contents during critical stages of lung development produces a mass effect that affects the growth of lung parenchyma, bronchial/bronchiolar branching, and the development of the pulmonary vasculature (3). On postmortem examination, ipsilateral lung has been found to be grossly hypoplastic (9). There is reduced bronchial and bronchiolar arborization, fewer alveoli, acinar hypoplasia, thick alveolar walls and increased interstitial tissue resulting in a reduced gas-exchange area (10). The abnormalities of pulmonary vasculature in CDH have been well described and include both structural and functional changes (3, 9, 10). Pulmonary arteries are decreased per unit lung volume. There is reduced pulmonary vascular branching accompanied by thickening of muscular media and adventitia (9–11). All these changes lead to a decreased anatomical vascular area for gas exchange. Multiple molecular pathways such as retinoic acid, nitric oxide, endothelin, vascular growth factors, micro RNAs, and impaired endothelial cell function have been implicated in the pulmonary vascular remodeling (3, 11). Functionally, the pulmonary vessels demonstrate abnormal vasoreactivity from an impaired endothelial response to vasoconstrictor and vasodilator signals and an imbalanced autonomic innervation (11, 12). Doppler studies of the fetal pulmonary flow across the pulmonary artery (PA) have shown increased pulsatility index consistent with increased pulmonary vascular resistance (PVR) (13). The severity of postnatal pulmonary hypertension is difficult to predict antenatally despite many efforts to review prenatal anatomy and relationship between the size of the defect and the size of the fetal lungs and heart (14). These structural and functional changes in pulmonary vessels prevent vasodilation and normal transition to postnatal circulation after birth resulting in increased pulmonary arterial pressure or pre-capillary pulmonary hypertension (15).
3.2 Right ventricular (RV) dysfunction
Increased afterload from persistent or severe PH in infants with CDH results in RV hypertrophy and dilatation. RV hypertrophy reduces compliance and diastolic filling whilst increasing oxygen demand. Increased RV pressure can also reduce coronary perfusion and lead to myocardial ischemia. Furthermore, a decreased preload of the right ventricle is frequent in ventilated infants after birth due to elevated mean airway pressure and poor venous return. RV dilatation can lead to shifting of interventricular septum towards the left ventricle impacting left ventricular (LV) performance. The net result is RV or biventricular dysfunction and further reduction in pulmonary and systemic blood flow (8, 16). Both systolic and diastolic dysfunction have been observed in studies on echocardiography early after birth and after surgery and have been correlated with need for extracorporeal membrane oxygenation (ECMO) and mortality (16–18).
3.3 Left ventricular (LV) hypoplasia and dysfunction
There is increasing recognition of LV dysfunction in infants with CDH due to severe pulmonary arterial hypertension and/or fetal LV hypoplasia (8).
Right heart dysfunction directly affects the left ventricle due to the interdependence of both structures that share anatomical elements such as muscular fibers, interventricular septum (IVS), and pericardium. Shifting of the IVS to the left directly impacts the filling of the LV affecting the cardiac output of the left heart (19–21).
Not all LV dysfunction in CDH is due to RV dysfunction. Recent studies have shown isolated or primary LV dysfunction without RV dysfunction (22, 23). Several factors may contribute to primary LV dysfunction as given below:
• Fetal LV hypoplasia. The congenital defect of the diaphragm with resulting visceral herniation during essential stages of development produces a mass effect in the chest that also causes variable degrees of hypoplasia of the heart, especially of the left ventricle. This effect seems to be independent of the size of the diaphragmatic defect (3, 4). Moreover, the decreased flow of blood through the PA during fetal life with subsequent reduced blood flow to the left heart affects the normal development of the left heart considerably (24). Another described factor is the redirection of blood flow from the ductus venosus and inferior vena cava due to the mediastinal shift. Normally, this flow is preferentially directed to the patent foramen ovale (PFO) and therefore acts as significant preload for the left ventricle. In infants with CDH, the redirection results in this blood streaming away from the PFO and going towards the right ventricle. A large portion of this blood subsequently goes through the patent ductus arteriosus (PDA) into the descending aorta. Both these factors (reduced pulmonary blood flow and streaming of blood away from PFO) are hypothesized to cause less growth of the left ventricle (25). Furthermore, it seems that infants with CDH have an abnormal embryonic and fetal development of the heart. The structural anatomy of the left heart of an infant with CDH includes a narrowing and elongated left ventricle (4).
• Acute changes in LV loading. Primary LV dysfunction is also related to acute changes in loading conditions after birth. The left ventricle is suddenly faced with a higher afterload with the removal of the low-resistance placenta. If the LV preload remains low due to reduced pulmonary blood flow, this combination of reduced preload and increased afterload, exacerbates LV dysfunction. The LV dysfunction is also self-perpetuating as the resultant hypotension leads to potential reduction in coronary blood flow (4).
• Acidosis and hypoxemia, as a result of severe PH, interatrial and ductal shunting or hypotension can cause LV dysfunction.
LV dysfunction usually presents immediately after birth and can cause decreased systemic blood flow and hypotension. In addition, LV dysfunction can increase left atrial and pulmonary venous pressure due to reduced LV end diastolic volume. This in turn will lead to pulmonary venous hypertension or post-capillary pulmonary hypertension.
4 Phenotypic profiles of PH in CDH
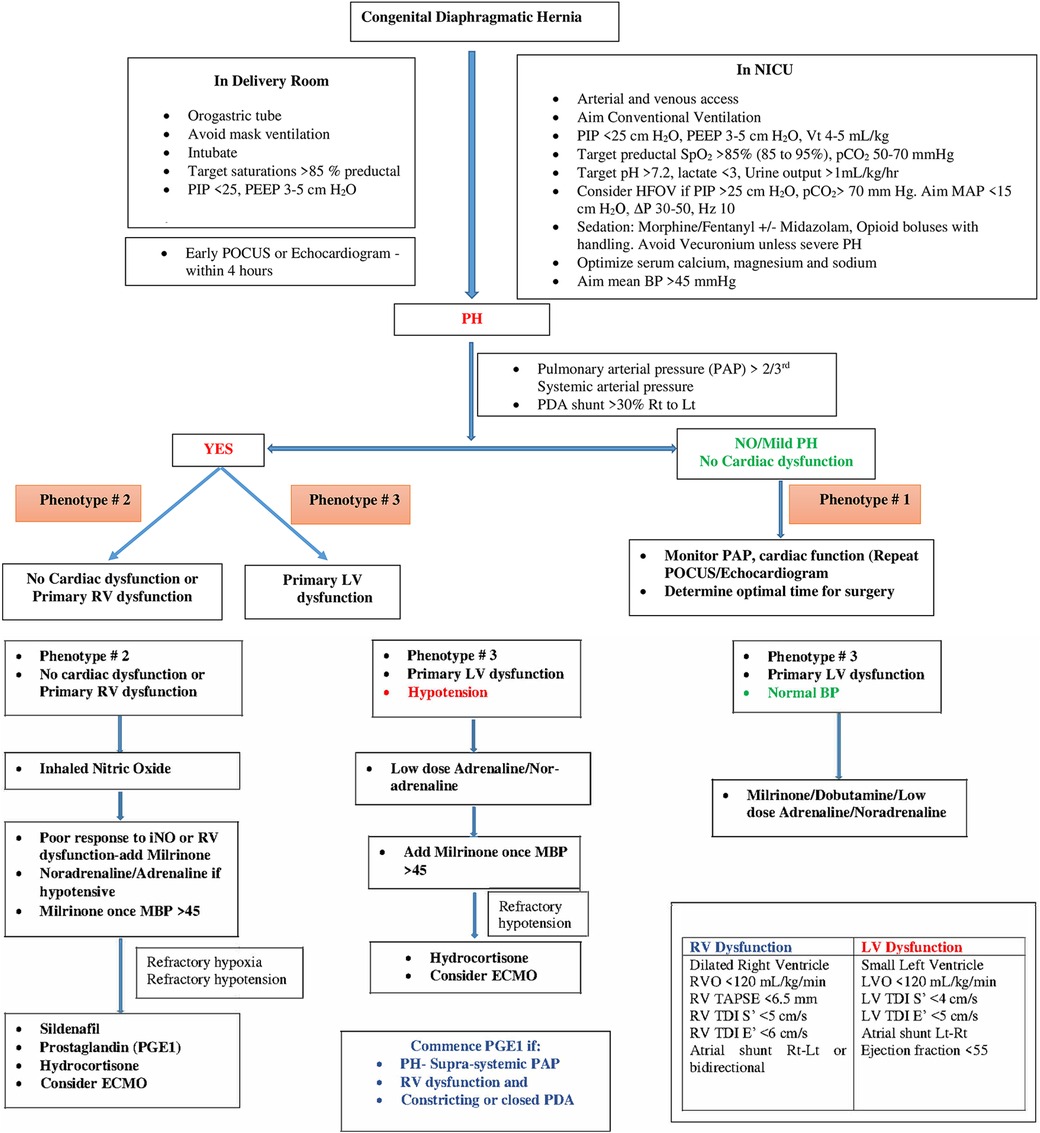
Phenotypic profiling of CDH-PH is based on contributing pathophysiological factors that are important to consider to guide physiology-based management and prognostication. In addition to clinical assessment, an echocardiographic evaluation is critical for phenotypic profiling of CDH-PH (1). A POCUS or echocardiography should be performed in all infants with CDH after initial stabilization. The scan should include structural assessment for congenital heart disease, assessment of PH, PAP measurements, ventricular size and function and shunts across the PDA and PFO.
Table 1 summarizes various echocardiographic measurements described in literature in infants with CDH (26–28). Each measurement has some limitations and there is no single gold standard measure (4). In our practice we use a combination of these measures to determine predominant phenotype and guide a physiology-based management (Figure 1).
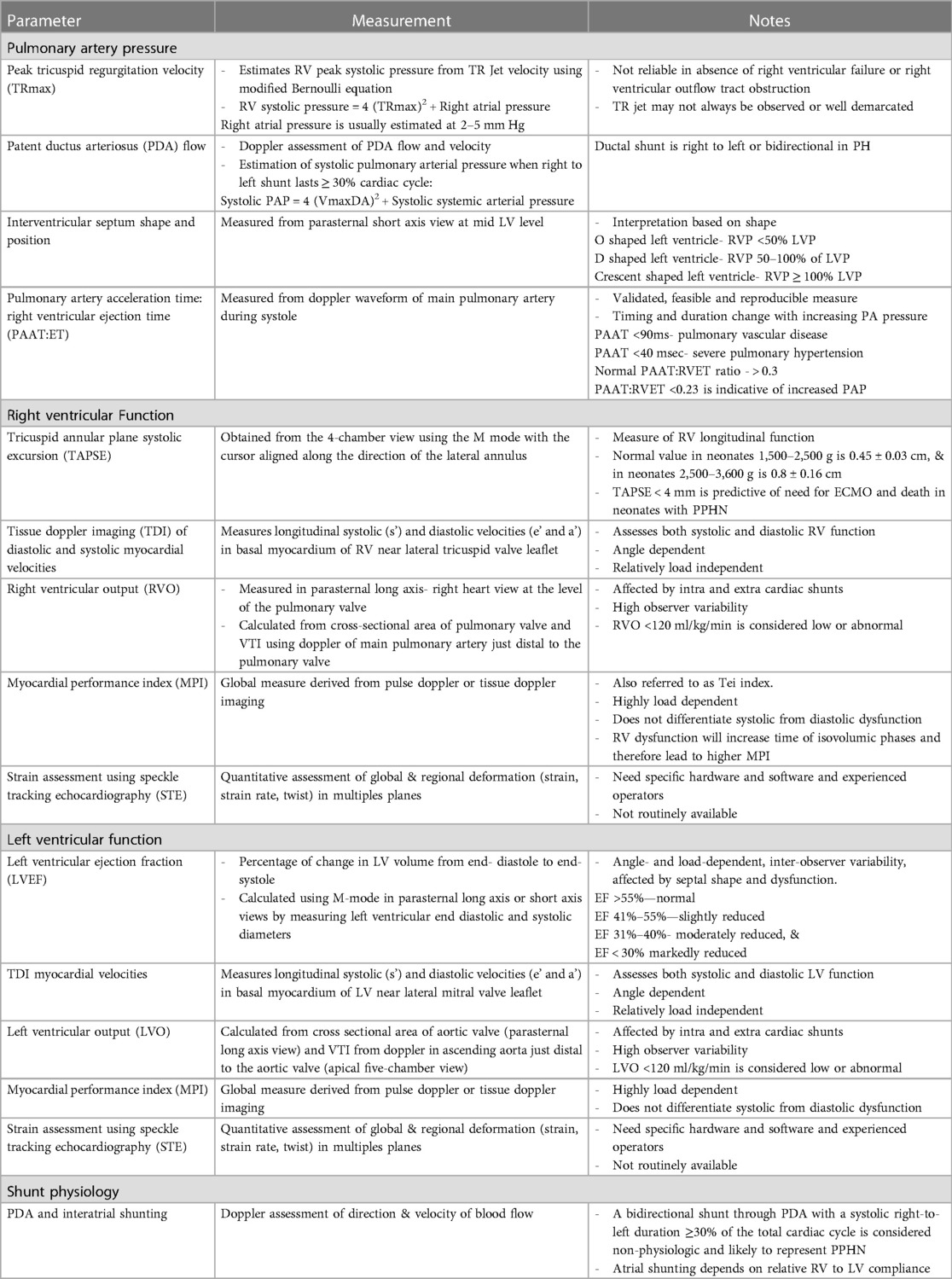
Table 1. Echocardiographic measurements to assess pulmonary hypertension and cardiac function in CDH.
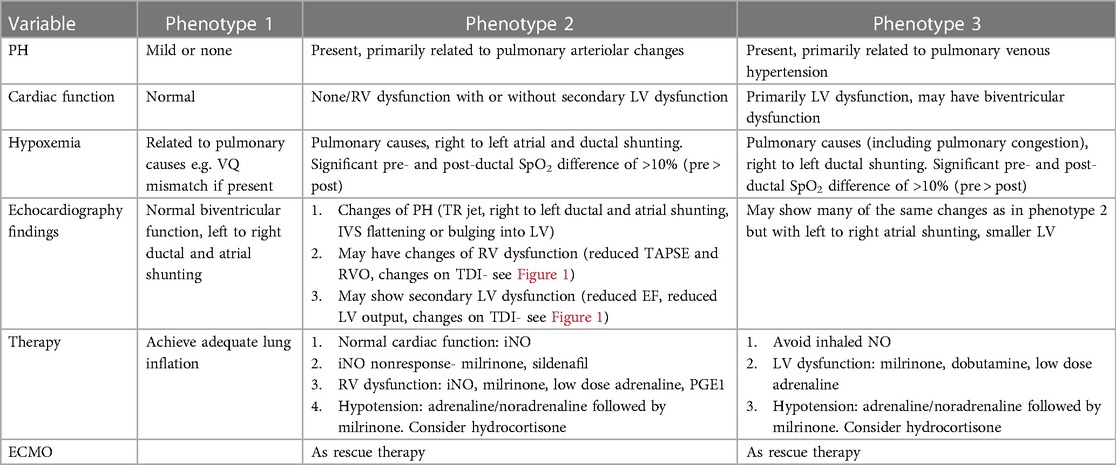
Based on the assessment of PH including atrial and ductal shunting, and cardiac function, three CDH-PH phenotypes have been described (Table 2).
4.1 Phenotype 1
4.1.1 Mild or no PH with normal cardiac function
In this phenotype, there is no clinically significant difference between pre-ductal and post-ductal oxygen saturation. If there is hypoxia, this is related to pulmonary causes such as atelectasis causing ventilation-perfusion (VQ) mismatch, and usually there is clinical response to appropriate changes in ventilatory settings.
On echocardiography, the shunting across the PDA and atria is predominantly left to right. There is either no evidence of PH, or features of only mild PH, and biventricular function is normal.
4.2 Phenotype 2
4.2.1 Pulmonary arterial hypertension (pre-capillary PH) with either no cardiac dysfunction, or primary RV dysfunction with or without secondary LV dysfunction
The PH in this phenotype is primarily related to the changes in the pulmonary arterioles. In this situation, there is likely to be significant difference (>10%) between pre- and post-ductal oxygen saturation (pre > post).
Echocardiography shows features of elevated pressure in the PA, such as tricuspid regurgitation (TR) jet with increased velocity in Doppler, dilated right ventricle, and septal flattening or bulging into the left ventricle. RV function may be preserved or there may be systolic and/or diastolic RV dysfunction. Secondary LV dysfunction may be seen as well as there is ventricular interdependence between the right and left ventricle. In addition, if the IVS bulges into the left ventricle due to elevated RV pressures, this may lead to LV diastolic dysfunction and impaired filling.
Depending on the level of pulmonary arterial pressure compared to the systemic pressure, the shunting through the PDA may be low velocity left to right, bidirectional, or pure right to left. In the setting of RV dysfunction and preserved LV function, shunting at atrial level is right to left and this shunting contributes to pre-ductal hypoxemia.
4.3 Phenotype 3
4.3.1 Pulmonary venous hypertension (post-capillary PH) with primary LV dysfunction
The post-capillary PH is primarily related to the underdevelopment of the left ventricle which affects left CDH more commonly than right CDH. The net effect of the LV dysfunction is an elevation in the left atrial pressures which leads to left to right flow at the PFO level. The ductal shunting could be bidirectional, or right to left due to increased pulmonary arterial pressure in relation to the systemic arterial pressure. This combination of bidirectional or right to left ductal shunting, and left to right PFO shunting, is important to recognize as pulmonary vasodilators such as inhaled nitric oxide (iNO) are unlikely to be helpful and may even cause harm by increasing the risk of pulmonary edema and hemorrhage. This is the most likely explanation for the lack of benefit shown for iNO in the treatment of CDH in a randomized controlled trial (RCT) published in 1997 (29). This is further supported by a more recent retrospective study (30) which concluded that treatment with iNO was associated with improved oxygenation and reduced need for extracorporeal membrane oxygenation (ECMO) only in a subpopulation of infants with CDH who have normal LV systolic function.
A publication from the CDH Study Group (23) that collected cardiac function prospectively (within 24–48 h of age) in 1,173 infants with CDH showed normal cardiac function in 61% of the cases, RV dysfunction in 15%, LV dysfunction in 5% and biventricular dysfunction in 19% of the cases. Supra-systemic PH was documented in 32% of the cases, but its frequency was more common (57%) when there was biventricular dysfunction. Survival varied according to the cardiac dysfunction with survival figures of 80%, 74%, 57% and 51% in the categories of normal cardiac function, RV dysfunction, LV dysfunction and biventricular dysfunction respectively.
Putnam et al. (31), investigated spectrum of iNO use in infants with CDH utilizing CDH Study Group data collected between January 2007 and December 2014. Their study showed that iNO use was very common (60.8%) and 36% of infants without PH received iNO. Nitric oxide use was significantly associated with increased mortality.
The above evidence suggests that early echocardiogram is essential to identify PH and its appropriate phenotype and that a targeted pathophysiology-based management may be beneficial and improve outcomes. We recommend performing an echocardiogram within the first 4 h after birth- ideally immediately after stabilization. Subsequent echocardiograms should be done every 6–12 h during the transitional period (24–48 h) or following clinical changes or treatment to determine changing physiology and assess response to treatment.
5 Therapeutic strategies to manage phenotypic profiles of PH in CDH
Given its multifactorial nature, management of CDH and CDH-PH is complex and depends on predominant pathophysiologic or phenotypic profile. Key principles of management applicable to all 3 phenotypes include maintaining oxygenation, blood pressure and cardiac output; optimizing lung recruitment (avoiding overdistension); and early diagnosis and management of PH and/or associated cardiac dysfunction.
5.1 General measures
Current observational evidence suggests better outcomes for infants with CDH managed medically and deferring surgical repair until their respiratory status and PH have improved (32, 33). For prenatally diagnosed CDH, a nasogastric tube should be placed immediately after delivery to decompress the stomach and intestines, and the infant should be intubated in the delivery room without mask ventilation. Avoidance of ventilator-induced lung injury with gentle ventilation strategies is the cornerstone of respiratory management in infants with CDH (Figure 1). A multicenter randomized trial (VICI trial) compared conventional mechanical ventilation to high-frequency oscillatory ventilation (HFOV) as the initial mode in infants with CDH. The study showed shorter ventilation time and lesser need for ECMO in the conventional ventilation group compared to HFOV group (34).
The approach to sedation is generally similar to the management of infants with non-CDH related PH. Sedation should be titrated to maintain spontaneous breathing if possible, and muscle relaxation should only be used when there is infant-ventilator dyssynchrony despite optimal sedation and adequate ventilatory settings (35).
5.2 Phenotype 1
5.2.1 Mild or no PH with normal cardiac function
As there is no or mild pulmonary hypertension, hypoxia is generally due to parenchymal lung disease or atelectasis and is managed with adequate lung recruitment in addition to general measures described above.
5.3 Phenotype 2
5.3.1 Pulmonary arterial hypertension (pre-capillary PH) with either no cardiac dysfunction, or primary RV dysfunction with or without secondary LV dysfunction
Management in this situation targets reduction in PVR with adequate sedation and lung recruitment (avoiding overdistension and derecruitment), and the use of pulmonary vasodilator therapies such as iNO, sildenafil and/or milrinone (4, 8). Use of prostaglandin E1 (PGE1) may also be considered if there is evidence of RV dysfunction as this reduces RV afterload, though this can result in post-ductal hypoxemia.
Inhaled nitric oxide is the most common pulmonary vasodilator used for CDH-PH (6). However, evidence from both RCT and CDH databases has failed to show an improvement in survival or decrease need for ECMO (29, 36). One possible explanation is the associated LV dysfunction observed in these infants. Pulmonary vasodilation from iNO could lead to LV failure and pulmonary edema by increasing the venous return (30). Other possible reasons for lack of iNO benefit could be the abnormal development of the pulmonary vasculature, blunted nitric oxide signaling pathway, or overexpression of vasoconstrictors such as endothelin 1 (37) in infants with CDH. Sildenafil, a phospho-diesterase 5 inhibitor, acts on the same pathway to prevent breakdown of cyclic GMP and augment action of nitric oxide. A recent international multicenter RCT (CoDiNOS trial) is currently comparing intravenous sildenafil and iNO for treatment of PH in infants with CDH (38).
Milrinone, a phosphodiesterase (PDE3) inhibitor, is another vasodilator that acts by targeting the cyclic AMP pathway. In addition, it has direct positive inotropic and lusitropic actions on the heart, which may benefit infants with coexisting RV dysfunction (39). Studies on use of milrinone in infants with CDH have shown improvement in oxygenation and cardiac function especially in combination with iNO (40, 41). A multicentre pilot RCT on impact of milrinone on oxygenation index in CDH is currently underway (42). It is important to consider that milrinone can cause systemic vasodilation and hypotension.
Systemic vasoconstrictors may be needed to support systemic vascular resistance (Figure 1). Noradrenaline has been demonstrated to reduce pulmonary/systemic pressure ratio in non-CDH neonatal PH and is our preferred inotrope to improve blood pressure in this phenotype (43).
Prostaglandin E1 (PGE1) is a potent pulmonary vasodilator and is also used to maintain ductal patency. In infants with severe PH, the ductus acts as a “pop-up” valve for a strained or failing right ventricle (44). In our practice we use PGE1 in infants with supra-systemic pulmonary hypertension and RV dysfunction with a constricting or closed duct.
We recommend commencing iNO as first line treatment for this phenotype. If there is no response within 30–60 min or if there is evidence of RV dysfunction, we recommend adding milrinone. If the baby is hypotensive, we recommend commencing low dose adrenaline or noradrenaline to improve mean blood pressure (MBP) > 45 mm Hg prior to adding milrinone. Sildenafil should be considered in patients with refractory pulmonary hypertension (i.e., unresponsive to inhaled nitric oxide) or as an adjunct when weaning inhaled nitric oxide.
5.4 Phenotype 3
5.4.1 Pulmonary venous hypertension (post-capillary PH) with primary LV dysfunction
With LV dysfunction, there is increase in the left ventricular end diastolic pressure resulting in increased left atrial pressure and pulmonary venous hypertension. In this phenotype, pulmonary vasodilator therapies could lead to clinical deterioration by increasing blood flow to a failing left ventricle (30). Therefore, the treatment of PH in this phenotype is directed towards improving LV function and avoiding excessive LV afterload using inotropes such as dobutamine and low dose adrenaline (4, 8). Fluid boluses should be avoided unless there is evidence of hypovolemia.
Milrinone may also improve LV function by a combination of factors such as reducing LV afterload and improving LV contractility and diastolic relaxation (39). Infants with LV dysfunction are often hypotensive. In our practice, we commence low dose adrenaline (0.05–0.1 microg/kg/min) or noradrenaline (0.05–1 microg/kg/min)to improve blood pressure prior to commencing milrinone (Figure 1). In addition to a synergistic inotropic effect, these systemic vasoconstrictors may improve coronary blood flow (8). Use of PGE1 may also assist with improving systemic flow to the lower half of the body albeit at the cost of reduced oxygenation (8, 44). It is important to closely monitor cardiac performance through serial ultrasound scans in infants on multiple inotropes.
Hydrocortisone is often used as an adjunct treatment for hypotension in infants with CDH. Cortisol insufficiency is very common in these infants and it is seen in up to 2/3rd of cases. Cortisol supplementation can improve both cardiac output and systemic vascular resistance (45).
ECMO use in infants with CDH should be considered in cases with severe PH or respiratory failure and severe cardiac dysfunction unresponsive to maximal medical treatment. LV dysfunction typically is a transient phenomenon and resolves in the first few days of life (46). ECMO can provide time for the heart function to recover. Infants with severe pulmonary hypoplasia and irreversible pulmonary vasculature abnormalities may be refractory to weaning from ECMO.
Infants with biventricular dysfunction are the most challenging to manage and have the least survival rate (23). Our approach is to commence a trial of treatment based on the worst affected ventricle with close hemodynamic monitoring. Treatment is then guided by appropriate clinical response. These babies should be referred for ECMO earlier.
6 Long term cardiovascular outcome in CDH-PH
PH is one of the most important prognostic factors in CDH and the prognosis is worse with greater severity of PH. In a prospective multicenter CDH birth cohort (47), echocardiography was performed at 1 and 3 months of age. PH was classified into mild/none (RV systolic pressure less than half systemic), moderate (RV systolic pressure half to two thirds systemic) and severe (RV systolic pressure more than two thirds systemic). In the absence of TR jet, ventricular septal position and movement as well as RV function and size, were used to estimate PH severity. The mortality at discharge with mild, moderate, and severe PH at 1 month of age was 1.4%. 7.4% and 56.1% respectively. Whilst PH is responsible for a substantial proportion of early mortality due to CDH, it is also linked to late mortality (death after 1 year of age). In a Swedish study encompassing 25 years (48), 49 of 251 (19.5%) CDH patients died. Of these deaths, 7 (14.3%) occurred after 1 year of age. Two of these 7 deaths were attributed to PH with the oldest PH-associated death observed in a 9-year-old child.
In a follow-up study during infancy (49), PH was assessed using echocardiography and ECG at 6 and 12 months of age. In this study cohort of 52 infants, whilst 71% were diagnosed with PH at hospital admission, only 8% of CDH infants had persistence of PH during later infancy. Those with persistent PH had a higher frequency of using supplemental oxygen, tube feeding and medications (diuretics, sildenafil, bosentan) at the age of 6 months, though by the age of 12 months, these differences were in general not significant (except for the use of sildenafil which remained higher in the persistent PH group). In another echocardiography-based study (50), PH was assessed at 1 and 3 months post-repair and then during follow-up with a median age of 26 months. PH was defined as RV systolic pressure >0.5 systemic systolic pressure. The prevalence of PH at 1 month, 3 months and at longer term follow-up was 38.6%, 25.6% and 16% respectively. These differences in PH prevalence may be due to differences in CDH severity and the criteria used in diagnosing PH.
In a recent systematic review (51), authors have investigated the prevalence of adverse cardiopulmonary outcomes in CDH survivors at >2 years of age. These outcomes included pulmonary function testing, indices of PH and RV function, risk of chronic obstructive pulmonary disease, functional outcomes (exercise tolerance, health related quality of life) and radiological outcomes. They found thirteen eligible studies which investigated PH in CDH survivors. Of these, six studies reported on prevalence of PH in CDH survivors between 2 and 5 years of age. Echocardiography was used in all these studies. Rates of PH were noted to be ranging from 4.5% to 38%. Importantly, none of the studies which included participants with an average age of >5 years reported any PH. The authors discussed that this reduction in PH after the age of 5 years may be due to true reduction in frequency of PH with increasing age; however, some of the reduction could also be due to late PH-associated deaths.
Follow-up studies have also looked at RV function in CDH survivors during early childhood. In a case-control study (52), authors investigated RV function at a mean age of about 6 years with strain analysis and tissue Doppler imaging (TDI). Impairment of RV systolic and diastolic function was noted in CDH survivors with significant reduction in TDI velocities. Schwartz et al. (53) evaluated 21 CDH survivors (all received ECMO in the newborn period) at a mean age of 3.2 years. Eight of 21 (38%) patients met echocardiographic criteria for PH. Six of 21 (29%) had either right axis deviation or right ventricular hypertrophy on ECG; two of these also had PH on echocardiography. In a similar follow-up study of “CDH treated with ECMO” survivors (54), 18 children were seen at 1 and 2 years of age. ECG showed features of RV hypertrophy in a third of these children.
In a case-control study, the authors compared MRI based cardiac function and pulmonary arterial flows in 12 babies with CDH with normal age and body size matched controls at a median age of 12 years. Interestingly, while the ECG and echocardiography in CDH cases was normal, there was evidence of ventricular dysfunction and reduction in stroke volume with compensatory increase in heart rate. Flow, acceleration time and cross-sectional area of the left pulmonary artery were reduced. Long-term significance of these changes remains unclear however (55).
Some studies have reported on medication use for PH during longer-term follow-up. In a cohort of 17 surviving patients (severely affected with predicted lung volume <15%) (56), two children had PH at the age of 3 years and were receiving supplemental oxygen and sildenafil therapy. In a Canadian follow-up study (57), 2 of 44 (4.5%) surviving patients had PH and were receiving IV pulmonary vasodilator therapy by 2 years of age. Similarly, in the follow-up study by Garcia et al. (50), all the infants with PH remained on long-term therapy with either sildenafil or inhaled/IV prostanoids.
In summary, PH is linked to a substantial proportion of mortality in CDH patients. The prevalence of PH in CDH survivors decreases over time and there are no reports describing significant PH in CDH survivors at school age or beyond.
7 Summary
In conclusion, cardiac dysfunction is an important component of CDH-PH pathophysiology. Pre- and post-capillary mechanisms of increased pulmonary vascular resistance contribute to variable clinical phenotypes of right and left ventricular dysfunction. Early and regular assessment of pulmonary pressures and cardiac function is important to understand the underlying predominant phenotype and guide targeted, individualized treatment and may lead to improved outcomes in this challenging condition.
Author contributions
TC: Funding acquisition, Conceptualization, Writing – original draft, Writing – review & editing. NS: Writing – original draft, Writing – review & editing. RM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The Canberra Hospital Private Practice Fund (TCHPPAF) provided funding to cover publication fees.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Politis MD, Bermejo-Sánchez E, Canfield MA, Contiero P, Cragan JD, Dastgiri S, et al.; international clearinghouse for birth defects surveillance and research. Prevalence and mortality in children with congenital diaphragmatic hernia: a multicountry study. Ann Epidemiol. (2021) 56:61–69.e3. doi: 10.1016/j.annepidem.2020.11.007
2. Mehollin-Ray AR. Congenital diaphragmatic hernia. Pediatr Radiol. (2020) 50:1855–71. doi: 10.1007/s00247-020-04718-w
3. Gupta VS, Harting MT. Congenital diaphragmatic hernia-associated pulmonary hypertension. Semin Perinatol. (2020) 44(1):151167. doi: 10.1053/j.semperi.2019.07.006
4. Patel N, Massolo AC, Kraemer US, Kipfmueller F. The heart in congenital diaphragmatic hernia: knowns, unknowns, and future priorities. Front Pediatr. (2022) 10:890422. doi: 10.3389/fped.2022.890422
5. Burgos CM, Frenckner B. Addressing the hidden mortality in CDH: a population-based study. J Pediatr Surg. (2017) 52(4):522–5. doi: 10.1016/j.jpedsurg.2016.09.061
6. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. (2019) 53(1):1801916. doi: 10.1183/13993003.01916-2018
7. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric pulmonary hypertension: guidelines from the American heart association and American thoracic society. Circulation. (2015) 132(21):2037–99. doi: 10.1161/CIR.0000000000000329 Erratum in: Circulation. 2016 Jan 26;133(4):e368.26534956
8. Bhombal S, Patel N. Diagnosis & management of pulmonary hypertension in congenital diaphragmatic hernia. Semin Fetal Neonatal Med. (2022) 27(4):101383. doi: 10.1016/j.siny.2022.101383
9. Meng CY, Zou JZ, Wang Y, Wei YD, Li JN, Liu C, et al. Pathological findings in congenital diaphragmatic hernia on necropsy studies: a single-center case series. Pediatr Pulmonol. (2023) 58(9):2628–36. doi: 10.1002/ppul.26565
10. Ameis D, Khoshgoo N, Keijzer R. Abnormal lung development in congenital diaphragmatic hernia. Semin Pediatr Surg. (2017) 26(3):123–8. doi: 10.1053/j.sempedsurg.2017.04.011
11. Montalva L, Antounians L, Zani A. Pulmonary hypertension secondary to congenital diaphragmatic hernia: factors and pathways involved in pulmonary vascular remodeling. Pediatr Res. (2019) 85:754–68. doi: 10.1038/s41390-019-0345-4
12. Lath NR, Galambos C, Rocha AB, Malek M, Gittes GK, Potoka DA. Defective pulmonary innervation and autonomic imbalance in congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. (2012) 302(4):L390–8. doi: 10.1152/ajplung.00275.2011
13. Cruz-Martinez R, Castañon M, Moreno-Alvarez O, Acosta-Rojas R, Martinez JM, Gratacos E. Usefulness of lung-to-head ratio and intrapulmonary arterial Doppler in predicting neonatal morbidity in fetuses with congenital diaphragmatic hernia treated with fetoscopic tracheal occlusion. Ultrasound Obstet Gynecol. (2013) 41(1):59–65. doi: 10.1002/uog.11212
14. Basurto D, Russo FM, Papastefanou I, Bredaki E, Allegaert K, Pertierra A, et al. Pulmonary hypertension in congenital diaphragmatic hernia: antenatal prediction and impact on neonatal mortality. Prenat Diagn. (2022) 42(10):1303–11. doi: 10.1002/pd.6207
15. Kipfmueller F, Akkas S, Pugnaloni F, Bo B, Lemloh L, Schroeder L, et al. Echocardiographic assessment of pulmonary hypertension in neonates with congenital diaphragmatic hernia using pulmonary artery flow characteristics. J Clin Med. (2022) 11(11):338. doi: 10.3390/jcm11113038
16. Evers PD, Scottoline B, Armsby LB. Acute right ventricular failure associated with pulmonary hypertension in pediatrics: understanding the hemodynamic profiles. Springer Nature J Perinatol. (2022) 42:139–42. doi: 10.1038/s41372-021-01231-2
17. Moenkemeyer F, Patel N. Right ventricular diastolic function measured by tissue Doppler imaging predicts early outcome in congenital diaphragmatic hernia. Pediatr Crit Care Med. (2014) 15(1):49–55. doi: 10.1097/PCC.0b013e31829b1e7a
18. Aggarwal S, Stockman PT, Klein MD, Natarajan G. The right ventricular systolic to diastolic duration ratio: a simple prognostic marker in congenital diaphragmatic hernia? Acta Paediatr 2011;100(10):1315–8. doi: 10.1111/j.1651-2227.2011.02302.x
19. Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res. (2017) 113(12):1474–85. doi: 10.1093/cvr/cvx160
20. Manders E, Bogaard HJ, Handoko ML, van de Veerdonk MC, Keogh A, Westerhof N, et al. Contractile dysfunction of left ventricular cardiomyocytes in patients with pulmonary arterial hypertension. J Am Coll Cardiol. (2014) 64(1):28–37. doi: 10.1016/j.jacc.2014.04.031
21. Massolo AC, Paria A, Hunter L, Finlay E, Davis CF, Patel N. Ventricular dysfunction, interdependence, and mechanical dispersion in newborn infants with congenital diaphragmatic hernia. Neonatol. (2019) 116(1):68–75. doi: 10.1159/000499347
22. Patel N, Massolo AC, Paria A, Stenhouse EJ, Hunter L, Finlay E, et al. Early postnatal ventricular dysfunction is associated with disease severity in patients with congenital diaphragmatic hernia. J Pediatr. (2018) 203:400–7. doi: 10.1016/j.jpeds.2018.07.062
23. Patel N, Lally PA, Kipfmueller F, Massolo AC, Luco M, van Meurs KP, et al. Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. Am J Respir Crit Care Med. (2019) 200(12):1522–30. doi: 10.1164/rccm.201904-0731OC
24. Bravo-Valenzuela NJM, Peixoto AB, Araujo Júnior E. Prenatal diagnosis of total anomalous pulmonary venous connection: 2D and 3D echocardiographic findings. J Clin Ultrasound. (2021) 49:240–7. doi: 10.1002/jcu.22973
25. Stressig R, Fimmers R, Eising K, Gembruch U, Kohl T. Preferential streaming of the ductus venosus and inferior caval vein towards the right heart is associated with left heart underdevelopment in human fetuses with left-sided diaphragmatic hernia. Heart. (2010) 96:1564–8. doi: 10.1136/hrt.2010.196550
26. de Boode WP, Singh Y, Molnar Z, Schubert U, Savoia M, Sehgal A, et al. Application of neonatologist performed echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr Res. (2018) 84(Suppl 1):68–77. doi: 10.1038/s41390-018-0082-0
27. More K, Soni R, Gupta S. The role of bedside functional echocardiography in the assessment and management of pulmonary hypertension. Semin Fetal Neonatal Med. (2022) 27(4):101366. doi: 10.1016/j.siny.2022.101366
28. Singh Y. Echocardiographic evaluation of hemodynamics in neonates and children. Front Pediatr. (2017) 5:201. doi: 10.3389/fped.2017.00201
29. Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The neonatal inhaled nitric oxide study group (NINOS). Pediatrics. 1997;99(6):838–45. doi: 10.1542/peds.99.6.838
30. Lawrence KM, Monos S, Adams S, Herkert L, Peranteau WH, Munson DA, et al. Inhaled nitric oxide is associated with improved oxygenation in a subpopulation of infants with congenital diaphragmatic hernia and pulmonary hypertension. J Pediatr. (2020) 219:167–72. doi: 10.1016/j.jpeds.2019.09.052
31. Putnam LR, Tsao K, Morini F, Lally PA, Miller CC, Lally KP, et al. Evaluation of variability in inhaled nitric oxide use and pulmonary hypertension in patients with congenital diaphragmatic hernia. JAMA Pediatr. (2016) 170(12):1188–94. doi: 10.1001/jamapediatrics.2016.2023
32. Harting MT, Jancelewicz T. Surgical management of congenital diaphragmatic hernia. Clin Perinatol. (2022) 49(4):893–906. doi: 10.1016/j.clp.2022.08.004
33. Gvetadze P, Adamia N, Kharashvili P, Manjavidze N, Ghughunishvili M. PS-294 Timing of surgery in newborns with congenital diaphragmatic hernia. Arch Dis Child. (2014) 99:A217–8. doi: 10.1136/archdischild-2014-307384.592
34. Snoek KG, Capolupo I, van Rosmalen J, Hout Lde J, Vijfhuize S, Greenough A, et al. Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomized clinical trial (the VICI-trial). Ann Surg. (2016) 263(5):867–74. doi: 10.1097/SLA.0000000000001533
35. Canadian Congenital Diaphragmatic Hernia Collaborative; Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ. 2018;190(4):E103–12. doi: 10.1503/cmaj.170206
36. Campbell BT, Herbst KW, Briden KE, Neff S, Ruscher KA, Hagadorn JI. Inhaled nitric oxide use in neonates with congenital diaphragmatic hernia. Pediatrics. (2014) 134(2):e420–6. doi: 10.1542/peds.2013-2644
37. Noh CY, Chock VY, Bhombal S, Danzer E, Patel N, Dahlen A, et al. Early nitric oxide is not associated with improved outcomes in congenital diaphragmatic hernia. Pediatr. Res. (2023) 93(7):1899–906. doi: 10.1038/s41390-023-02491-8. Erratum in: Pediatr Res. (2023). PMID: 3672590836725908
38. Cochius-den Otter S, Schaible T, Greenough A, van Heijst A, Patel N, Allegaert K, et al. The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia. BMJ Open. (2019) 9(11):e032122. doi: 10.1136/bmjopen-2019-032122
39. Stocker CF, Shekerdemian LS, Nørgaard MA, Brizard CP, Mynard JP, Horton SB, et al. Mechanisms of a reduced cardiac output and the effects of milrinone and levosimendan in a model of infant cardiopulmonary bypass. Crit Care Med. (2007) 35:252–9. doi: 10.1097/01.CCM.0000251123.70632.4E
40. Kumar VHS, Dadiz R, Koumoundouros J, Guilford S, Lakshminrusimha S. Response to pulmonary vasodilators in infants with congenital diaphragmatic hernia. Pediatr Surg Int. (2018) 34:735–42. doi: 10.1007/s00383-018-4286-5
41. Patel N. Use of milrinone to treat cardiac dysfunction in infants with pulmonary hypertension secondary to congenital diaphragmatic hernia: a review of six patients. Neonatol. (2012) 102(2):130–6. doi: 10.1159/000339108
42. Lakshminrusimha S, Keszler M, Kirpalani H, Van Meurs K, Chess P, Ambalavanan N, et al. Milrinone in congenital diaphragmatic hernia—a randomized pilot trial: study protocol, review of literature and survey of current practices. Matern Health Neonatol Perinatol. (2017) 3:27. doi: 10.1186/s40748-017-0066-9
43. Tourneux P, Rakza T, Bouissou A, Krim G, Storme L. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J Pediatr. (2008) 153(3):345–9. doi: 10.1016/j.jpeds.2008.03.007
44. Lawrence K, Berger K, Herker L, Franciscovich C, O’Dea C, Waqar L, et al. Use of prostaglandin E1 to treat pulmonary hypertension in congenital diaphragmatic hernia. J Pediatr Surg. (2019) 54:55–9. doi: 10.1016/j.jpedsurg.2018.10.039
45. Kamath BD, Fashaw L, Kinsella JP. Adrenal insufficiency in newborns with congenital diaphragmatic hernia. J Pediatr. (2010) 156:495–7.e1. doi: 10.1016/j.jpeds.2009.10.044
46. Bo B, Balks J, Gries K, Holdenrieder S, Mueller A, Kipfmueller F. Increased N-terminal pro-B-type natriuretic peptide during extracorporeal life support is associated with poor outcome in neonates with congenital diaphragmatic hernia. J Pediatr. (2021) 241:83–9. doi: 10.1016/j.jpeds.2021.09.034
47. Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, Stolar CJ, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr. (2013) 163(1):114–9 e1. doi: 10.1016/j.jpeds.2012.12.036
48. Burgos CM, Modee A, Ost E, Frenckner B. Addressing the causes of late mortality in infants with congenital diaphragmatic hernia. J Pediatr Surg. (2017) 52(4):526–9. doi: 10.1016/j.jpedsurg.2016.08.028
49. Kraemer US, Leeuwen L, Krasemann TB, Wijnen RMH, Tibboel D, Hanneke IJ. Characteristics of infants with congenital diaphragmatic hernia who need follow-up of pulmonary hypertension. Pediatr Crit Care Med. 2018;19(5):e219–e26. doi: 10.1097/PCC.0000000000001464
50. Garcia AV, Fingeret AL, Thirumoorthi AS, Hahn E, Leskowitz MJ, Aspelund G, et al. Lung to head ratio in infants with congenital diaphragmatic hernia does not predict long term pulmonary hypertension. J Pediatr Surg. (2013) 48(1):154–7. doi: 10.1016/j.jpedsurg.2012.10.031
51. Lewis L, Sinha I, Kang SL, Lim J, Losty PD. Long term outcomes in CDH: cardiopulmonary outcomes and health related quality of life. J Pediatr Surg. (2022) 57(11):501–9. doi: 10.1016/j.jpedsurg.2022.03.020
52. Egan MJ, Husain N, Stines JR, Moiduddin N, Stein MA, Nelin LD, et al. Mid-term differences in right ventricular function in patients with congenital diaphragmatic hernia compared with controls. World J Pediatr. (2012) 8(4):350–4. doi: 10.1007/s12519-012-0380-2
53. Schwartz IP, Bernbaum JC, Rychik J, Grunstein M, D'Agostino J, Polin RA. Pulmonary hypertension in children following extracorporeal membrane oxygenation therapy and repair of congenital diaphragmatic hernia. J Perinatol. (1999) 19(3):220–6. doi: 10.1038/sj.jp.7200141
54. Van Meurs KP, Robbins ST, Reed VL, Karr SS, Wagner AE, Glass P, et al. Congenital diaphragmatic hernia: long-term outcome in neonates treated with extracorporeal membrane oxygenation. J Pediatr. (1993) 122(6):893–9. doi: 10.1016/S0022-3476(09)90013-0
55. Abolmaali N, Koch A, Götzelt K, Hahn G, Fitze G, Vogelberg C. Lung volumes, ventricular function and pulmonary arterial flow in children operated on for left-sided congenital diaphragmatic hernia: long-term results. Eur Radiol. (2010) 20(7):1580–9. doi: 10.1007/s00330-010-1723-9
56. Shieh HF, Wilson JM, Sheils CA, Smithers CJ, Kharasch VS, Becker RE, et al. Does the ex utero intrapartum treatment to extracorporeal membrane oxygenation procedure change morbidity outcomes for high-risk congenital diaphragmatic hernia survivors? J Pediatr Surg. (2017) 52(1):22–5. doi: 10.1016/j.jpedsurg.2016.10.010
Keywords: congenital diaphragmatic hernia, pulmonary hypertension, pulmonary hypoplasia, ventricular dysfunction, left ventricular hypoplasia, ductal shunt, atrial shunt, cardiac output
Citation: Chaudhari T, Schmidt Sotomayor N and Maheshwari R (2024) Diagnosis, management and long term cardiovascular outcomes of phenotypic profiles in pulmonary hypertension associated with congenital diaphragmatic hernia. Front. Pediatr. 12:1356157. doi: 10.3389/fped.2024.1356157
Received: 15 December 2023; Accepted: 26 February 2024;
Published: 25 March 2024.
Edited by:
Kiran More, Bai Jerbai Wadia Hospital for Children, IndiaReviewed by:
Arno van Heijst, Self-employed, Millingen aan de Rijn, NetherlandsThomas Schaible, University of Heidelberg, Germany
© 2024 Chaudhari, Schmidt Sotomayor and Maheshwari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tejasvi Chaudhari dGVqYXN2aS5jaGF1ZGhhcmlAYWN0Lmdvdi5hdQ==
Abbreviations CDH, congenital diaphragmatic hernia; Cyclic AMP, cyclic adenosine monophosphate; Cyclic GMP, cyclic guanosine monophosphate; ECMO, extracorporeal membrane oxygenation; FS, fractional shortening; iNO, inhaled nitric oxide; IVS, interventricular septum; LV, left ventricular; LVEF, left ventricular ejection fraction; LVP, left ventricular pressure; MBP, mean blood pressure; MPI, myocardial performance index; PA, pulmonary artery; PAAT, pulmonary artery acceleration time; PAP, pulmonary arterial pressure; PDA, patent ductus arteriosus; PDE 3, phosphodiesterase 3; PDE 5, phosphodiesterase 5; PFO, patent foramen ovale; PGE1, prostaglandin E1; PH, pulmonary hypertension; POCUS, point of care ultrasound; PVR, pulmonary vascular resistance; RCT, randomized controlled trial; RV, right ventricular; RVET, right ventricular ejection time; RVP, right ventricular pressure; STE, speckle tracking echocardiography; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue doppler imaging; TR, tricuspid regurgitation; VQ, ventilation-perfusion; VTI, velocity time integral.
 Tejasvi Chaudhari
Tejasvi Chaudhari Nadia Schmidt Sotomayor
Nadia Schmidt Sotomayor Rajesh Maheshwari
Rajesh Maheshwari