
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 06 June 2024
Sec. Pediatric Endocrinology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1338404
Introduction: Hyponatremia is one of the most prevalent water-electrolyte disturbances encountered in clinical practice in pediatrics and can arise from various conditions. However, there are limited reports on hyponatremia in hospitalized infants. The objective of this study was to provide an overview of the incidence, etiologies, and clinical characteristics of hyponatremia in hospitalized babies (from birth to 3 years old) at a tertiary hospital.
Method: Computer records of all hospitalized babies (from birth to 3 years old) with hyponatremia were extracted from the First Affiliated Hospital of Guangxi Medical University's clinical databases.
Results: 801 patients from 39,019 hospital admissions were found to have hyponatremia and the overall prevalence of this condition was 2.05% in babies. Patients with hyponatremia due to aldosterone signaling abnormalities, neurological disorders, and liver diseases exhibited more severe outcomes than those with other etiologies.
Conclusions: Various conditions can result in hyponatremia in hospitalized babies. Aldosterone signaling abnormalities were not that uncommon and it could lead to severe hyponatremia in babies.
A balance of water and electrolytes is essential for maintaining the normal biological processes in the human body. Sodium, the major electrolyte in extracellular fluid, plays a crucial role in stabilizing intracellular and extracellular volumes, as well as regulating acid-base balance (1, 2). Water balance and sodium homeostasis are closely interconnected. Thirst and the levels of antidiuretic hormone (ADH), which is secreted by the pituitary gland, are the principal mechanisms for regulating water metabolism. However, the concentration of sodium is mainly controlled by aldosterone. An excess of ADH can occur as a result of either hypovolemia or an inappropriate secondary secretion due to neurological disorders or pulmonary diseases, and these can increase the relative excess of water in the total body fluid, leading to hyponatremia. Aldosterone, the major mineralocorticoid hormone in humans, is responsible for the re-absorption of sodium as well as the excretion of potassium via the distal nephrons (3). Aldosterone signaling defects including defects in its biosynthesis [e.g., congenital adrenal hyperplasia (CAH), primary adrenal insufficiency (PAI) and isolated hypoaldosteronism] or aldosterone functional resistance (pseudo-hypoaldosteronism, PHA) may result in hyponatremia, especially in in babies (4, 5).
Hyponatremia is the most common electrolyte imbalance encountered in clinical practice, accounting for up to 20% of emergency hospital admissions, and it is a biochemical manifestation of various diseases (6, 7). In certain disorders, hyponatremia has been associated with disease severity, including increased mortality and hospital stay duration (8–10). Both acute hyponatremia (occurring within <48 h) and severe hyponatremia (serum sodium concentration <125 mmol/L) are considered as medical emergencies, since they can lead to serious neurological complications such as brain edema. Moreover, even mild hyponatremia has been linked to increased morbidity and mortality (11). Compared to adults, the occurrence of hyponatremia in infants is considered more dangerous due to their higher water and electrolyte requirements, as well as their weaker abilities to reabsorb sodium and water in urine. However, there are limited reports on hyponatremia in infants and young children.
The objective of this study was to assess all hospitalized babies (aged 0–36 months) in a tertiary hospital who presented with hyponatremia upon admission, in order to provide an overview of the incidence, etiologies, and clinical characteristics of this condition in this specific age group.
A retrospective study was conducted in the First Affiliated Hospital of Guangxi Medical University, which is a tertiary medical center. Medical files of all hospitalized babies aged 0–36 months, who had at least once electrolyte concentration measurement from January 2012 to January 2023, were screened. All the patients with serum sodium concentration <135 mmol/L were included in our study. For those with several values, the lowest value was used for this study.
The exclusion criteria were patients with the following: hemolysis of blood specimen, known hyperglycemia or hyperlipoproteinemia and the impossibility of obtaining essential data from the patient's medical records.
All data regarding the patients' clinical and biological characteristics were collected from their medical records in order to identify the etiology of hyponatremia. The following information was gathered: demographics (age at admission, age at onset of hyponatremia, and sex), personal and previous medical histories (such as incidence of preterm birth, intestinal surgery, congenital malformation, or other disorders), family medical history (e.g., consanguinity and childhood deaths), in-hospital details (e.g., chief complaints and duration of hospitalization), clinical events preceding hyponatremia (e.g., gastrointestinal loss, sepsis, heart failure, urinary tract disorders, and neurological disease), ongoing therapy (e.g., diuretics, intravenous fluid administration, and chemotherapy drugs), biochemical tests (e.g., electrolytes, glucose, routine blood measurements, liver and renal functions), and discharge status. Additionally, imaging test results, hormonal characteristics, and genetic mutation analysis for patients suspected of having endocrine disorders were also included.
Hyponatremia was defined as a serum sodium concentration <135 mmol/L, with mild hyponatremia defined as levels between 130 and 135 mmol/L. Moderate hyponatremia was categorized as a serum sodium concentration between 125 and 129 mmol/L, and levels below 125 mmol/L were classified as severe hyponatremia (7, 12).
Aldosterone signaling abnormalities, including impairment in its biosynthesis (e.g., CAH, PAI and isolated hypoaldosteronism) as well as functional resistance (e.g., pseudo-hypoaldosteronism). Diagnosis of a disease was based on its practical guidelines (13, 14). For rare cases with no guidelines, this mainly depended on the patient's clinical symptoms, biochemical parameters and molecular genetic tests.
Patients with urinary tract infections (UTIs) such as obstructive uropathy (OUP), urinary tract malformations (UTM) and intestinal tract disease (e.g., ileostomy and jejunal membrane-associated complications) who present with hyponatremia, metabolic acidosis as well as hyperkalemia would usually be suspected of having transient pseudo-hypoaldosteronism (PHA).This would subsequently be confirmed by elevated serum levels of aldosterone.
All statistical analyses were performed with SPSS 26.0 software (IBM Corp., Armonk, NY, USA). Normally distributed data with were expressed as means ± standard deviations and were compared using either a two-independent sample t-test or one-way analysis of variance. Data not normally distribution were expressed as medians (with the minimum and maximum ranges) and were compared by using either the Whitney U or Kruskal–Wallis H tests. P-values <0.05 were considered to be statistically significant.
A total of 39,019 patients aged under 36 months were admitted to our hospital from January 2012 to January 2023. Among them, 1,890 patients were found to have electrolyte disturbances, with 829 of these experiencing an episode of hyponatremia. However, 28 patients were later excluded from the study: 27 individuals had highly hemolyzed blood samples, and subsequent biochemical tests conducted in the following days showed normal serum sodium levels. Additionally, one infant was diagnosed with familial hyperlipidemia due to a genetic disorder. Ultimately, 801 patients who presented with an episode of hyponatremia were included in this study.
Among the 801 patients enrolled in the study (515 males and 286 females), the median age at onset of hyponatremia was 2.57 months (range: 0.00–36.00 months). Of these patients, 404 (50.4%), 109 (13.6%), and 288 (36.0%) fell into the age groups of 0–3, 3–12, and 12–36 months, respectively (Table 1). Within 48 h of hospitalization, 215 patients (26.8%) experienced an episode of hyponatremia. The serum sodium concentrations ranged from 94.1 to 134.9 mmol/L, with a median value of 128.8 mmol/L. Among the patients, 320 (39.9%), 273 (34.1%), and 208 (26.0%) were diagnosed with mild, moderate, and severe hyponatremia, respectively. Infants aged 0–3 months constituted the largest proportion in all hyponatremia groups (Figure 1). Additionally, 56 cases (7.0%) presented with hyponatremia accompanied by convulsions, and 118 patients (14.7%) had poor prognoses, including death or discharge for palliative care. Furthermore, two patients had prognoses closely associated with severe hyponatremia.
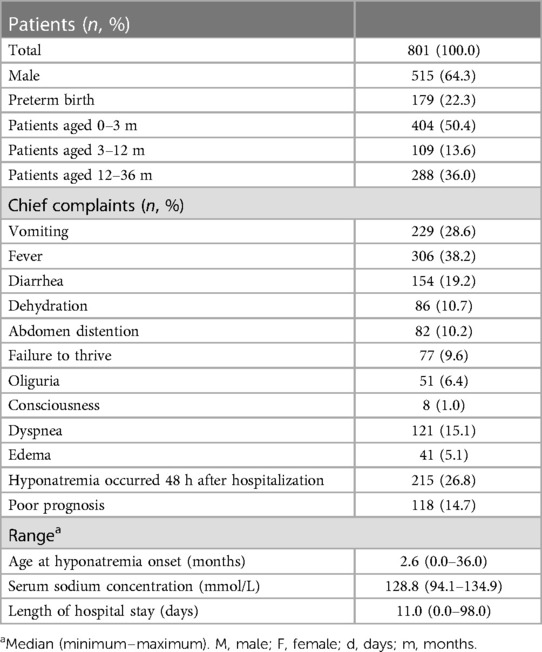
Table 1. The characteristics of the babies aged 0–3 years who presented with an episode of hyponatremia.
Hyponatremia could have resulted from a wide spectrum of conditions in infants and young children including aldosterone signaling abnormalities, sepsis and neurological disorders as well as diseases of the respiratory, hematological, hepatic, urinary, gastrointestinal and cardiovascular systems in our study (Table 2). Multiple systems disorders were also included. In addition, medical interventions including those of iatrogenic origin (e.g., diuretics, chemotherapeutic agents and hypotonic fluid infusion) could also lead to hyponatremia. The commonest three etiologies of hyponatremia in this study were gastrointestinal system disease, sepsis and respiratory system disease which occurred in 284 (35.5%), 93 (11.6%) and 87 patients (10.9%), respectively. Among patients with hyponatremia younger than 3 months of age, the top three causes were gastrointestinal diseases (180/404, 44.6%), respiratory diseases (71/404, 17.6%), and aldosterone signaling abnormalities (39/404, 9.7%). Among patients with severe hyponatremia, gastrointestinal diseases (57/208, 27.4%) and aldosterone signaling abnormalities (36/208, 17.3%) accounted for the largest proportion, respectively.
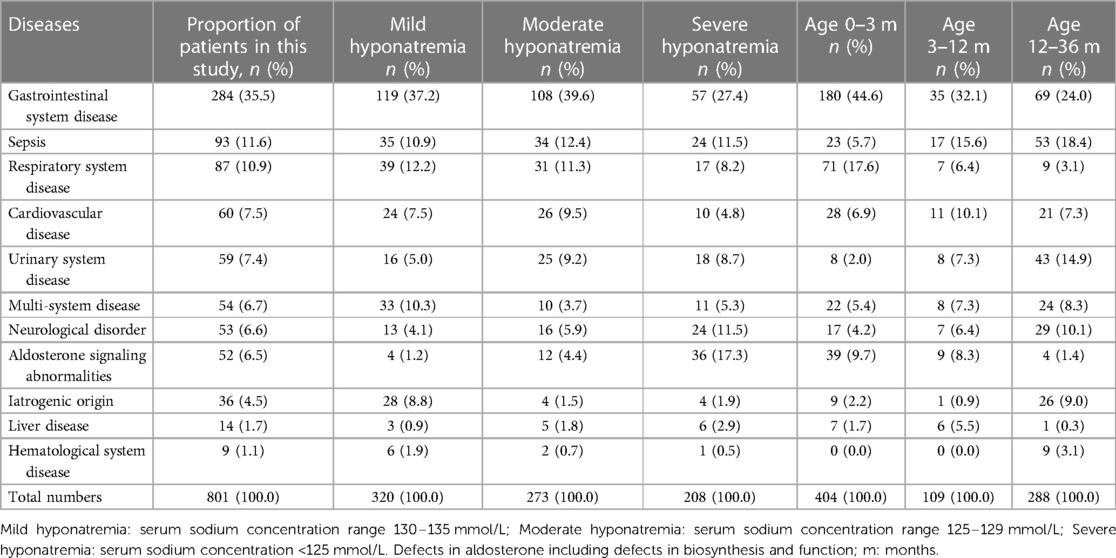
Table 2. Characteristics of hospitalized babies presented with hyponatremia in different etiologies.
Aldosterone signaling abnormalities including biosynthesis defects and functional resistance were identified in 52 (6.5%) patients, of which CAH due to 21 hydroxylase deficiency and PAI occurred in 32 and 4 patients, respectively. Isolated hypoaldosteronism due to novel compound heterozygous mutations of the CYP11B2 gene and PHA type 1 due to a novel heterozygous mutation of NR3C2 gene each occurred in one patient. 13 patients were considered as transient PHA (in 6 infants this was secondary to urinary tract disorders and in 7 patients this due to intestinal tract disorders). Additionally, one young child presented with difficulties in walking as well as a high creatine kinase level and low serum cortisol and hyponatremia was finally confirmed with Xp21 contiguous gene deletion syndrome.
Serum sodium and potassium concentrations were significantly different between the different etiologies of hyponatremia (Figure 2). Patients with aldosterone signaling abnormalities had more severe hyponatremia than those with other diseases except for neurological disorders and liver diseases. Serum potassium concentrations were also significantly higher in patients due to aldosterone signaling abnormalities than in those with all other etiologies (P < 0.05).
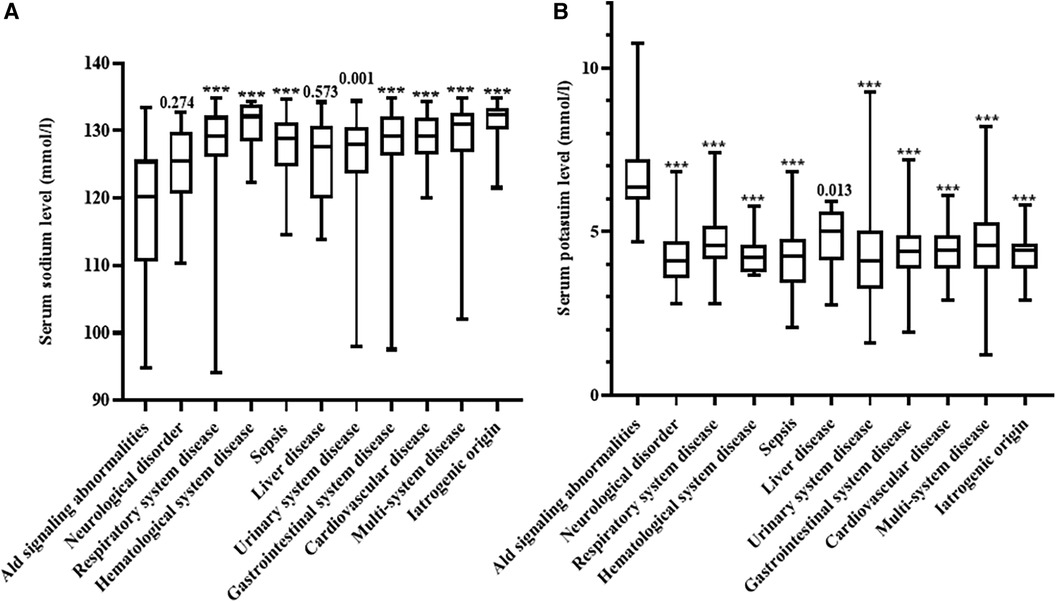
Figure 2. Comparison of serum sodium and potassium concentrations in the different disorders noted in our patients. (A) Comparison of serum sodium in the different disorders noted in our patients; (B) comparison of serum potassium concentrations in the different disorders noted in our patients. Ald, aldosterone; ns, not significant. ***P < 0.001.
Hyponatremia was found to be a relatively common disorder in hospitalized patients. The incidence of hyponatremia in hospitalized babies aged 0–36 months was 2.05% (801/39,019 patients). Wattad et al. (15) conducted a study on hospitalized children over a 12-month period and the results showed that the overall frequency of hyponatremia was 1.38%. Moreover, Storey et al. (16) found that neonatal hyponatremia had a prevalence of 4.3%. Our study is the first study to describe the incidence, etiologies and clinical characteristics of hyponatremia in babies. Hyponatremia was found to be highly heterogeneous and based on causes with numerous different conditions (15–17). In our study, the top three causes of hyponatremia in babies were gastrointestinal system disease, sepsis and respiratory system disease. This was probably due to the incompletely development of the digestive and gastrointestinal systems in infants and young children. Recurrent vomiting and diarrhea due to gastrointestinal system disease can result in hyponatremia and these clinical symptoms were commonly encountered in our cohort. Sepsis can also reduce the circulating blood volume and it may cause baroreceptor activation and ADH release, leading to hyponatremia (6, 18).
Our findings confirmed the findings of some previous studies (19, 20). Hyponatremia usually results from respiratory system diseases including respiratory tract infections, pneumonia and bronchiolitis in children. Additionally, hyponatremia due to an iatrogenic origin is not unusual. Diuretics and hypotonic fluid infusion were the main iatrogenic causes of hyponatremia. However, in our cohort, hyponatremia also occurred in 10 cases with acute lymphoblastic leukemia (ALL) following vincristine therapy. It is reported that vincristine may have some neurotoxic effects which are towards the hypothalamus and pituitary glands, resulting in hyponatremia (21). Similar results were found in several other ALL pediatric cohorts (22, 23). This indicates that intensive monitoring of the serum sodium levels is an essential requirement for children on vincristine therapy and diuretic treatments. Moreover, the use of hypotonic fluid infusion should be carefully assessed in order to prevent iatrogenic hyponatremia.
Aldosterone biosynthesis defects or aldosterone resistance was also relatively common in our cohort. Besides the salt-losing associated with CAH and PAI, we also identified some rare etiologies including isolated hypoaldosteronism, PHA type 1, transient PHA. PHA type 1 and transient PHA are described as mineralocorticoid resistance. These two diseases have similar clinical features including hyponatremia, hyperkalemia acidosis and elevated serum aldosterone during infancy. However, PHA type 1 is a genetic disorder and transient PHA is secondary to disorders of urinary and intestinal tracts and the resultant hyponatremia that arise from these conditions, can be corrected with improvement of the primary disease (5, 24).
To date, more than 130 cases of transient PHA have been reported and these are mainly secondary to urinary tract infections (UTIs) and UTMs during infancy (25–27). However, up to now, only a few cases secondary to UTIs and/or UTMs have been documented in China (28, 29). In this study, we identified six infants in UTIs and/or UTMs presenting with transient PHA and the oldest patient was 6.43 months. The incidence of transient PHA is still unclear. A survey of the Irish population found that transient PHA occurs at a rate of 1 per 13,200 total live births per year (30). However, the authors only included the patients who had disorders of the urinary tract (31–37). Intestinal tract disorders can also result in transient PHA, and we reviewed these cases in Supplementary Table S1. When compared with UTIs and UTMs, transient PHA secondary to intestinal tract disorders can occur at any age, especially in patients with ileostomy. The mechanism of transient PHA is still unclear. Bacterial endotoxins and several cytokines including transforming factor-β1, IL-1 and TNF-α may act on the relatively immature young kidneys and this may be the underlying mechanism in patients with transient PHA secondary to UTIs and UTMs (26, 38). Whereas, the hypothesis in PHA secondary to intestinal is probably very different. A high over output of electrolytes probably results in chronic sodium depletion and hypovolemia leading to severe secondary hyperaldosteronism. This would eventually trigger PHA and cause the down-regulation of mineralocorticoid receptors which may result in the development of secondary PHA due to intestinal tract disorders (31, 34).
More experiments are needed to corroborate these hypotheses. In addition, the four of the six infants with PHA secondary to UTIs in our cohort, showed no fever. This reminds us that pediatricians seeing any infant presenting with unexplained hyponatremia should perform urine test cultures and renal ultrasound.
Our study found that the serum sodium concentrations differed considerably between the different diseases. Hyponatremia due to aldosterone signaling abnormalities, neurological disorders and liver diseases were generally more severe conditions when compared to the other diseases (Figure 2A). In addition, serum potassium concentrations were also significantly different between the different causes of hyponatremia (Figure 2B). These results remind pediatricians should consider aldosterone signaling abnormalities in babies presented with severe hyponatremia and hyperkalemia. Furthermore, we further propose a specific diagnostic flow chart to facilitate the accurate diagnosis of babies who present with hyponatremia where aldosterone signaling abnormalities are suspected (Figure 3).
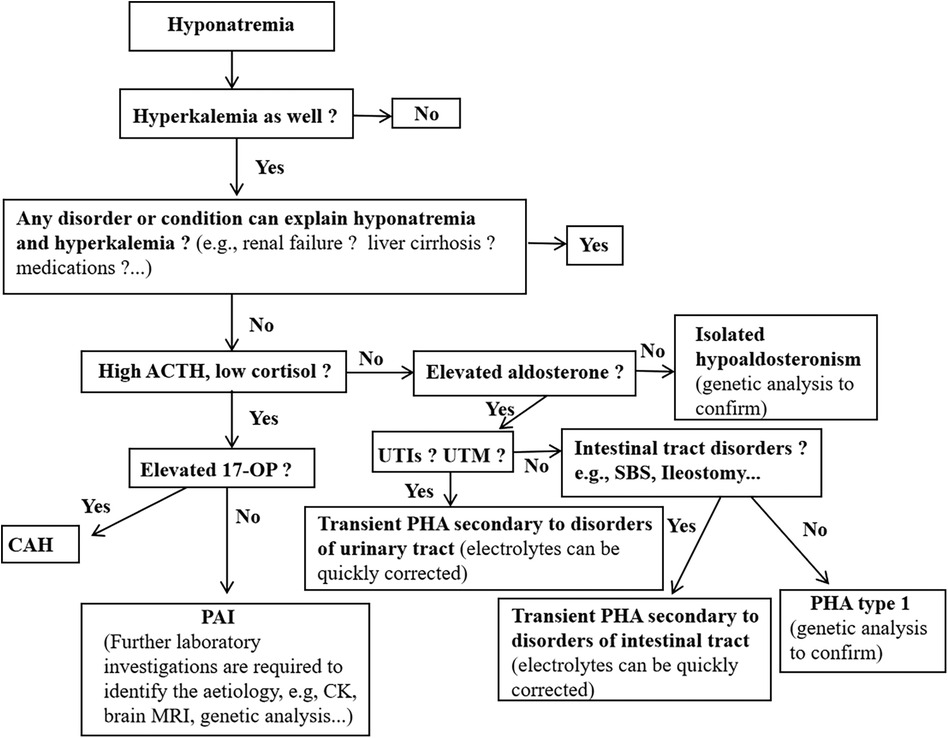
Figure 3. Proposed a brief diagnostic flow chart for inants and young children presenting with hyponatremia who is suspected of aldosterone signaling abnormalities. Ald, aldosterone; CAH, congenital adrenal hyperplasia; PHA, pseudohypoaldosteronism; PAI, primary adrenal insufficiency; 17-OHP, 17-hydroxyprogesterone; CK, creatine kinase; UTIs, urinary tract infections; UTM, urinary tract malformations.
This study described the prevalence, etiologies and clinical characteristics of hyponatremia in hospitalized infants and young children. The findings showed that hyponatremia is a relatively common electrolyte disorder in hospitalized babies and it can result from a wide spectrum of conditions. The top three causes of hyponatremia in babies were found to be gastrointestinal system disease, sepsis and diseases of the respiratory system. Additionally, hyponatremia due to aldosterone signaling abnormalities was not unusual. We further proposed a specific flow chart to help pediatricians to investigate the aldosterone pathway abnormalities in babies with hyponatremia. This study had many limitations due to its retrospective nature. Larger sample sizes as well as prospective and long-term follow-up studies are required to fully understand the mechanisms and the outcomes of hyponatremia in young children.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
XL: Data curation, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft. YX: Data curation, Writing – review & editing. JT: Methodology, Supervision, Writing – review & editing. JZ: Data curation, Writing – review & editing. DL: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
We thank the Scientific research Foundation of the First Affiliated Hospital of Guangxi Medical University (grant no. 2010219) and the Guangxi Clinical Research Center of Pediatric Diseases (grant no: GUI KE AD22035219) for funding this project.
We are very grateful to the patient and families. The authors also thank Dr. Dev Sooranna of Imperial College London for editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1338404/full#supplementary-material
1. Bizzarri C, Pedicelli S, Cappa M, Cianfarani S. Water balance and ‘Salt wasting’ in the first year of life: the role of aldosterone-signaling defects. Horm Res Paediatr. (2016) 86:143–53. doi: 10.1159/000449057
2. Bockenhauer D, Zieg J. Electrolyte disorders. Clin Perinatol. (2014) 41:575–90. doi: 10.1016/j.clp.2014.05.007
3. White PC. Aldosterone synthase deficiency and related disorders. Mol Cell Endocrinol. (2004) 217:81–7. doi: 10.1016/j.mce.2003.10.013
4. Memoli E, Lava SAG, Bianchetti MG, Vianello F, Agostoni C, Milani GP. Prevalence, diagnosis, and management of secondary pseudohypoaldosteronism. Pediatr Nephrol. (2020) 35:713–4. doi: 10.1007/s00467-019-04419-z
5. Furgeson SB, Linas S. Mechanisms of type I and type II pseudohypoaldosteronism. J Am Soc Nephrol. (2010) 21:1842–5. doi: 10.1681/ASN.2010050457
6. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. (2014) 170:G1–G47. doi: 10.1530/EJE-13-1020
7. Thompson CJ. Hyponatraemia: new associations and new treatments. Eur J Endocrinol. (2010) 162(Suppl 1):S1–3. doi: 10.1530/EJE-10-0374
8. Luu R, DeWitt PE, Reiter PD, Dobyns EL, Kaufman J. Hyponatremia in children with bronchiolitis admitted to the pediatric intensive care unit is associated with worse outcomes. J Pediatr. (2013) 163:1652–1656.e1. doi: 10.1016/j.jpeds.2013.06.041
9. Don M, Valerio G, Korppi M, Canciani M. Hyponatremia in pediatric community-acquired pneumonia. Pediatr Nephrol. (2008) 23:2247–53. doi: 10.1007/s00467-008-0910-2
10. Layton JA, Le Jeune IR, Hall IP. Severe hyponatraemia in medical in-patients: aetiology, assessment and outcome. QJM. (2006) 99:505–11. doi: 10.1093/qjmed/hcl071
11. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. (2009) 122:857–65. doi: 10.1016/j.amjmed.2009.01.027
12. Mazzolai M, Apicella A, Marzuillo P, Rabach I, Taddio A, Barbi E, et al. Severe hyponatremia in children: a review of the literature through instructive cases. Minerva Pediatr (Torino). (2022) 74:616–9. doi: 10.23736/S2724-5276.21.05856-4
13. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. (2017) 390:2194–210. doi: 10.1016/S0140-6736(17)31431-9
14. Kirkgoz T, Guran T. Primary adrenal insufficiency in children: diagnosis and management. Best Pract Res Clin Endocrinol Metab. (2018) 32:397–424. doi: 10.1016/j.beem.2018.05.010
15. Wattad A, Chiang ML, Hill LL. Hyponatremia in hospitalized children. Clin Pediatr (Phila). (1992) 31:153–7. doi: 10.1177/000992289203100305
16. Storey C, Dauger S, Deschenes G, Heneau A, Baud O, Carel JC, et al. Hyponatremia in children under 100 days old: incidence and etiologies. Eur J Pediatr. (2019) 178:1353–61. doi: 10.1007/s00431-019-03406-8
17. Elliman MG, Vongxay O, Soumphonphakdy B, Gray A. Hyponatraemia in a Lao paediatric intensive care unit: prevalence, associations and intravenous fluid use. J Paediatr Child Health. (2019) 17(55):695–700. doi: 10.1111/jpc.14278
19. Lavagno C, Milani GP, Uestuener P, Simonetti GD, Casaulta C, Bianchetti MG, et al. Hyponatremia in children with acute respiratory infections: a reappraisal. Pediatr Pulmonol. (2017) 52:962–7. doi: 10.1002/ppul.23671
20. Park SW, Shin SM, Jeong M, Cho DH, Lee KH, Eisenhut M, et al. Hyponatremia in children with respiratory infections: a cross-sectional analysis of a cohort of 3938 patients. Sci Rep. (2018) 8:16494. doi: 10.1038/s41598-018-34703-1
21. Berghmans T. Hyponatremia related to medical anticancer treatment. Support Care Cancer. (1996) 4:41–350. doi: 10.1007/BF01788840
22. Salvador C, Salvador R, Willeit P, Kuntner C, Haid A, Müller T, et al. Hyponatremia during induction therapy in distinct pediatric oncological cohorts: a retrospective study. Front Oncol. (2021) 11:708875. doi: 10.3389/fonc.2021.708875
23. Janczar S, Zalewska-Szewczyk B, Mlynarski W. Severe hyponatremia in a single-center series of 84 homogenously treated children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. (2017) 39:e54–8. doi: 10.1097/MPH.0000000000000758
25. Watanabe T. Reversible secondary pseudohypoaldosteronism. Pediatr Nephrol. (2003) 18:486. doi: 10.1007/s00467-003-1104-6
26. Bogdanović R, Stajić N, Putnik J, Paripović A. Transient type 1 pseudo-hypoaldosteronism: report on an eight-patient series and literature review. Pediatr Nephrol. (2009) 24:2167–75. doi: 10.1007/s00467-009-1285-8
27. Delforge X, Kongolo G, Cauliez A, Braun K, Haraux E, Buisson P. Transient pseudohypoaldosteronism: a potentially severe condition affecting infants with urinary tract malformation. J Pediatr Urol. (2019) 15:265.e1–e7. doi: 10.1016/j.jpurol.2019.03.002
28. Tuoheti Y, Zheng Y, Lu Y, Li M, Jin Y. Transient pseudohypoaldosteronism in infancy mainly manifested as poor appetite and vomiting: two case reports and review of the literature. Front Pediatr. (2022) 10:895647. doi: 10.3389/fped.2022.895647
29. Watanabe T, Yamazaki A. Pneumothorax and transient pseudohypoaldosteronism in an infant with hydronephrosis. Pediatr Nephrol. (2003) 18:62–4. doi: 10.1007/s00467-002-1005-0
30. Kaninde A, Grace ML, Joyce C, Taylor NF, Ghataore L, Riordan MF, et al. The incidence of transient infantile pseudohypoaldosteronism in Ireland: a prospective study. Acta Paediatr. (2021) 110:1257–63. doi: 10.1111/apa.15688
31. Nakasone R, Fujioka K, Nishida K, Nozu K, Iijima K. Three Cases of pseudohypoaldosteronism following ileostomy in preterm infants. Pediatr Neonatol. (2020) 62:119–21. doi: 10.1016/j.pedneo.2020.09.006
32. Ou CY, Chen YJ, Lin GB, Chen MF, Chia ST. Case Report: newborns with pseudohypoaldosteronism secondary to excessive gastrointestinal losses through high output stoma. Front Pediatr. (2021) 9:773246. doi: 10.3389/fped.2021.773246
33. Nissen M, Dettmer P, Thränhardt R, Winter K, Niemeyer T, Tröbs RB. Congenital jejunal membrane causing transient pseudohypoaldosteronism and hypoprothrombinemia in a 7-week-old infant. Klin Padiatr. (2017) 229:302–3. doi: 10.1055/s-0043-113570
34. Vantyghem MC, Hober C, Evrard A, Ghulam A, Lescut D, Racadot A, et al. Transient pseudo-hypoaldosteronism following resection of the ileum: normal level of lymphocytic aldosterone receptors outside the acute phase. J Endocrinol Invest. (1999) 22:122–7. doi: 10.1007/BF03350891
35. Sugawara M, Lebron BA, Calabria R. Pseudohypoaldosteronism following resection of ileum and colon. Nephron. (1989) 51:567–8. doi: 10.1159/000185403
36. Niyazov D, Shawa H. A case of postileostomy hypovolemia presenting as pseudohypoaldosteronism with complete resolution after ostomy reversal. AACE Clin Case Rep. (2017) 3:e5–7. doi: 10.4158/EP151011.CR
37. Alassaf A, Abdelghani T, Jabour HAA, Odeh R, Badran E. Pseudohypoaldosteronism secondary to high output ileostomy: a unique report in an infant. Pediatr Ther. (2015) 5:1–53. doi: 10.4172/2161-0665.1000224
Keywords: hyponatremia, aldosterone, pseudo-hypoaldosteronism, mineralocorticoid receptor, babies
Citation: Liu X, Xie Y, Tang J, Zhong J and Lan D (2024) Hyponatremia in babies: a 11-year single-center study. Front. Pediatr. 12:1338404. doi: 10.3389/fped.2024.1338404
Received: 14 November 2023; Accepted: 15 May 2024;
Published: 6 June 2024.
Edited by:
Eli Hershkovitz, Soroka Medical Center, IsraelReviewed by:
Orkun Tolunay, University of Health Sciences, Türkiye© 2024 Liu, Xie, Tang, Zhong and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Lan, bGFuZGFuX2xkQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.