
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 23 May 2024
Sec. Pediatric Cardiology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1340495
 Johannes Hofer1,2*
Johannes Hofer1,2* Marina Blum1
Marina Blum1 Regina Wiltsche1
Regina Wiltsche1 Nikoletta Deluggi1
Nikoletta Deluggi1 Daniel Holzinger1,2,3
Daniel Holzinger1,2,3 Johannes Fellinger1,2,4
Johannes Fellinger1,2,4 Gerald Tulzer5
Gerald Tulzer5 Gina Blum1
Gina Blum1 Raphael Oberhuber1,5,6
Raphael Oberhuber1,5,6
Background: Children with congenital heart defects (CHD) are at risk for a range of developmental disabilities that challenge cognition, executive functioning, self-regulation, communication, social-emotional functioning, and motor skills. Ongoing developmental surveillance is therefore key to maximizing neurodevelopmental outcome opportunities. It is crucial that the measures used cover the spectrum of neurodevelopmental domains relevant to capturing possible predictors and malleable factors of child development.
Objectives: This work aimed to synthesize the literature on neurodevelopmental measures and the corresponding developmental domains assessed in children aged 1−8 years with complex CHD.
Methods: PubMed was searched for terms relating to psycho-social, cognitive and linguistic-communicative outcomes in children with CHD. 1,380 papers with a focus on complex CHD that reported neurodevelopmental assessments were identified; ultimately, data from 78 articles that used standardized neurodevelopmental assessment tools were extracted.
Results: Thirty-nine (50%) of these excluded children with syndromes, and 9 (12%) excluded children with disorders of intellectual development. 10% of the studies were longitudinal. The neurodevelopmental domains addressed by the methods used were: 53% cognition, 16% psychosocial functioning, 18% language/communication/speech production, and 13% motor development-associated constructs.
Conclusions: Data on social communication, expressive and receptive language, speech motor, and motor function are underrepresented. There is a lack of research into everyday use of language and into measures assessing language and communication early in life. Overall, longitudinal studies are required that include communication measures and their interrelations with other developmental domains.
One in 100 newborns is affected by a congenital heart defect (CHD) (1). Twenty-five percent of them have a severe CHD that requires early corrective heart surgery within the first year of life (1). In about one third of these severely affected children, a genetic-syndromic disease causes the heart defect(s) (2). Since surgical and cardiological pediatric therapy protocols have improved over the last few years, around 80%–90% of children with CHD now survive to adulthood (3, 4).
Children with CHD are at risk for a range of developmental disabilities that challenge cognition, executive functioning, self-regulation, communication, social-emotional functioning, and motor skills (5–8). Studies have reported a prevalence of learning disability in 20% of children (9, 10), autism spectrum disorder in up to 10% (9, 11, 12), ADHD in up to 5% (13) and visual impairment in around 5% (14).
Despite the extensive knowledge gained about the developmental profiles of children with CHD, little is known about predictive and moderating developmental factors related to quality of life and psychosocial well-being. While only 5% of the variance in cognitive outcomes can be explained by surgical factors (15–17) and 1% by the choice of a cardiopulmonary bypass (18), up to 33% is determined by innate patient- and family-related variables (19, 20). Numerous studies have shown that the total length of stay in hospital is another predictor of cognitive outcome (21). However, with regard to developmental trajectories, a large proportion of the variance currently remains unexplained. There is great diversity in children with CHD, for instance, in the type of heart defect and its pathophysiological consequences, the palliation needed and its cardiocirculatory consequences, the variety of etiologies and the family resources available.
In 2012, the American Heart Association/American Academy of Pediatrics (5) highlighted the increased developmental risk for children with CHD and the need for ongoing developmental surveillance to maximize neurodevelopmental outcome opportunities. In 2020, the Cardiac Neurodevelopmental Outcome Collaborative established a consensus-based, standardized battery for the content and timing of neurodevelopmental assessments for children with complex CHD with the goal of promoting consistent neurodevelopmental care and quality improvement (22, 23). The recommendations include core and extended versions of age-specific assessment batteries.
This scoping review aims to provide a concise snapshot of the measures used to investigate neurodevelopmental domains of children with complex CHD between the ages of one and eight years. The research question was: To what extent do the standardized neurodevelopmental assessment tools cover the spectrum of neurodevelopmental domains relevant for capturing possible predictors and malleable factors of child development?
The methodological framework by Arksey and O'Malley (24) was used for this scoping review: (1) identify the research question; (2) identify relevant studies; (3) select studies; (4) chart the data; and (5) collect, summarize and report results.
The data synthesis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Studies were identified by searching the electronic database PubMed. Initial searches were conducted to identify relevant literature. Each search used the keywords “congenital heart disease” and one or more of the keywords from the following list: “mental health,” “quality of life,” “psychosocial outcome,” “neurodevelopmental outcome,” “social communication,” “parent-child interaction,” “language,” “social cognition,” “family,” “self-esteem,” “anxiety,” “depression”, “functional outcome.”
The quality of the studies was assessed using an adapted version of the Newcastle-Ottawa Scale (25) with a maximum score of 7 points. One point was allocated to each of the following categories:
a) representativeness of the sample (e.g., all syndromes were included, no exclusion due to ethnicity, language or cognitive function);
b) sample size (adequate);
c) adequate control group available;
d) clear definition of the neurodevelopmental measures used;
e) clear description of the domains assessed
f) the study reported on outcomes; and
g) appropriate statistical analysis was conducted and included.
Original articles were included in the review if all of the following criteria were met: (i) study participants were diagnosed with complex CHD and (ii) children aged between 1 and 8 years were included; (iii) the article was in English, peer-reviewed and from the period 1980–2021; (iv) standardized quantitative methods were used to evaluate neurodevelopment; and (v) the study had a fair to good quality [adapted Newcastle-Ottawa Scale (NOS) score >2]. Studies based exclusively on questionnaires or interviews without direct assessment were excluded.
Four authors (Raphael Oberhuber (RO), Nikoletta Deluggi (ND), Regina Wiltsche (RW), Marina Blum (MB)) conducted the initial searches, and after initial exclusion at the title and abstract levels, 270 articles remained. These 270 articles were again independently screened at the title, abstract and text levels. The remaining 170 articles were then reviewed by two researchers [Johannes Hofer (JH) and MB] at the text level, which resulted in a final selection of 78 articles with 100% agreement. The reference lists of the selected articles were examined for the possibility of additional relevant studies; however, no additional studies were found. Figure 1 shows the flow diagram of the review process.
A standardized data extraction form was used to extract data from the included articles. For each article, the following data were extracted: (i) study title, (ii) names of authors, (iii) Digital Object Identifier, (iv) year of publication, (v) journal, (vi) details of the study population, (vii) number of participants, (viii) percentage of male participants, (ix) ethnicity of participants, (x) inclusion criteria, (xi) exclusion criteria, (xii) study type, (xiii) outcome measures, (xiv) methods/tests used, (xv) main findings of the study. The standardized neurodevelopmental measures extracted were categorized into four main domains: cognition, psychosocial functioning, language/communication/speech production and motor functioning. Table 1 lists the domains assigned to the set constructs.
Seventy-eight articles met the inclusion criteria of the study. The majority of these (57/78; 73%) were published in the United States, and the remaining 21 in other high-income countries. No study was conducted in a middle- or low-income country.
The publication years of the articles ranged from 1995 to 2021, with a median of 2013 (SD = 5.7). All studies were published within the past 28 years, and 60% in the last ten years.
Most studies (58%) used a retrospective, cross-sectional, or mixed study design, while the remaining 42% used a prospective and/or longitudinal design. The adapted NOS scores for the studies included ranged from 3 to 7 for a maximum of seven points, with a mean of 5.4. Table 2 lists all longitudinal studies included and all other studies with a NOS score >4. A total of 12.125 participants, ranging in age from 1 month to 15 years, were included across the articles. All extracted measures referred to the age group 1–8 years. The number of participants ranged from 10 to 1.770, with a mean of 155 (SD = 236.5). Thirteen different patient cohorts were found (Table 3). Forty-seven (59%) articles used data taken from these 13 cohorts.
Overall, eight longitudinal cohorts were included in the articles reviewed, with follow-ups conducted after 1–5 years.
The neurodevelopmental domains measured were distributed as follows: Cognition-associated constructs (53%) formed the main domain tested, followed by speech/language/communication (18%), psychosocial functioning (16%) and motor development (13%). Overall, a variety of standardized measures were used to describe neurodevelopmental functioning; however, the Bayley scales most prominent.
Tables 4A–C lists all extracted measures that were used in three or more publications according to domain, construct and target age group.
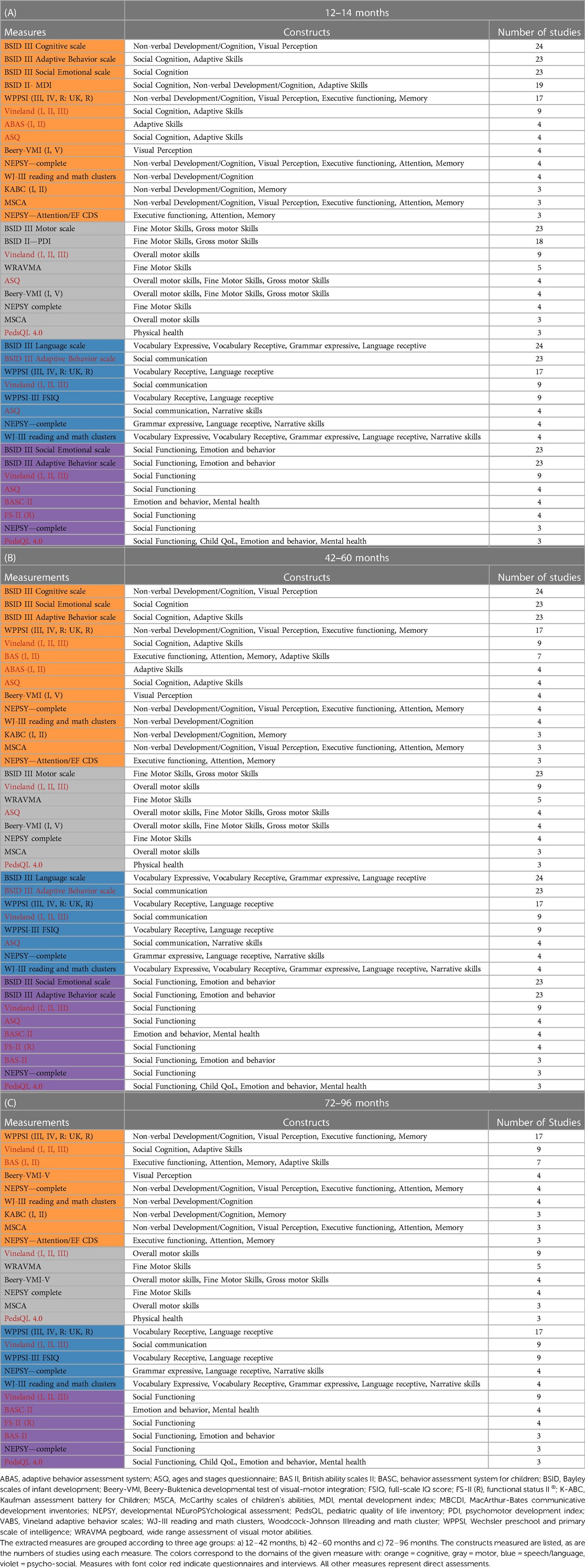
Table 4. (A)–(C) extracted standardized measures used in a minimum of 3 of the extracted publications.
Thirty-nine of the measures (54%) were used in only one study. Thirty-nine (50%) of the 78 articles excluded children with syndromes, and nine (12%) excluded children with cognitive disabilities.
The goal of this review was to generate a concise overview of the methodology used to document neurodevelopment in young children aged 1–8 years with CHD, identify factors that are modifiable by intervention and to identify research gaps that need to be addressed by future studies.
Seventy-eight studies met the inclusion criteria, all of which were conducted in high-income countries within the past 28 years. That no study from a low- or middle-income country was found demonstrates the compelling need to extend rigorous science and innovative clinical practice by focusing on stepped care processes at the global level.
The quality of the studies included, assessed by means of an adapted Newcastle-Ottawa Scale, ranged from fair to excellent. Most studies (58%) used a retrospective, cross-sectional, or mixed study design, while the remaining 42% used a prospective and/or longitudinal design. Less than half of the studies (30/78, 38%) included representative samples that did not exclude severely affected children (28–31, 33–35, 37–45, 48–52, 56, 60–63, 74, 79, 82, 84, 85). Notably, 50% of the 78 articles excluded children with various syndromes, and 12% excluded children with cognitive disabilities. This is especially true for the few larger longitudinal studies (26, 27): Children with chromosomal changes and/or additional impairments were excluded. Insufficient recruitment of children with poor cardiac outcomes and disproportionate inclusion of privileged children and families are common. Including children with special needs requires additional time and knowledge. It is challenging to combine age-appropriate testing with testing that is appropriate to the individual child's level of development. This finding is not surprising and is well known for other well-studied patient cohorts, for instance, children with hearing loss (86) and children with autism spectrum disorders (87). Epidemiological study designs are needed to ensure that all children with CHD are included in our understanding of neurodevelopment and neurodevelopmental trajectories and their potential malleability.
Longitudinal data on the neurodevelopment of children with CHD are restricted to 8 different study cohorts, where follow-ups were conducted over a maximum of five years. Despite their high quality and their scene-setting impact, these studies did not use cohorts that were broadly representative. Considering the recommendations from the Cardiac Neurodevelopmental Outcome Collaborative (22), the measures chosen for neurodevelopmental trajectories were not sufficiently fine-tuned to allow malleable predictors of good neurodevelopmental outcomes to be identified.
Overall, trajectories of structural language development, especially social communication and speech production, have been less researched than other domains of cognitive functioning. With increasing age of the children studied, published data on (i) language and communication and (ii) speech motor and motor function decrease, and neurodevelopmental measures within the cognitive domain become even more predominant.
Considering the predominant use of the Bayley Scales for early infant development and the current literature pointing towards an overestimation of neurodevelopment using the Bayley III scales and possible underestimation by using the Bayley II scales (88–92), there is a need for supplementary measures of early childhood neurodevelopment. However, the Bayley III language scales provide a good estimate of language development (88, 91, 92).
There remains a lack of research into measures of language and communication early in life and into everyday use of language, although social communication is expected to impact language development, social cognition, peer interaction, and mental health later in life. In addition, standardized measures of child self-regulation and parent-child interaction are missing and need to be addressed in future studies, as they represent important, potentially malleable predictors.
Overall, a variety of standardized measures have been used to describe neurodevelopmental functioning, with the Bayley scales dominating. Comparing the results of this scoping review to the recent recommendations for neurodevelopmental assessment (22, 23) highlights the importance of a clear and standardized guidance for neurodevelopmental assessments.
Given the known risks associated with CHD and the demonstrated benefit of early intervention in other populations (93–96), regular monitoring and periodic neurodevelopmental assessment are critical throughout childhood in order to optimize the neurodevelopmental outcomes of patients with CHD.
Standard application of well-balanced neurodevelopmental assessment batteries across cardiac neurodevelopmental sites holds enormous promise for both clinical care and research within the CHD population.
The findings discussed above must be considered in the light of specific limitations of this scoping review and the limitations of scoping reviews in general:
Including studies only by searching the electronic database PubMed may exclude further relevant published literature and grey literature, which leads to potential bias in the findings. This can result in an incomplete representation of the evidence available. The used search terms lack infant specific formulations like regulation or attention.
In conclusion, our systematic review of the neurodevelopmental assessment tools used in children with complex CHD identified the following research gaps:
➢ No data on low- or middle-income countries,
➢ There is a lack of representative studies of the whole cohort of children with severe CHD: children with syndromes or intellectual disability are in most cases excluded,
➢ Need for longitudinal studies that focus on a balanced use of measures for all important neurodevelopmental domains,
➢ Data on social communication, expressive and receptive language, speech motor, and motor function are underrepresented,
➢ Presently, there remains a lack of (i) research into the everyday use of language and language and communication measures early in life and (ii) tools to measure early social communication skills.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JH: Conceptualization, Writing – original draft, Writing – review & editing. MB: Project administration, Writing – original draft, Writing – review & editing. RW: Data curation, Writing – review & editing. ND: Data curation, Methodology, Writing – review & editing. DH: Conceptualization, Writing – review & editing. JF: Conceptualization, Writing – review & editing. GT: Supervision, Writing – review & editing. GB: Formal Analysis, Visualization, Writing – review & editing. RO: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Funded by the parent organization „Kinderherzverein “sterreich”. Article processing charge was funded by the Johannes Kepler University Open Access Publishing Fund.
The authors thank Michaela Altendorfer, the president of the “Herzkinderverein Österreich”, for her support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39(12):1890–900. doi: 10.1016/S0735-1097(02)01886-7
2. Sun R, Liu M, Lu L, Zheng Y, Zhang P. Congenital heart disease: causes, diagnosis, symptoms, and treatments. Cell Biochem Biophys. (2015) 72:857–60. doi: 10.1007/s12013-015-0551-6
3. Gerstle M, Beebe DW, Drotar D, Cassedy A, Marino BS. Executive functioning and school performance among pediatric survivors of complex congenital heart disease. J Pediatr. (2016) 173:154–9. doi: 10.1016/j.jpeds.2016.01.028
4. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. (2001) 37(5):1170–5. doi: 10.1016/S0735-1097(01)01272-4
5. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. (2012) 126(9):1143–72. doi: 10.1161/CIR.0b013e318265ee8a
6. Sarrechia I, Miatton M, De Wolf D, François K, Gewillig M, Meyns B, et al. Neurocognitive development and behaviour in school-aged children after surgery for univentricular or biventricular congenital heart disease. Eur J Cardiothorac Surg. (2016) 49(1):167–74. doi: 10.1093/ejcts/ezv029
7. Oberhuber RD, Huemer S, Mair R, Sames-Dolzer E, Kreuzer M, Tulzer G. Cognitive development of school-age hypoplastic left heart syndrome survivors: a single center study. Pediatr Cardiol. (2017) 38(6):1089–96. doi: 10.1007/s00246-017-1623-8
8. Huisenga D, La Bastide-Van Gemert S, Van Bergen A, Sweeney J, Hadders-Algra M. Developmental outcomes after early surgery for complex congenital heart disease: a systematic review and meta-analysis. Dev Med Child Neurol. (2021) 63(1):29–46. doi: 10.1111/dmcn.14512
9. Razzaghi H, Oster M, Reefhuis J. Long-term outcomes in children with congenital heart disease: National Health Interview Survey. J Pediatr. (2015) 166:119–24. doi: 10.1016/j.jpeds.2014.09.006
10. Ryberg C, Sunnegårdh J, Thorson M, Broberg M. Intellectual functioning in children with congenital heart defects treated with surgery or by catheter interventions. Front Pediatr. (2016) 4:113. doi: 10.3389/fped.2016.00113
11. Tan A, Semmel ES, Wolf I, Hammett B, Ilardi D. Implementing standard screening for autism spectrum disorder in CHD. Cardiol Young. (2020) 30(8):1118–25. doi: 10.1017/S1047951120001626
12. Loblein HJ, Vukmirovich PW, Donofrio MT, Sanz JH. Prevalence of neurodevelopmental disorders in a clinically referred sample of children with CHD. Cardiol Young. (2023) 33:619–26. doi: 10.1017/S1047951122001469
13. Gonzalez VJ, Kimbro RT, Cutitta KE, Shabosky JC, Bilal MF, Penny DJ, et al. Mental health disorders in children with congenital heart disease. Pediatrics. (2021) 147(2):e20201693. doi: 10.1542/peds.2020-1693
14. Vilela MA, Colossi CG, Freitas HP, Del Valle G, Pellanda LC. Ocular alterations associated with primary congenital heart disease–A cross-sectional study. Middle East Afr J Ophthalmol. (2020) 27(1):28. doi: 10.4103/meajo.MEAJO_89_19
15. Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics. (1999) 103(2):402–8. doi: 10.1542/peds.103.2.402
16. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. (2007) 133(5):1344–53. doi: 10.1016/j.jtcvs.2006.10.087
17. Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. (2012) 27(2):82–91. doi: 10.1097/HCO.0b013e328350197b
18. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. (2015) 135(5):816–25. doi: 10.1542/peds.2014-3825
19. McCusker CG, Doherty NN, Molloy B, Casey F, Rooney N, Mulholland C, et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch Dis Child. (2007) 92(2):137–41. doi: 10.1136/adc.2005.092320
20. McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child Care Health Dev. (2010) 36(1):110–7. doi: 10.1111/j.1365-2214.2009.01026.x
21. Mussatto KA, Hoffmann R, Hoffman G, Tweddell JS, Bear L, Cao Y, et al. Risk factors for abnormal developmental trajectories in young children with congenital heart disease. Circulation. (2015) 132(8):755–61. doi: 10.1161/CIRCULATIONAHA.114.014521
22. Ware J, Butcher JL, Latal B, Sadhwani A, Rollins CK, Soto CLB, et al. Neurodevelopmental evaluation strategies for children with congenital heart disease aged birth through 5 years: recommendations from the cardiac neurodevelopmental outcome collaborative. Cardiol Young. (2020) 30(11):1609–22. doi: 10.1017/S1047951120003534
23. Ilardi D, Sanz JH, Cassidy AR, Sananes R, Rollins CK, Shade CU, et al. Neurodevelopmental evaluation for school-age children with congenital heart disease: recommendations from the cardiac neurodevelopmental outcome collaborative. Cardiol Young. (2020) 30(11):1623–36. doi: 10.1017/S1047951120003546
24. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8(1):19–32. doi: 10.1080/1364557032000119616
25. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. (2012). Available online at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp
26. Hoskoppal A, Roberts H, Kugler J, Duncan K, Needelman H. Neurodevelopmental outcomes in infants after surgery for congenital heart disease: a comparison of single-ventricle vs. Two-ventricle physiology. Congenit Heart Dis. (2010) 5(2):90–5. doi: 10.1111/j.1747-0803.2009.00373.x
27. Sananes R, Manlhiot C, Kelly E, Hornberger LK, Williams WG, MacGregor D, et al. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. (2012) 93(5):1577–83. doi: 10.1016/j.athoracsur.2012.02.011
28. Wray J, Radley-Smith R. Longitudinal assessment of psychological functioning in children after heart or heart–lung transplantation. J Heart Lung Transplant. (2006) 25(3):345–52. doi: 10.1016/j.healun.2005.09.018
29. Brosig CL, Bear L, Allen S, Simpson P, Zhang L, Frommelt M, et al. Neurodevelopmental outcomes at 2 and 4 years in children with congenital heart disease. Congenit Heart Dis. (2018) 13(5):700–5. doi: 10.1111/chd.12632
30. Bucholz EM, Sleeper LA, Sananes R, Brosig CL, Goldberg CS, Pasquali SK, et al. Trajectories in neurodevelopmental, health-related quality of life, and functional status outcomes by socioeconomic status and maternal education in children with single ventricle heart disease. J Pediatr. (2021) 229:289–93. doi: 10.1016/j.jpeds.2020.09.066
31. Hiraiwa A, Ibuki K, Tanaka T, Hirono K, Miya K, Yoshimura N, et al. Toddler neurodevelopmental outcomes are associated with school-age IQ in children with single ventricle physiology. Semin Thorac Cardiovasc Surg. (2020) 32(2):302–10. doi: 10.1053/j.semtcvs.2019.10.017
32. McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A randomized controlled trial of interventions to promote adjustment in children with congenital heart disease entering school and their families. J Pediatr Psychol. (2012) 37(10):1089–103. doi: 10.1093/jpepsy/jss092
33. Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. (2014) 133(3):e570–7. doi: 10.1542/peds.2013-2309
34. Sananes R, Goldberg CS, Newburger JW, Hu C, Trachtenberg F, Gaynor JW, et al. Six-year neurodevelopmental outcomes for children with single-ventricle physiology. Pediatrics. (2021) 147(2):e2020014589. doi: 10.1542/peds.2020-014589
35. Atallah J, Dinu IA, Joffe AR, Robertson CM, Sauve RS, Dyck JD, et al. Two-year survival and mental and psychomotor outcomes after the Norwood procedure: an analysis of the modified blalock-taussig shunt and right ventricle–to–pulmonary artery shunt surgical eras. Circulation. (2008) 118(14):1410–8. doi: 10.1161/CIRCULATIONAHA.107.741579
36. Barbosa MDG, Castelo PM, Ferreira CLP, Haddad DS, Chiari BM, Santana MV, et al. Congenital heart disease in children: orofacial myofunctional aspects, eating behavior and facial temperature. Int J Pediatr Otorhinolaryngol. (2020) 131:109883. doi: 10.1016/j.ijporl.2020.109883
37. Bayram AK, Kütük MS, Doganay S, Özgün MT, Gümüş H, Başbuğ M, et al. An analysis of 109 fetuses with prenatal diagnosis of complete agenesis of corpus callosum. Neurol Sci. (2020) 41:1521–9. doi: 10.1007/s10072-019-04224-4
38. Brosig CL, Bear L, Allen S, Hoffmann RG, Pan A, Frommelt M, et al. Preschool neurodevelopmental outcomes in children with congenital heart disease. J Pediatr. (2017) 183:80–6. doi: 10.1016/j.jpeds.2016.12.044
39. Bucholz EM, Sleeper LA, Goldberg CS, Pasquali SK, Anderson BR, Gaynor JW, et al. Socioeconomic status and long-term outcomes in single ventricle heart disease. Pediatrics. (2020) 146(4):e20201240. doi: 10.1542/peds.2020-1240
40. Butts RJ, Zak V, Hsu D, Cnota J, Colan SD, Hehir D, et al. Factors associated with serum B-type natriuretic peptide in infants with single ventricles. Pediatr Cardiol. (2014) 35:879–87. doi: 10.1007/s00246-014-0872-z
41. Chorna O, Baldwin HS, Neumaier J, Gogliotti S, Powers D, Mouvery A, et al. Feasibility of a team approach to complex congenital heart defect neurodevelopmental follow-up: early experience of a combined cardiology/neonatal intensive care unit follow-up program. Circ Cardiovasc Qual Outcomes. (2016) 9(4):432–40. doi: 10.1161/CIRCOUTCOMES.116.002614
42. Cottrell SM, Morris KP, Davies P, Bellinger DC, Jonas RA, Newburger JW. Early postoperative body temperature and developmental outcome after open heart surgery in infants. Ann Thorac Surg. (2004) 77(1):66–71. doi: 10.1016/S0003-4975(03)01362-6
43. Friedland-Little JM, Uzark K, Yu S, Lowery R, Aiyagari R, Hirsch-Romano JC. Functional status and quality of life in survivors of extracorporeal membrane oxygenation after the Norwood operation. Ann Thorac Surg. (2017) 103(6):1950–5. doi: 10.1016/j.athoracsur.2016.11.015
44. Goldberg CS, Hu C, Brosig C, Gaynor JW, Mahle WT, Miller T, et al. Behavior and quality of life at 6 years for children with hypoplastic left heart syndrome. Pediatrics. (2019) 144(5):e20191010. doi: 10.1542/peds.2019-1010
45. Goldberg CS, Lu M, Sleeper LA, Mahle WT, Gaynor JW, Williams IA, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. (2014) 165(3):490–6. doi: 10.1016/j.jpeds.2014.05.019
46. Goldsworthy M, Franich-Ray C, Kinney S, Shekerdemian L, Beca J, Gunn J. Relationship between social-emotional and neurodevelopment of 2-year-old children with congenital heart disease. Congenit Heart Dis. (2016) 11(5):378–85. doi: 10.1111/chd.12320
47. Hallioglu O, Gurer G, Bozlu G, Karpuz D, Makharoblidze K, Okuyaz C. Evaluation of neurodevelopment using bayley-III in children with cyanotic or hemodynamically impaired congenital heart disease. Congenit Heart Dis. (2015) 10(6):537–41. doi: 10.1111/chd.12269
48. Hamrick SE, Gremmels DB, Keet CA, Leonard CH, Connell JK, Hawgood S, et al. Neurodevelopmental outcome of infants supported with extracorporeal membrane oxygenation after cardiac surgery. Pediatrics. (2003) 111(6):e671–5. doi: 10.1542/peds.111.6.e671
49. Hövels-Gürich HH, Bauer SB, Schnitker R, Messmer BJ, Seghaye MC, Huber W. Long-term outcome of speech and language in children after corrective surgery for cyanotic or acyanotic cardiac defects in infancy. Eur J Paediatr Neurol. (2008) 12(5):378–86. doi: 10.1016/j.ejpn.2007.10.004
50. Kern JH, Hinton VJ, Nereo NE, Hayes CJ, Gersony WM. Early developmental outcome after the Norwood procedure for hypoplastic left heart syndrome. Pediatrics. (1998) 102(5):1148–52. doi: 10.1542/peds.102.5.1148
51. Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Gender differences in the developmental outcomes of children with congenital cardiac defects. Cardiol Young. (2012) 22(5):514–9. doi: 10.1017/S1047951111002071
52. Martin BJ, De Villiers Jonker I, Joffe AR, Bond GY, Acton BV, Ross DB, et al. Hypoplastic left heart syndrome is not associated with worse clinical or neurodevelopmental outcomes than other cardiac pathologies after the norwood–sano operation. Pediatr Cardiol. (2017) 38:922–31. doi: 10.1007/s00246-017-1598-5
53. Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Do parental ratings on cognition reflect neuropsychological outcome in congenital heart disease? Acta Paediatr. (2008) 97(1):41–5. doi: 10.1111/j.1651-2227.2007.00530.x
54. Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr. (2007) 151(1):73–8. doi: 10.1016/j.jpeds.2007.02.020
55. Naef N, Liamlahi R, Beck I, Bernet V, Dave H, Knirsch W, et al. Neurodevelopmental profiles of children with congenital heart disease at school age. J Pediatr. (2017) 188:75–81. doi: 10.1016/j.jpeds.2017.05.073
56. Ricci MF, Alton GY, Ross DB, Dicken BJ, Moddemann DM, Robertson CM, et al. Gastrostomy tube feeding after neonatal complex cardiac surgery identifies the need for early developmental intervention. J Pediatr. (2016) 169:160–5. doi: 10.1016/j.jpeds.2015.10.087
57. Sadhwani A, Asaro LA, Goldberg C, Ware J, Butcher J, Gaies M, et al. Impact of tight glycemic control on neurodevelopmental outcomes at 1 year of age for children with congenital heart disease: a randomized controlled trial. J Pediatr. (2016) 174:193–8. doi: 10.1016/j.jpeds.2016.03.048
58. Sarajuuri A, Lönnqvist T, Mildh L, Rajantie I, Eronen M, Mattila I, et al. Prospective follow-up study of children with univentricular heart: neurodevelopmental outcome at age 12 months. J Thorac Cardiovasc Surg. (2009) 137(1):139–45. doi: 10.1016/j.jtcvs.2008.06.025
59. Yoshida T, Hiraiwa A, Ibuki K, Makimoto M, Inomata S, Tamura K, et al. Neurodevelopmental outcomes at 3 years for infants with congenital heart disease and very-low birthweight. Pediatr Int. (2020) 62(7):797–803. doi: 10.1111/ped.14160
60. Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, et al. Neurodevelopmental outcome of patients after the fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. (2000) 137(5):646–52. doi: 10.1067/mpd.2000.108952
61. Heye KN, Knirsch W, Latal B, Scheer I, Wetterling K, Hahn A, et al. Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before fontan completion. Pediatr Res. (2018) 83(1):63–70. doi: 10.1038/pr.2017.203
62. Puosi R, Korkman M, Sarajuuri A, Jokinen E, Mildh L, Mattila I, et al. Neurocognitive development and behavioral outcome of 2-year-old children with univentricular heart. J Int Neuropsychol Soc. (2011) 17(6):1094–103. doi: 10.1017/S135561771100110X
63. Werner H, Latal B, Buechel EV, Beck I, Landolt MA. Health-related quality of life after open-heart surgery. J Pediatr. (2014) 164(2):254–8. doi: 10.1016/j.jpeds.2013.10.022
64. Atallah J, Joffe AR, Robertson CM, Leonard N, Blakley PM, Nettel-Aguirre A, et al. Two-year general and neurodevelopmental outcome after neonatal complex cardiac surgery in patients with deletion 22q11.2: a comparative study. J Thorac Cardiovasc Surg. (2007) 134(3):772–9. doi: 10.1016/j.jtcvs.2007.03.007
65. Garcia Guerra G, Robertson CM, Alton GY, Joffe AR, Cave DA, Yasmin F, et al. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Pediatr Anesth. (2014) 24(3):257–65. doi: 10.1111/pan.12257
66. Garcia Guerra G, Robertson CM, Alton GY, Joffe AR, Cave DA, Dinu IA, et al. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Pediatr Anesth. (2011) 21(9):932–41. doi: 10.1111/j.1460-9592.2011.03581.x
67. Grasty MA, Ittenbach RF, Knightly C, Solot CB, Gerdes M, Bernbaum JC, et al. Hearing loss after cardiac surgery in infancy: an unintended consequence of life-saving care. J Pediatr. (2018) 192:144–51. doi: 10.1016/j.jpeds.2017.09.049
68. Fuller S, Rajagopalan R, Jarvik GP, Gerdes M, Bernbaum J, Wernovsky G, et al. Deep hypothermic circulatory arrest does not impair neurodevelopmental outcome in school-age children after infant cardiac surgery. Ann Thorac Surg. (2010) 90(6):1985–95. doi: 10.1016/j.athoracsur.2010.08.005
69. Gaynor JW, Ittenbach RF, Gerdes M, Bernbaum J, Clancy RR, McDonald-McGinn DM, et al. Neurodevelopmental outcomes in preschool survivors of the fontan procedure. J Thorac Cardiovasc Surg. (2014) 147(4):1276–83. doi: 10.1016/j.jtcvs.2013.12.019
70. Schultz AH, Ittenbach RF, Gerdes M, Jarvik GP, Wernovsky G, Bernbaum J, et al. Effect of congenital heart disease on 4-year neurodevelopment within multiple-gestation births. J Thorac Cardiovasc Surg. (2017) 154(1):273–81. doi: 10.1016/j.jtcvs.2017.02.022
71. Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. (2012) 125(17):2081–91. doi: 10.1161/CIRCULATIONAHA.111.064113
72. Peyvandi S, Chau V, Guo T, Xu D, Glass HC, Synnes A, et al. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J Am Coll Cardiol. (2018) 71(18):1986–96. doi: 10.1016/j.jacc.2018.02.068
73. Reich B, Heye KN, Tuura ROG, Beck I, Wetterling K, Hahn A, et al. Interrelationship between hemodynamics, brain volumes, and outcome in hypoplastic left heart syndrome. Ann Thorac Surg. (2019) 107(6):1838–44. doi: 10.1016/j.athoracsur.2018.12.012
74. Knirsch W, Mayer KN, Scheer I, Tuura R, Schranz D, Hahn A, et al. Structural cerebral abnormalities and neurodevelopmental status in single ventricle congenital heart disease before fontan procedure. Eur J Cardiothorac Surg. (2017) 51(4):740–6. doi: 10.1093/ejcts/ezw399
75. Heye KN, Knirsch W, Scheer I, Beck I, Wetterling K, Hahn A, et al. Health-related quality of life in pre-school age children with single-ventricle CHD. Cardiol Young. (2019) 29(2):162–8. doi: 10.1017/S1047951118001993
76. Roberts SD, Kazazian V, Ford MK, Marini D, Miller SP, Chau V, et al. The association between parent stress, coping and mental health, and neurodevelopmental outcomes of infants with congenital heart disease. Clin Neuropsychol. (2021) 35(5):948–72. doi: 10.1080/13854046.2021.1896037
77. Fourdain S, St-Denis A, Harvey J, Birca A, Carmant L, Gallagher A, et al. Language development in children with congenital heart disease aged 12–24 months. Eur J Paediatr Neurol. 2019;23(3):491–9. doi: 10.1016/j.ejpn.2019.03.002
78. Liamlahi R, Latal B. Neurodevelopmental outcome of children with congenital heart disease. Handb Clin Neurol. (2019) 162:329–45. doi: 10.1016/B978-0-444-64029-1.00016-3
79. Sarajuuri A, Jokinen E, Puosi R, Eronen M, Mildh L, Mattila I, et al. Neurodevelopmental and neuroradiologic outcomes in patients with univentricular heart aged 5 to 7 years: related risk factor analysis. J Thorac Cardiovasc Surg. (2007) 133(6):1524–32. doi: 10.1016/j.jtcvs.2006.12.022
80. Gunn JK, Beca J, Hunt RW, Goldsworthy M, Brizard CP, Finucane K, et al. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child. (2016) 101(11):1010–6. doi: 10.1136/archdischild-2015-309449
81. Gunn JK, Beca J, Hunt RW, Olischar M, Shekerdemian LS. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. (2012) 38:1539–47. doi: 10.1007/s00134-012-2608-y
82. Wolfe KR, Brinton J, Di Maria MV, Meier M, Liptzin DR. Oxygen saturations and neurodevelopmental outcomes in single ventricle heart disease. Pediatr Pulmonol. (2019) 54(6):922–7. doi: 10.1002/ppul.24275
83. Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, et al. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. (2013) 162(2):250–6. doi: 10.1016/j.jpeds.2012.07.048
84. Williams IA, Fifer WP, Andrews H. Fetal growth and neurodevelopmental outcome in congenital heart disease. Pediatr Cardiol. (2015) 36:1135–44. doi: 10.1007/s00246-015-1132-6
85. Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Gender differences in use of stress testing and coronary heart disease mortality: a population-based study in Olmsted County, Minnesota. J Am Coll Cardiol. (1998) 32(2):345–52. doi: 10.1016/S0735-1097(98)00229-0
86. Dall M, Kiblböck S, Müllegger D, Fellinger J, Hofer J, Kapplmüller R, et al. Understanding the impact of child, intervention, and family factors on developmental trajectories of children with hearing loss at preschool age: design of the AChild study. J Clin Med. (2022) 11(6):1508. doi: 10.3390/jcm11061508
87. Lord C, Charman T, Havdahl A, Carbone P, Anagnostou E, Boyd B, et al. The lancet commission on the future of care and clinical research in autism. Lancet. (2022) 399(10321):271–334. doi: 10.1016/S0140-6736(21)01541-5
88. Torras-Mañá M, Guillamón-Valenzuela M, Ramírez-Mallafré A, Brun-Gasca C, Fornieles-Deu A. Usefulness of the bayley scales of infant and toddler development,third edition, in the early diagnosis of language disorder. Psicothema. (2014) 26(3):349–56. doi: 10.7334/psicothema2014.29
89. Acton BV, Biggs WS, Creighton DE, Penner KA, Switzer HN, Thomas JH, et al. Overestimating neurodevelopment using the Bayley-III after early complex cardiac surgery. Pediatrics. (2011) 128(4):e794–800. doi: 10.1542/peds.2011-0331
90. Goldstone AB, Baiocchi M, Wypij D, Stopp C, Andropoulos DB, Atallah J, et al. The Bayley-III scale may underestimate neurodevelopmental disability after cardiac surgery in infants. Eur J Cardiothorac Surg. (2020) 57(1):63–71. doi: 10.1093/ejcts/ezz123
91. Rasheed MA, Kvestad I, Shaheen F, Memon U, Strand TA. The predictive validity of Bayley Scales of infant and toddler development-III at 2 years for later general abilities: findings from a rural, disadvantaged cohort in Pakistan. PLOS Glob Public Health. (2023) 3(1):e0001485. doi: 10.1371/journal.pgph.0001485
92. Flynn RS, Huber MD, DeMauro SB. Predictive value of the BSID-II and the Bayley-III for early school age cognitive function in very preterm infants. Glob Pediatr Health. (2020) 7:2333794X20973146. doi: 10.1177/2333794X20973146
93. Anderson PJ, Treyvaud K, Spittle AJ. Early developmental interventions for infants born very preterm—what works? Semin Fetal Neonatal Med. (2020) 25(3):101119. doi: 10.1016/j.siny.2020.101119
94. Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. (2015) 2015(11):CD005495. doi: 10.1002/14651858.CD005495
95. Sandbank M, Bottema-Beutel K, Crowley S, Cassidy M, Dunham K, Feldman JI, et al. Project AIM: autism intervention meta-analysis for studies of young children. Psychol Bull. (2020) 146(1):1–29. doi: 10.1037/bul0000215
Keywords: complex congenital heart defects (CHD), neurodevelopment, social communication, speech motor, malleable predictors, assessment
Citation: Hofer J, Blum M, Wiltsche R, Deluggi N, Holzinger D, Fellinger J, Tulzer G, Blum G and Oberhuber R (2024) Research gaps in the neurodevelopmental assessment of children with complex congenital heart defects: a scoping review. Front. Pediatr. 12:1340495. doi: 10.3389/fped.2024.1340495
Received: 21 November 2023; Accepted: 15 April 2024;
Published: 23 May 2024.
Edited by:
Eva Goossens, University of Antwerp, BelgiumReviewed by:
Kathleen Mussatto, Milwaukee School of Engineering, United States© 2024 Hofer, Blum, Wiltsche, Deluggi, Holzinger, Fellinger, Tulzer, Blum and Oberhuber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Hofer, am9oYW5uZXMuaG9mZXJAamt1LmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.