- 1Department of Maternal and Child Health, Policlinico Umberto I, Sapienza University of Rome, Rome, Italy
- 2Pediatrics Unit, Department of Neuroscience, Mental Health and Sense Organs (NESMOS), Faculty of Medicine and Psychology, Sant’ Andrea Hospital, Sapienza University of Rome, Rome, Italy
Introduction: Prolonged mechanical ventilation, commonly used to assist preterm newborns, increases the risk of developing bronchopulmonary dysplasia (BPD). In recent decades, studies have demonstrated that systemic corticosteroids play a significant role in the prevention and management of BPD. In this systematic review of randomized controlled trials (RCTs), we evaluated the association between the administration of systemic corticosteroids in preterm infants and its long-term outcomes, such as neurodevelopment, growth, extubation rate, and related adverse effects.
Methods: We conducted an electronic search in Medline, Scopus, and PubMed using the following terms: “premature infants” and “corticosteroids.” We considered all RCTs published up to June 2023 as eligible. We included all studies involving preterm newborns treated with systemic corticosteroids and excluded studies on inhaled corticosteroids.
Results: A total of 39 RCTs were evaluated. The influence of steroids administered systemically during the neonatal period on long-term neurological outcomes remains unknown, with no influence observed for long-term growth. The postnatal administration of systemic corticosteroids has been found to reduce the timing of extubation and improve respiratory outcomes. Dexamethasone appears to be more effective than hydrocortisone, despite causing a higher rate of systemic hypertension and hyperglycemia. However, in the majority of RCTs analyzed, there were no differences in the adverse effects related to postnatal corticosteroid administration.
Conclusion: Dexamethasone administered during the neonatal period appears to be more effective than hydrocortisone in terms of respiratory outcomes; however, caution should be taken when administering dexamethasone. Data derived from current evidence, including meta-analyses, are inconclusive on the long-term effects of the administration of systemic steroids in preterm infants or the possibility of neurodevelopmental consequences.
Introduction
The survival rate of preterm newborns has improved over the last 20 years due to advances in neonatal care (1). However, the improvement in survival has been associated with an increased morbidity rate and reduced long-term neurodevelopmental (NDV) outcomes (2, 3). Two of the most important innovations in neonatal care are the introduction of surfactant therapy and the improvement in mechanical ventilation (MV). However, prolonged MV (PMV) is harmful and increases the risk of developing bronchopulmonary dysplasia (BPD) (4, 5). BPD is a chronic inflammatory lung disease of premature neonates characterized by impaired lung development (4, 6). It has a multifactorial pathogenesis, wherein prolonged oxygen exposure induces a destructive local inflammatory response in the lung alveoli, associated with a simultaneous impaired repair response (6). In addition, it has been demonstrated that BPD is associated with impaired long-term NDV and pulmonary function outcomes (7, 8). Thus, neonatologists aim to extubate preterms as soon as possible, albeit not always possible, especially for extremely low gestational age newborns (ELGAN). Corticosteroids (e.g., dexamethasone and hydrocortisone) are currently administered intravenously (IV) or orally (PO) for the treatment and prevention of BPD. Studies have related their beneficial effects to their anti-inflammatory activity (9–11). The authors researched inhaled corticosteroids (12); however, no beneficial effects were found on the risk of neurological disability although the mortality rate was higher in the treated group, thus not allowing the routine administration (13, 14). However, whether the use of dexamethasone or hydrocortisone IV or PO improves or reduces long-term neurological outcomes is still debated. In addition, there are concerns regarding the prophylactic use of systemic corticosteroid therapy for possible adverse effects (i.e., sepsis, infection, and metabolic side effects).
In this systematic review of randomized controlled trials (RCTs), we studied the association between the administration of systemic corticosteroids during the neonatal period and its long-term outcomes, in terms of NDV and growth. Additionally, we evaluated the respiratory outcomes and possible adverse effects.
Material and methods
Studies, population, and intervention
We considered all RCTs published up to June 2023 as eligible. We included all studies involving preterm newborns treated with systemic (IV or PO) corticosteroids and excluded studies on inhaled corticosteroids.
Outcomes
Our primary outcome was the long-term effects such as NDV and growth. Our secondary outcomes were as follows: the rate of extubation, reintubation, BPD, other respiratory outcomes (considering the duration of invasive or non-invasive MV, supplemental oxygen therapy, FiO2, or other specific ventilatory/respiratory data), and steroid-related adverse effects. We considered the following as adverse effects: systemic hypertension, hyperglycemia (HG), sepsis or other infections, patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP).
Research methods and study selection
We performed a systematic review of the published RCTs, in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (15). We conducted an electronic search in Medline, Scopus, and PubMed using the following medical subject headings and terms: “premature infants” and “corticosteroids.” Only English manuscripts and RCTs were considered. Two authors (G.B. and F.L.) independently assessed the study eligibility according to the pre-established criteria and performed an accurate check to exclude duplicates. A discussion with a third part researcher (G.T.) resolved different in opinion, to achieve consensus. We performed a manual search of the reference list of the systematic reviews and meta-analyses published and excluded them from this review.
Data extraction, management, and risk of bias
Two authors (G.B. and F.L.) independently extracted the data from the selected articles using specifically designed data forms. For each selected RCT, the form summarized data on authorship, year of publication, population, inclusion and exclusion criteria, doses of steroids, days to extubation, duration of therapy, more than one cycle of steroids, and administration of other steroids. Another specific data form summarized the outcomes (e.g., extubation, reintubation, BPD, other respiratory outcomes, systemic hypertension, HG, sepsis/infection, PDA, IVH, NEC, ROP, and long-term outcomes). These data were checked for missing information, errors, and inconsistencies with published reports. If evidenced, differences were resolved by discussion and consensus between the researchers. The corresponding authors were contacted when the eligibility criteria of their papers were unclear.
The risk of bias was assessed independently by two researchers (G.B. and G.T.) using a specific form. We considered bias as selection bias (random sequence generation and allocation concealment), performance bias (blinding of the study personnel as to which intervention a neonate had received), detection bias (blinding of personnel evaluating outcomes), attrition bias (completeness of reporting data, reason, and balance across groups of missing data), reporting bias (reporting of the study's prespecified or expected outcomes of interest to the review), and other sources of bias (early interruption of the trial due to data-dependent process or bias related to the specific study design). We categorized the risks of bias as high, low, or unclear for each study, using standard methods (15). The selection bias was judged as unclear when these aspects were not available. The differences in opinion were resolved by discussion and consensus.
Results
Study description
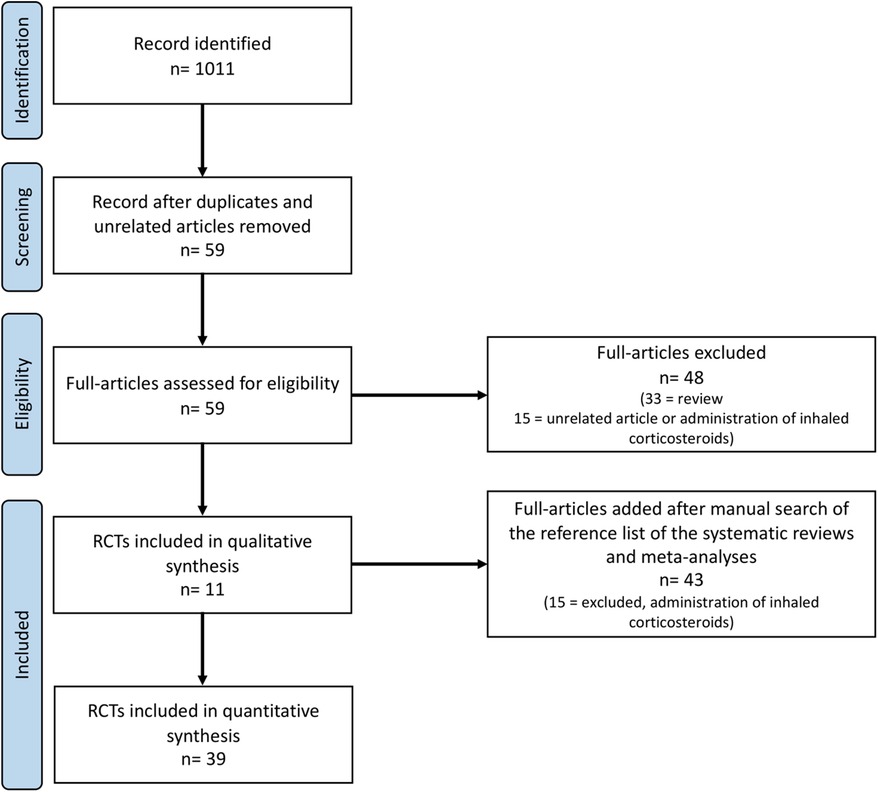
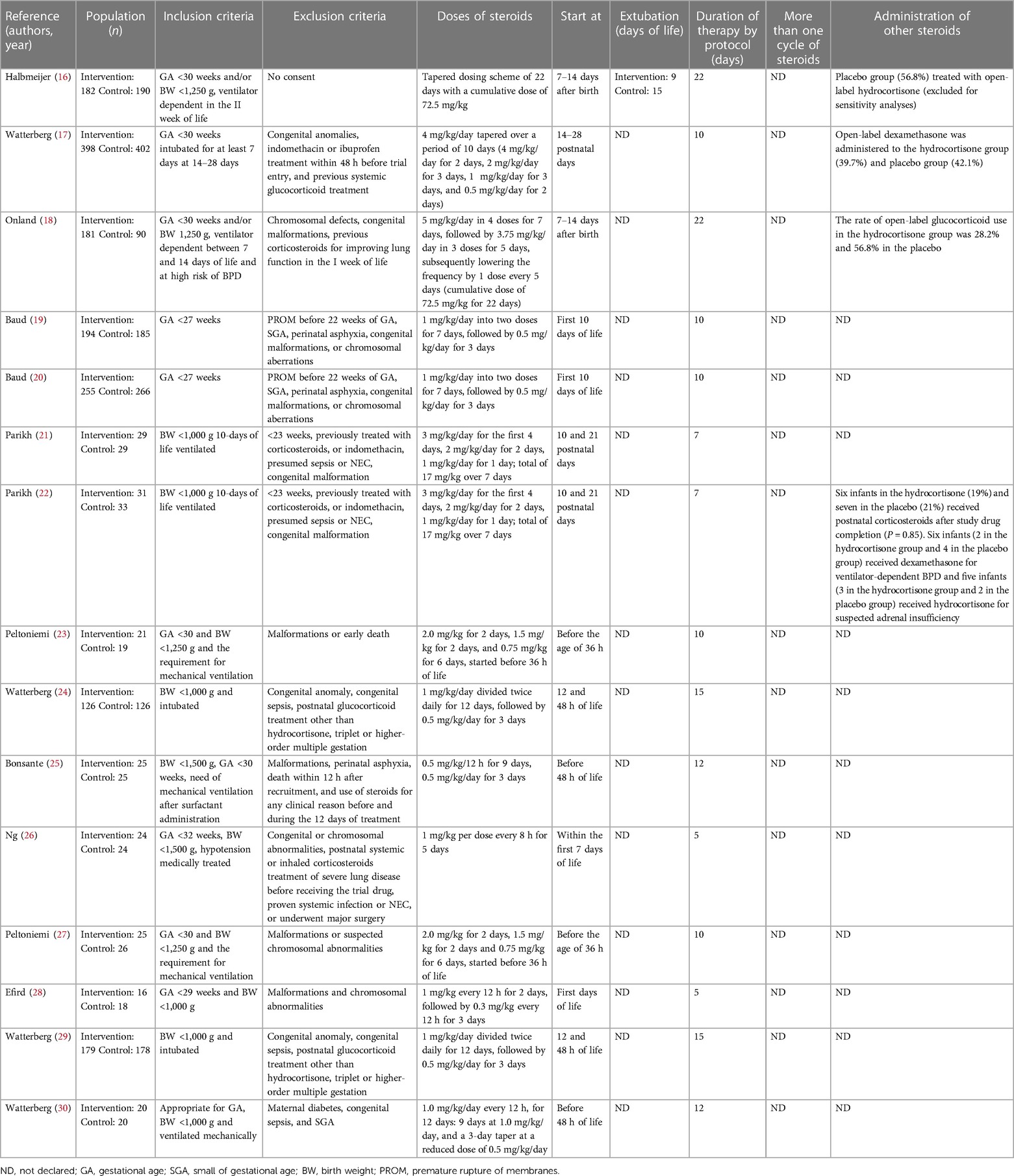
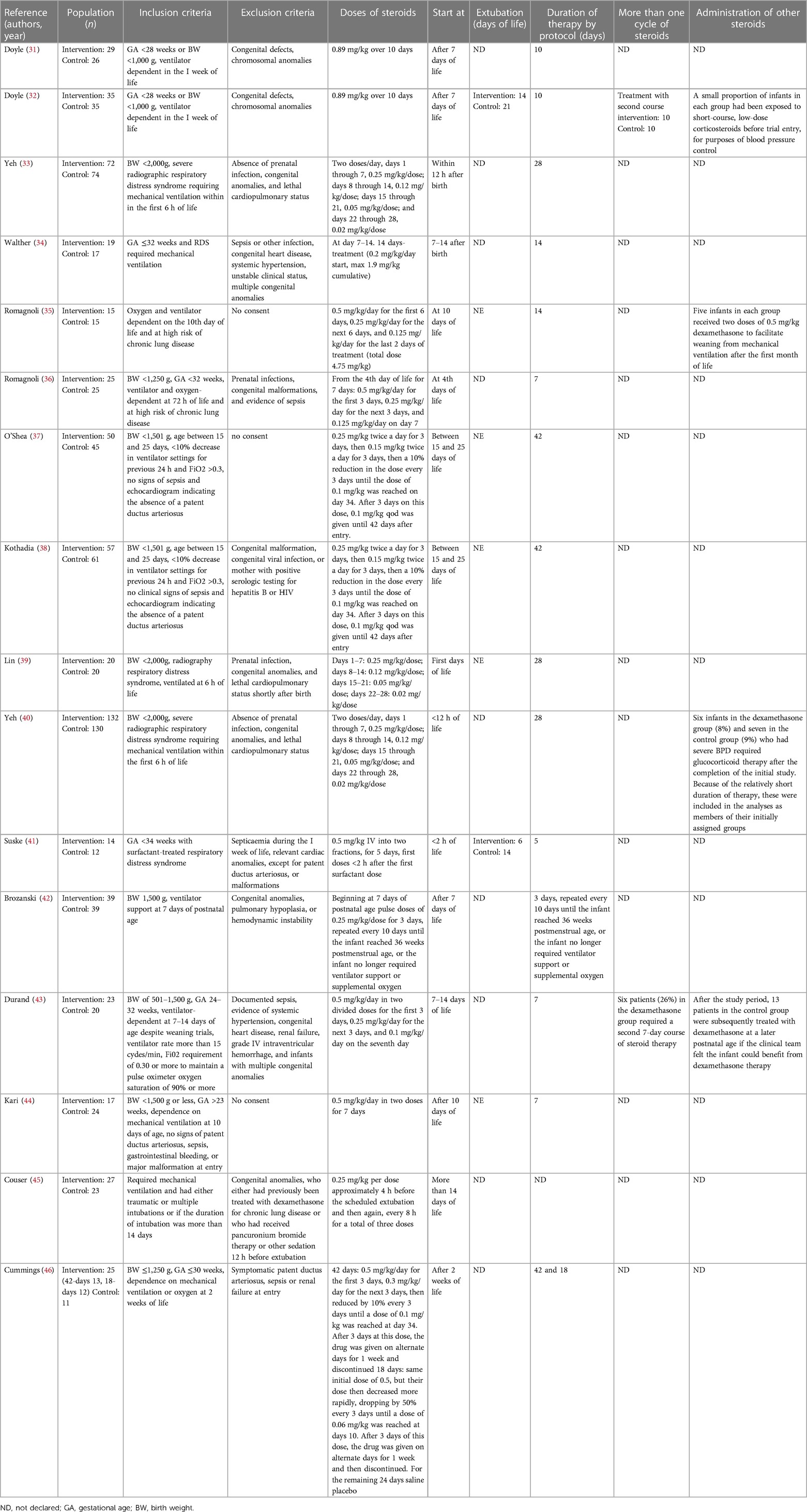
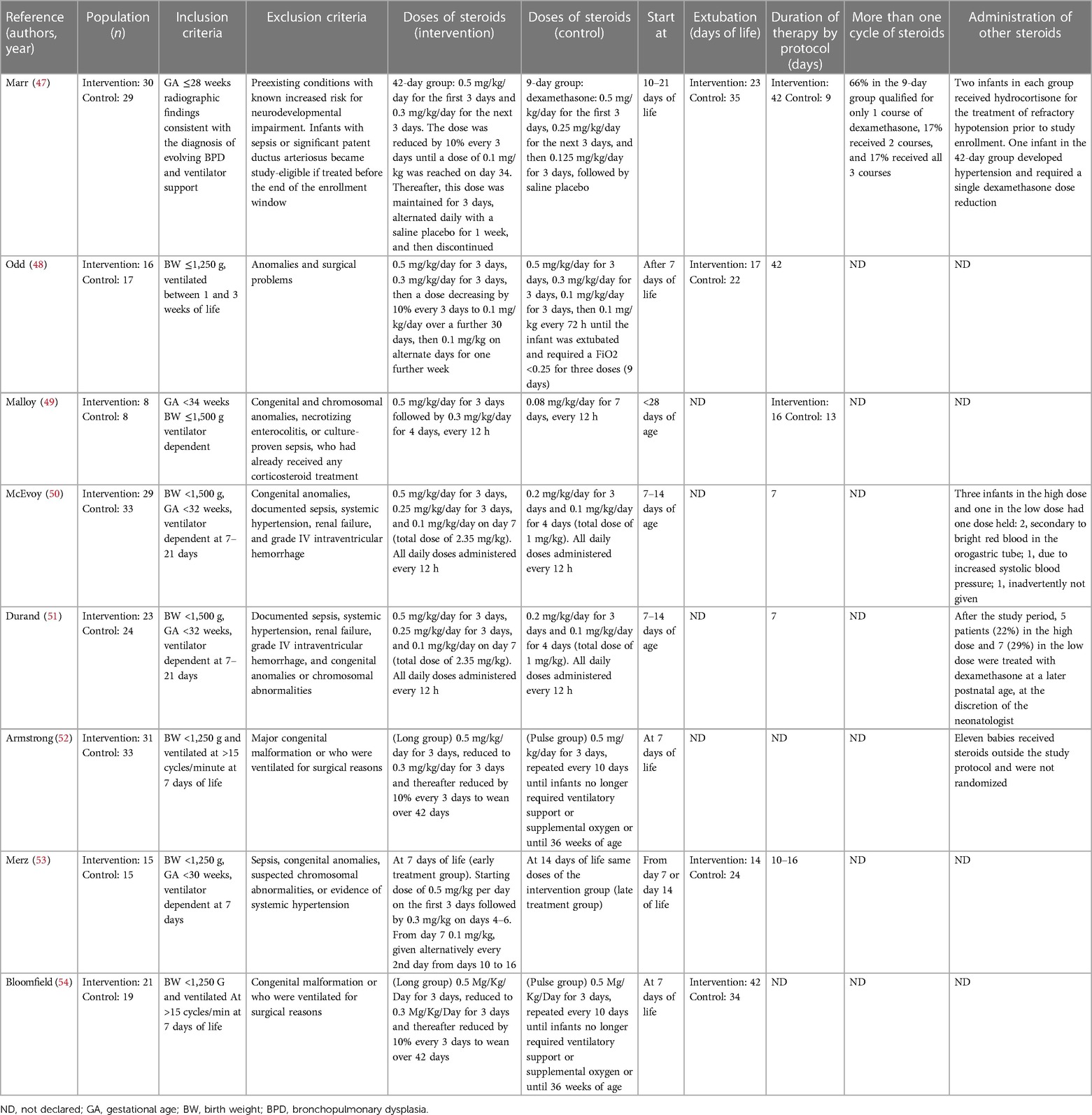
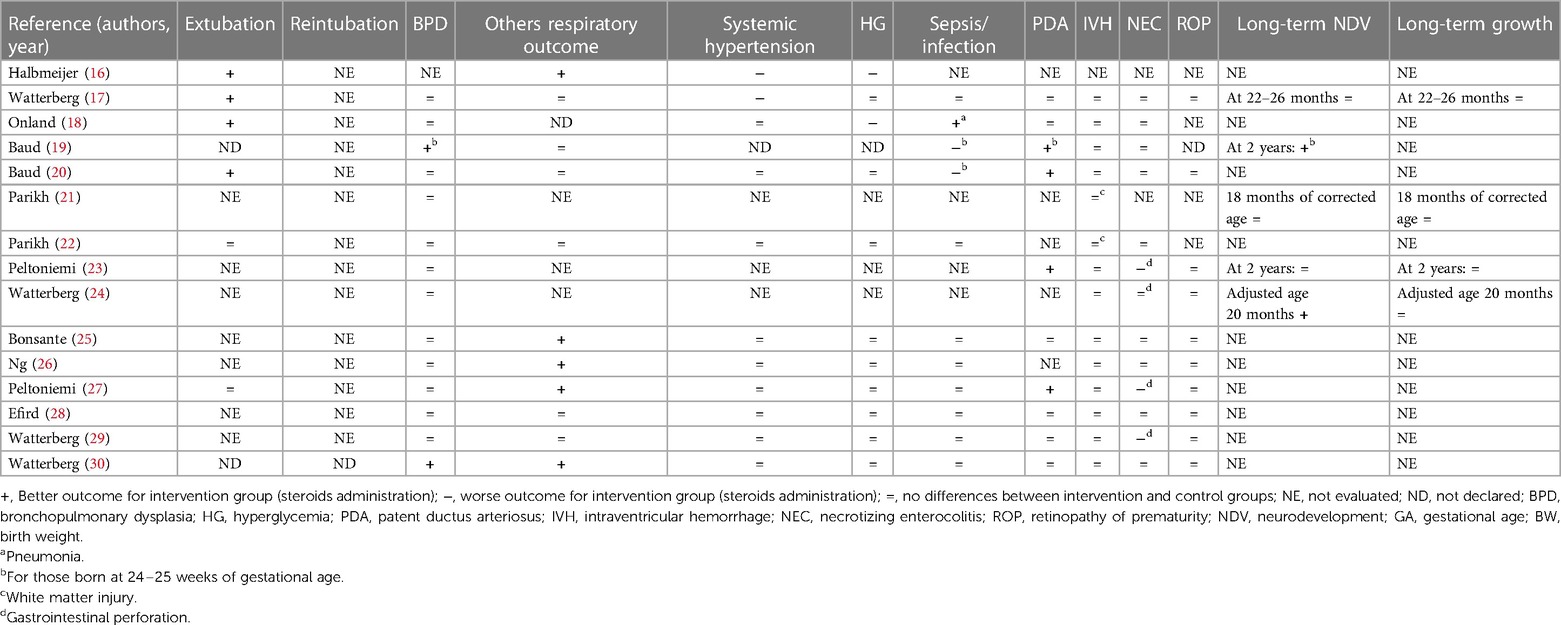
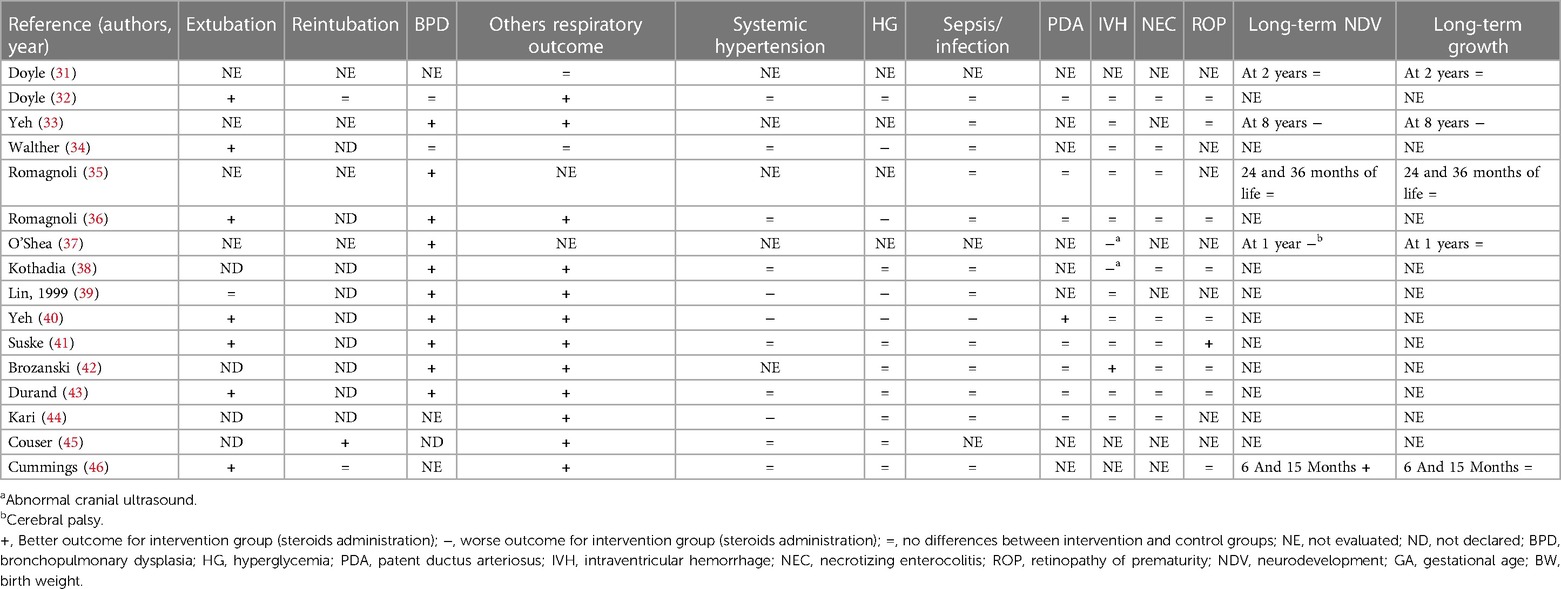
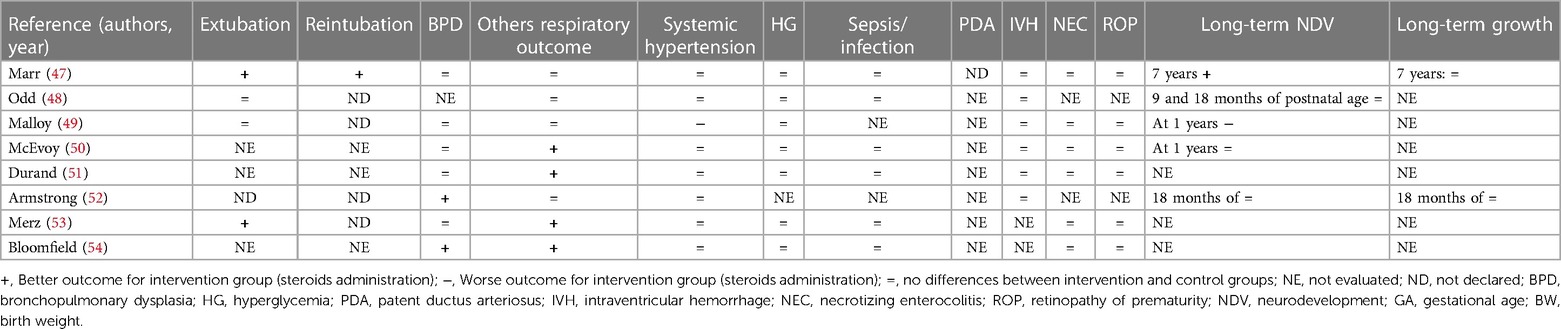
During the research process using the mesh term described in the Materials and methods section, we found 1,011 articles, and 11 RCTs were selected in the first qualitative synthesis after the screening process (Figure 1). After a manual search of the reference list of the systematic reviews and meta-analyses analyzed in the previous stages, we added 28 RCTs, and 39 RCTs were analyzed in the final step of this systematic review (Figure 1). Data extracted are summarized in Tables 1–6 (16–54).
Primary outcome: long-term NDV effects and growth
A graphical representation of the percentage of the studies evaluating the outcomes of interest for this study is shown in Figure 2 (16–54).
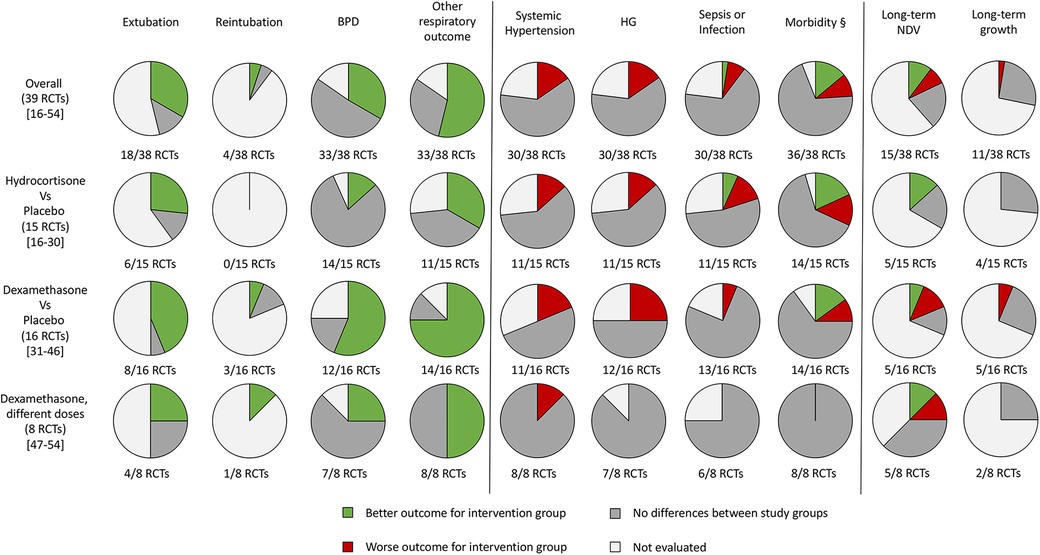
Figure 2. Graphical representation of the randomized controlled trials that evaluated the outcomes of the systematic review. RCTs, randomized controlled trials; BPD, bronchopulmonary dysplasia; HG, hyperglycemia; NDV, neurodevelopment; §, at least one between patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis (or gastrointestinal perforation) and retinopathy of prematurity.
Of the 38 RCTs, 15 performed a follow-up program considering NDV, growth, or both (17, 19, 21, 23, 24, 31, 33, 35, 37, 46–50, 52).
Eight studies did not find statistically significant long-term neurological differences (17, 21, 23, 31, 35, 48, 50, 52). Malloy et al. (49) found an increased risk of NDV delay, and O'Shea et al. (37) showed an increased risk of cerebral palsy at 1 year in the groups receiving dexamethasone (different doses vs. placebo, respectively). A long-term follow-up study performed by Yeh et al. (33) showed worse effects on neuromotor and cognitive function at school age in newborns who received dexamethasone compared to the placebo group. Watterberg et al. (24) (hydrocortisone vs. placebo), Cummings et al. (46) (dexamethasone vs. placebo), and Marr et al. (47) (different doses of dexamethasone) found an improved long-term NDV. Baud et al. (19) found better NDV outcomes at 2 years in newborns who received hydrocortisone if they were born at 24–25 weeks of GA, while no statistical difference considering those born at 26–27 weeks of GA. All the RCTs that considered growth parameters did not find differences between the two groups (17, 23, 24, 31, 35, 37, 46, 47, 52). Only Yeh et al. (33) found that newborns who received dexamethasone were significantly shorter than the controls and had a significantly smaller head circumference, evaluated at school age. However, the long-term NDV and growth outcomes evaluated in these 15 RCTs were measured at different time points (Tables 4–6).
To better characterize the effects of corticosteroids on long-term neurological outcomes, we performed also a sub-analysis separating the studies for early (before 7 days of life) and late (after 7 days of life) administration. The graphical representation of this sub-analysis is reported in Supplementary Figure S1. We excluded the studies of Baud et al. (19, 20) for this analysis, because the starting age of the intervention was before 10 days of life, and based on the definition of early and late administration of corticosteroids considered, we were not able to add these studies in the analysis.
Secondary outcome: respiratory outcome, metabolic effects, and morbidity during hospital stay
Despite most of the studies not finding differences for all the outcomes evaluated for both hydrocortisone and dexamethasone, systemic hypertension and HG appear to be the most frequent side effects, especially for dexamethasone compared with hydrocortisone (Figure 2). Both have an important effect on respiratory outcome and time to extubation (Figure 2). The reintubation rate has been rarely evaluated (Figure 2).
We found that 18 studies evaluated the early extubation rate (16–18, 20, 22, 27, 32, 34, 36, 39–41, 43, 46–49, 53). Five of 18 RCTs found no differences between intervention and control groups (2 hydrocortisone vs. placebo, 1 dexamethasone vs. placebo, and 2 with different doses of dexamethasone) (22, 27, 39, 48, 49), while 13 found an early time of extubation in newborns who received system corticosteroids (4 hydrocortisone vs. placebo, 7 dexamethasone vs. placebo, and 2 with different doses of dexamethasone) (16–18, 20, 32, 34, 36, 40, 41, 43, 46, 47, 53). A total of 21RCTs did not evaluate this outcome or declare the rate of extubation in relation to the administration of systemic corticosteroids (19, 21, 23–26, 28–31, 35, 37, 38, 42, 44, 45, 50–52, 54). Only four studies declared the rate of reintubation, specifically two RCTs found a better reintubation rate for newborns treated with systemic corticosteroids (one dexamethasone vs. placebo and one with different doses of dexamethasone) (45, 47), and two studies found no differences for newborns treated with dexamethasone (32, 36). A total of 33 studies evaluated the rate of BPD (17–30, 32–43, 47, 49–54). Thirteen of 33 found a better outcome (2 hydrocortisone vs. placebo, 9 dexamethasone vs. placebo, and 2 with different doses of dexamethasone) (19, 30, 35–40, 42, 43, 52, 54), while 20 studies did not find differences (17, 18, 20–29, 32, 34, 41, 47, 49–51, 53). Baud et al. found a better outcome for babies born at 24–25 weeks of GA whereas no differences for those born at 26–27 weeks of GA (19, 20). No studies found an increased rate of BPD between the groups. Thirty-three of 39 studies evaluated the pulmonary function (16, 17, 19, 20, 22, 25–34, 36, 38–54). In addition, 21 of 33 RCTs found an improved respiratory outcome for intervention groups (5 hydrocortisone vs. placebo, 12 dexamethasone vs. placebo, and 4 with different doses of dexamethasone) (16, 25–27, 30, 32, 33, 36, 38–46, 50, 51, 53, 54). Twelve did not find a difference (17, 19, 20, 22, 28, 29, 31, 34, 47–49, 52), whereas six did not evaluate this outcome (18, 21, 23, 24, 35, 37).
Systemic hypertension was evaluated in 30/39 studies (16–18, 20, 21, 25–30, 32, 34, 36, 38–41, 43–54). Six of 30 found an increased rate of systemic hypertension in the intervention group (2 hydrocortisone vs. placebo, 3 dexamethasone vs. placebo, and 1 with different doses of dexamethasone) (16, 17, 39, 40, 44, 49). Nine did not evaluate this outcome (19, 21, 23, 24, 31, 33, 35, 37, 42), while the rest of the 24 RCTs found no differences between the study groups (9 hydrocortisone vs. placebo, 8 dexamethasone vs. placebo and 7 with different doses of dexamethasone) (18, 20, 22, 25–30, 32, 34, 36, 38, 41, 43, 45–48, 50–54). As with systemic hypertension, a quasi-totality of the study (30/39) evaluated the outcome HG (16–18, 20, 22, 25–30, 32, 34, 36, 38–51, 53, 54). Six of 30 found an increased rate of HG in intervention groups (2 hydrocortisone vs. placebo and 4 dexamethasone vs. placebo) (16, 18, 34, 35, 39, 40) compared with control, while 24 did not find differences (17, 20, 22, 25–30, 32, 38, 41–51, 53, 54). Brozanski et al. (42) found no differences in the rate of HG, but newborns in the intervention group (dexamethasone vs. placebo) received more statistically significant insulin therapy. Ng et al. (26) and Couser et al. (45) found an increased incidence of glycosuria in the intervention group (hydrocortisone vs. placebo and dexamethasone vs. placebo, respectively), despite no difference in HG rate. Thirty studies considered the risk of sepsis or other infections (17–20, 22, 25–30, 32–36, 38–44, 46–48, 50, 51, 53, 54). Despite the almost total of the study (26/30) did not find differences (17, 22, 25–30, 32–36, 38, 39, 41–44, 46–48, 50, 51, 53, 54), the two RCTs of Baud et al. found a statistically increased risk for babies that received hydrocortisone, born at 24–25 weeks of GA (19, 20). Onland et al. found a statistically reduced incidence of pneumonia for the group of babies that received hydrocortisone (18). Only one study that compared dexamethasone vs. placebo, found an increased risk for the intervention group (40). For the outcome PDA, we considered the incidence and the treatment (medical or surgery ligation) as PDA. Eighteen RCTs considered this outcome (17–20, 23, 25, 27–30, 32, 35, 36, 40–44). Five/18 found a better outcome for treated newborns (4 hydrocortisone vs. placebo and 1 dexamethasone vs. placebo) (19, 20, 23, 27, 40). Specifically, Baud et al. found a better outcome for babies born at 24–25 weeks of GA, treated with hydrocortisone (19, 20). Thirteen RCTs did not find differences between treated and not treated newborns (17, 18, 25, 28–30, 32, 35, 36, 41–44), whereas no studies found a worse outcome. None of the studies that compared different doses of dexamethasone evaluated this outcome. IVH was considered in 33 of the 39 studies (17–30, 32, 34–44, 47–52). Only O'Shea et al. (37) and Kothadia et al. (38) demonstrated an increased risk of abnormal cranial ultrasound for neonates that received corticosteroids, whereas Brozanski et al. (42) showed a reduced risk of IVH in intervention groups. All three studies compared dexamethasone vs. placebo (37, 38, 42). The rest of the 29 studies found no statistical differences for IVH or white matter injury (17–26, 28–30, 32, 34–36, 39–41, 43, 44, 47–52). Of the 29 studies that evaluated NEC, the studies of Watterberg et al. and Peltoniemi et al. found an increased risk of gastrointestinal perforation in neonates that received corticosteroids (specifically hydrocortisone) (23, 27, 29). The other 26 RCTs did not find statistically significant differences between the two groups for NEC or gastrointestinal perforation (17–20, 22, 24–26, 28, 30, 32, 34–36, 38, 40–44, 47, 49–51, 53, 54). The risk of ROP was evaluated by 25/39 RCTs (17, 20, 23–30, 32, 33, 36, 38, 40–43, 46, 47, 49–51, 53, 54). Twenty-four did not find any statistical differences between the two trial groups (17, 20, 23–30, 32, 33, 36, 38, 40, 42, 43, 46, 47, 49–51, 53, 54), but only Suske et al. (41) demonstrated a reduced risk of ROP for newborns of the intervention groups, who were administered dexamethasone.
Risk of bias
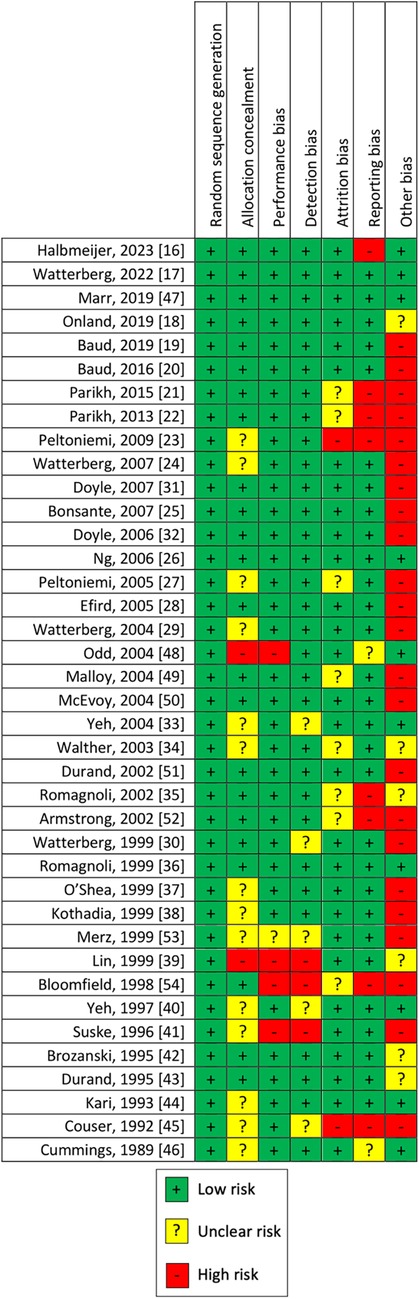
The quality of the studies was assessed by risks of bias, as shown in Figure 3. We judged the risk of selection bias as low in all uncontrolled studies, unclear for 14, and high for 2 RCTs. Performance bias and detection bias were high for four and three studies, respectively, and unclear for one and five, respectively. The rest of the 34 and 31 RCTs were judged as low risk of performance and detection bias, respectively. Attrition bias was judged as low for 29 studies, high for 2, and unclear for 8 RCTs. Considering our outcome, reporting bias was judged high for 8, low for 29, and unclear for the remaining 2 RCTs. Other sources of bias were not evaluable for 6 controlled trials, while was high for 23 studies and low only for 10.
Discussion
Despite many studies exploring the effects of corticosteroid administration in postnatal life for preterm newborns, the optimal modality of administration remains to be defined. The high heterogeneity of the included RCTs regarding dosages, timing of administration, and outcome measures, discourages the mathematical analysis of the data (55). A recent meta-analysis and network meta-analysis included only one study for a therapeutic regiment or more than one study but with different timing of administration and were focused primarily on BPD (11). In this systematic review, we evaluated the efficacy of steroid therapy on other respiratory outcomes, including extubation rate, reintubation, BPD, and related side effects, considering separately hydrocortisone, dexamethasone, and different doses of dexamethasone. Despite the analyzed studies suggesting an overall favorable effect of steroids on respiratory outcomes, a well-designed large RCT is urgently needed to establish the optimal indication and modalities of administration. Among the steroids used for preterm newborns, early and high doses of dexamethasone have a better impact on the respiratory outcome, while hydrocortisone is related to fewer side effects such as systematic hypertension or HG.
Long-term NDV effects and growth
A major limitation in the analysis of the studies including the use of steroids in the neonatal period is represented by the lack of data for long-term outcomes. Only a few studies evaluated neurological and growth outcomes of preterm newborns receiving steroids in early life (17, 19, 21, 23, 24, 31, 35, 37, 46–50, 52). Only one-third of the trials on hydrocortisone evaluated the long-term effects on NDV up to 2 years of life (17, 19, 21, 23, 24). In these studies, neurological outcomes seem to be not influenced by the use of hydrocortisone. Two RCTs found an improvement in neurological outcomes, particularly in ELGAN and newborns with extremely low birth weight (19, 24). Almost half of studies on dexamethasone evaluated neurological outcomes up to 1–2 years of life (31, 35, 37, 46–50, 52). The results of these studies are controversial. If the majority of the RCTs demonstrated that there is no influence of dexamethasone on long-term NDV (31, 35, 48, 50, 52), O'Shea et al. (37) reported an increased rate of cerebral palsy at 1 year for newborns treated with dexamethasone (42 days of therapy, starting doses at 0.25 mg/kg twice a day for 3 days) compared with placebo. Malloy et al. (49) found a worse NDV long-term outcome for newborns in the high doses of dexamethasone groups (0.5 mg/kg/day for 3 days followed by 0.3 mg/kg/day for 4 days every 12 h vs. 0.08 mg/kg/day for 7 days every 12 h). In addition, Yeh et al. (33) showed that children treated with two doses per day of dexamethasone for 28 days (0.25 mg/kg/dose up to 7 days and then the dose was tapered) had significantly poorer motor skills, motor coordination, visual–motor integration, and significantly lower full IQ, verbal IQ, and performance IQ scores. The frequency of clinically significant disabilities was significantly higher among children in the dexamethasone group compared with controls (39% vs. 22%, p value 0.04) (33). However, O'Shea et al. did not power the study for long-term NDV and selection bias because differential survival rates across the two study groups could explain the greater risk of cerebral palsy among the intervention group; Malloy et al. performed a trial with a very low sample size (8 vs. 8). On the other hand, Cummings et al. and Marr et al. found a better long-term neurological outcome for babies treated with dexamethasone (42 days of therapy, starting dose at 0.5 mg/kg/day for the first 3 days, 0.3 mg/kg/day for the next 3 days, and then reduced by 10% every 3 days until a dose of 0.1 mg/kg was reached at day 34) (46, 47). They enrolled newborns born ≤30 weeks of GA (and ≤1,250 g) and ≤27 weeks of GA, respectively. Thus, it could be possible that ELGAN should benefit more than other newborns from steroid treatment. In addition, Marr et al. performed a study with a long time follow-up evaluation of 7 years. Most of the studies did not consider long-term growth as an outcome. The available studies suggest a low impact of early steroid treatment and long-term growth (17, 23, 24, 31, 35, 37, 46, 47, 52). Only Yeh et al. demonstrated that dexamethasone could negatively influence height and head circumference, evaluated at school age (33).
Respiratory outcome, metabolic effects, and morbidity during hospital stay
Postnatal steroid treatment is beneficial for respiratory outcomes, such as extubation, reduced risk of BPD or duration of invasive or non-invasive MV, supplemental oxygen therapy, FiO2, and/or other specific ventilatory/respiratory data. Dexamethasone has a better impact compared to hydrocortisone, but the optimal therapeutic regiment remains to be defined. For the reintubation rate outcome, a conclusion cannot be made, since it has been evaluated only in a few, unpowered studies (32, 45–47). In addition, in all these studies, dexamethasone was used as an intervention, while none of them considered hydrocortisone.
Our analysis showed that BPD is the most studied respiratory outcome. The majority of the studies demonstrated that dexamethasone had a positive effect on the BPD rate, whereas hydrocortisone appears to not improve this outcome. The study with a lower risk of bias showed that the therapeutic regiment of dexamethasone in early life (0.5 mg/kg/day for the first 3 days, 0.25 mg/kg/day for the next 3 days, and 0.125 mg/kg/day on the day 7) had the best impact on BPD (36).
Steroids work as anti-inflammatory agents, which can explain their rationale in the prevention of BPD. One of the main risk factors for developing BPD is prolonged oxygen exposure and MV, which induce a pulmonary local inflammatory response (4, 5). Postnatal corticosteroids decrease inflammation and edema, improving gas exchange and lung protective mechanisms (56, 57).
To the best of our knowledge, there are no RCTs that evaluated the long-term pulmonary outcome of preterm newborns treated with corticosteroids in neonatal life. On the other hand, studies aimed at investigating long-term respiratory function in preterm babies were mainly focused on BPD and did not independently evaluate the role of postnatal steroid treatment on the final outcome (58).
Not all studies evaluated side effects associated with the use of steroids in preterm newborns.
Despite the majority of the RCTs found no difference between the study groups (17, 18, 20, 22, 25–30, 32, 34, 36, 38, 41–54), some studies suggested an increased risk of systemic hypertension and/or HG.
Systemic hypertension was analyzed in the majority of the studies included in this systematic review. Few studies (∼16%) reported an increased risk of hypertension in newborns treated with steroids. The administration of dexamethasone has been associated with systemic hypertension more frequently compared to hydrocortisone. Treatment regimens of more than 0.25 mg/kg of dexamethasone, especially for more than 10 days, appear to increase the risk of systemic hypertension.
HG is an independent risk factor for mortality and NDV delay in newborns (59, 60); thus, all efforts should be made to reduce the risk of this condition. A higher number of studies on dexamethasone reported an increased risk of HG compared with studies on hydrocortisone. However, all the studies that compared two different doses or timing for dexamethasone administration found no difference for HG (47–54). When steroid treatment is needed, neonatologists should minimize the other conditions that induce an increased risk of HG (such as nutrition) (60, 61), or they should improve continuous glucose monitoring to maintain euglycemia (62).
Sepsis remains the major cause of morbidity and mortality in preterm newborns (63). Our analysis showed a relevant increased risk of sepsis in newborns treated with steroids, more for hydrocortisone compared with dexamethasone. However, a recent study demonstrated a reduced risk of pneumonia (18) in newborns treated with hydrocortisone, probably due to an improvement in respiratory outcome and a reduced time of invasive ventilation support.
Data regarding the relationship between steroid administration and morbidity conditions are controversial. Most studies suggested no relation (about 69%), while others demonstrated an increased rate of prematurity-related morbidities (about 14%); finally, about 10% suggested a better outcome. We speculated that this result depends on the different modalities of administration of corticosteroids, different therapeutic regiments, and different morbidity definitions of the studies. Based on our findings, more than 42 days of dexamethasone therapy, more than 1 mg/kg starting dose, or more than 10 days of hydrocortisone therapy might increase the risk of morbidity.
We speculate that part of the reduction in morbidity might be related to the improvement in PDA closure, associated with steroid use. Some studies demonstrated that steroids (especially hydrocortisone) could improve the PDA outcome, reducing the need for medical or surgical treatment (19, 20, 23, 27, 40). Several reasons may explain this effect: (1) in vitro studies demonstrated that hydrocortisone treatment decreases the sensitivity of the ductus arteriosus to the relaxing action of prostaglandin E2, which explains the beneficial effects in vivo of steroids (64, 65), and (2) the relationship between PDA and BPD, especially for ELGAN, has been demonstrated (66). Despite this topic is not well evaluated, it could be possible that the effects of steroid administration on PDA also improve BPD. However, future studies should evaluate this aspect.
Brozanski et al. (42) found a reduced risk of IVH for preterm receiving steroids. The authors administered a pulse dose of 0.25 mg/kg/dose of dexamethasone to newborns at 7 days of life for 3 days, repeated every 10 days until 36 weeks of postmenstrual age or up to weaning of ventilation/oxygen support. In addition, they affirmed that the decreased rate of IVH in the intervention (pulse) group could be associated with a better stabilization of capillary membranes or alteration of cerebral blood flow by corticosteroids or by an improvement in the ventilatory status of the infants (42). However, two RCTs underline the effects of steroids on early brain damage (37, 38). They administered 0.25 mg/kg twice a day for 3 days, 0.15 mg/kg twice a day for other 3 days, and then a 10% reduction in the dose every 3 days until the dose of 0.1 mg/kg was reached on day 34 and after 3 days on this dose. However, all three studies are not powered for this outcome. Thus, considering the different therapeutic regiments and this limitation, further studies are needed to clarify this aspect.
Concerns remain regarding the risk of spontaneous gastrointestinal perforations in newborns treated with hydrocortisone (23, 27, 29). In particular, Peltomieni et al. (23, 27) administered hydrocortisone 2.0 mg/kg for 2 days, 1.5 mg/kg for 2 days, and 0.75 mg/kg for 6 days, started before 36 h of life (duration therapy 10 days), and Wetterberg et al. (29) 1 mg/kg/day divided twice daily for 12 days starting at randomization (12–48 h of life), followed by 0.5 mg/kg/day for 3 days (duration therapy 15 days). In both studies, The authors stopped the studies because of the higher rate of spontaneous gastrointestinal perforation, limiting the power of the studies (23, 27, 29). Studies with similar treatment did not find differences. In addition, the majority of the analyzed RCTs did not find differences in terms of NEC or spontaneous gastrointestinal perforations between treated and placebo groups (17–20, 22, 24–26, 28, 30, 32, 34–36, 38, 40–44, 47, 49–51, 53, 54).
Risk of bias
Given that some studies with concerns about the overall risk of bias have been included, our results need to be confirmed by further RCTs with a low risk of bias. Blinding bias was low for most of the studies analyzed. Attrition and reporting bias were judged low for most of the studies reviewed. Major concerns are about the risk of other bias (early interruption of the trial due to data-dependent process or bias related to the specific study design), which was judged high for 23 of 38 studies. In addition, the studies presented a high heterogeneity of inclusion criteria (e.g., GA and/or BW) and intervention (different doses, timing of administration, and duration therapy), which could have influenced the results. We included only RCTs, despite some of these enrolling a small number of patients with low power of the study. Some of the studies included adopted a non-optimal blinding method or were unclear.
Strengths and limitations
Our results should be interpreted considering the limitations of the studies analyzed and of the review process. First, we decided not to perform a meta-analysis because of the extreme variability in methodology, modality of administration of steroids, and outcome of the studies (55). We systematically collected evidence and after a deep evaluation and discussion between the authors, we decided not to make a meta-analysis considering the wide differences in methodology used in different studies included in this manuscript. In particular, the studies vary regarding inclusion and exclusion criteria, enrollment, dose of treatment, starting days and duration of steroids, type of steroids administered, timing of follow-up, and assessment scales (Tables 1–3). The data deriving from current evidence, including meta-analyses, are inconclusive on the long-term effects either to exclude completely that there may be consequences on the central nervous system. Thus, we believe that steroids should be used in trial settings and to collect data in large databases to verify the consequences of this therapy.
We believe that there are no minimal criteria to perform a meta-analysis and that conclusions deriving from published meta-analyses were not supported by robust statistical data. Our data might contribute to better define the modality of steroid therapy and the target population to reduce the risk of brain damage. Whether meta-analyses suggested deleterious effects of steroids on NDV, our study demonstrated that further well-designed studies are needed to reach conclusions regarding the relationship between steroid treatment in preterm newborns and long-term NDV.
We synthesized the results of different studies on NDV. However, the long-term outcomes were not analyzed at the same time point and with different NDV assessment scales. We selected articles published in the English language; thus, it is possible that some gray literature has not been analyzed. In addition, studies showing positive results have a greater likelihood of being published. Finally, in some studies, other medications were administered, based on the clinical conditions of patients, in some studies treatment was interrupted prematurely, and others were not powered for the long-term outcomes.
Conclusion
Postnatal administration of systemic corticosteroids is an important tool for neonatologists to improve respiratory outcomes. Based on published RCTs, dexamethasone appears to be more effective than hydrocortisone for extubation, prevention of BPD, and improvement of respiratory outcomes. However, considering the deleterious effects such as HG, caution should be made during administration of dexamethasone. In addition, long-term effects on NDV and growth remain undefined. Considering that data deriving from current evidence, including meta-analyses, are inconclusive on the long-term effects to exclude completely that there may be consequences on the central nervous system, further studies are advocated to define the optimal therapeutic regiment, to improve the positive effects and reduce the side effects of steroid administration in preterm newborns.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
GB: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, writing – review and editing. VC: writing – review & editing. MGC: validation, writing – review & editing. FL: data curation, investigation, writing – review and editing. PR: writing – review and editing. PP: writing – review and editing. GT: conceptualization, data curation, formal analysis, methodology, project administration, software, supervision, validation, visualization, writing – original draft, writing – review and editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1344337/full#supplementary-material
Supplementary Figure S1
Graphical representation of the randomized controlled trials that evaluated neurological outcomes in relation to early (before 7 days of life) or late (after 7 days of life) administration of steroids. RCTs, randomized controlled trials; *the studies of Baud et al. were excluded (19, 20).
References
1. World Health Organization. Survive and thrive: transforming care for every small and sick newborn. (2019). Available online at: https://www.unicef.org/reports/transforming-care-for-every-small-and-sick-newborn-2020 (accessed May 2020).
2. Morniroli D, Tiraferri V, Maiocco G, De Rose DU, Cresi F, Coscia A, et al. Beyond survival: the lasting effects of premature birth. Front Pediatr. (2023) 11:1213243. doi: 10.3389/fped.2023.1213243
3. Terrin G, Boscarino G, Di Chiara M, Iacobelli S, Faccioli F, Greco C, et al. Nutritional intake influences zinc levels in preterm newborns: an observational study. Nutrients. (2020) 12:529. doi: 10.3390/nu12020529
4. Nuthakki S, Ahmad K, Johnson G, Cuevas Guaman M. Bronchopulmonary dysplasia: ongoing challenges from definitions to clinical care. JCM. (2023) 12:3864. doi: 10.3390/jcm12113864
5. Boscarino G, Conti MG, De Luca F, Di Chiara M, Deli G, Bianchi M, et al. Intravenous lipid emulsions affect respiratory outcome in preterm newborn: a case-control study. Nutrients. (2021) 13:1243. doi: 10.3390/nu13041243
6. Naeem A, Ahmed I, Silveyra P. Bronchopulmonary dysplasia: an update on experimental therapeutics. Eur Med J (Chelmsf). (2019) 4:20–9. doi: 10.33590/emj/10313109
7. Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, Sauve RS, Whitfield MF, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. (2003) 289:1124. doi: 10.1001/jama.289.9.1124
8. Davidson L, Berkelhamer S. Bronchopulmonary dysplasia: chronic lung disease of infancy and long-term pulmonary outcomes. JCM. (2017) 6:4. doi: 10.3390/jcm6010004
9. Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. (2021) 11:CD001145. doi: 10.1002/14651858.CD001145.pub5
10. Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Early (<7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. (2021) 10:CD001146. doi: 10.1002/14651858.CD001146.pub6
11. Ramaswamy VV, Bandyopadhyay T, Nanda D, Bandiya P, Ahmed J, Garg A, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA Pediatr. (2021) 175:e206826. doi: 10.1001/jamapediatrics.2020.6826
12. Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates. Cochrane Database Syst Rev. (2017) 1:CD001969. doi: 10.1002/14651858.CD001969.pub4
13. Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau P-H, Carnielli V, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med. (2015) 373:1497–506. doi: 10.1056/NEJMoa1501917
14. Bassler D, Shinwell ES, Hallman M, Jarreau P-H, Plavka R, Carnielli V, et al. Long-term effects of inhaled budesonide for bronchopulmonary dysplasia. N Engl J Med. (2018) 378:148–57. doi: 10.1056/NEJMoa1708831
15. Higgins JPT, Cochrane Collaboration, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edn. Hoboken, NJ: Wiley-Blackwell (2020). ISBN: 978-0-470-51845-8.
16. Halbmeijer NM, Onland W, Cools F, Kroon A, van der Heide-Jalving M, Dijk P, et al. Short-term pulmonary and systemic effects of hydrocortisone initiated 7–14 days after birth in ventilated very preterm infants: a secondary analysis of a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2023) 108:20–5. doi: 10.1136/archdischild-2022-323882
17. Watterberg KL, Walsh MC, Li L, Chawla S, D’Angio CT, Goldberg RN, et al. Hydrocortisone to improve survival without bronchopulmonary dysplasia. N Engl J Med. (2022) 386:1121–31. doi: 10.1056/NEJMoa2114897
18. Onland W, Cools F, Kroon A, Rademaker K, Merkus MP, Dijk PH, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. JAMA. (2019) 321:354–63. doi: 10.1001/jama.2018.21443
19. Baud O, Trousson C, Biran V, Leroy E, Mohamed D, Alberti C. Two-year neurodevelopmental outcomes of extremely preterm infants treated with early hydrocortisone: treatment effect according to gestational age at birth. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F30–5. doi: 10.1136/archdischild-2017-313756
20. Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet. (2016) 387:1827–36. doi: 10.1016/S0140-6736(16)00202-6
21. Parikh NA, Kennedy KA, Lasky RE, Tyson JE. Neurodevelopmental outcomes of extremely preterm infants randomized to stress dose hydrocortisone. PLoS One. (2015) 10:e0137051. doi: 10.1371/journal.pone.0137051
22. Parikh NA, Kennedy KA, Lasky RE, McDavid GE, Tyson JE. Pilot randomized trial of hydrocortisone in ventilator-dependent extremely preterm infants: effects on regional brain volumes. J Pediatr. (2013) 162:685–90.e1. doi: 10.1016/j.jpeds.2012.09.054
23. Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA, et al. Rial of early neonatal hydrocortisone: two-year follow-up. Neonatology. (2009) 95:240–7. doi: 10.1159/000164150
24. Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. (2007) 120:40–8. doi: 10.1542/peds.2006-3158
25. Bonsante F, Latorre G, Iacobelli S, Forziati V, Laforgia N, Esposito L, et al. Early low-dose hydrocortisone in very preterm infants: a randomized, placebo-controlled trial. Neonatology. (2007) 91:217–21. doi: 10.1159/000098168
26. Ng PC, Lee CH, Bnur FL, Chan IHS, Lee AWY, Wong E, et al. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics. (2006) 117:367–75. doi: 10.1542/peds.2005-0869
27. Peltoniemi O, Kari MA, Heinonen K, Saarela T, Nikolajev K, Andersson S, et al. Pretreatment cortisol values may predict responses to hydrocortisone administration for the prevention of bronchopulmonary dysplasia in high-risk infants. J Pediatr. (2005) 146:632–7. doi: 10.1016/j.jpeds.2004.12.040
28. Efird MM, Heerens AT, Gordon PV, Bose CL, Young DA. A randomized-controlled trial of prophylactic hydrocortisone supplementation for the prevention of hypotension in extremely low birth weight infants. J Perinatol. (2005) 25:119–24. doi: 10.1038/sj.jp.7211193
29. Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. (2004) 114:1649–57. doi: 10.1542/peds.2004-1159
30. Watterberg KL, Gerdes JS, Gifford KL, Lin HM. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics. (1999) 104:1258–63. doi: 10.1542/peds.104.6.1258
31. Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, and the DART Study Investigators. Outcome at 2 years of age of infants from the DART study: a multicenter, international, randomized, controlled trial of low-dose dexamethasonef. Pediatrics (2007) 119:716–21. doi: 10.1542/peds.2006-2806
32. Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, and the DART Study Investigators. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics (2006) 117:75–83. doi: 10.1542/peds.2004-2843
33. Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. (2004) 350:1304–13. doi: 10.1056/NEJMoa032089
34. Walther FJ, Findlay RD, Durand M. Adrenal suppression and extubation rate after moderately early low-dose dexamethasone therapy in very preterm infants. Early Hum Dev. (2003) 74:37–45. doi: 10.1016/s0378-3782(03)00082-3
35. Romagnoli C, Zecca E, Luciano R, Torrioli G, Tortorolo G. A three year follow up of preterm infants after moderately early treatment with dexamethasone. Arch Dis Child Fetal Neonatal Ed. (2002) 87:F55–8. doi: 10.1136/fn.87.1.f55
36. Romagnoli C, Zecca E, Vento G, De Carolis MP, Papacci P, Tortorolo G. Early postnatal dexamethasone for the prevention of chronic lung disease in high-risk preterm infants. Intensive Care Med. (1999) 25:717–21. doi: 10.1007/s001340050935
37. O’Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG, et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics. (1999) 104:15–21. doi: 10.1542/peds.104.1.15
38. Kothadia JM, O’Shea TM, Roberts D, Auringer ST, Weaver RG, Dillard RG. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants. Pediatrics. (1999) 104:22–7. doi: 10.1542/peds.104.1.22
39. Lin YJ, Yeh TF, Hsieh WS, Chi YC, Lin HC, Lin CH. Prevention of chronic lung disease in preterm infants by early postnatal dexamethasone therapy. Pediatr Pulmonol. (1999) 27:21–6. doi: 10.1002/(sici)1099-0496(199901)27:1%3C21::aid-ppul5%3E3.0.co;2-y
40. Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY, et al. Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics. (1997) 100:E3. doi: 10.1542/peds.100.4.e3
41. Suske G, Oestreich K, Varnholt V, Lasch P, Kachel W. Influence of early postnatal dexamethasone therapy on ventilator dependency in surfactant-substituted preterm infants. Acta Paediatr. (1996) 85:713–8. doi: 10.1111/j.1651-2227.1996.tb14132.x
42. Brozanski BS, Jones JG, Gilmour CH, Balsan MJ, Vazquez RL, Israel BA, et al. Effect of pulse dexamethasone therapy on the incidence and severity of chronic lung disease in the very low birth weight infant. J Pediatr. (1995) 126:769–76. doi: 10.1016/s0022-3476(95)70410-8
43. Durand M, Sardesai S, McEvoy C. Effects of early dexamethasone therapy on pulmonary mechanics and chronic lung disease in very low birth weight infants: a randomized, controlled trial. Pediatrics. (1995) 95:584–90. doi: 10.1542/peds.95.4.584
44. Kari MA, Heinonen K, Ikonen RS, Koivisto M, Raivio KO. Dexamethasone treatment in preterm infants at risk for bronchopulmonary dysplasia. Arch Dis Child. (1993) 68:566–9. doi: 10.1136/adc.68.5_spec_no.566
45. Couser RJ, Ferrara TB, Falde B, Johnson K, Schilling CG, Hoekstra RE. Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. J Pediatr. (1992) 121:591–6. doi: 10.1016/s0022-3476(05)81154-0
46. Cummings JJ, D’Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med. (1989) 320:1505–10. doi: 10.1056/NEJM198906083202301
47. Marr BL, Mettelman BB, Bode MM, Gross SJ. Randomized trial of 42-day compared with 9-day courses of dexamethasone for the treatment of evolving bronchopulmonary dysplasia in extremely preterm infants. J Pediatr. (2019) 211:20–6.e1. doi: 10.1016/j.jpeds.2019.04.047
48. Odd DE, Armstrong DL, Teele RL, Kuschel CA, Harding JE. A randomized trial of two dexamethasone regimens to reduce side-effects in infants treated for chronic lung disease of prematurity. J Paediatr Child Health. (2004) 40:282–9. doi: 10.1111/j.1440-1754.2004.00364.x
49. Malloy CA, Hilal K, Rizvi Z, Weiss M, Muraskas JK. A prospective, randomized, double-masked trial comparing low dose to conventional dose dexamethasone in neonatal chronic lung disease. Internet J Pediatr Neonatol. (2004) 5(1).
50. McEvoy C, Bowling S, Williamson K, McGaw P, Durand M. Randomized, double-blinded trial of low-dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol. (2004) 38:55–63. doi: 10.1002/ppul.20037
51. Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics. (2002) 109:262–8. doi: 10.1542/peds.109.2.262
52. Armstrong DL, Penrice J, Bloomfield FH, Knight DB, Dezoete JA, Harding JE. Follow up of a randomised trial of two different courses of dexamethasone for preterm babies at risk of chronic lung disease. Arch Dis Child Fetal Neonatal Ed. (2002) 86:F102–7. doi: 10.1136/fn.86.2.f102
53. Merz U, Peschgens T, Kusenbach G, Hörnchen H. Early versus late dexamethasone treatment in preterm infants at risk for chronic lung disease: a randomized pilot study. Eur J Pediatr. (1999) 158:318–22. doi: 10.1007/s004310051081
54. Bloomfield FH, Knight DB, Harding JE. Side effects of 2 different dexamethasone courses for preterm infants at risk of chronic lung disease: a randomized trial. J Pediatr. (1998) 133:395–400. doi: 10.1016/s0022-3476(98)70277-x
55. Onland W, van de Loo M, Offringa M, van Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. (2023) 3:CD010941. doi: 10.1002/14651858.CD010941.pub3
56. Jobe AH. Postnatal corticosteroids for bronchopulmonary dysplasia. Clin Perinatol. (2009) 36:177–88. doi: 10.1016/j.clp.2008.09.016
57. Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. (2016) 33:1076–8. doi: 10.1055/s-0036-1586107
58. Manti S, Galdo F, Parisi GF, Napolitano M, Decimo F, Leonardi S, et al. Long-term effects of bronchopulmonary dysplasia on lung function: a pilot study in preschool children’s cohort. J Asthma. (2021) 58:1186–93. doi: 10.1080/02770903.2020.1779289
59. Stensvold HJ, Strommen K, Lang AM, Abrahamsen TG, Steen EK, Pripp AH, et al. Early enhanced parenteral nutrition, hyperglycemia, and death among extremely low-birth-weight infants. JAMA Pediatr. (2015) 169:1003. doi: 10.1001/jamapediatrics.2015.1667
60. Boscarino G, Conti MG, Gasparini C, Onestà E, Faccioli F, Dito L, et al. Neonatal hyperglycemia related to parenteral nutrition affects long-term neurodevelopment in preterm newborn: a prospective cohort study. Nutrients. (2021) 13:1930. doi: 10.3390/nu13061930
61. Boscarino G, Conti MG, Di Chiara M, Bianchi M, Onestà E, Faccioli F, et al. Early enteral feeding improves tolerance of parenteral nutrition in preterm newborns. Nutrients. (2021) 13:3886. doi: 10.3390/nu13113886
62. Galderisi A, Facchinetti A, Steil GM, Ortiz-Rubio P, Cavallin F, Tamborlane WV, et al. Continuous glucose monitoring in very preterm infants: a randomized controlled trial. Pediatrics. (2017) 140:e20171162. doi: 10.1542/peds.2017-1162
63. Conti MG, Angelidou A, Diray-Arce J, Smolen KK, Lasky-Su J, De Curtis M, et al. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr Res. (2020) 87:399–405. doi: 10.1038/s41390-019-0647-6
64. Clyman RI, Mauray F, Roman C, Rudolph AM, Heymann MA. Glucocorticoids alter the sensitivity of the lamb ductus arteriosus to prostaglandin E2. J Pediatr. (1981) 98:126–8. doi: 10.1016/s0022-3476(81)80558-6
65. Clyman RI, Mauray F, Roman C, Heymann MA, Ballard PL, Rudolph AM, et al. Effects of antenatal glucocorticoid administration on ductus arteriosus of preterm lambs. Am J Physiol. (1981) 241:H415–20. doi: 10.1152/ajpheart.1981.241.3.H415
Keywords: hydrocortisone, dexamethasone, neurodevelopment, growth, extubation, pulmonary outcome, systemic hypertension, hyperglycemia
Citation: Boscarino G, Cardilli V, Conti MG, Liguori F, Repole P, Parisi P and Terrin G (2024) Outcomes of postnatal systemic corticosteroids administration in ventilated preterm newborns: a systematic review of randomized controlled trials. Front. Pediatr. 12:1344337. doi: 10.3389/fped.2024.1344337
Received: 25 November 2023; Accepted: 30 January 2024;
Published: 14 February 2024.
Edited by:
Karel Allegaert, KU Leuven, BelgiumReviewed by:
Anne Greenough, King’s College London, United KingdomNoah H. Hillman, Saint Louis University, United States
Shalin Parikh, Ganpat University, India
© 2024 Boscarino, Cardilli, Conti, Liguori, Repole, Parisi and Terrin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianluca Terrin Z2lhbmx1Y2EudGVycmluQHVuaXJvbWExLml0
Abbreviations BPD, bronchopulmonary dysplasia; ELGAN, extremely low gestational age newborns; GA, gestational age; HG, hyperglycemia; IV, intravenous; IVH, intraventricular hemorrhage; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NDV, neurodevelopment; PO, orally; PDA, patent ductus arteriosus; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCTs, randomized controlled trials; ROP, retinopathy of prematurity.
†ORCID Giovanni Boscarino orcid.org/0000-0003-2481-0692 Maria Giulia Conti orcid.org/0000-0001-8659-4652 Pasquale Parisi orcid.org/0000-0001-9042-8120 Gianluca Terrin orcid.org/0000-0003-3541-2876
 Giovanni Boscarino
Giovanni Boscarino Viviana Cardilli1
Viviana Cardilli1 Maria Giulia Conti
Maria Giulia Conti Pasquale Parisi
Pasquale Parisi Gianluca Terrin
Gianluca Terrin